Abstract
Background
Rapid-acting analog insulin is used increasingly for continuous subcutaneous insulin infusion therapy (CSII). As the choice of insulin may be a determinant of catheter occlusion, we compared rates of early and late occlusion of a standard CSII catheter with three insulin analogs in a laboratory-based setting.
Methods
Twenty-four pumps were used for the study. Each insulin analog (glulisine, lispro, and aspart) was assigned to eight pumps in a randomized order for each of nine runs of 5-day duration. Pumps were primed to receive a basal dose of 0.1 IU/h with a bolus dose of 2 IU given three times each day. Pumps were placed in an incubator to maintain temperature in the range of 32 to 36 °C.
Results
Over the entire study period, there were 48 occlusions. Early occlusions (within 72 hours) occurred during five of the nine runs with no evidence of any difference between insulins (p = .27); there were no occlusions before 48 hours. Over the whole of the 5-day infusion period, the probabilities of overall occlusion for each insulin were 40.9% [28 to 55%, 95% confidence interval (CI)] for glulisine, 9.2% (4 to 19.5%, 95% CI) for aspart, and 15.7% (8.1 to 28.1%, 95% CI) for lispro. All occlusions, except for three, occurred during a bolus infusion.
Conclusions
During CSII under laboratory conditions, early catheter occlusions (within 72 hours) are rare and independent of the choice of insulin analog. For patients using insulin pump therapy, the importance of catheter change within 72 hours should be emphasized irrespective of the insulin used. Beyond 72 hours, the risk of occlusion differs between insulins, being more common with glulisine.
Keywords: analogs, CSII, occlusions, insulin, temperature
Introduction
For patients with type 1 diabetes, continuous sub-cutaneous insulin infusion (CSII) may be an alternative form of insulin delivery compared to multiple daily injections.1 Nowadays, the insulin of choice for CSII appears to be a rapid-acting analog preparation, although this remains controversial.2
During the education of patients starting CSII, patients are encouraged to change the infusion catheters every 2–3 days, although anecdotally, some individuals change less often to reduce wastage.3 The type of insulin used in CSII has been reported to be one factor related to the risk of catheter occlusion and subsequent unstable glycemic control.4 The aim of this study was to compare rates of occlusion of a standard CSII catheter occurring with each of three insulin analogs, in a nonclinical, laboratory setting using a randomized, controlled trial design.
Methods
Pilot study data suggested that occlusion rates were potentially higher with low dose bolus and infusion rates, and venting of the cannulae into air rather than water. In order to maximize the chance of occlusion occurring, and hence to enable any differences between insulins to be detected, the combination of low dose insulin and venting into air was chosen for this study. All studies were performed using ACCU-CHEK® Rapid-D Link infusion sets (Roche, Burgdorf, Switzerland) with a length of 100 cm.
Pumps were placed on two trays with a flask to collect effluent and placed in an incubator with a temperature in the range of 32 to 36 °C. This was chosen to mimic the pumps' normal working environment: worn under clothing close to the body. Humidity was not controlled. Occlusions were determined by the pump alarms.
The three insulins for comparison were lispro (Humalog©, Eli Lilly, Basingstoke UK) aspart (NovoRapid©, Novo Nordisk, Crawley, UK), and glulisine (Apidra©, sanofi-aventis, Guildford, UK). All insulins were within their expiration dates. The study ran over nine separate periods of 5 days. At the start of the first day of each period, cannulae were primed with insulin to deliver a basal dose of 0.1 IU/h using identical CSII devices (ACCU-CHEK®Spirit, Roche, Burgdorf, Switzerland). A bolus dose of 2 IU was given three times a day. This would represent the low dose infusions required in pediatric patients and individuals with marked insulin sensitivity.
The pumps were primed and started at similar times and left running for 5 days, unless an occlusion developed. With complete occlusion, the pump alarmed and the time was recorded automatically. Twenty-four pumps were used in the study (all ACCU-CHEK Spirit pumps). For every run, each insulin was assigned randomly to eight pumps, and each pump used insulin on three occasions over the course of the study. The allocation of insulin to pumps was randomized subject to these constraints. The infusion periods were observed by a single observer who was blind to the insulin allocation. Pumps were checked every 2–4 hours during the day and first thing each morning.
Statistical Methods
The study was designed to detect a difference in the occlusion rates at 48 hours, based on data from previous pilot studies. However, since there were no occlusions seen in this study before 48 hours, the incidence of occlusion at 72 hours was investigated. Each insulin was labelled A, B, or C for the purposes of statistical analyses with the code broken only after completion.
For each insulin, 72 runs were completed (comprising eight pumps for each insulin on nine separate run sessions). This was planned to give 80 to 90% power of detecting a difference in occlusion rates at 48 hours, of 2.5% to 5% for glulisine compared to 20% for lispro, based on experience during pilot studies. The incidence of occlusion and time to occlusion were compared for the three insulins.
The incidence of occlusion, both at 72 hours and overall (i.e., occlusion occurring at any time during the 5-day study period), was compared between the three insulins using logistic regression. The run session and the insulin were fitted into the model. The odds of occlusion were calculated for each of glulisine and aspart relative to lispro, and for glulisine relative to aspart, with 95% confidence intervals (CI). Since the incidence of occlusion within 72 hours was low, exact methods were also used to derive estimates of treatment differences.
For the analysis of time to occlusion, the time until complete occlusion occurred was calculated as the time from when the prime infusion was set until the alarm indicating occlusion went off. If occlusion did not occur, the end time was taken as the time of switching off the pump on the fifth day, and the data were considered censored at that point. Data were analyzed using survival analysis methods to compare the time until occlusion occurred. The Gehan-Wilcoxon test was used for the comparison, which places more emphasis on the early part of the study, as the study was designed to detect differences in rates of early occlusion.
For the purposes of this analysis, each pump on each run was taken to be a separate entity, thus its performance in one run of the study was assumed not to affect subsequent runs. The analysis was repeated using the number of boluses that had been given when occlusion occurred as the time variable instead of the number of hours. Where occlusion occurred during a bolus, that bolus was counted as having been given.
Results
During the third run, the incubator temperature was raised from 34 °C to 40 °C in error on the second day. It was returned to normal levels on the fourth day.
Incidence of Occlusion
There were no occlusions during the eighth run of the study, and 18 on the third run (when the temperature was raised accidentally). Over the entire study period, comprising nine 5-day periods, there were 48 occlusions: 9 (12.5%) for aspart, 13 (18%) for lispro, and 26 (36%) for glulisine.
No occlusions occurred within 48 hours for any insulin. The upper 95% confidence limit for the estimate of occlusion within 48 hours, given that none occurred, was 5%. Within 72 hours, occlusions occurred during five of the nine runs. Overall there were five early occlusions with lispro, three with glulisine, and one with aspart. There was no difference between insulins (p = .27). The estimates of the odds ratios for each comparison are given in Table 1. The confidence intervals are wide due to the small number of occlusions.
Table 1.
Estimated Odds Ratios for Early Occlusion(within 72 hours)
| Insulin | Estimated odds ratio | 95% CI for odds ratio | p value |
|---|---|---|---|
| Glulisine:aspart | 3.06 | 0.24 to 165.7 | .62 |
| Lispro:glulisine | 1.72 | 0.30 to 11.7 | .72 |
| Lispro:aspart | 5.32 | 0.57 to 259.7 | .21 |
Estimates of the probabilities of early occlusion for each insulin, with 95% confidence intervals are given in Table 2.
Table 2.
Estimated Probability of Early Occlusion(within 72 hours)
| Insulin | Estimated probability | 95% CI for probability | |
|---|---|---|---|
| Glulisine | 6.9% | 2.5 to 17.5 | |
| Aspart | 2.3% | 0.0 to 12.3 | |
| Lispro | 11.6% | 4.3 to 27.6 |
Over 5 days, there were differences between insulins in terms of the risk of occlusion (p = .0005) and between runs (p <.0001). Omitting the third run, in which the occlusion rate was exceptionally high (5 of 8 for aspart, 6 of 8 for lispro, and 7 of 8 for glulisine), also yielded significance levels of p = .0009 and p = .29 for insulin and run, respectively.
For each insulin, the odds of occlusion relative to the others are shown in Table 3 with and without the third run.
Table 3.
Estimated Odds Ratios for Overall Occlusion
| Analysis | Insulin | Estimated odds ratio | 95% CI for odds ratio | p value |
|---|---|---|---|---|
| All runs | Glulisine:aspart | 6.84 | 2.39 to 19.58 | .0003 |
| Glulisine:lispro | 3.72 | 1.47 to 9.45 | .006 | |
| Lispro:aspart | 1.84 | 0.61 to 5.51 | .28 | |
| Omitting third run | Glulisine:aspart | 7.39 | 2.25 to 24.24 | .001 |
| Glulisine:lispro | 3.90 | 1.44 to 10.59 | .008 | |
| Lispro:aspart | 1.89 | 0.51 to 7.14 | .34 |
The estimates of the probabilities of overall occlusion for each insulin over the 5 days, with 95% confidence intervals, are given in Table 4. Estimates are given from the analysis omitting the eighth run (inclusion of this run led to problems with estimating standard errors). The confidence intervals suggest that the chance of occlusion is greater than zero for all the insulins. The estimates from the analysis excluding the third run led to similar conclusions, although all the occlusion probabilities were slightly lower.
Table 4.
Estimated Probability of Occlusion at Any Time
| Analysis | Insulin | Estimated probability | 95% CI for probability |
|---|---|---|---|
| Omitting eighth run | Glulisine | 40.9% | 28.0 to 55.0 |
| Aspart | 9.2% | 4.0 to 19.5 | |
| Lispro | 15.7% | 8.1 to 28.1 |
Time to Complete Occlusion
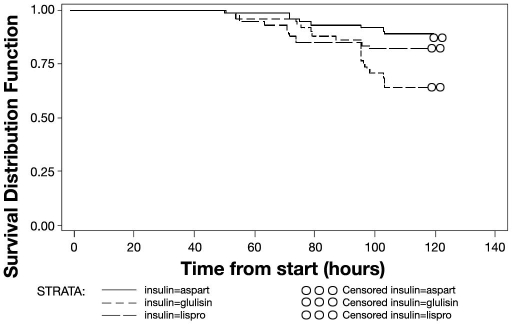
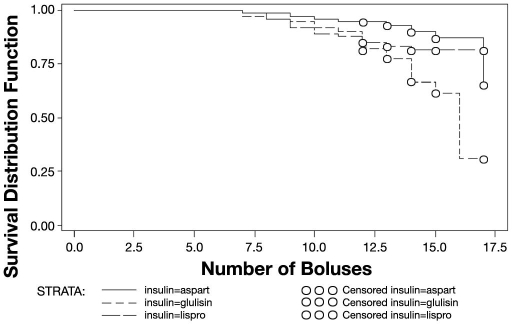
There was a statistically significant difference between insulins in terms of the time to occlusion (Gehan-Wilcoxon test, p = .005). No occlusion occurred before 48 hours, the earliest occurring after 51 hours (seven boluses). Median times to occlusion could not be estimated since the occlusion rate was below 50%. A plot of the hazard rates (i.e., probability of occlusion at a particular time) indicates that the risk of occlusion was greatest just after 72 hours. Repeating the analysis using the number of boluses before occlusion instead of time, yielded similar results (Gehan-Wilcoxon test, p = .006). The plots in Figures 1 and 2 show how the estimated probability of no occlusion occurring changes with time (Figure 1) or number of boluses (Figure 2).
Figure 1.
Estimate of probability of having no occlusion related to time (hours).
Figure 2.
Estimate of probability of having no occlusion related to number of boluses
All occlusions during the third run occurred after the temperature had been reduced back down to 34 °C, apart from two with lispro that occurred after 55 hours. All occlusions, except for three, occurred during a bolus infusion. Of these three, one was on aspart after 51.5 hours (7 boluses), one on lispro after 64.4 hours (8 boluses) and one with glulisine after 88.2 hours (13 boluses).
Discussion
Worldwide, the number of patients with type 1 diabetes using insulin pump therapy is increasing, mostly as a result of the perceived benefits of reduced rates of severe hypoglycemia, return of hypoglycemic warning symptoms, modest reductions in glycated hemoglobin levels, and a general improvement in quality of life with this method of insulin delivery.1 Compared to the early years of pump therapy, technical failures including unexpected episodes of diabetic ketoacidosis are nowadays rare.5 Previously, small clinical studies and case reports have suggested that the type of insulin used during CSII may impact on the achieved standard of glycemic control. Anecdotally, most large pump centres use analog insulin for CSII. Although one short-term comparison of aspart, lispro, and buffered regular insulin failed to show differences in occlusion rates,6 case reports have been published suggesting greater risk of occlusion with lispro.7 More recently, in patients with well-controlled type 1 diabetes with tubing changed every 2 days, catheter occlusion rates were not different with either insulin glulisine or aspart.8 In that study, the frequency of catheter changes was similar (14.1 changes per month for glulisine, and 14.8 per month for aspart). Previous in vitro studies have also examined the stability of lispro and aspart in infusion pumps exposed to a higher temperature (37 °C).9,10 In those studies, potency and stability of insulin did not change significantly over 7 days, but importantly, both used continuous mechanical agitation of the pumps as part of the protocol. In our study, the pumps were not in continuous motion which is more in keeping with the clinical situation, for example, during sleep. Furthermore, there are no instructions from the manufacturers to suggest that motion is a factor in ensuring optimum pump performance.
Whatever insulin is used, advice from pump manufacturers is that the catheters should be changed every 2–3 days to avoid unexpected occlusions as a consequence of insulin crystallization. We found that under laboratory conditions, the risk of early occlusion i.e., within 72 hours, was very small. There was no difference between insulins in the incidence of early (before 72 hours) occlusion, although the observed incidence for the three insulins was low (less than 10% in every case) and the study was not powerful enough to detect such small differences. The study was designed assuming a 48-hour occlusion rate of approximately 5% for glulisine and 20% for lispro (derived from pilot studies). Although the 48-hour rate for glulisine was consistent with that assumed (the upper confidence limit was 5%), the rate for lispro was considerably lower than the assumed rate. This affected the power of the study to detect differences in early occlusions. The confidence intervals suggested that at 72 hours, the occlusion rates tended to be higher for lispro than for glulisine, and higher for glulisine than for aspart, although the study was not sufficiently powerful for these differences to be statistically significant. However, when considering the entire 5-day study period, the odds of occlusion occurring with glulisine were approximately seven times higher than for aspart, and around four times higher than with lispro. These findings are consistent with the statistically significant difference between the insulins in the time to occlusion, whether defined in terms of number of hours or number of boluses.
The markedly increased rate of occlusions during the third run was probably related to a temporary rise in temperature in the incubator in which the pumps were contained. It has been recognized for many years that formation of aggregates of insulin molecules (fibrillation) is more likely with increments in temperature as well as being influenced by insulin species, purity, and concentration.11 We found that most occlusions occurred during a bolus infusion. It may be that if aggregates form, they are associated loosely with the interior wall of the lumen, and the bolus may slough them off to cause the occlusion.
There are a number of limitations to our study. Firstly, the low-dose basal infusion rates and two-unit boluses would normally be used in a very small number of patients, mostly in the pediatric population. We do not know if alternative infusion and bolus rates influence the potential for occlusion. Secondly, unlike the clinical situation, our pumps were left stationary for considerable lengths of time and at a constant temperature. Nevertheless, there is no evidence that insulin pumps require constant movement to perform efficiently, and the temperature used would be similar to that achieved if the pump is worn next to skin under garments. Thirdly, we were infusing into air without resistance. In the clinical situation, resistance at the subcutaneous level may be important, but this will only be resolved with clinical studies.
Conclusions
Results from this laboratory-based study suggest that very early catheter occlusions during continuous subcutaneous insulin infusion (within 48 hours) are extremely rare. Subsequently, occlusions do occur and are mainly during boluses, although the risk of occlusion may also be affected by temperature. Late occlusions appear to be more common with glulisine than with aspart or lispro insulin, although given the limitations expressed above, care needs to be exercised in the interpretation of this observation. For patients using insulin pump therapy, the importance of catheter change within 72 hours should be emphasised irrespective of the insulin used.
Abbreviations
- CI
confidence interval
- CSII
continuous subcutaneous insulin infusion therapy
References
- 1.NICE Technology Appraisal Guidance No. 57: Guidance on the use of continuous subcutaneous insulin infusion for diabetes. National Institute for Clinical Excellence. 2003 Feb [Google Scholar]
- 2.Plank J, Siebenhofer A, Berghold A, Jeitler K, Horvath K, Mrak P, Pieber TR. Systematic review and meta-analysis of short-acting insulin analogues in patients with diabetes mellitus. Arch Intern Med. 2005;165(12):1337–1344. doi: 10.1001/archinte.165.12.1337. [DOI] [PubMed] [Google Scholar]
- 3.Medtronic Diabetes UK [homepage on the Internet] Watford UK: Medtronic MiniMed, Inc.; 2008. [cited 2007 September 30]. Effective infusion site management. Available from: http://www.medtronic-diabetes.co.uk/pdf/MedtronicUK/ [Google Scholar]
- 4.Guilhem I, Leguerrier AM, Lecordier F, Poirier JY, Maugendre D. Technical risks with subcutaneous insulin infusion. Diabetes Metab. 2006;32(3):279–284. doi: 10.1016/s1262-3636(07)70281-1. [DOI] [PubMed] [Google Scholar]
- 5.Weissberg-Benchell J, Antisdel-Lomaglio J, Seshadri R. Insulin pump therapy: a meta-analysis. Diabetes Care. 2003;26(4):1079–1087. doi: 10.2337/diacare.26.4.1079. [DOI] [PubMed] [Google Scholar]
- 6.Bode B, Weinstein R, Bell D, McGill J, Nadeau D, Raskin P, Davidson J, Henry R, Huang W, Reinhardt RR. Comparison of insulin aspart with buffered regular insulin and insulin lispro in continuous subcutaneous insulin infusion: a randomized study in type 1 diabetes. Diabetes Care. 2002;25(3):439–444. doi: 10.2337/diacare.25.3.439. [DOI] [PubMed] [Google Scholar]
- 7.Wolpert HA, Faradji RN, Bonner-Weir S, Lipes MA. Metabolic decompensation in pump users due to lispro insulin precipitation. BMJ. 2002;324(7348):1253. doi: 10.1136/bmj.324.7348.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoogma RP, Schumicki D. Safety of insulin glulisine when given by continuous subcutaneous infusion using an external pump in patients with type 1 diabetes. Horm Metab Res. 2006;38(6):429–433. doi: 10.1055/s-2006-944549. [DOI] [PubMed] [Google Scholar]
- 9.DeFelippis MR, Bell MA, Heyob JA, Storms SM. In vitro stability of insulin lispro in continuous subcutaneous insulin infusion. Diabetes Technol Ther. 2006;8(3):358–368. doi: 10.1089/dia.2006.8.358. [DOI] [PubMed] [Google Scholar]
- 10.Senstius J, Harboe E, Westermann H. In vitro stability of insulin aspart in simulated continuous subcutaneous insulin infusion using a MiniMed 508 insulin pump. Diabetes Technol Ther. 2007;9(1):75–79. doi: 10.1089/dia.2006.0041. [DOI] [PubMed] [Google Scholar]
- 11.Brange J, Andersen L, Laursen ED, Meyn G, Rasmussen E. Toward understanding insulin fibrillation. J Pharma Sci. 1997;86(5):517–525. doi: 10.1021/js960297s. [DOI] [PubMed] [Google Scholar]