Abstract
Background
In silico testing was used extensively in the European Commission-funded Closed Loop Insulin Infusion for Critically Ill Patients (Clinicip) project, which aimed to develop prototype systems for closed loop glucose control in the critically ill. This article presents two examples of how the simulation environment was utilized in this project.
Methods
The in silico simulation environment was used to simulate a 48-hour clinical trial in a surgical intensive care unit to achieve tight glycemic control. A set of 10 critically ill synthetic subjects was selected for two different studies. In the first study, two sets of clinical trials were simulated using two versions of a model predictive control (MPC)-based glucose control algorithm: MPC Version 0.1.5 with hourly glucose measurements and updated MPC Version 1.4.3 with variable 1- to 4-hour glucose sampling. In the second study, four sets of clinical trials were simulated with four levels of measurement error at 2, 5, 7, and 15% coefficient of variation corresponding to the measurement error of commercially available glucose measuring devices.
Results
In the first study, more frequent glucose measurements associated with MPC Version 0.1.5 facilitated more efficacious and safer glucose control compared to that obtained with the prolonged and variable glucose sampling rate associated with MPC Version 1.4.3. In the second study, a marked deterioration in safety measures was observed in studies performed with a measurement error of 15%.
Conclusions
The presented simulation studies highlighted two important uses of in silico simulation environment in the Clinicip project. The impressive progress and successful completion of the Clinicip project would not be possible without computer-based simulations.
Keywords: critical illness, glucose control, simulation environment
Introduction
Computer simulations have become an inherent part of the design process of complex engineering systems, resulting in dramatic technological progress of the last two decades. A typical example comes from the aviation industry. Modern airplanes are efficiently designed and tested in a computer simulation environment. This virtual design has eliminated the need for many costly experiments and accelerated the development process.
In general, simulations aim to model a real-life or hypothetical process in order to assess how it works. Depending on the purpose, simulations in biology can be subdivided into two categories, conceptual and “variability inclusive.” As the name suggests, the former type is helpful in assessing the conceptual behavior by modeling the average patient/organ/process. The latter also includes the representation of variability to evaluate system behavior across a feasible range of possibilities—this may include representation of between and within subject variability as well as modeling variability induced by the measurement process. The variability-inclusive type of simulation is most suitable to support the development of approaches for tight glycemic control (TGC).
It has been demonstrated that TGC within a narrow range of 4.4 to 6.1 mM (80 to 110 mg/dl) reduces mortality and morbidity in the critically ill population.1,2 TGC is based on intensive intravenous insulin infusion and frequent blood glucose sampling, potentially increasing the workload of the intensive care (ICU) nursing staff. However, TGC is not easily achievable with standard treatment practices at ICUs and increases the risk of hypoglycemia.3 The need for novel glucose protocols, controllers, and treatment systems is therefore rising. Numerous glucose management protocols and computer-based algorithms have been developed to achieve TGC.4–7
The aim of the European Commission (EC)-funded project “Closed Loop Insulin Infusion for Critically Ill Patients” (Clinicip), which started in 2004 and ran for 4 years until the end of 2007, was to develop prototype automated systems for blood glucose control in ICUs. A computer-based algorithm for the TGC based on model predictive control (MPC) was developed and evaluated as part of the Clinicip project. Following experience from another EC-funded project, “Advanced Insulin Infusion using a Control Loop,”8 the development and use of an in silico simulation environment was considered crucial in order to facilitate rational, timely, and efficient development and evaluation of glucose controllers.
This article presents two examples of how the simulation environment was used in the Clinicip project and shows the impact of the results on the development of glucose controllers and the Clinicip system as a whole. We exclude the detailed description of the synthetic population or the control algorithm and focus on the assessment of the utility of the simulation environment. In the first example, two versions of the glucose control algorithm are compared. The original version of the controller uses regular hourly glucose measurements, whereas the updated version, preferred by clinicians as less resource demanding, adopts variable, 1- to 4-hour glucose sampling. Safety and efficacy measures provided by the simulations allowed preclinical comparison of the two controllers. The objective of the second study was to determine an acceptable level of the measurement error of the glucose measuring device to achieve TGC with the MPC.
Materials and Methods
Simulation Environment
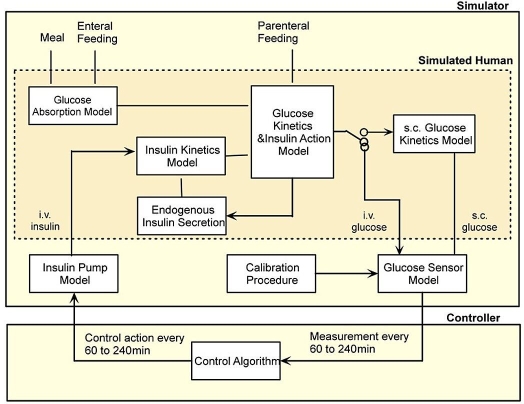
The simulation environment reflects the setup of a real clinical trial. Figure 1 presents the overall structure of the simulation environment. The simulated experiment comprises the experimental protocol, the simulated critically ill subject, the model of the glucose sensor, the model of the insulin pump, the glucose controller, and finally the metrics summarizing the results.
Figure 1.
Simulation environment—main components.
Subjects are represented by individual parameter sets, which characterize the physiological model of glucose regulation.
Figure 2 shows that the simulated critically ill subject is represented by a set of interacting submodels. The model of glucose kinetics and insulin action described by Hovorka et al.9 represents the core of the simulation model. Other submodels include a model of endogenous insulin secretion developed using hourly insulin and C-peptide concentrations collected in the critically ill treated by intravenous insulin to achieve TGC, a model of insulin kinetics where saturable hepatic insulin extraction is acknowledged10 and a single compartment is used to represent a whole body insulin kinetics, and finally a two-compartment submodel of enteral glucose absorption9 with added saturability of the gut absorption rate. External inputs to those submodels include enteral and parenteral feeding and an insulin infusion by the insulin pump. The rate of insulin infusion is calculated by the glucose control algorithm and is updated at 1- to 4-hour intervals. At each of these time intervals the controller requires information about glucose concentration, which is provided by the glucose measuring device.
Figure 2.
Simulator design—the simulated human (dark shaded) is represented by a set of interacting submodels. i.v., intravenous; s.c., subcutaneous.
Virtual Population of Critically Ill Subjects
The complete virtual population consisted of 55 critically ill subjects; 29 of those subjects came from the medical ICU and 26 from the surgical ICU. The majority of the subject parameters were generated in the process of fitting the glucoregulatory model to the rich set of clinical data using Bayesian parameter estimation. The remaining parameters were drawn from informed probability distributions. The number of subjects was dictated by the availability of clinical data to determine the core of the carbohydrate metabolism model, i.e., submodels of glucose kinetics and insulin action. For the purpose of this study, a subset of 10 simulated surgical ICU subjects was selected.
Model Predictive Control
The MPC algorithm has been described previously by Hovorka and colleagues.9 The main component of the MPC is a model representing the glucoregulatory system. As the original algorithm was developed for the treatment of type 1 diabetes, the MPC model had to be modified for the purpose of the critically ill. The main modifications included addition of an endogenous insulin secretion model and introduction of time variant insulin resistance. Further details of these and other modifications can be found in Hovorka et al.12 The model enables prediction of the glucose excursion by a dose optimizer. The dose optimizer proposes future insulin infusion rates and optimizes the rates until the predicted glucose excursion fits into the desired glucose excursion, where excursion is a slow normalization in case of hyperglycemia, a fast recovery in case of hypoglycemia, or maintenance of normoglycemia. The glucoregulatory model of the MPC has individual parameters, which are adapted online. Glucose concentration, insulin dosage, and carbohydrate intake are the input variables for the MPC. Entry of the glucose concentration is required every 60 to 240 minutes and triggers the online adaptation of the parameters and the calculation of the insulin infusion rate for the next 60–240 minutes.
In the course of the Clinicip project, several versions of the MPC have been developed and tested in clinical trials. The early versions adopted regular hourly glucose measurements, such as that reported by Plank et al.11 (MPC Version 0.1.5 and similar). Following a clinical review of these early trials it was suggested that the glucose sampling rate should be extended to reduce the workload of the ICU staff and the aggressiveness increased to facilitate faster achievement of the target glucose concentration of 4.4 to 6.1 mM (80 to 110 mg/dl). The MPC was modified and optimized on the simulation environment to allow 1- to 4-hour glucose sampling; clinical testing of MPC Version 1.4.3 adopting a variable sampling interval was described by Hovorka and colleagues.12 In later versions, the MPC also provided advice on the time to the next glucose measurement. The sampling interval is extended if glucose excursions are predictable and reduced if glucose swings occur, for example.
Measurement Error
In the studies performed during the Clinicip project, the coefficient of variation (CV) of the measurement error associated with blood glucose measurements ranged from 2% for commercial laboratory equipment such as ABL (ABL 700™, Radiometer Medical, Copenhagen, Denmark) to 15% reported for the OneTouch® II glucose meter (LifeScan, Milpitas, CA).13 Two intermediate levels of 5 and 7% corresponded to other commercially available devices, the Accutrend® (Boehringer Mannheim, Mannheim, Germany) and Accu-Check® Inform (Roche Diagnostics, Burgdorf, Switzerland), respectively.
In Silico Study Design
Ten synthetic subjects from a surgical ICU were selected for the purpose of this analysis. An in silico simulation environment was used to simulate a 48-hour-long clinical trial in surgical ICU subjects to achieve TGC. In the first study, two sets of 10 clinical trials were simulated: one set was simulated with MPC Version 0.1.5 and the other with updated MPC Version 1.4.3.
In the second study, four sets of 10 clinical trials were simulated using updated MPC Version 1.4.3 with the variable sampling rate. Each of the four sets had a different level of the measurement error at 2, 5, 7, and 15% CV.
Glucose Control Measures
The mean blood glucose concentration, the standard deviation (SD) of the glucose concentration, the hyper-glycemic index (HGI)14 defined as the area under the curve above glucose level of 6.0 mM divided by the duration of the experiment, the time to reach the target glucose range of 4.4 to 6.1 mM (80 to 110 mg/dl), and finally the percentage of time spent in the target range were used to assess efficacy of the TGC. The number of hypoglycemia episodes (<2.8 mM; <50 mg/dl) and the number of subjects experiencing hypoglycemia were used to assess the safety of the TGC.
Results
Comparison of Two Versions of Glucose Controller
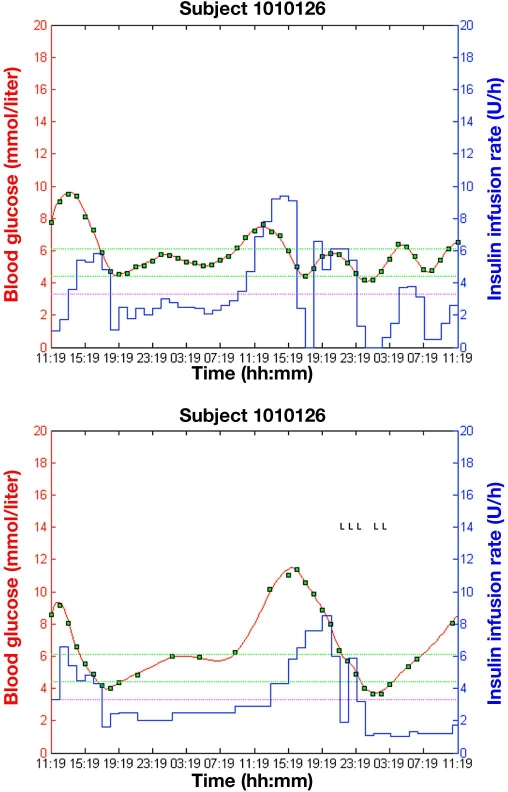
An example of graphical output of two simulation studies with synthetic subject 1010126 utilizing MPC Version 0.1.5 with the regular hourly glucose measurements and updated MPC Version 1.4.3 with the variable, controller-determined, glucose measurements is shown in Figure 3.
Figure 3.
Graphical output from simulation studies with synthetic subject 1010126 using two versions of the control algorithm: MPC Version 0.1.5 with a regular hourly sampling interval (top) and MPC Version 1.4.3 with a variable sampling interval (bottom); green squares represent glucose measurements, the blue line (piecewise constant) represents the insulin infusion rate, and the red solid line represents simulated blood glucose. Green dashed lines represent the target glucose range (4.4–6.1 mmol/liter; 80–110 mg/dl), and the magenta dashed line represents the level of mild hypoglycemia (3.3 mmol/liter; 60 mg/dl). Letters L seen in the background on the right are used as diagnostic symbols.
Results of the comparison between the original versus the updated version of the MPC are shown in Table 1. The mean glucose sampling interval for updated MPC Version 1.4.3 was 111 minutes.
Table 1.
Glucose Control Measures for Original MPC Version 0.1.5 (Regular Sampling) and Updated MPC Version 1.4.3 (Variable Sampling) of the MPC (N =10; Median Values Are Reported)
| Original MPC Version 0.1.5 | Updated MPC Version 1.4.3 | |
|---|---|---|
| Mean glucose (mM) | 5.55 | 5.99 |
| Glucose SD (mM) | 0.87 | 1.59 |
| Time in targeta (%) | 76 | 56 |
| Time to target (min) | 360 | 177 |
| Hyperglycemic index (mM) | 0.17 | 0.40 |
| Hypoglycemic episodesb (unitless) | 0 | 2 |
| Subjects with hypoglycemia (unitless) | 0 | 2 |
Target glucose range from 4.4 to 6.1 mM (80 to 110 mg/dl).
Blood glucose <2.8 mM (<50 mg/dl).
Comparison of Selected Glucose Measuring Devices
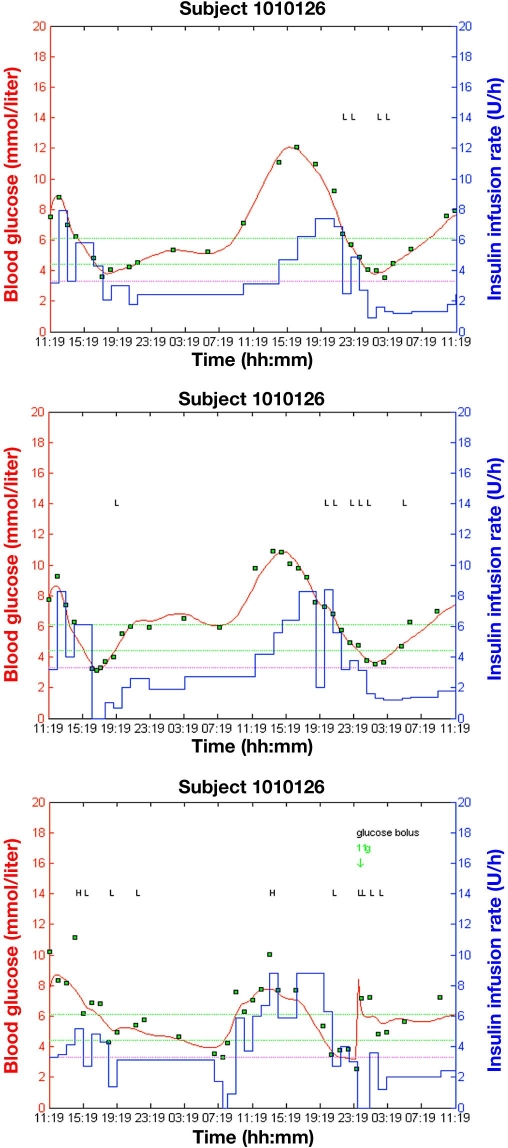
An example of graphical output from three simulation studies with three different levels of the measurement error of 5, 7, and 15% CV is presented in Figure 4. Updated MPC Version 1.4.3 was used in these studies. Figure 3 (bottom) shows graphical output from the simulation study with the reference measurement error 2%, which corresponds to the commercial laboratory equipment ABL. Table 2 lists results obtained with updated MPC Version 1.4.3 at four different levels of the measurement error.
Figure 4.
Graphical output from three simulation studies with subject 1010126 using different levels of the measurement error: 5% (top), 7% (middle), and 15% (bottom); symbols as in Figure 1. Letters L and H seen in the background are diagnostic symbols.
Table 2.
Glucose Control Measures with Updated MPC Version 1.4.3 (Variable Sampling) at Four Levels of Measurement Error (N = 10; Median Values Are Reported)
| Measurement error (CV) | |||||
|---|---|---|---|---|---|
| 2% | 5% | 7% | 15% | ||
| Mean glucose (mM) | 6.01 | 5.98 | 6.08 | 6.31 | |
| Glucose SD (mM) | 1.49 | 1.45 | 1.68 | 1.98 | |
| Time in targeta (%) | 54 | 48 | 50 | 44 | |
| Time to target (min) | 178 | 150 | 178 | 181 | |
| Hyperglycemic index (mM) | 0.42 | 0.49 | 0.55 | 0.68 | |
| Hypoglycemic episodesb (unitless) | 2 | 2 | 2 | 7 | |
| Subjects with hypoglycemia (unitless) | 2 | 2 | 2 | 5 | |
Target glucose range 4.4 to 6.1 mM (80 to 110 mg/dl).
Blood glucose <2.8 mM (<50 mg/dl).
Discussion
This study highlighted two important uses of the simulation environment in the Clinicip project. In the first example, two versions of the MPC controller were tested on 10 synthetic ICU subjects and the simulation results were compared. A trade-off between less frequent sampling and efficacy and safety of glucose control by the MPC has been demonstrated. As the sampling interval increased, deterioration in most of the efficacy performance measures was observed (see Table 1). The mean glucose and glucose SD increased with the updated version of the MPC. More than a twofold increase was noted in the HGI. The percentage of time spent in the target glucose range was reduced by 26%. The reduction of time needed to reach the target range from 360 to 177 minutes is explained by a more aggressive insulin treatment in the initial phase with updated MPC Version 1.4.3. The two safety measures were also affected. While the simulation studies with the original MPC version did not demonstrate any hypoglycemia events, studies with the updated version resulted in two hypoglycemia events observed in 2 out of 10 synthetic subjects. Although more frequent glucose measurements associated with the original version of the MPC facilitated more efficacious and safer glucose control compared to that obtained with the prolonged and variable glucose sampling rate associated with the updated version of the MPC, the latter version was accepted by the clinicians and consequently progressed to the implementation stage. The benefits of reduced workload at the ICU gained with the updated version of the controller outweighed the loss of performance observed in the simulation studies.
It is worth mentioning that the results of this simulation study differ from the clinical study results reported by Plank et al.11 for MPC Version 0.1.5 and, similarly, Hovorka et al.12 for MPC Version 1.4.3. The reason for the discrepancy stems from the fact that the simulation studies were carried out on a small sample of 10 synthetic subjects. Five of those subjects came from clinical studies conducted in Graz, Austria, and the remaining 5 came from studies conducted in Prague, Czech Republic; both studies are reported by Plank et al.11 The subjects studied in London, United Kingdom,11 were not included. None of the subjects reported by Hovorka et al.12 were represented in our synthetic population.
The objective of the second study was to determine an acceptable level of the measurement error of the glucose measuring device to achieve TGC with the MPC. Four levels of the measurement error were selected corresponding to four commercially available devices ranging from 2% for laboratory equipment such as ABL to 15% for the One Touch II glucose meter. The simulation results obtained for 10 synthetic subjects demonstrated some degree of deterioration in the efficacy of glucose control observed mainly in glucose SD, HGI, and the percentage of time spent in the target glucose range (see Table 2). However, a marked deterioration was observed in the safety measures. The number of hypoglycemia events increased over threefold, and the number of subjects who experienced hypoglycemia more than doubled in the studies performed with the measurement error of 15% (see Table 2). Consequently, this level of the measurement error was considered unacceptable.
In summary, realistic computer simulations can provide invaluable information about safety, efficacy, and limitations of control algorithms. Various control algorithms can be tested efficiently and compared in a variety of control scenarios. However, computer simulations can only be used to reject inappropriate or inefficient algorithms. Simulations alone cannot assess the safety and efficacy of control algorithms. Ultimately this is the role of clinical trials.
Conclusion
The simulation environment has been used routinely throughout the Clinicip project, serving as an invaluable tool to assess preclinically the performance of different versions of the MPC controller prior to clinical testing. The simulation environment played an important role in making important decisions about the experimental setup, such as selecting an appropriate type of a bloods glucose measuring device. The impressive progress and successful completion of the Clinicip project would not be possible without computer-based simulations.
Acknowledgment
Support by EC-funded Clinicip Project (FP7 IST-2002-506965) and NIHR Cambridge Biomedical Research Centre is acknowledged.
Abbreviations
- Clinicip
Closed Loop Insulin Infusion for Critically Ill Patients
- CV
coefficient of variation
- EC
European Commission
- HGI
hyperglycemic index
- ICU
intensive care unit
- MPC
model predictive control
- SD
standard deviation
- TGC
tight glycemic control
References
- 1.Van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, Vlasselaers D, Ferdinande P, Lauwers P, Bouillon R. Intensive insulin therapy in the critically ill patients. N Engl J Med. 2001;345(19):1359–1367. doi: 10.1056/NEJMoa011300. [DOI] [PubMed] [Google Scholar]
- 2.Van den Berghe G, Wilmer A, Hermans G, Meersseman W, Wouters PJ, Milants I, Van Wijngaerden E, Bobbaers H, Bouillon R. Intensive insulin therapy in the medical ICU. N Engl J Med. 2006;354(5):449–461. doi: 10.1056/NEJMoa052521. [DOI] [PubMed] [Google Scholar]
- 3.Brunkhorst FM, Engel C, Bloos F, Meier-Hellmann A, Ragaller M, Weiler N, Moerer O, Gruendling M, Oppert M, Grond S, Olthoff D, Jaschinski U, John S, Rossaint R, Welte T, Schaefer M, Kern P, Kuhnt E, Kiehntopf M, Hartog C, Natanson C, Loeffler M, Reinhart K German Competence Network Sepsis (SepNet) Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N Engl J Med. 2008;358(2):125–139. doi: 10.1056/NEJMoa070716. [DOI] [PubMed] [Google Scholar]
- 4.Chee F, Fernando T, van Heerden PV. Closed-loop glucose control in critically ill patients using continuous glucose monitoring system (CGMS) in real time. IEEE Trans Inf Technol Biomed. 2003;7(1):43–53. doi: 10.1109/titb.2003.808509. [DOI] [PubMed] [Google Scholar]
- 5.Goldberg PA, Siegel MD, Sherwin RS, Halickman JI, Lee M, Bailey VA, Lee SL, Dziura JD, Inzucchi SE. Implementation of a safe and effective insulin infusion protocol in a medical intensive care unit. Diabetes Care. 2004;27(2):461–467. doi: 10.2337/diacare.27.2.461. [DOI] [PubMed] [Google Scholar]
- 6.Lonergan T, Le Compte A, Willacy M, Chase JG, Shaw GM, Wong XW, Lotz T, Lin J, Hann CE. A simple insulin-nutrition protocol for tight glycemic control in critical illness: development and protocol comparison. Diabetes Technol Ther. 2006;8(2):191–206. doi: 10.1089/dia.2006.8.191. [DOI] [PubMed] [Google Scholar]
- 7.Boulkina LS, Braithwaite SS. Practical aspects of intensive insulinization in the intensive care unit. Curr Opin Clin Nutr Metab Care. 2007;10(2):197–205. doi: 10.1097/MCO.0b013e3280141ff4. [DOI] [PubMed] [Google Scholar]
- 8.Hovorka R, Chassin LJ, Wilinska ME, Canonico V, Akwi JA, Federici MO, Massi-Benedetti M, Hutzli I, Zaugg C, Kaufmann H, Both M, Vering T, Schaller HC, Schaupp L, Bodenlenz M, Pieber TR. Closing the loop: the adicol experience. Diabetes Technol Ther. 2004;6(3):307–318. doi: 10.1089/152091504774197990. [DOI] [PubMed] [Google Scholar]
- 9.Hovorka R, Canonico V, Chassin LJ, Haueter U, Massi-Benedetti M, Orsini Federici M, Pieber TR, Schaller HC, Schaupp L, Vering T, Wilinska ME. Nonlinear model predictive control of glucose concentration in subjects with type 1 diabetes. Physiol Meas. 2004;25(4):905–920. doi: 10.1088/0967-3334/25/4/010. [DOI] [PubMed] [Google Scholar]
- 10.Hovorka R, Powrie JK, Smith GD, Sonksen PH, Carson ER, Jones RH. Five-compartment model of insulin kinetics and its use to investigate action of chloroquine in NIDDM. Am J Physiol. 1993;265(1 Pt 1):E162–E175. doi: 10.1152/ajpendo.1993.265.1.E162. [DOI] [PubMed] [Google Scholar]
- 11.Plank J, Blaha J, Cordingley J, Wilinska ME, Chassin LJ, Morgan C, Squire S, Haluzik M, Kremen J, Svacina S, Toller W, Plasnik A, Ellmerer M, Hovorka R, Pieber TR. Multicentric, randomized, controlled trial to evaluate blood glucose control by the model predictive control algorithm versus routine glucose management protocols in intensive care unit patients. Diabetes Care. 2006;29(2):271–276. doi: 10.2337/diacare.29.02.06.dc05-1689. [DOI] [PubMed] [Google Scholar]
- 12.Hovorka R, Kremen J, Blaha J, Matias M, Anderlova K, Bosanska L, Roubicek T, Wilinska ME, Chassin LJ, Svacina S, Haluzik M. Blood glucose control by a model predictive control algorithm with variable sampling rate versus a routine glucose management protocol in cardiac surgery patients: a randomized controlled trial. J Clin Endocrinol Metab. 2007;92(8):2960–2964. doi: 10.1210/jc.2007-0434. [DOI] [PubMed] [Google Scholar]
- 13.Glasmacher AG, Brennemann W, Hahn C, Marko C, Wehnert S, Löhr U, Kutschkow R. Evaluation of five devices for self-monitoring of blood glucose in the normoglycaemic range. Exp Clin Endocrinol Diabetes. 1998;106(4):360–364. doi: 10.1055/s-0029-1211998. [DOI] [PubMed] [Google Scholar]
- 14.Vogelzang M, van der Horst IC, Nijsten MW. Hyperglycaemic index as a tool to assess glucose control: a retrospective study. Crit Care. 2004;8(3):R122–R127. doi: 10.1186/cc2840. [DOI] [PMC free article] [PubMed] [Google Scholar]