Abstract
Background
This proof of concept study was designed to evaluate the safety and effectiveness of a computerized insulin program, the Clarian GlucoStabilizer™ Subcutaneous Insulin Program (CGS-SQ). This paper discusses the CGS-SQ's impact on the glycemic control of hospitalized patients with hyperglycemia.
Methods
Patients at Methodist and Indiana University Hospitals requiring subcutaneous insulin were treated using the CGS-SQ. This program calculates subcutaneous bolus insulin doses based on the current blood glucose (BG), using an insulin sensitivity factor, the number of grams of carbohydrates eaten, and an insulin-to-carbohydrate ratio, with a goal of maintaining the patient's BG in a prespecified target range. The target range, insulin sensitivity factor, and insulin-to-carbohydrate ratio are established by the physician.
Results
From April 2006 to September 2007, the CGS-SQ treated 1772 patients at Methodist and Indiana University Hospitals, with 46,575 BGs in its database. For these patients, the average BG was 158.3 mg/dl, 40.5% percent of BGs were in the default target range of 100–150 mg/dl, and 69.8% were in the wider range of 70–180 mg/dl. The hypoglycemia (BG <40 mg/dl) rate was 0.18%.
Conclusions
The CGS-SQ provided a means to deliver insulin in a standardized manner, resulting in satisfactory BG control with a low hypoglycemia rate, thus serving as a tool for safe and effective insulin therapy for hospitalized patients.
Keywords: computers, diabetes, dosing, hyperglycemia, insulin, subcutaneous, protocol
Introduction
In June of 2003, a glycemic control strategy, the Systematic Utilization of Glucose Assessment and Response (SUGAR™) program, was introduced at two Clarian Health hospitals in Indianapolis: (1) Methodist Hospital (Methodist), a 763-bed semiprivate hospital, where the physicians are private practice doctors and Indiana University faculty and residents; and (2) Indiana University Hospital (University), a 317-bed academic medical center, where the attending physicians are all Indiana University faculty and residents. This program, a collaborative effort of Indiana University and Clarian Health, was developed in response to the growing body of evidence linking glycemic control to reduced morbidity and mortality in critically ill patents.1–8
One of the key components of SUGAR was the development of standardized tools to facilitate the safe administration of insulin. As a first step, new paper protocols were created for both intravenous (IV) and subcutaneous insulin dosing. However, the manual calculation of insulin doses was seen by many as burdensome and potentially error-prone. There was also concern that more aggressive insulin dosing would lead to increased hypoglycemia. To address these concerns, a computerized IV insulin dosing program, the Clarian GlucoStabilizer™ (CGS-IV) program, was introduced in late 2004. With the demonstration of the CGS-IV's safety and effectiveness in the pilot ICUs, the CGS-IV was rolled out to all ICUs at Methodist and University during 2005. The CGS-IV is described by Juneja et al.9
Despite the success of the CGS-IV, it was realized that a computerized subcutaneous insulin dosing program was also needed. Glycemic control on the general medical/surgical units presented numerous challenges. Although a standardized subcutaneous insulin order set (protocol) was available, it was not being used regularly. In many cases, sliding scale insulin, confusing physician insulin orders, and inconsistent nursing execution of these orders led to medical errors. Even when insulin was ordered correctly, incorrect administration would occur, since BG testing was performed at prespecified clock times rather than at mealtimes. In other words, insulin administration was not always well synchronized with meals. At Clarian Health, mealtimes are highly variable, since patients can eat whenever they choose; in some cases, insulin doses were given after meals, even when patients were too sick to eat predictably. In other cases, insulin doses might have been omitted altogether when the patient's BG level was “normal.” To correct these shortcomings and to standardize treatment, a computerized subcutaneous insulin program, the Clarian GlucoStabilizer™ Subcutaneous Insulin Program (CGS-SQ) was developed. It was introduced at University Hospital and subsequently rolled out in stages throughout both University and Methodist Hospitals. Naked sliding scale insulin protocols, that is, rapid-acting insulin without basal insulin coverage, and mixed insulins, long the standard means of insulin treatment, were omitted purposely from the program. The CGS-SQ would be the next piece needed to automate insulin dosing across all hospital units. This paper describes our initial experience with the CGS-SQ.
Subjects and Methods
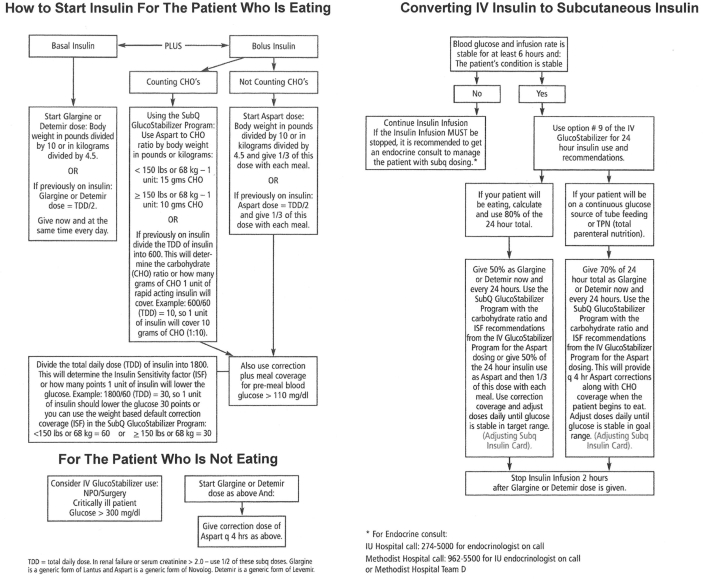
The CGS-SQ is initiated by physician order set (Figure 1). The use of this protocol is currently optional and under physician discretion. The CGS-SQ can be used to manage all aspects of subcutaneous insulin dosing—transition from the CGS-IV to the CGS-SQ, basal insulin, prandial insulin, correction insulin, insulin dose adjustment, and recovery from hypoglycemia. It is encouraged that oral antidiabetic drugs are discontinued at admission, as these medications are usually inappropriate in the acute care setting.
Figure 1.
Adult SubQ Insulin physician order set used at Methodist and University Hospitals.
Patient treatment under the CGS-SQ is stopped if a patient transitions from subcutaneous insulin to IV insulin (for example, as the patient's condition worsens) and may be started again on resumption of subcutaneous insulin (as the patient's condition improves). Each initiation of a CGS-SQ treatment is the beginning of a run.
Transition from IV Insulin to Subcutaneous Insulin
Patients are transitioned from IV insulin to subcutaneous insulin only if their condition is stable and their BG levels and infusion rate have been stable for at least 6 hours. The CGS-IV provides a drip weaning report to assist in the transition. This report calculates a total daily dose (TDD) by extrapolating to a 24-hour period the amount of insulin used in the immediately preceding hours for which the insulin drip has been stable. It then recommends an insulin sensitivity factor (ISF) and an insulin-to-carbohydrate ratio (ICR) to enter on the CGS-SQ. The patient is given basal insulin, and IV insulin is discontinued 2 hours later. The procedures for transitioning from IV insulin to subcutaneous insulin are included in the eight-page pocket guide for adult inpatient glucose management (Figure 2).
Figure 2.
Pocket guide for adult inpatient glucose management.
Basal Insulin Dosing
The CGS-SQ does not calculate basal insulin doses. However, all patients treated with the CGS-SQ are on basal insulin, either glargine, detemir, or neutral protamine Hagedorn, unless the physician decides that basal needs are being met by regular insulin in total parenteral nutrition (TPN). The initial basal insulin dosage and any subsequent dosage changes are under physician control. Both the order set (Figure 1) and the pocket guide (Figure 2) give recommendations for initial and subsequent dosing.
Prandial Insulin Dosing
For prandial insulin dosing, the CGS-SQ calculates a dose of rapid-acting insulin, based on the current BG and the midpoint of the target range (MidPoint), using an ISF, the total number of grams of carbohydrate (CHO) consumed in the meal or bolus tube feeding, and an ICR. The ISF is the estimated reduction in blood glucose (mg/dl) that would occur with one unit of rapid-acting insulin. The ICR is the estimated number of grams of carbohydrate covered by one unit of rapid-acting insulin. The CGS-SQ provides default values for the target range (100–150 mg/dl) and weight-based defaults for the ISF and ICR. The ISF defaults to 30 for patients ≥150 pounds (68 kg) and 60 for patients <150 pounds. The ICR defaults to 10 for patients ≥150 pounds and 15 for patients <150 pounds. On the order set, these defaults can be accepted, or the target range, ISF, and/or ICR can be specified by the physician for entry into the program. For the insulin-naïve patient, the weight-based values for ISF and ICR may be used. When the weight-based default for ISF is inappropriate because of extremes in body mass index, the physician may adjust the default value. For those previously on insulin at home, a more appropriate ISF can be determined by dividing the patient's previous TDD into 1800; ICR can be determined by dividing the patient's previous TDD into 600. After initial entry, values for ISF and ICR can be changed by the physician, based on patient response. The order set and the pocket guide include recommendations for the initial and subsequent values of ISF and ICR. The CGS-SQ calculates the prandial insulin dose, in units, as the sum of a carbohydrate coverage factor and a correction factor. The carbohydrate coverage factor is calculated as CHO/ICR. The correction factor is calculated when the BG is greater than the midpoint of the target range, as (BG – MidPoint)/ISF. The final calculated dose is rounded to the nearest whole unit.
For patients who are eating, carbohydrates can be covered at any time. For example, if a patient ate lunch and then decided 2 hours later to eat a snack, this would be covered with a rapid-acting insulin dose. Since it is too soon after eating lunch to test the patient's BG level meaningfully, only the number of grams of carbohydrate eaten would be entered into the program, which would calculate the rapid-acting insulin dose needed to cover the dietary carbohydrates.
Correction Insulin Dosing
In some instances, correction insulin doses may be required at times other than mealtimes. For example, if a patient who is normally eating skips a meal, then the blood glucose is obtained and entered into the program for a calculated correction dose. However, if the patient has received a correction dose in the previous 3 hours, the program will not calculate a dose. This is a safety feature to prevent insulin stacking.
Blood Glucose Testing Orders
Timely BG testing and appropriately timed insulin delivery are critical to the success of insulin administration regimens. The CGS-SQ issues audible reminder alarms when it is time for the basal insulin dose, and when it is time to measure a patient's BG for prandial and/or correction insulin. The timing of these alarms and the associated actions depend on the patient's feeding status. For patients who are eating or receiving bolus tube feedings, alarms are typically not needed. Alarms will be generated, however, at 10:00 a.m., 2:00 p.m., or 7:00 p.m., if a BG was not entered in the preceding periods of 5:00 a.m. to 10:00 a.m., 10:01 a.m. to 2:00 p.m., or 2:01 p.m. to 7:00 p.m., respectively. The patient's BG is measured prior to feeding. This BG and the number of grams of carbohydrate eaten or given by bolus tube feeding are entered into the program immediately after feeding, whereupon the program calculates the prandial plus correction insulin dosage. For patients who are not eating but are on a continuous glucose source (that is, TPN, tube feeding, or dextrose IV fluids), the CGS-SQ issues alarms every 4 hours, at which time the caregiver measures the patient's BG, enters it into the program, and provides the correction insulin dose calculated by the program.
Insulin Dose Adjustment
Insulin doses can be adjusted by the physician as often as desired, usually daily. The CGS-SQ provides the patient's BG and treatment history to assist in dose adjustment. If BG levels are above target, the physician can reduce ISF and/or ICR to increase rapid-acting insulin doses; if BG levels are below target, the physician can increase ISF and/or ICR to reduce rapid-acting insulin doses. Basal insulin dosage may need to be increased if too much correction insulin is being used, and can also be adjusted to bring any significant (>30 mg/dl) change in the bedtime or fasting BG into range. The pocket guide provides adjustment recommendations.
Recovery from Hypoglycemia
If the patient's BG is ≤70 mg/dl, the CGS-SQ provides the caregiver with hypoglycemia recovery instructions. For patients with IV access, the CGS-SQ calculates a dextrose 50% dosage as 0.4 x (100 – BG) grams; for patients without IV access who are able to drink liquids, the CGS-SQ calculates the amount of juice or 7-Up™ (soft drink) needed to supply approximately 5 carbohydrate grams for every 10 mg/dl below 100 mg/dl; for patients without IV access who are non per os (nothing by mouth), the CGS-SQ calculates a weight-based glucagon dose—1 mg subcutaneous glucagon for patients ≥100 pounds and 0.5 mg for patients <100 pounds. The CGS-SQ will issue reminder alarms every 15 minutes until BG >70 mg/dl. For any BG ≤70 mg/dl, the nurse is reminded to notify the physician after treating the hypoglycemia, immediately if the reaction was clinically severe, otherwise before the next dose of insulin is given. If appropriate, the ISF, ICR, and/or basal insulin dose may be adjusted to forestall future hypoglycemic events. These adjustments require physician input.
Program Safeguards
Patient safety is the primary focus of the CGS-SQ. Numerous safeguards are built into the software. The program issues warnings for potentially unsafe insulin doses. Basal insulin doses ≥100 units and bolus insulin doses ≥30 units are flagged for physician confirmation. The program also alerts the nurse to call the physician when hyperglycemia presents, as a single BG >350 mg/dl or two consecutive BGs >220 mg/dl. Warning thresholds can be changed, if desired.
System Usability
Because of its features, the CGS-SQ requires users to be trained. At the most fundamental level, the physician and nurses need to understand just three elements to drive the system: ISF, ICR, and TDD. These parameters control basal, bolus, and correction insulin dosing. Weight-based defaults or simple weight-based calculations can be used for setting the initial values for these parameters; when transitioning from IV insulin to subcutaneous insulin, the CGS-IV provides the initial values for these parameters. Daily adjustment of the ISF and ICR and the basal insulin dose allows the physician to tailor the insulin dose to individual patient response. After training on these principles of subcutaneous insulin and after hands-on experience with the CGS-SQ's user interface, clinicians and nurses become comfortable with the system.
Statistical Analysis
Descriptive statistics were obtained using SPSS 15.0 (SPSS Inc., Chicago, IL).
Results
From April 26, 2006 to September 25, 2007, 1772 patients in both ICUs and general medical/surgical units at Methodist and University Hospitals were treated using the CGS-SQ, with 46,575 BG measurements in its database. There were a total of 2490 runs. (See Methods.)
The average BG at the start of a run was 182.2 mg/dl. The average BG over all CGS-SQ measurements was 158.3 mg/dl, the percentage of measurements in the default target range of 100–150 mg/dl was 40.5%, and the percentage of measurements in a wider range of 70–180 mg/dl was 69.8%. The hypoglycemia rates for ranges of <40, <50, <60, and <70 mg/dl, were 0.18, 0.52, 1.07, and 2.33%, respectively; the hyperglycemia rates for ranges of >180 and >220 mg/dl were 27.8 and 14.7%, respectively. No adverse clinical events were associated with hypoglycemic episodes. Figure 3 shows the number, mean, and 95% confidence intervals of the mean by month for all BGs in the CGS-SQ database from April 2006 to September 2007.
Figure 3.
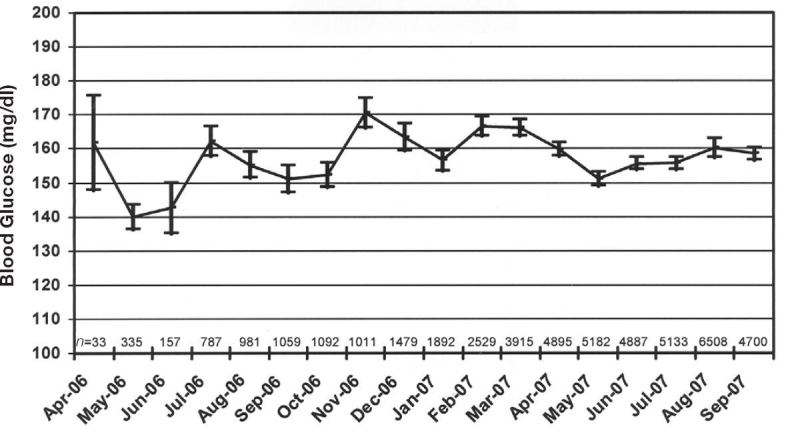
Mean blood glucose by month, for all measurements taken with the CGS-SQ at Methodist and University Hospitals from April 2006 to September 2007. Bars represent 95% confidence intervals of the mean; n is the number of CGS-SQ measurements per month.
Each run of the CGS-SQ extends over several days of treatment. Because treatment days are the same length and include BGs taken at similar times for basal and prandial insulin dosing, they are a more easily interpretable unit of analysis than the individual BG measurements. There were 12,679 treatment days. For each treatment day, the daily average BG and the presence of hypoglycemic or hyperglycemic measurements were determined. The daily average BG, averaged over all treatment days, was 161.9 mg/dl. The percentage of treatment days with hypoglycemic episodes for the ranges <40, <50, <60, and <70 mg/dl were 0.50, 1.47, 2.87, and 5.86%, respectively. The percentage of treatment days with hyperglycemic episodes for ranges of >180 and >220 mg/dl were 48.9 and 30.0%, respectively.
Discussion
Following the successful implementation of a computerized IV insulin dosing program at Methodist and University Hospitals, it was a natural next step to extend computerized insulin dosing to subcutaneous insulin. From our previous experience, we knew that the computerization of paper protocols by itself would have immediate benefits: rapid, automatic, and error-free calculation with reminder alarms prompting treatment. Even though we had a pocket guide to help physicians calculate insulin doses, we struggled with the on-time delivery of the insulin. We had the opportunity to standardize best practices for glycemic control in both ICUs and general medical/surgical units by creating a computerized alarm-driven program to facilitate the timely delivery of insulin—the CGS-SQ.
Initial experience with the CGS-SQ program in ICUs and general medical/surgical units has been encouraging. Looked upon simply as a process improvement tool, the CGS-SQ has been an unquestioned success. The CGS-SQ, like any innovation that significantly changes daily work habits, requires education, both prior to and after introduction. Comprehensive ongoing education on the principles of subcutaneous insulin and an increasing familiarity with the CGS-SQ have led to a growing acceptance. There are now entire floors that use the CGS-SQ as their ordering mechanism. Nurses appreciate that they no longer have to calculate insulin doses using carbohydrate ratios and sliding scales. Physicians find the order set easier to use than the previous subcutaneous insulin paper order set.
Selection of patients for CGS-SQ treatment was under physician discretion—neither explicit criteria nor informal guidelines were used. As a result, we were unable to define a population of concurrent or historical controls to compare with our CGS-SQ patients. Despite the lack of a controlled study, glycemic control with the CGS-SQ has met our expectations. The daily average BG, averaged over all treatment days, was 161.9 mg/dl; and the hyper-glycemia (BG >180 mg/dl) rate was 48.9% per treatment day. The CGS-SQ also appears to be safe, with a low hypoglycemia (BG <40 mg/dl) rate of 0.18% per measurement and 0.50% per treatment day. None of the hypoglycemic events occurring with the CGS-SQ were felt to be clinically significant and all were reversible.
The CGS-SQ currently operates under several limitations. Although its bolus insulin dose calculations use patient-specific insulin sensitivity factors and insulin-to-carbohydrate ratios, these parameters are adjustable only by physician order. Unlike the CGS-IV, which independently adjusts its insulin sensitivity factors based on the patient's response to insulin, the CGS-SQ depends on external adjustment. The CGS-SQ is also limited by an inability to recommend changes to basal insulin dosages. We are striving to modify the program to suggest adjustments to basal and prandial doses.
Conclusions
The primary purpose of the CGS-SQ was to standardize insulin administration practices, in order to improve our hospitals' quality of care. Standardization leads to greater understanding of best practices, increased awareness of expectations for patient care, and ultimately, lasting change in practice culture. Standardization of insulin practice is especially important, given the prevalence of medication errors associated with insulin and the dangers of hypoglycemia. This proof of concept study suggests that subcutaneous insulin dosing calculators, like the CGS-SQ, can be used safely and effectively in both ICUs and general medical/surgical units.
Acknowledgements
The authors would like to thank the nursing staff and Decision Support staff at Clarian Health for their assistance with this study. We would also like to thank The Epsilon Group Virginia, LLC for assistance with the statistical analysis.
Abbreviations
- BG
blood glucose
- CGS-IV
Clarian GlucoStabilizer™ program
- CGS-SQ
Clarian GlucoStabilizer™ Subcutaneous Insulin Program
- ICR
insulin-to-carbohydrate ratio
- ISF
insulin sensitivity factor
- IV
intravenous
- SUGAR
Systematic Utilization of Glucose Assessment and Response
- TDD
total daily dose
- TPN
total parenteral nutrition
References
- 1.Langouche L, Vanhorebeek I, Vlasselaers D, Vander Perre S, Wouters PJ, Skogstrand K, Hansen TK, Van den Berghe G. Intensive insulin therapy protects the endothelium of critically ill patients. J Clin Invest. 2005;115(8):2277–2286. doi: 10.1172/JCI25385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lazar HL, Chipkin SR, Fitzgerald CA, Bao Y, Cabral H, Apstein CS. Tight glycemic control in diabetic coronary artery bypass graft patients improves perioperative outcomes and decreases recurrent ischemic events. Circulation. 2004;109(12):1497–1502. doi: 10.1161/01.CIR.0000121747.71054.79. [DOI] [PubMed] [Google Scholar]
- 3.Krinsley JS. Effect of an intensive glucose management protocol on the mortality of critically ill adult patients. Mayo Clin Proc. 2004;79(8):992–1000. doi: 10.4065/79.8.992. [DOI] [PubMed] [Google Scholar]
- 4.Preiser JC, Devos P, Van den Berghe G. Tight control of glycaemia in critically ill patients. Curr Opin Clin Nutr Metab Care. 2002;5(5):533–537. doi: 10.1097/00075197-200209000-00012. [DOI] [PubMed] [Google Scholar]
- 5.Van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, Vlasselaers D, Ferdinande P, Lauwers P, Bouillon R. Intensive insulin therapy in critically ill patients. N Engl J Med. 2001;345(19):1359–1367. doi: 10.1056/NEJMoa011300. [DOI] [PubMed] [Google Scholar]
- 6.Capes SE, Hunt D, Malmberg K, Gerstein HC. Stress hyperglycaemia and increased risk of death after myocardial infarction in patients with and without diabetes: a systematic overview. Lancet. 2000;355(9206):773–778. doi: 10.1016/S0140-6736(99)08415-9. [DOI] [PubMed] [Google Scholar]
- 7.Furnary AP, Zerr KJ, Grunkemeier GL, Starr A. Continuous intravenous insulin infusion reduces the incidence of deep sternal wound infection in diabetic patients after cardiac surgical procedures. Ann Thorac Surg. 1999;67(2):352–360. doi: 10.1016/s0003-4975(99)00014-4. discussion 360–362. [DOI] [PubMed] [Google Scholar]
- 8.Zerr KJ, Furnary AP, Grunkemeier GL, Bookin S, Kanhere V, Starr A. Glucose control lowers the risk of wound infection in diabetics after open heart operations. Ann Thorac Surg. 1997;63(2):356–361. doi: 10.1016/s0003-4975(96)01044-2. [DOI] [PubMed] [Google Scholar]
- 9.Juneja R, Roudebush C, Kumar N, Macy A, Golas A, Wall D, Wolverton C, Nelson D, Carroll J, Flanders SJ. Utilization of a computerized intravenous insulin infusion program to control blood glucose in the intensive care unit. Diabetes Technol Ther. 2007;9(3):232–240. doi: 10.1089/dia.2006.0015. [DOI] [PubMed] [Google Scholar]