Abstract
Devices that measure glucose on a near-continuous basis may provide a better insight into glycemic profiles, allowing patients with diabetes to make therapeutic adjustments to improve metabolic control, thereby reducing the risk of diabetic complications. Motivated and technologically adept patients with brittle diabetes, hypoglycemia unawareness, diabetic pregnancy, or who use pumps might benefit.
Current evidence of continuous glucose monitoring (CGM) on health outcome in patients with diabetes is critically reviewed. No data are available on chronic complications or mortality. Therefore, surrogate endpoints need to be investigated, particularly HbA1c, number of hypo- and hyperglycemic episodes, time within normal, high, or low glucose concentrations, glycemic variability, and quality of life.
Randomized controlled trials (RCTs) using CGM in a retrospective way did not show metabolic improvement. In contrast, most RCTs applying real-time CGM showed a decrease in HbA1c, reduced glycemic variability, and a diminished number and length of hypo- and hyperglycemic events. Using accurate, real-time CGM devices improves quality of life by reducing the fear of unexpected hypoglycemic events. These beneficial effects were observed despite the fact that in most studies no clear treatment algorithm based on CGM results was provided to the patients. However, most trials were too short in duration, with a variable use of CGM, and were performed in small study samples.
In conclusion, real-time CGM systems can improve metabolic control, reduce hypoglycemic episodes, and improve quality of life. Whether this holds true for longer time periods and in the majority of patients remains to be proven. In the long term, CGM might help to reduce chronic diabetes complications and perhaps also mortality, thereby reducing health care costs.
Keywords: continuous glucose monitoring, diabetes, metabolic control
Introduction
Longterm diabetic complications can be prevented or reduced by achieving good metabolic control, reflected by HbA1c <6.5–7.0%.1–3 Glucose excursions might also contribute to the development of diabetic complications.4,5 Fear of hypoglycemia limits our ability to reach strict glycemic control, because it is usually accompanied by reluctance of the patient to intensify insulin therapy. Indeed, the goal of intensive therapy is to normalize HbA1c and control fasting and postprandial glycemia, while concurrently limiting the number and severity of hypoglycemic events. To reach tight glycemic control, frequent self-monitoring of blood glucose (SMBG) must be performed.6 SMBG devices provide the patient with accurate but discrete blood glucose levels. However, they do not provide trend information, nor do they reflect glycemic fluctuations, which is possible by using continuous glucose monitoring (CGM) systems. Thus, implementation of strict glycemic control may be facilitated by a CGM device. This manuscript critically reviews the proposed benefits and indications of CGM and the current evidence of CGM on health outcome in diabetic patients.
Proposed Advantages of CGM
Current CGM systems display the glucose level, the direction and magnitude of change of glucose levels, and can be used as a tool to predict impending glucose excursions (hypo- and hyperglycemia), and to assess glycemic variability.7,8 In addition, reliable alarm signals of low or high glucose values warn the patient to take action.9 All this is being executed on a near-continuous basis, throughout the day, and this for several days, thereby facilitating pattern recognition, and helping the patient (and physician) to optimize therapy and improve metabolic control. In the future, by means of complex mathematical trend analysis, the course of glycemic excursions may be predicted for longer time periods ahead, allowing the patient to take preventative actions in case of impending hypoglycemia.10 Quality of life might also improve by using real-time CGM via reducing the fear of unexpected hypoglycemia.
A number of therapeutic recommendations can be made using CGM to improve metabolic control and to avoid hypoglycemia. These could include changing the insulin regimen (from regular to rapid-acting insulin analogs, from neutral protamine Hagedorn to long-acting insulin analogs, changing the number of daily injections, starting insulin pump with appropriate basal and bolus insulin dosages), adapting the mealtime insulin bolus dosage, changing the insulin-to-glucose correction algorithm, changing the carbohydrate content of the meal, altering the insulin dosage for exercise, adapting the nighttime insulin dosage to avoid the dawn phenomenon, etc.8,11–13 CGM data also show the effect of exercise and food composition on glucose levels. Use of CGM in adolescent outpatients with diabetes achieved a significant improvement in metabolic control, not only by providing accurate data for adjustment of insulin treatment but also by promoting patient communication and motivation.12,14
Indications for CGM
Use of CGM devices can be indicated for:
patients with brittle diabetes who are in poor metabolic control and/or have high glucose variability
patients with hypoglycemia unawareness or fear of hypoglycemia
patients with gastroparesis
pregnant women with diabetes (type 1, type 2, or gestational diabetes)
critically ill patients with or without diabetes
Despite extensive efforts, the efficacy of the various insulin regimens in achieving good metabolic control is limited in patients with wide inter- and intraday glycemic variability/fluctuations (so-called brittle diabetes). Real-time CGM systems can be used as a tool to show trends and predict impending glucose excursions (hypo- and hyperglycemia), and to monitor glycemic variability. By making therapeutic adjustments based on these trends, CGM may enable patients to reduce glycemic variability and increase the time spent in normoglycemia.14,15 For some patients, a decreased amount of glycemic instability alone, even without any improvement in HbA1c, might represent an improved outcome. Glycemic control may be better reflected by glucose variability, or a new measure derived from the duration of normo-, hypo-, and hyperglycemia, in conjunction with HbA1c, rather than by HbA1c alone,5 because in a patient with brittle diabetes, many hypo- and hyperglycemic spikes may cancel each other out in terms of altering HbA1c.
CGM devices can also be used as an educational tool to document the incidence and magnitude of hypoglycemia. A rather high prevalence of hypoglycemia, particularly nocturnal hypoglycemia, has been observed in patients either on multiple daily injections or treated by means of an insulin pump.16–18 This disturbing frequency of nocturnal hypoglycemia often remains undetected by standard SMBG because fingersticks are rarely done at night. Half of these episodes were asymptomatic. Real-time CGM systems can be used as a tool to identify (nocturnal) hypoglycemia and predict impending glucose excursions (hypo- and hyperglycemia). Alarm signals of hypo- and hyperglycemic values warn the patient to take preventative actions. This particular characteristic represents a major advantage for the diabetic patient with hypoglycemia unawareness, allowing the patient to feel more confident and to improve metabolic control and quality of life.
CGM highlights different contributions of fasting and postprandial glucose values at different HbA1c levels in contrast to SMBG measurements, and can be used as a tool to assess the effect of a meal on postprandial glycemia. CGM can detect high postprandial glucose levels more reliably than SMBG. Indeed, the optimal timing of postprandial glucose measurement varies according to the composition of each meal, and single postprandial measurements can miss the highest peak values, which are only detectable with CGM. Diabetic gastroparesis, affecting nearly half of all patients with diabetes, may cause hypoglycemia soon after the meal, followed by hyperglycemia and further delays in gastric emptying. Improved metabolic control can be achieved by lowering postprandial glycemia, possibly by using rapid-acting insulin analogs. This can be evaluated using CGM.19–21
Strict glycemic control in diabetic pregnancy reduces the risk of macrosomia, fetal malformations, spontaneous preterm delivery, Caesarean section, and other complications. Likely advantages of using CGM in pregnancy include developing a normoglycemic target for pregnant women with diabetes to aim for during their pregnancy,22 helping start or adjust insulin treatment,23 improving overall glucose profile,24–28 and decreasing the risks of poor fetal outcomes. Large-scale studies are needed to evaluate whether CGM-guided initiation of antihyperglycemic therapy results in less complications. Murphy et al. reported a prospective randomized study of CGM in 57 women with pregestational type 1 (n = 40) or type 2 (n = 17) diabetes with 7-day CGM profiles during each trimester.28 During the first trimester, women with diabetes spent only 50% or 12 h/day in the euglycemic range (70–140 mg/dl). Furthermore, despite intensive multidisciplinary team advice, including the use of CGMS® (Medtronic MiniMed, Northridge, CA) as an educational and therapeutic tool, the proportion of time spent euglycemic has risen to only 66%, or 16 h/day by the end of pregnancy. During the critical stages of organogenesis, up to 8 weeks' gestation, women with type 2 diabetes were spending as much time hyper-glycemic as those with type 1 diabetes. The reduction in hyperglycemia achieved by the end of the first trimester may therefore be too late to reduce rates of malformation.
Provided that CGM systems give accurate results in critically ill patients (admitted in the intensive care unit (ICU), coronary care unit, or medium care unit), these devices can save time for nursing staff and ensure that measurements are made on time. Stress hyperglycemia recently became a major therapeutic target in the ICU since it occurs in most critically ill patients and is associated with adverse outcome, including increased mortality. Intensive insulin therapy to achieve normoglycemia may reduce mortality, morbidity, and the length of ICU and in-hospital stay. However, the inherent clinical perturbations in critically ill patients (fluctuating severity of illness, changes in nutritional delivery, administration of drugs, etc.) result in frequent changes in insulin requirements. Thus, obtaining normoglycemia requires extensive efforts from the medical staff, including frequent glucose monitoring and adjustment of insulin dose. Current insulin titration is based upon intermittent glucose measurements, which may miss fast changes in glycemia and do not give a full picture of overall glycemic control. Recent evidence suggests that CGM may help to signal glycemic excursions and eventually to optimize insulin titration in the ICU. In the future, the development of a closed-loop control system that automatically regulates the dose of insulin based on continuous glucose measurements and a good algorithm could permit tight glycemic control without increasing nursing workload.29
CGM and Health Outcome
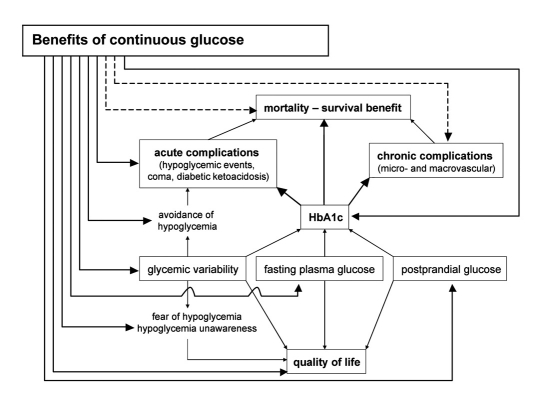
To our knowledge there are no randomized controlled trials (RCTs) that have studied the effect of CGM on diabetes-related morbidity (chronic micro- and macrovascular complications) and on mortality (Medline last searched January 1, 2008). Since no hard endpoints have been investigated, probably due to the fact that CGM systems have only recently become available, one has to focus on surrogate endpoints, including HbA1c, glycemic variability, number and length of hypo- and hyperglycemic episodes, and quality of life. The outline of this article is represented in Figure 1. Nonrandomized, uncontrolled trials of CGM have demonstrated improvement in HbA1c and glycemic excursions11,12,14,30–36 (Table 1), but what is the evidence from RCTs?
Figure 1.
Possible benefits of continuous glucose monitoring systems. Mortality and morbidity of a patient with diabetes are largely determined by the level of metabolic control (HbA1c), which is in turn largely determined by fasting and postprandial glucose control and glycemic variability. There is no evidence of any benefit of CGM on hard endpoints such as mortality, chronic complications, only limited evidence on acute complications (number of hypoglycemic episodes), and more evidence on surrogate endpoints including HbA1c, fasting and postprandial glycemia, glycemic variability, fear of hypoglycemia, and quality of life.
Table 1.
Observational Trials of CGM on Metabolic Outcome
| Study | CGM device | Use of CGM | Number of patients | Length of study | ΔHbA1c | Hypoglycemia |
|---|---|---|---|---|---|---|
| Bode et al. 1999 | CGMS | retrospective | 9 t1 DM adults | 10 weeks | −1.3% (p = .019) | |
| Kaufman et al. 2001 | CGMS | retrospective | 47 children | 6 months | −0.3% (p <.04) | |
| Salardi et al. 2002 | CGMS | retrospective | 44 t1DM | 6 months | −0.43% (p = .032) | |
| Schiaffini et al. 2002 | CGMS | retrospective | 18 children | 6 weeks | Δ fructosamine: −19 mmol/liter (p <.05) | number of events: −1.4 event/72h |
| Schaepelynck-Bélicar et al. 2003 | CGMS | retrospective | 12 adolescents | 2 months | −1.55% (p <.05) | |
| Garg et al. 2004 | DexCom implantable | real-time | 15 t1 DM adults | 3 months | 47% less time in hypo- and 25% less time in hyperglycemia | p <.05 |
| Garg et al. 2006 | DexCom STS | real-time | 86 t1 and t2 DM adults | 21 days | 33% less time in hypo- and 28% less time in hyperglycemia | p <.05 |
| DirecNet 2007 | FreeStyle Navigator | real-time | 30 insulin pump t1 DM children | 13 weeks | −0.3% (p = .02) | |
| Garg et al. 2007 | DexCom STS | real-time | 47 t1 DM adults | 12 weeks | −0.4 vs +0.3% (p = .039) | |
| Bailey et al. 2007 | DexCom STS | real-time | 140 t1 and t2 DM adults | 12 weeks | −0.4% (p <.0001) |
Notes: DM, diabetes mellitus; NS, not significant.
Randomized Controlled Trials of CGM and HbA1c
A total of 12 RCTs of CGM on metabolic control have been performed so far37–49 (Table 2). Of the 11 RCTs of CGM systems that used HbA1c as the primary endpoint, 7 used CGM in a retrospective, Holter-like manner, and 4 in a real-time modus. Only one of out these 7 CGM studies making therapeutic adjustments based on retrospective analysis of CGM data showed improved mean HbA1c levels as compared to standard SMBG monitoring.40 In contrast, except for one trial,42,43 in all studies making dynamic adjustments based on real-time CGM data and verified by SMBG, CGM was associated with improved mean HbA1c levels compared to standard monitoring (Table 2). Real-time CGM improved glycemic excursions, reduced glycemic variability, decreased time spent in hypo- and hyperglycemia, and improved HbA1c.38,45,46,49 Many studies are, however, limited by small sample size and variation in the number of CGM uses per week, contributing to variation in outcomes.
Table 2.
Randomized Controlled Trials of CGM on Metabolic Outcome
| Study | Type of study | CGM device | Use of CGM | Number of patients | Length of study | ΔHbA1c (intervention vs control) | Hypoglycemia |
|---|---|---|---|---|---|---|---|
| Chase et al. 2001 | RCT | CGMS | retrospective | 11 children | 1 month | −0.36 vs −0.20% (NS) | |
| Chase et al. 2003 | RCT | GW2B | real-time | 40 children | 3 months | −0.5 vs + 0.4% (p <.05) | |
| Chico et al. 2003 | RCT | CGMS | retrospective | 75 t1 DM adults | 3 months | −0.8 vs −0.5% (NS) | |
| Ludvigsson and Hanas 2003 | RCT / crossover | CGMS | retrospective | 27 t1DM adults | 3 + 3 month crossover | −0.41 vs −0.1% (p = .011) | |
| Tanenberg et al. 2004 | RCT | CGMS | retrospective | 128 t1 and t2 DM adults | 3 months | −0.8 vs −0.7% (NS) | reduced duration in hypo: 49 vs 81 min (p = .009) |
| DirecNet 2005 | RCT | GW2B | real-time | 200 children | 6 months | +0.1 vs −0.1% (NS) | NS |
| Lagarde et al. 2006 | RCT | CGMS | retrospective | 27 children | 6 months | −0.61 vs −0.28% (NS) | |
| Garg et al. 2006 | RCT | DexCom STS | real-time | 91 t1 and t2 DM adults | 10 days | 21% less time in hypo- and 23% less time in hyperglycemia | p <.0001 |
| Deiss et al. 2006 | RCT | Guardian RT | real-time | 81 children and 81 adults | 3 months | −1.0 vs −0.4% (p = .003) | |
| Deiss et al. 2006 | RCT / crossover | CGMS | retrospective | 30 children and adolescents | 3 + 3 month crossover | +0.1 vs −0.1% (NS) | |
| Yates et al. 2006 | RCT | CGMS | retrospective | 36 children and adolescents | 12 weeks | −0.4 vs −0.4% (NS) | |
| Lee et al. 2007 | RCT | Paradigm RT (sensor-augmented pump) | real-time | 16 t1DM adults | 15 weeks | −2.05 vs −1.08% (p = .02) |
Notes: DM, diabetes mellitus; NS, not significant.
In 2001, Chase et al. reported a 1-month pilot RCT of the CGMS® (Medtronic MiniMed, Northridge, CA) in 11 children as an adjunct to SMBG (≥4/day) to improve HbA1c.37 Patients in the CGM-plus-SMBG group (n = 6) underwent 18 days of CGM. The CGMS group experienced a decrease in mean HbA1c of 0.36% (p <.01), compared to a nonsignificant decrease in HbA1c of 0.2% in the control group.
In 2003, Chase et al. conducted a single-blinded RCT in 40 children, to evaluate the use of the GlucoWatch® G2™ Biographer system (GW2B) (Cygnus, Inc., Redwood City, CA) as an adjunct to SMBG.38 Subjects in the GW2B group used it for 3 months (intervention phase) and were then followed for another 6 months (observational phase). An alarm sounded during CGM if blood glucose was ≥70 mg/dl. After 3 months, HbA1c values improved from 8.9 to 8.4% in GW2B users and worsened from 8.6 to 9.0% in control subjects (difference between subgroups, p <.05). During the observation phase, the HbA1c in the GW2B group remained lower than in the control group, but differences were not statistically significant.
Chico et al. reported the results of a 3-month RCT of 75 subjects with type 1 diabetes, evaluating the use of the CGMS (n = 40) as an adjunct to SMBG, versus intensive (≥8/day) SMBG measurements (n = 35).39 HbA1c decreased in both the CGMS (from 8.3 to 7.5%, p <.01) and the control (from 8.0 to 7.5%, p <.01) groups. CGMS detected unrecognized hypoglycemias in half of the patients, with >70% of all events occurring at night. However, the CGMS did not result in better outcomes compared with SMBG. However, performing an eight-point SMBG profile is not common practice in daily life.
Ludvigsson and Hanas published a controlled, crossover trial comparing the effect of CGM or 7-point glucose profiles on HbA1c.40 During the open arm of the trial, 27 subjects with type 1 diabetes wore the CGMS for 3 days every 2 weeks for 3 months, and during the blinded (to CGMS data) arm, the 7-point glucose profiles were done every week for 3 months. At 3 months, the two study arms were crossed over. HbA1c levels decreased in the open arm using the CGMS (from 7.70 to 7.31%; p = .013), but not in the blinded arm (7.75 to 7.65%, not significant). The difference in change in HbA1c between study arms was significant (p = .011).
In 2004, Tanenberg et al. performed a 12-week trial comparing CGMS with standard SMBG in 128 insulin-treated subjects with diabetes.41 In both groups, HbA1c levels decreased significantly compared with baseline values, but the difference was not statistically significant between groups. One must realize that CGMS data were used in a retrospective way and that both groups performed nearly 7 SMBG measurements per day. The CGMS group, however, had a shorter mean duration of sensor readings: ≤60 mg/dl at week 12 of the study (49 vs 81 min, p = .009). Thus, the same improvement in HbA1c was accomplished with a shorter duration of hypoglycemic events.
In 2005, DirecNet (Diabetes Research in Children Network) reported a 6-month multicenter RCT, studying 200 children with diabetes, assessing the use of the GW2B in addition to SMBG compared with 8-point SMBG alone. No benefits on glycemic control and on the incidence of hypoglycemia were observed when using the GW2B.42,43
In 2006, Garg et al. performed a multicenter RCT in 91 insulin-requiring patients with diabetes. They used the DexCom™ Short-Term CGM System (STS) (DexCom, San Diego, CA) for three consecutive periods of 3 days in all subjects, but in the control group real-time data were not displayed to the subjects (blinded fashion).45 When compared with control subjects, the CGM subgroup that was able to see the glucose data spent 21% less time in hypoglycemia, 23% less time in hyperglycemia, and 26% time more in target glycemia (81–140 mg/dl). Nocturnal hypoglycemia was also reduced by 38%. Real-time CGM thus improved glycemic excursions by reducing hypo- and hyperglycemic episodes.
Deiss et al. reported a 3-month, multicenter RCT in 81 children and 81 adults with type 1 diabetes.46 Patients were randomized into three groups. The first group used the Guardian® REAL-Time (RT) CGM System (Medtronic MiniMed, Northridge, CA) continuously, the second group used the Guardian RT biweekly for 3-day periods every 2 weeks, and the last group used SMBG (5/day). HbA1c, which was high at the start of the study, was reduced in all groups, but significantly more in the group that used the Guardian RT on a continuous basis as compared to the control group.
Deiss et al. also performed a double-blinded crossover study in 30 children and adolescents with type 1 diabetes.47 CGMS was used openly in a 3-month period and then in a blinded fashion, or in reversed order. Patients received 3 days of CGMS at the beginning, at 3 and at 6 months, totaling 9 days. No benefits of using CGMS were shown. However, even though CGMS provided information over a few days, it is possible that it is not sufficiently representative of overall glycemic control, particularly in patients with marked day-to-day glycemic variability. In such patients, CGM should probably be used at least 5 days a week. Yates et al. published a 12-week RCT performed in 36 children and adolescents, comparing the effects of CGMS versus SMBG on glycemic control. In both groups, HbA1c decreased in a comparable way. However, in the CGMS group, improved HbA1c was at the expense of increased duration of hypoglycemia.48
Sensor-augmented insulin pump therapy is a convergence of two technologies: continuous insulin pump therapy and real-time CGM. Lee et al. evaluated the Guardian REAL-Time and Paradigm® CGM System (Medtronic MiniMed, Northridge, CA) as compared to multiple daily injections with SMBG.49 This was a 15-week treat-to-target RCT in 16 patients with type 1 diabetes. Metabolic control improved in both groups (HbA1c -2.05% in the intervention group, and -1.08% in the control group). The reduction in HbA1c was greater in the intervention group (p = .02), but this group had a higher initial HbA1c level and a greater number of clinician visits. However, the proof-of-concept was established.
The Juvenile Diabetes Research Foundation is currently sponsoring a RCT in which 330 patients with type 1 diabetes are enrolled to assess the efficacy of real-time CGM.50 Outcome measures that will be investigated include HbA1c, episodes of severe hypoglycemia, percentage of sensor values in target range (70–180 mg/dl), measures of variability, and quality of life indices at 6 months and 1 year (http://clinicaltrials.gov/ct/show/NCT00406133).
CGM and Hypoglycemia
CGM has been used to assess hypoglycemia perception and to evaluate the impact of treatment changes on hypoglycemia awareness,11,51 and as a therapeutic tool to decrease the incidence and magnitude of hypoglycemia.32–34,52 Schiaffini et al. reported a reduced number of hypoglycemic events per 72 h after adapting therapy according to CGM results, compared with the incidence rate among baseline measurements (2.5 vs 3.9 episodes per 72 h, p <.05).32 Garg et al. used a longterm, investigational, subcutaneously implanted CGM sensor (DexCom, San Diego, CA) in 15 patients with type 1 diabetes. Subjects spent 47% less time <60 mg/dl (p <.05) and 25% less time >240 mg/dl (p <.05) during the nonblinded study period compared with during the blinded control period.33 As described above, only two RCTs have shown a decrease in incidence and/or length of hypoglycemic episodes,41,45 whereas one RCT observed no benefit of CGM42 (Table 2).
CGM and Glycemic Excursions/Variability
Reducing glycemic variability might also be an important aspect of glucose management. In 2007, the DirecNet study group investigated the use of the FreeStyle Navigator™ CGM System (Abbott Laboratories, Alameda, CA) in 30 insulin pump-using children with type 1 diabetes.14 Patients were asked to wear the Navigator daily for 13 weeks. HbA1c improved from 7.1 to 6.8% at week 13 (p = .02). Glycemic variability also decreased during the study period. Kovatchev and Clarke also observed decreased glycemic variability using real-time CGM.15
CGM and Quality of Life
In addition to glycemic benefits, clinical use of CGM devices could substantially affect diabetes management and generate both beneficial and adverse psychological reactions. Motivational benefits include reinforcements of concepts taught in diabetes education, enhanced diabetes self-efficacy, decreased anxiety about unexpected hypoglycemia, increased flexibility in daily life, and enhanced motivation for improved glycemic control.53 Conversely, CGM may also lead to information overload and increased treatment burden. Indeed, a potential concern with real-time CGM is that patients might not be able to deal with all the additional data, or might over-correct each increase in glycemia.
DirecNet performed a psychological assessment in a RCT of the GlucoWatch G2 Biographer system in 200 children with type 1 diabetes. The continuous glucose monitor satisfaction scale, CGM-SAT, was developed.42 Diabetes treatment adherence, diabetes-specific quality of life, and diabetes-related anxiety were assessed. The results indicated neither adverse nor beneficial psychological effects of CGM use. However, it is important to note that patients were more dissatisfied with the technical functioning of the device [local skin irritation, excessive (false) alarms, and suboptimal accuracy] than about the psychological ramifications of using the device.54 Using the FreeStyle Navigator CGM System, the DirecNet study group observed very positive comments by patients and parents.14 Specifically, most subjects used the Navigator on an almost daily basis, and more than 70% of both patients and parents agreed that the use of the Navigator made adjusting insulin easier, made them more sure about making diabetes decisions, showed patterns in blood glucose not seen before, and clarified how eating habits affected glycemia. Quality of life improved by reducing the fear of hypoglycemic events.
Limitations of Current CGM
First of all, it is of paramount importance to select the proper patients. Patients that are likely to benefit and safely use CGM systems are those who are motivated to participate in the care of their diabetes, are well informed about the importance of strict metabolic control, and are technologically adept. In contrast, patients who have poor control because of reluctance to perform SMBG will not comply with CGM and will not be helped by use of a CGM device if they are not interested in using it.36,55,56 All this is even more important for real-time than for retrospective use of the CGM system. A linear relationship was indeed observed between CGM compliance and lower HbA1c levels: each 10% increase in sensor utilization was associated with a 41% increased probability of a 0.5% HbA1c reduction.55 Bailey et al., applying real-time CGM in 140 adults with type 1 and type 2 diabetes, also noticed that increased use of CGM was associated with greater reductions in HbA1c.36
Second, currently available real-time CGM systems are approved as an adjunct to SMBG and should not be used to make therapeutic decisions. Instead, CGM readings that indicate hypo- or hyperglycemic events must be verified by SMBG, before taking action.
Third, patients, but also physicians, need to be educated and instructed how to use the large amount of data generated by the real-time CGM system. They must also be flexible in adapting their insulin therapy and must be aware of the pharmacodynamic profile of the insulin preparation they use. This last topic is very important in order to avoid repeated and excessive injections of insulin-correction boluses.
Fourth, to successfully implement CGM in daily practice, it is very important that the treating physician and the patient are aware of the limitations of current CGM systems, which originate from physiological and technical aspects including lag time and calibration issues. For CGM systems to be used in daily practice, their technical performance and accuracy must prove reliable. Accuracy depends partly on physiological and device-specific lag time and on calibration issues of the system.7
Fifth, currently available CGM systems are minimally invasive and require insertion of a needle or microdialysis catheter into the subcutaneous adipose tissue to measure glucose in the interstitial fluid. The sensor or microdialysis catheter needs to be firmly attached to the skin using tape, which may cause skin irritation or infection, and does not allow the patient to take a bath. For patients, the CGM system should be as small as possible, should not interfere with their daily life, should have a warning system for impending hypo- or hyperglycemic excursions, and should offer real-time glucose values without delay. Also, replacement of the sensor should be easy and painless.
Sixth, a major disadvantage of CGM is that the functional operability is limited to 2 to 7 days, which may be too short to detect recurrent glycemic patterns throughout the day or night. The sensor signal should also be stable and without drift.
Only after all this has been considered will these new CGM "toys" become "tools" to improve the life of the patient with diabetes.
Many trials performed in the past had one or more deficiencies, either being uncontrolled, not blinded, only observational, inadequately powered, too short in duration, or not statistically or clinically significant. Well-designed randomized controlled trials are critically needed to address the likely benefits of CGM.
Conclusions and Perspective
At present, a number of accurate, minimally invasive CGM systems are available that measure glucose in the interstitial fluid, that can either be used in a retrospective way, or operate in real-time. CGM devices provide information about the direction, magnitude, duration, and frequency of glycemic fluctuations, and may facilitate specific therapeutic adjustments that need to be made to avoid hypo- and hyperglycemic excursions, thereby improving metabolic control. Patients who are motivated to participate in the care of their diabetes and are technologically adept are likely to safely use CGM systems. In particular, pregnant women, pump users, and patients with brittle diabetes, hypoglycemia unawareness, or gastroparesis may benefit from CGM. However, to successfully implement CGM in daily practice, the patient and the treating physician must be aware of the limitations of current CGM systems, which originate from physiological and technical aspects. Only a few randomized controlled trials using real-time CGM have provided us with limited evidence in favor of improved metabolic control, reductions in HbA1c, reductions in hypo- and hyperglycemic episodes, and improved quality of life. Whether this is a transient effect or will persist for longer periods, with sustained reduction in HbA1c, remains to be proven. If a CGM system was proven to be accurate, reliable under different conditions, and with sufficient longevity under daily life conditions, then it could reduce the incidence of devastating longterm micro- and macrovascular complications, and might reduce hospitalizations due to diabetic ketoacidosis or hypoglycemic coma, and their associated economic costs. If all this proves to be the case, then CGM systems will be cost-efficient as well, and will be reimbursed by several health care systems. If a reliable and long-lasting CGM system can be used in the future to construct a (semi-) closed loop system—a step towards the artificial pancreas—this would represent a major breakthrough in diabetes care.
Acknowledgments
The content of this manuscript was orally presented at the AIDPIT/EuDTT 2008 Meeting (Artificial Insulin Delivery, Pancreas and Islet Transplanttion/European Diabetes Technology and Transplantation Meeting), Igls, Austria, January 27–29, 2008.
Abbreviations
- CGM
continuous glucose monitoring
- GW2B
GlucoWatch G2 Biographer
- ICU
intensive care unit
- RCT
randomized controlled trial
- RT
real time
- SMBG
self-monitoring of blood glucose
- STS
short-term system
References
- 1.Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329(14):977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 2.UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352(9131):837–853. [PubMed] [Google Scholar]
- 3.Nathan DM, Cleary PA, Backlund JY, Genuth SM, Lachin JM, Orchard TJ, Raskin P, Zinman B. Diabetes Control and Complications Trial / Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353(25):2643–2653. doi: 10.1056/NEJMoa052187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Monnier L, Mas E, Ginet C, Michel F, Villon L, Cristol J, Colette C. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA. 2006;298(14):1681–1687. doi: 10.1001/jama.295.14.1681. [DOI] [PubMed] [Google Scholar]
- 5.Brownlee M, Hirsch IB. Glycaemic variability: a hemoglobin A1c-independent risk factor for diabetic complications. JAMA. 2006;295(14):1707–1708. doi: 10.1001/jama.295.14.1707. [DOI] [PubMed] [Google Scholar]
- 6.Schütt M, Kern W, Krause U, Busch P, Dapp A, Grziwotz R, Mayer I, Rosenbauer J, Wagner C, Zimmermann A, Kerner W, Holl RW. DPV Initiative. Is the frequency of self-monitoring of blood glucose related to long-term metabolic control? Multicenter analysis including 24,500 patients from 191 centers in Germany and Austria. Exp Clin Endocrinol Diabetes. 2006;114(7):384–388. doi: 10.1055/s-2006-924152. [DOI] [PubMed] [Google Scholar]
- 7.Koschinsky T, Heinemann L. Sensors for glucose monitoring: technical and clinical aspects. Diabetes Metab Res Rev. 2001;17(2):113–123. doi: 10.1002/dmrr.188. [DOI] [PubMed] [Google Scholar]
- 8.Klonoff DC. Continuous glucose monitoring: roadmap for 21st century diabetes therapy. Diabetes Care. 2005;28(5):1231–1239. doi: 10.2337/diacare.28.5.1231. [DOI] [PubMed] [Google Scholar]
- 9.Bode B, Gross K, Rikalo N, Schwartz S, Wahl T, Page C, Gross T, Mastrototaro J. Alarms based on real-time sensor glucose values alert patients to hypo- and hyperglycemia: the guardian continuous monitoring system. Diabetes Technol Ther. 2004;6(2):105–113. doi: 10.1089/152091504773731285. [DOI] [PubMed] [Google Scholar]
- 10.Sparacino G, Zanderigo F, Corazza S, Maran A, Facchinetti A, Cobelli C. Glucose concentration can be predicted ahead in time from continuous glucose monitoring sensor time-series. IEEE Trans Biomed Eng. 2007;54(5):931–937. doi: 10.1109/TBME.2006.889774. [DOI] [PubMed] [Google Scholar]
- 11.Kaufman FR, Gibson LC, Halvorson M, Carpenter S, Fisher LK, Pitukcheewanont P. A pilot study of the continuous glucose monitoring system: clinical decisions and glycaemic control after its use in pediatric type 1 diabetic subjects. Diabetes Care. 2001;24(12):2030–2034. doi: 10.2337/diacare.24.12.2030. [DOI] [PubMed] [Google Scholar]
- 12.Schaepelynck-Bélicar P, Vague P, Simonin G, Lassmann-Vague V. Improved metabolic control in diabetes adolescents using the continuous glucose monitoring system (CGMS) Diabete Metab. 2003;29(6):608–612. doi: 10.1016/s1262-3636(07)70076-9. [DOI] [PubMed] [Google Scholar]
- 13.Halvorson M, Carpenter S, Kaiserman K, Kaufman FR. A pilot trial in pediatrics with the sensor-augmented pump: combining real-time glucose monitoring with the insulin pump. J Pediatr. 2007;150(1):103–105. doi: 10.1016/j.jpeds.2006.08.069. [DOI] [PubMed] [Google Scholar]
- 14.Buckingham B, Beck RW, Tamborlane WV, Xing D, Kollman C, Fiallo-Scharer R, Maurus N, Ruedy KJ, Tansey M, Weinzimer SA, Wysocki T. Continuous glucose monitoring in children with type 1 diabetes. J Pediatr. 2007;151(4):388–393. doi: 10.1016/j.jpeds.2007.03.047. Diabetes Research in Children Network (DirecNet) Study Group. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kovatchev B, Clarke W. Continuous glucose monitoring (CGM) reduces risks for hypo- and hyperglycemia and glucose variability in diabetes. Diabetes. 2007;56(Suppl 1):A23. [Google Scholar]
- 16.Bode BW, Schwartz S, Stubbs HA, Block JE. Glycemic characteristics in continuously monitored patients with type 1 and type 2 diabetes: normative values. Diabetes Care. 2005;28(1):2361–2366. doi: 10.2337/diacare.28.10.2361. [DOI] [PubMed] [Google Scholar]
- 17.Wentholt IME, Maran A, Masurel N, Heine RJ, Hoekstra JBL, DeVries JH. Nocturnal hypoglycaemia in type 1 diabetic patients, assessed with continuous glucose monitoring: frequency, duration and associations. Diabet Med. 2007;24(5):527–532. doi: 10.1111/j.1464-5491.2007.02107.x. [DOI] [PubMed] [Google Scholar]
- 18.Amin R, Ross K, Acerini CL, Edge JA, Warner J, Dunger DB. Hypoglycaemia prevalence in prepubertal children with type 1 diabetes on standard insulin regimen: use of continuous glucose monitoring system. Diabetes Care. 2003;26(3):662–667. doi: 10.2337/diacare.26.3.662. [DOI] [PubMed] [Google Scholar]
- 19.Manuel-y-Keenoy B, Vertommen J, Abrams P, Van Gaal L, De Leeuw I, Messeri D, Poscia A. Postprandial glucose monitoring in type 1 diabetes mellitus: use of a continuous subcutaneous monitoring device. Diabetes Metab Res Rev. 2004;20(Suppl.2):S24–S31. doi: 10.1002/dmrr.516. [DOI] [PubMed] [Google Scholar]
- 20.Tanenberg RJ, Pfeifer MA. Continuous glucose monitoring system: a new approach to the diagnosis of diabetic gastroparesis. Diabetes Technol Ther. 2000;2(Suppl.1):S73–S80. doi: 10.1089/15209150050214168. [DOI] [PubMed] [Google Scholar]
- 21.Boland E, Monsod T, Delucia M, Brandt CA, Fernando S, Tamborlane WV. Limitations of conventional methods of self-monitoring of blood glucose: lessons learned from 3 days of continuous glucose sensing in pediatric patients with type 1 diabetes. Diabetes Care. 2001;24(11):1858–1862. doi: 10.2337/diacare.24.11.1858. [DOI] [PubMed] [Google Scholar]
- 22.Yogev Y, Ben-Haroush A, Chen R, Rosenn B, Hod M, Langer O. Diurnal glycemic profile in obese and normal weight nondiabetic pregnant women. Am J Obstet Gynecol. 2004;191(3):949–953. doi: 10.1016/j.ajog.2004.06.059. [DOI] [PubMed] [Google Scholar]
- 23.Kestilä KK, Ekblad UU, Rönnemaa T. Continuous glucose monitoring versus self-monitoring of blood glucose in the treatment of gestational diabetes mellitus. Diabetes Res Clin Pract. 2007;77(2):174–179. doi: 10.1016/j.diabres.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 24.Yogev Y, Ben-Haroush A, Chen R, Kaplan B, Phillip M, Hod M. Continuous glucose monitoring for treatment adjustment in diabetic pregnancies--a pilot study. Diabet Med. 2003;20(7):558–562. doi: 10.1046/j.1464-5491.2003.00959.x. [DOI] [PubMed] [Google Scholar]
- 25.Chen R, Yogev Y, Ben-Haroush A, Jovanovic L, Hod M, Phillip M. Continuous glucose monitoring for the evaluation and improved control of gestational diabetes mellitus. J Matern Fetal Neonatal Med. 2003;14(4):256–260. doi: 10.1080/jmf.14.4.256.260. [DOI] [PubMed] [Google Scholar]
- 26.Kerssen A, de Valk HW, Visser GH. Day-to-day glucose variability during pregnancy in women with type 1 diabetes mellitus: glucose profiles measured with the continuous glucose monitoring system. BJOG. 2004;111(9):919–924. doi: 10.1111/j.1471-0528.2004.00203.x. [DOI] [PubMed] [Google Scholar]
- 27.McLachlan K, Jenkins A, O'Neal D. The role of continuous glucose monitoring in clinical decision-making in diabetes in pregnancy. Aust N Z J Obstet Gynaecol. 2007;47(3):186–190. doi: 10.1111/j.1479-828X.2007.00716.x. [DOI] [PubMed] [Google Scholar]
- 28.Murphy HR, Rayman G, Duffield K, Lewis KS, Kelly S, Johal B, Fowler D, Temple RC. Changes in the glycemic profiles of women with type 1 and type 2 diabetes during pregnancy. Diabetes Care. 2007;30(11):2785–2791. doi: 10.2337/dc07-0500. [DOI] [PubMed] [Google Scholar]
- 29.De Block C, Manuel-y-Keenoy B, Van Gaal L, Rogiers P. Intensive insulin therapy in the intensive care unit: assessment by continuous glucose monitoring. Diabetes Care. 2006;29(8):1750–1756. doi: 10.2337/dc05-2353. [DOI] [PubMed] [Google Scholar]
- 30.Bode BW, Gross TM, Thornton KR, Mastrototaro JJ. Continuous glucose monitoring used to adjust diabetes therapy improves glycosylated hemoglobin: a pilot study. Diabetes Res Clin Pract. 1999;46(3):183–190. doi: 10.1016/s0168-8227(99)00113-8. [DOI] [PubMed] [Google Scholar]
- 31.Salardi S, Zucchini S, Santoni R, Ragni L, Gualandi S, Cicognani A, Cacciari E. The glucose area under the profiles obtained with continuous glucose monitoring system relationship with HbA(1c) in pediatric type 1 diabetic patients. Diabetes Care. 2002;25(1):1840–1844. doi: 10.2337/diacare.25.10.1840. [DOI] [PubMed] [Google Scholar]
- 32.Schiaffini R, Ciampalini P, Fierabracci A, Spera S, Borrelli P, Bottazzo GF, Crino A. The Continuous Glucose Monitoring System (CGMS) in type 1 diabetic children is the way to reduce hypoglycemic risk. Diabetes Metab Res Rev. 2002;18(4):324–329. doi: 10.1002/dmrr.309. [DOI] [PubMed] [Google Scholar]
- 33.Garg SK, Schwartz S, Edelman SV. Improved glucose excursions using an implantable real-time continuous glucose sensor in adults with type 1 diabetes. Diabetes Care. 2004;27(3):734–738. doi: 10.2337/diacare.27.3.734. [DOI] [PubMed] [Google Scholar]
- 34.Garg S, Jovanovic L. Relationship of fasting and hourly blood glucose levels to HbA1c values: safety, accuracy, and improvements in glucose profiles obtained using a 7-day continuous glucose sensor. Diabetes Care. 2006;29(12):2644–2649. doi: 10.2337/dc06-1361. [DOI] [PubMed] [Google Scholar]
- 35.Garg SK, Kelly W, Voelmle M, Ritchie P, Gottlieb P, McFann K, Ellis SL. Continuous home monitoring of glucose: improved control with real-life use of continuous glucose sensors in adult subjects with type 1 diabetes. Diabetes Care. 2007;30(12):3023–3025. doi: 10.2337/dc07-1436. [DOI] [PubMed] [Google Scholar]
- 36.Bailey TS, Zisser HC, Garg SK. Reduction in hemoglobin A1c with real-time continuous glucose monitoring: results from a 12-week observational study. Diab Technol Ther. 2007;9(3):203–210. doi: 10.1089/dia.2007.0205. [DOI] [PubMed] [Google Scholar]
- 37.Chase HP, Kim LM, Owen SL, MacKenzie TA, Klingensmith GJ, Murtfeldt R, Garg SK. Continuous subcutaneous glucose monitoring in children with type 1 diabetes. Pediatrics. 2001;107(2):222–226. doi: 10.1542/peds.107.2.222. [DOI] [PubMed] [Google Scholar]
- 38.Chase HP, Roberts MD, Wightman C, Klingensmith G, Garg SK, Van Wyhe M, Desai S, Harper W, Lopatin M, Bartkowiak M, Tamada J, Eastman RC. Use of the GlucoWatch biographer in children with type 1 diabetes. Pediatrics. 2003;111(4 Pt 1):790–794. doi: 10.1542/peds.111.4.790. [DOI] [PubMed] [Google Scholar]
- 39.Chico A, Vidal-Rios P, Subira MN, Novials A. The continuous glucose monitoring system is useful for detecting unrecognized hypoglycemias in patients with type 1 and type 2 diabetes but is not better than frequent capillary glucose measurements for improving metabolic control. Diabetes Care. 2003;26(4):1153–1157. doi: 10.2337/diacare.26.4.1153. [DOI] [PubMed] [Google Scholar]
- 40.Ludvigsson J, Hanas R. Continuous subcutaneous glucose monitoring improved metabolic control in pediatric patients with type 1 diabetes: a controlled crossover study. Pediatrics. 2003;111(5):933–938. doi: 10.1542/peds.111.5.933. [DOI] [PubMed] [Google Scholar]
- 41.Tanenberg R, Bode B, Lane W, Levetan C, Mestman J, Harmel AP, Tobian J, Gross T, Mastrototaro J. Use of the Continuous Glucose Monitoring System to guide therapy in patients with insulin-treated diabetes: a randomized controlled trial. Mayo Clin Proc. 2004;79(12):1521–1526. doi: 10.4065/79.12.1521. [DOI] [PubMed] [Google Scholar]
- 42.Chase HP, Beck R, Tamborlane W, Buckingham B, Mauras N, Tsalikian E, Wysocki T, Weinzimer S, Kollman C, Ruedy K, Xing D DirecNet Study Group. A randomized multicenter trial comparing the GlucoWatch Biographer with standard glucose monitoring in children with type 1 diabetes. Diabetes Care. 2005;28(5):1101–1106. doi: 10.2337/diacare.28.5.1101. [DOI] [PubMed] [Google Scholar]
- 43.Fiallo-Scharer R Diabetes Research in Children Network Study Group. Eight-point glucose testing versus the continuous glucose monitoring system in evaluation of glycemic control in type 1 diabetes. J Clin Endocrinol Metab. 2005;90(6):3387–3391. doi: 10.1210/jc.2004-2510. [DOI] [PubMed] [Google Scholar]
- 44.Lagarde WH, Barrows FP, Davenport ML, Kang M, Guess HA, Calikoglu AS. Continuous subcutaneous glucose monitoring in children with type 1 diabetes mellitus: a single-blind, randomized, controlled trial. Pediatric Diabetes. 2006;7(3):159–164. doi: 10.1111/j.1399-543X.2006.00162.x. [DOI] [PubMed] [Google Scholar]
- 45.Garg S, Zisser H, Schwartz S, Bailey T, Kaplan R, Ellis S, Jovanovic L. Improvement in glycemic excursions with a transcutaneous, real-time continuous glucose sensor: a randomized controlled trial. Diabetes Care. 2006;29(1):44–50. doi: 10.2337/diacare.29.01.06.dc05-1686. [DOI] [PubMed] [Google Scholar]
- 46.Deiss D, Bolinder J, Riveline JP, Battelino T, Bosi E, Tubiana-Rufi N, Kerr D, Phillip M. Improved glycemic control in poorly controlled patients with type 1 diabetes using real-time continuous glucose monitoring. Diabetes Care. 2006;29(12):2730–2732. doi: 10.2337/dc06-1134. [DOI] [PubMed] [Google Scholar]
- 47.Deiss D, Hartmann R, Schmidt J, Kordonouri O. Results of a randomised controlled cross-over trial on the effect of continuous subcutaneous glucose monitoring (CGMS) on glycaemic control in children and adolescents with type 1 diabetes. Exp Clin Endocrinol Diabetes. 2006;114(2):63–67. doi: 10.1055/s-2006-923887. [DOI] [PubMed] [Google Scholar]
- 48.Yates K, Milton AH, Dear K, Ambler G. Continuous glucose monitoring-guided insulin adjustments in children and adolescents on near-physiological insulin regimens. Diabetes Care. 2006;29(7):1512–1517. doi: 10.2337/dc05-2315. [DOI] [PubMed] [Google Scholar]
- 49.Lee SW, Sweeney T, Clausen D, Kolbach C, Hassen A, Firek A, Brinegar C, Petrofsky J. Combined insulin pump therapy with real-time continuous glucose monitoring significantly improves glycemic control compared to multiple daily injection therapy in pump naïve patients with type 1 diabetes; single center pilot study experience. J Diabetes Sci Technol. 2007;1(3):400–404. doi: 10.1177/193229680700100313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tamborlane W, Ruedy K, Wysocki T, O'Grady M, Kollman C, Block J, Chase HP, Hirsch I, Huang E, Beck R, Wilson D, Lawrence J, Laffel L. JDRF randomized clinical trial to assess the efficacy of real-time continuous glucose monitoring in the management of type 1 diabetes: research design and methods. Diabetes Technol Ther. 2008;10 doi: 10.1089/dia.2007.0302. (in press) [DOI] [PubMed] [Google Scholar]
- 51.Kubiak T, Hermanns N, Schreckling HJ, Kulzer B, Haak T. Assessment of hypoglycaemia awareness using continuous glucose monitoring. Diabet Med. 2004;21(5):487–490. doi: 10.1111/j.1464-5491.2004.1136.x. [DOI] [PubMed] [Google Scholar]
- 52.Weintrob N, Schechter A, Benzaquen H, Shalitin S, Lilos P, Galatzer A, Phillip M. Glycemic patterns detected by continuous subcutaneous glucose sensing in children and adolescents with type 1 diabetes mellitus treated by multiple daily injections vs continuous subcutaneous insulin infusion. Arch Pediatr Adolesc Med. 2004;158(7):677–684. doi: 10.1001/archpedi.158.7.677. [DOI] [PubMed] [Google Scholar]
- 53.Kruger D, Marcus AO. Psychological motivation and patient education: a role for continuous glucose monitoring. Diabetes Technol Ther. 2000;2(Suppl 1):S93–S97. doi: 10.1089/15209150050214195. [DOI] [PubMed] [Google Scholar]
- 54.Diabetes Research in Children Network (DirecNet) Study Group. Youth and parent satisfaction with clinical use of the GlucoWatch G2 Biographer in the management of pediatric type 1 diabetes. Diabetes Care. 2005;28(8):1929–1935. doi: 10.2337/diacare.28.8.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mitka M. Poor patient adherence may undermine the aim of continuous glucose monitoring. JAMA. 2007;298(6):614–615. doi: 10.1001/jama.298.6.614. [DOI] [PubMed] [Google Scholar]
- 56.Hirsch IB, Bode BW, Abelseth J, Fischer JS, Kaufman FR, Mastrototaro J, Wolpert HA, Buckingham BA. Sensor augmented pump therapy: results of the first treat-to-target study. Diabetes. 2007;56(Suppl 1):A24. doi: 10.1089/dia.2008.0068. [DOI] [PubMed] [Google Scholar]