Abstract
Background
Cardiovascular autonomic neuropathy (CAN) is a disorder of progressive autonomic dysfunction (AD) associated with diabetes and other chronic diseases. Orthostatic hypotension (OH) is one of the most incapacitating symptoms of CAN and AD. AD in OH can include sympathetic withdrawal (SW). To detect and diagnose SW, parasympathetic and sympathetic changes must be clearly differentiated from each other. This is accomplished by means of the novel autonomic nervous system (ANS) method based on the simultaneous spectral analyses of respiratory activity (RA) and heart rate variability (HRV).
Methods
We performed autonomic profiling of 184 (142 females) consecutive, arrhythmia-free patients with type 2 diabetes using the ANX-3.0 autonomic monitoring system. The patient cohort included 86 (64 female) patients for whom an α1-agonist was the only drug changed and increased from one test to the next; 37 (33 female) for whom the α1-agonist was discontinued; and 61 (45 female) who were on an α1-agonist, but for whom no drug changes were made. The tests averaged 3.1 ± 1.4 months apart; midodrine (ProAmatine) was the α1-agonist prescribed. Of the group, 99 patients also had hypertension and 47 also had cardiovascular disease. No patient had supine hypertension.
Results
Changes in parameters from the HRV (without respiration) and ANS methods were compared with changes in heart rate and blood pressure (BP) as measured from one test (test N) to the next (test N + 1). SW with a BP drop of less than the clinical definition may be a trend that can be an early indicator of orthostasis. In this study, patients were treated with low-dose, short-term α1-agonist (vasopressor) therapy, which tended to correct the abnormal trend of SW with a drop in BP. Included in the findings was a systolic BP trend in response to vasopressor therapy of an (expected) initial increase in BP followed by an eventual decrease in systolic BP as SW was reversed.
Conclusions
The ANS method enables quantitative assessment of CAN by independently and simultaneously quantifying the two branches of the ANS, sympathetic and parasympathetic. The ANS method modifies standard spectral analysis of HRV (without RA analysis) by incorporating spectral analysis of RA.
The ANS method appears to model the normal and abnormal responses to upright posture and changes in vasopressor therapy with greater fidelity than the HRV method. Independent, simultaneous assessment of progressive parasympathetic and sympathetic dysfunction, autonomic imbalance, and responses of the two ANS branches to therapy seems to enable early detection and early intervention. Orthostasis, by way of example, illustrates that frequent, sensitive assessments of both ANS branches can improve the negative outcomes associated with CAN.
Keywords: autonomic nervous system, cardiovascular autonomic neuropathy, orthostatic hypotension, postural orthostatic tachycardia syndrome, respiratory activity analysis, vasopressor
Introduction
Cardiovascular autonomic neuropathy (CAN) is progressive and starts without symptoms. The pre-symptomatic state is defined as “autonomic dysfunction” (AD). As AD increases in severity, it leads to peripheral autonomic neuropathy, then diabetic autonomic neuropathy (DAN), and, finally, symptoms associated with end-organ failure. This final stage is also known as CAN.1–3 In patients with diabetes, autonomic assessment is often based on three time-domain heart rate variability (HRV) ratios: the exhalation/inhalation (E/I) ratio from a deep breathing challenge, the Valsalva ratio, and the 30:15 ratio from an upright posture challenge.4 DAN is indicated if two of the three ratios are abnormally low. CAN is indicated if all three ratios are low. DAN is a risk factor for CAN, and both DAN and CAN are associated with blood pressure (BP) anomalies, including orthostatic hypotension (OH).5–8
Orthostatic hypotension is one of the most incapacitating symptoms of autonomic failure, including CAN,9 and may be used as a marker for CAN. In diabetes and other chronic diseases, AD and CAN are also associated with orthostatic symptoms.10 Orthostasis presents earlier in patients with diabetes than in those without.11,12 The earlier onset of orthostasis is partly due to the fact that diabetes can accelerate AD and therefore CAN onset. There is a wide variability in the diagnosis of orthostasis,13 especially in earlier stages when therapy can be lower dose and shorter term.14
Orthostasis may be difficult to detect and diagnose13 because both autonomic nervous system (ANS) branches are actively changing in a coordinated fashion during a normal response to postural change: the parasympathetics decrease or withdrawal and the sympathetics increase or surge.9,15,16 Therefore, diagnosing orthostasis requires a test that independently and simultaneously measures the response of both ANS branches, the sympathetic nervous system and the parasympathetic nervous system. Because CAN is treatable in all stages11,17 and because orthostasis is often treatable in parallel with the other effects of AD,11 the American Diabetes Association and the American Heart Association have recommended testing diabetic patients more than once per year to detect symptoms as early as possible to slow progression of AD.6–8,18,19
Background
Sympathetic insufficiency or sympathetic withdrawal (SW) upon assuming an upright posture (standing), as observed in orthostasis, is abnormal and can present as occasional or frequent dizziness or lightheadedness upon standing. The symptoms of frank SW are associated with improper BP or heart rate (HR) changes. A significant decrease in BP upon standing can lead to OH,20 and a significant increase in BP upon standing can lead to vasoconstrictor syndrome or orthostatic hypertension.21,22 A significant increase in HR upon standing can lead to postural orthostatic tachycardia syndrome (POTS).
The abnormal response of a parasympathetic surge upon assuming an upright posture can force the reactionary sympathetics to increase further to maintain proper BP. This inflated sympathetic reaction not only masks SW but also leads to overcompensation. Overcompensation is associated with abnormal increases in BP, potentially leading to hypertension or increases in HR, and possibly tachycardia.23 To detect and diagnose such a masked SW condition, parasympathetic surges must be clearly differentiated from SW. This differentiation requires simultaneous and independent measures of both parasympathetic and sympathetic branches of the ANS.
Bradley and Davis20 documented the current algorithm for assessing and treating OH. Currently, several tests are performed to assess the effects of assuming an upright posture on the cardiovascular system.24 Diagnoses can include syncope, OH, and POTS. The tilt-table study is one standard test for postural difficulties25 in which a diagnosis is based on symptoms elicited. Since not all patients experience symptoms, an incorrect diagnosis is possible. BP changes resulting from a change in position from supine to standing also reveal postural difficulties.26 However, they do not sufficiently differentiate autonomic involvement.
An autonomic laboratory typically combines tilt and other studies with HRV measures obtained during deep breathing and Valsalva.6,25,27–29 In these cases, waveform analysis of HRV measures alone requires highly trained and experienced physicians to assess the underlying AD.30 Spectral-domain HRV measures (without analysis of respiratory activity) are not very sensitive or specific, and time-domain HRV measures are mixed measures of activity in both ANS branches.30
The literature specifies that spectral analysis of HRV, combined with an independent spectral analysis of respiratory activity (RA), is the only way to obtain quantitative, independent, simultaneous, noninvasive measures of both autonomic branches.1,4,25,30–38
Independently measured and analyzed RA is critical. Fundamentally, two independent measures are required to fully characterize a system with two independent components. HRV is only one such measure. Clinically, HRV is based on a cardiogram, which includes two components: mean HR (mHR) changes over time and respiratory sinus arrhythmia (RSA) changes. These coupled signals are generated from the sympathetics and parasympathetics, respectively, and cannot be uncoupled by studying HRV without respiration.30 A second independent measure of variability is required to uncouple the two components precisely and quantify them accurately. RA is the second independent measure. The method of spectral analysis of HRV, together with spectral analysis of RA, is called ANS monitoring. This is differentiated from the method of spectral analysis of HRV (alone), which is called HRV monitoring.
Autonomic nervous system monitoring can detect preclinical forms of orthostasis on the basis of sympathetic insufficiency or SW prior to changes in BP or HR.13,23,39 Although the long-term benefits of presymptomatic autonomic therapy have yet to be proven, data in this article suggest that treatment of SW can correct orthostatic symptoms prior to the clinical presentation of orthostasis. Treatments include pharmacologic and nonpharmocologic therapies40,41; however, pharmacologic therapies are more common. These can include pyridostygmine,42 volume-expanding agents such as fludrocortisone, or α1-adrenergic agonists (α1-agonist) such as midodrine.9,11–13 SW, prior to clinically abnormal BP changes, can be corrected using low-dose, short-term pharmaceutical therapies.
This article presents spectral analyses of RA and HRV (ANS method) to assess the responses of both ANS branches to postural change before and after α1-agonist (vasopressors) therapy. The objective is to effectively diagnose OH, a potential early marker for CAN. To highlight the efficacy of the ANS method, this article also compares the obtained results with selected standard time-domain and spectral-domain HRV (alone) measures (HRV method), mean HR, and BP.
Methods
We performed autonomic profiling of 184 (142 females) consecutive, arrhythmia-free patients with type 2 diabetes mellitus, serially recruited from ambulatory clinics. Autonomic profiling was performed with the ANX-3.0 autonomic monitoring system (ANSAR Medical Technologies, Inc., Philadelphia, PA). Patients were either receiving an α1-agonist prior to ANS testing or had an α1-agonist introduced immediately following ANS testing. The patient cohort (Table 1) included 86 (64 female) patients for whom an α1-agonist was the only therapy introduced from one test to the next; 37 (33 female) patients for whom the α1-agonist was the only therapy discontinued; and 61 (45 female) patients who had an α1-agonist on board and for whom no therapy changes were made. Forty-five of the 184 patients in the cohort had a change in BP from sitting to standing that qualified as clinical OH (a decrease of 20/10 mm Hg or more). The clinical diagnosis of OH based on abnormal BP was made prior to dosing for 19 of these patients during this study and for 26 of these patients prior to this study. All patients reported symptoms of OH prior to dosing. The tests averaged 3.1 ± 1.4 months apart; midodrine (ProAmatine) was the α1-agonist prescribed. Of the group, 99 patients with type 2 diabetes also had hypertension and 47 also had cardiovascular disease. Patients with supine hypertension were excluded from this study.
Table 1.
Patient Demographicsa
| N | Females | HTN | CVD | Weight (pounds) | Height (inches) | Age (years) | |
|---|---|---|---|---|---|---|---|
| Totals | 184 | 142 | 99 | 47 | 150.6 | 63.6 | 57.5 |
| Midodrine (− +) | 86 | 64 | 52 | 19 | 153.1 | 63.8 | 60.7 |
| Midodrine (+ −) | 37 | 33 | 15 | 10 | 145.1 | 63.1 | 52.4 |
| Midodrine (+ +) | 61 | 45 | 32 | 18 | 153.4 | 63.9 | 59/3 |
The patient population is also sorted by gender and disease state. For the latter, of these 184 patients with type 2 diabetes, 99 were also diagnosed with hypertension (HTN) and 47 were also diagnosed with a cardiovascular disease (CVD). For row headers with midodrine, plus and minus symbols indicate the presence (“+”) or absence (“−”) of the α1-agonist in the two consecutive tests being compared. For example, “midodrine (+ −)” indicates that this subpopulation of patients had midodrine on board (“+”) during the first of two consecutive tests (test N) for which a 5-minute seated to a 5-minute standing postural change was performed. Then midodrine was discontinued (“−”) and approximately 3 months later another seated-to-standing postural change was performed in a second of two consecutive tests (test N + 1). See text for details. Patients with diabetes, without SW, and not on an α1-agonist during this study, “midodrine (− −)” patients, had no abnormal changes in BP or HR with postural change and were not included in this study population, as they did not have more than two serial ANS tests over the time of the study.
The ANX-3.0 uses U.S. Food and Drug Administration-certified software that computes sympathetic [low frequency area (LFa)14] and parasympathetic [respiratory frequency area (RFa)14] activity using spectral analysis of RA concurrent with spectral analysis of HRV (the ANS method). RA was recorded by impedance plethysmography and HRV was computed from a rhythm strip according to standard methods.30 The spectral-analysis method is a continuous wavelet transform (CWT) using the normalized complex Morlet wavelet.12,14,43 The ANS method parameters, including the LFa/RFa ratio or sympathovagal balance (SB), were compared with mHR, BP, and the following classical HRV method parameters (also computed by the ANX-3.0 according to the 1996 standards article30): (1) spectral-domain parameters of low frequency (LF), high frequency (HF), and LF/HF ratio [using a standard fast Fourier transform (FFT), with LF-and HF-normalized (nu) to total spectral power (TSP)]; (2) time-domain parameters (e.g., sdNN); (3) time-domain ratios (e.g., E/I ratio); and (4) statistical parameters (e.g., max/min HR and range HR [rHR = (max - min)HR]). All parameters were computed over a 5-minute seated (resting) baseline and a 5-minute period that included a quick postural change (in under 5 seconds) followed by quiet standing for the remainder of the second 5 minutes.
A normal, healthy, resting cardiogram is dominated by RSA coupled with slower mHR changes over time.14 RSA is mediated via the vagus nerve (a significant part of the parasympathetic nervous system), and mHR changes are mediated primarily by the sympathetic nervous system. This coupling represents sympathetic activity (mHR) as modulated by parasympathetic activity (RSA).30 Uncoupling these components and analyzing them individually using spectral analysis result in independent and simultaneous measures of parasympathetic (vagus) and sympathetic activity. Since the ANS has two independent components, there must be two independent measures to fully characterize it. HRV is only one independent measure. RA is the second measure. Spectral analysis of RA makes uncoupling possible by identifying the average frequency of RSA over a 4-second analysis interval. This frequency is the fundamental respiratory frequency (FRF).
Akselrod and colleagues31–34 determined the bandwidth of the parasympathetic portion of the HRV spectrum, and that the parasympathetic band is centered on the FRF. The computed area under the HRV spectral curve within this band represents parasympathetic activity over the analysis interval. This area is labeled the respiratory frequency area (RFa). The parasympathetic measure is based on a moving frequency band that follows the patient's breathing and is narrower than the HF band used in the HRV method.
The low-frequency band29 is fixed from 0.04 to 0.10 Hz.30 The area under the HRV spectral curve over this band is the LF parameter from the HRV method. In the ANS method, the RFa frequency band can include portions of the LF band, and for the ANS method, the area under the HRV spectral curve over the remaining portion of the LF band is computed as the measure of the sympathetic activity (LFa). For example, when the patient's breathing rate slows and the area under the HRV spectral curve over the LF region changes, the parasympathetic component is identified and removed from the sympathetic activity computations. This separation relieves the problem of the LF being a mixed measure.30,35–37 These two spectra, along with the ANS and HRV components, are recomputed and updated every 4 seconds.12,14,43
The described patients had more than two serial ANS tests over time. Tests between which medication was not introduced served as the basis for comparison with those tests between which medication was changed: introduced or discontinued. In this manner the patients were their own controls. Because more than two serial tests were considered in this study, but only two consecutive tests were analyzed at any one time, the first test of an analysis couplet is labeled “test N” and the second “test N + 1. ”
Test couplets between which the α1-agonist midodrine was introduced were labeled as “midodrine (− +).” Tests between which midodrine was already on board and no change was made were labeled “midodrine (+ +).” Tests between which midodrine was already on board and then discontinued were labeled “midodrine (+ -).” Patients with diabetes, without SW, and not on an α1-agonist throughout this study, e.g., “midodrine (− −)” patients, had no abnormal changes in BP and were not included in this study population, as they did not have more than two serial ANS tests over the time of the study. An example of putting the test abbreviations all together is as follows: midodrine (+ −), test N to test N + 1. This population of patients performed a seated-to-standing postural change with ( “+”) midodrine on board in the first of two consecutive tests ( “test N”) and then approximately 3 months later performed another seated-to-standing postural change ( “test N + 1”) without ( “− ”) midodrine on board (see Table 2).
Table 2.
A Description of Patient Subpopulations and Test Conditions for the Study
| Test N to test N + 1 | |
|---|---|
| Midodrine (− +) | This population of patients performed a seated-to-standing postural change without ("−") midodrine on board in the first of two consecutive tests (“test N”) of a series of four or more tests. Midodrine was dosed immediately following the test, and then approximately 3 months later the patients performed another seated-to-standing postural change (“test N + 1”) with (“+”) midodrine on board. |
| Midodrine (+ −) | This population of patients performed a seated-to-standing postural change with ("+") midodrine on board in the first of two consecutive tests (“test N”) of a series of four or more tests. Midodrine was discontinued immediately following the test, and then approximately 3 months later the patients performed another seated-to-standing postural change (“test N + 1”) without (“−”) midodrine on board. |
| Midodrine (+ +) | This population of patients performed a seated-to-standing postural change with (“+”) midodrine already on board in the first of two consecutive tests (“test N”) of a series of four or more tests. The midodrine dose was not changed after the test, and then approximately 3 months later the patients performed another seated-to-standing postural change (“test N + 1”) with (“+”) midodrine still on board. |
| Midodrine (− −) | This population of patients with diabetes, without sympathetic withdrawal and not on an α1-agonist throughout this study, had no abnormal changes in BP and were not symptomatic. They were not included in this study population, as they did not have more than two serial ANS tests in the time period of the study. |
Data were analyzed using SPSS; all correlations were controlled for age. Significant correlations are indicated (*P < 0.0100 and **P < 0.0010). Two-tailed Student t tests (α = 0.10) were performed between female and male populations assuming equal variance.
Results
Figure 1 shows the average responses of the ANS (left) and HRV (right) parameters from the predosing test (test N). Figure 1 (left) shows that the LFa decreased significantly (P < 0.001) from sitting to standing; systolic BP also decreased upon standing. Both responses are abnormal. In normals, LFa and systolic BP are expected to increase. RFa decreased as expected, and mHR and SB both increased, as expected. Figure 1 (right) shows that the average HRV responses from this cohort included an increase in rHR, LFnu, and LF/HF ratio and a decrease in HFnu and TSP.
Figure 1.
Average ANS (left) and HRV (right) responses from 5-minutes seated to a quick stand followed by 5 minutes of standing from the predosing test (test N). (Left) Sympathetic (LFa) and parasympathetic (RFa) measures, sympathovagal balance (SB = LFa/RFa), systolic BP, and mean HR. (Right) Normalized low- and high-frequency parameters (LFnu and HFnu, respectively), total spectral power (TSP), and range HR. Data represent results from patients presenting with orthostatic symptoms as evidenced by the average decrease in systolic BP (P < 0.0010). Midodrine was introduced following this test in this subpopulation of patients. See text for details.
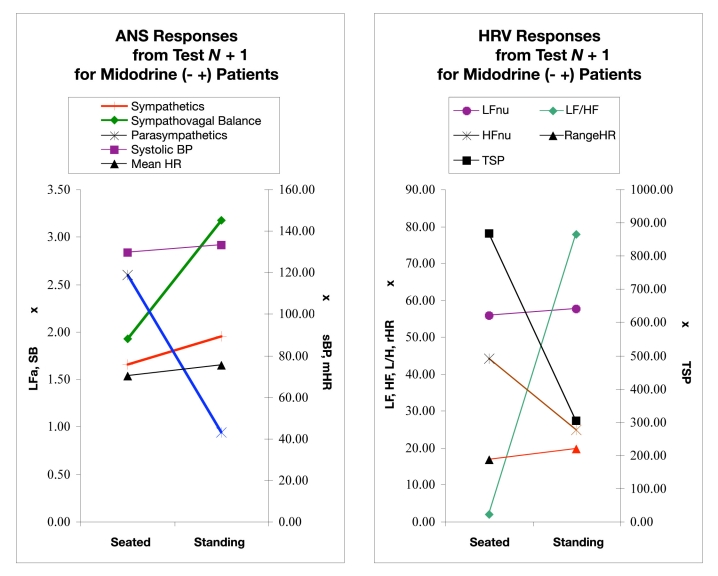
Figure 2 shows average responses of the ANS and HRV parameters from the postdosing test (test N + 1). Figure 2 (left) indicates that LFa increased significantly (P < 0.001) from a seated to a standing position—this is an expected normal response. The mHR, systolic BP, and SB also increased as expected. The average SB increase was larger than for the test N results. RFa decreased as expected, and this average change was larger than that in test N. Figure 2 (right) shows that the average HRV responses among this cohort included little change in rHR and LFnu, an increase in the LF/HF ratio, and a decrease in HFnu and TSP.
Figure 2.
ANS (left) and HRV (right) responses from 5 minutes seated to a quick stand followed by 5 minutes of standing during a follow-up test (test N + 1), approximately 3 months postdosing. (Left) Sympathetic (LFa) and parasympathetic (RFa) measures, sympathovagal balance (SB = LFa/RFa), systolic BP, and mean HR. (Right) Normalized low- and high-frequency parameters (LFnu and HFnu, respectively), total spectral power (TSP), and range HR. These data are from patients first presenting with orthostatic symptoms and for whom midodrine was introduced and are reporting significantly fewer orthostatic symptoms, as evidenced by the change from an average decrease in systolic BP during the first test (Figure 1) to an average increase in systolic BP presented in this figure (P < 0.0010). See text for details.
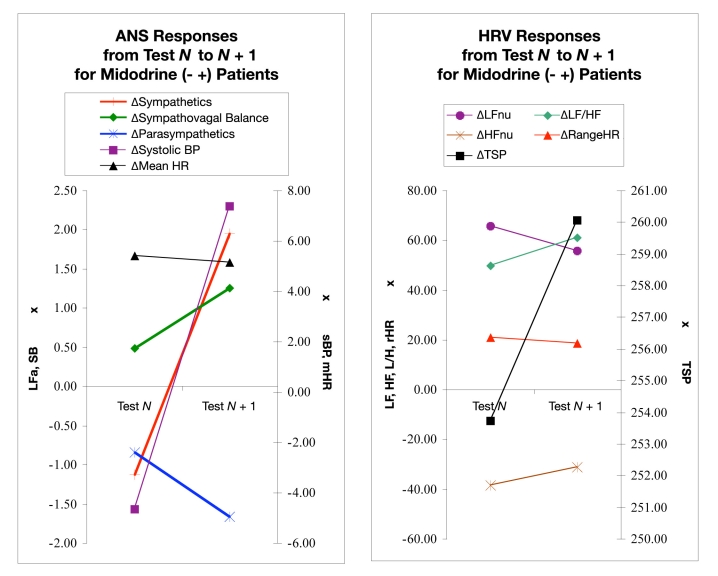
Figure 3 displays the changes in ANS and HRV responses to midodrine introduction between test N and test N + 1. On average, in this population, LFa decreased when the patients’ posture changed from seated to upright in test N. As discussed, this is abnormal. After midodrine introduction there was a significant (P < 0.001) change in the response. In test N + 1, the response became normal, an expected increase. This is well correlated (99.34%) with a significant (P < 0.001) change in systolic BP response from an average decrease upon standing (abnormal) to an average increase upon standing (normal) over the same period. SB also increased significantly over this period (P < 0.001), and parasympathetic activity and mHR decreased but not significantly.
Figure 3.
ANS (left) and HRV (right) responses from predosing (test N) to postdosing (test N + 1), where midodrine was introduced immediately after test N. Each data point represents a parameter response to a 5-minute seated to a 5-minute standing postural change. Each pair of points shows the change in the postural response from a pair of consecutive tests, “test N” to a follow-up “test N + 1” where midodrine was introduced immediately after test N. Test N + 1 is approximately 3 months after dosing. (Left) Sympathetic (LFa) and parasympathetic (RFa) measures, sympathovagal balance (SB = LFa/RFa), systolic BP, and mean HR. (Right) Normalized low- and high-frequency parameters (LFnu and HFnu, respectively), total spectral power (TSP), and range HR. See text for details.
Heart rate variability changes shown in Figure 3 (right) include an average increase in LFnu during the first test with a lesser average increase in LFnu during the second test. The same pattern was observed for rHR. On average, the LF/HF ratio increased during test N and increased more during test N + 1. Conversely, the HFnu decreased, on average, during test N and decreased less during test N + 1. TSP showed a small decrease during test N but increased during test N + 1. None of the HRV changes were statistically significant.
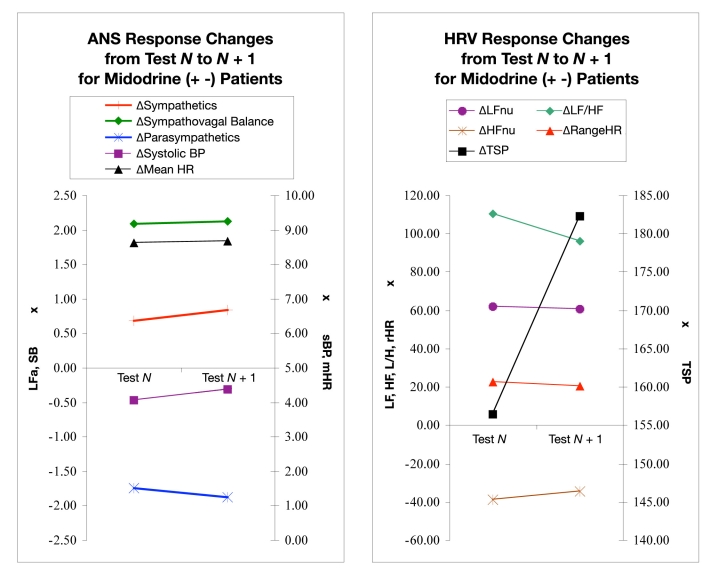
Figure 4 displays ANS and HRV responses among patients receiving midodrine before test N and discontinuing the agent before test N + 1. On average in these patients, the sympathetic response to posture change from seated to upright was normal, i.e., LFa increased. After midodrine was discontinued, an increase remained. Although this increase was slightly higher than in test N, it was not significant (P = −0.023). Similarly, there was an increase in systolic BP and SB before midodrine was discontinued (test N) and a slightly higher increase after the agent was discontinued (test N + 1). The parasympathetic and mHR responses decreased; none of these changes were significant (P > 0.100).
Figure 4.
ANS (left) and HRV (right) responses from test N to test N + 1, where midodrine was introduced prior to test N and discontinued before test N + 1. Each data point represents a parameter response to a 5-minute seated to a 5-minute standing postural change. Each pair of points shows the change in the postural response from a pair of consecutive tests, “test N” to a follow-up “test N + 1” where midodrine was discontinued immediately after test N. Test N + 1 is approximately 3 months later. (Left) Sympathetic (LFa) and parasympathetic (RFa) measures, sympathovagal balance (SB = LFa/RFa), systolic BP, and mean HR. (Right) Normalized low- and high-frequency parameters (LFnu and HFnu, respectively), total spectral power (TSP), and range HR. See text for details.
The HRV responses shown Figure 4 (right) include an average decrease in the change in LFnu following midodrine discontinuation. The same pattern was observed for rHR and LF/HF ratio. HFnu and TSP both increased from test N to test N + 1 as midodrine was discontinued; however, none of the changes were significant (P > 0.100).
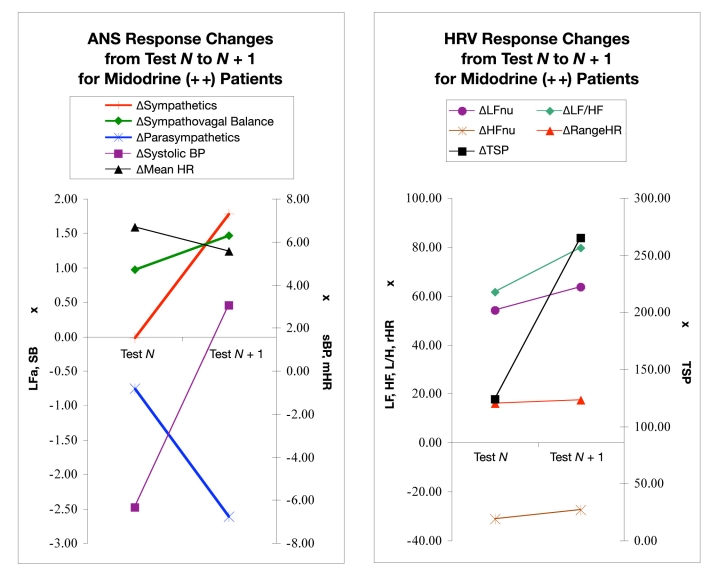
Figure 5 displays changes in the ANS and HRV responses of those patients with midodrine already on board before test N and continued through test N + 1. Among these patients, the sympathetic response on average changed from a slightly positive response in test N to a greater positive response in test N + 1 (P = 0.010). Note that a positive response indicates the expected increase in sympathetic activity after a seated-to-standing postural change. SB and systolic BP also increased from test N to test N + 1. The change in SB response was not statistically significant (P = 0.124); however, the change in systolic BP response was (P < 0.01). Parasympathetic and mHR responses decreased but not significantly (P > 0.100) in this period. The HRV responses shown in Figure 5 include an average increase in the LFnu, HFnu, LF/HF ratio, and TSP responses. Only rHR decreased from test N to test N + 1 while patients received midodrine. None of the changes HRV responses were significant (P > 0.040).
Figure 5.
ANS (left) and HRV (right) responses from test N to test N + 1, where midodrine was introduced prior to test N and the patient continued to have it on board through test N + 1. Each data point represents a parameter response to a 5-minute seated to a 5-minute standing postural change. Each pair of points shows the change in the postural response from a pair of consecutive tests, “test N” to a follow-up “test N + 1.” Test N + 1 is approximately 3 months later. (Left) Sympathetic (LFa) and parasympathetic (RFa) measures, sympathovagal balance (SB = LFa/RFa), systolic BP, and mean HR. (Right) Normalized low- and high-frequency parameters (LFnu and HFnu, respectively), total spectral power (TSP), and range HR. See text for details.
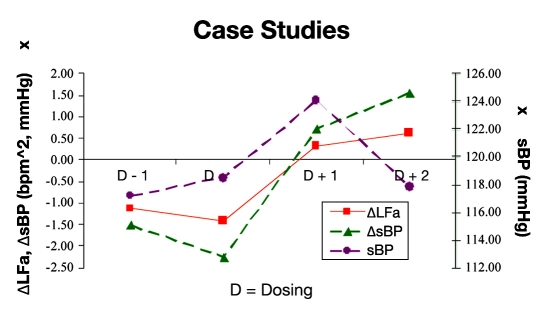
Figure 6 presents results from 22 cases that had more than four tests. The solid line in Figure 6 shows the sympathetic response (ΔLFa) to a change from seated to standing for each test. The trend from pre- to postdosing appears to indicate a normalization of the sympathetic response. In response to normalization of sympathetic activity, the average systolic BP (ΔsBP; Figure 6, broken line with triangles) response upon standing changed from negative (abnormal drop in systolic BP) to positive (expected rise in systolic BP). The associated trend in resting systolic BP is represented by the broken line with filled asterisks in Figure 6. The trend indicates a rise in resting systolic BP immediately after midodrine dosing, followed by a decrease in resting systolic BP several months thereafter.
Figure 6.
Changes in response to postural change from predosing to postdosing (D − 1 to D + 1) in the sympathetic measure (ΔLFa, solid line with squares) and systolic BP (ΔsBP, broken line with triangles). Also shown is the resting systolic BP (broken line with filled asterisks; the scale is on the right). These are averages from 22 of the patients from the cohort who had more than four serial tests. The test series are centered on the test just prior to which midodrine was introduced (designated on the abscissa as “D”). See text for details.
Overall changes—the change from test to test in the responses to standing up, abbreviated as ΔΔ—for the three medication states are presented in Table 3 (ANS method, mHR and BP responses) and Table 4 (HRV method). The average mHR responses were not statistically significant and included a decrease in response to an introduction [“midodrine (- +)”] or a continuation of midodrine [ “midodrine (+ +)”] and a slight increase in response to discontinuation of midodrine [ “midodrine (+ -)”]. As expected, changes in BP responses to upright posture with midodrine were statistically significant. On average, both systolic and diastolic measures increased with the introduction of midodrine and decreased when midodrine was discontinued. Among patients already receiving midodrine and no change in dosing was made, the average systolic pressure response to upright posture increased from test N to test N + 1 and the average diastolic pressure remained approximately the same. The overall average changes in RFa responses to upright posture were only statistically significant when midodrine was continued. The overall average changes in mHR and in the HRV (alone) measures (Table 4) were not statistically significant.
Table 3.
Changes in ANS, BP, and mHR Responses from Predosing (Test N) to Postdosing (Test N + 1), including Sympathetics (S), Parasympathetics (P), SB, Systolic BP (sBP), and Diastolic BP (dBP)a
| ΔΔS | ΔΔP | ΔΔSB | ΔΔmHR | ΔΔsBP | ΔΔdBP | |
|---|---|---|---|---|---|---|
| Midodrine (− +) | 1.42** | −0.81 | 0.77 | −0.24 | 2.63** | 1.53* |
| Midodrine (+ −) | −0.16* | −0.13 | 0.04* | 0.05 | −6.41* | −2.84* |
| Midodrine (+ +) | 0.43 | −1.86* | 0.49 | −1.11 | 1.64* | 0.02 |
The “ΔΔ” symbol indicates that a change in the response is represented. For example, an abnormal stand response in a single test is a decrease in sympathetic activity as compared to the resting seated position. This response may then change and become normalized as an increase in sympathetic activity upon standing. As a result, the change in the response (ΔΔ) would be positive. Changes marked with an asterisk are significant (*P < 0.01 or **P < 0.001). See text for details.
Table 4.
Overall HRV Changes from Predosing (Test N) to Postdosing (Test N + 1), including rHR, LFnu and HFnu, LF/HF Ratio, and TSPa
| ΔΔrHR | ΔΔLFnu | ΔΔHFnu | ΔΔLF/HF | ΔΔTSP | |
|---|---|---|---|---|---|
| Midodrine (− +) | −2.51 | −10.14 | 7.45 | 11.11 | 6.34 |
| Midodrine (+ −) | −1.90 | −1.39 | 4.36 | −14.24 | 25.92 |
| Midodrine (+ +) | 1.08 | 9.44 | 3.64 | 18.20 | 141.08 |
The “ΔΔ” symbol indicates that a change in the change is represented (see Table 2 legend). None were statistically significant. See text for details.
There is an unintentional female bias to the sample population. Student t tests were performed on the sample subpopulations [midodrine “(- +),” “(+ -),” “(+ +) ”], and case data. All t test results were insignificant (all results were greater than 0.425), suggesting that the two populations are uniform. Therefore, data suggest that these results extrapolate to males.
Discussion
The ANS method modifies standard spectral analysis of HRV by incorporating a second independent measure of the ANS: spectral analysis of RA. The ANS method further advances the current state of the art by substituting the CWT spectral analysis technique for the FFT technique. This substitution relieves the time-frequency compromise and the assumption of signal stationarity inherent in FFT spectral analysis. As presented herein, the ANS method (unlike the HRV method) yields independent and simultaneous measures of sympathetic (LFa) and parasympathetic (RFa) nervous system activity. The HRV method offers a mixed measure (LF) of the two branches30 and the HF. HF is a broad, fixed frequency band that can only quantify parasympathetic activity if the breathing frequency is high enough and is computed from an analysis window that is wider than needed for any given analysis period. Therefore, the ANS method is an improvement in the fidelity with which it can individually categorize expected changes in sympathetic and parasympathetic activity. When used to assess patients with type 2 diabetes with orthostasis, the ANS method accurately represents the autonomic changes in response to the upright postural challenge (as correlated with changes in BP and HR) and changes in vasopressor therapy.
Results showed that in orthostatic patients responding to postural change, the abnormal decreases in sympathetic and systolic BP responses will normalize (increase with a change in posture) with the introduction of α1-agonist (vasopressor) therapy. Data also showed that the trend in systolic BP over time in response to vasopressor therapy did increase initially, but eventually decreased (Figure 6). Extrapolating this trend to the physiology, the eventual decrease in systolic BP may be the result of normalizing the vascular response to upright posture. This normalization may then reduce the workload of the heart by more appropriately supporting blood in the abdomen while standing and ensuring proper brain perfusion. This normalization may also be associated with the statistically significant and expected decrease in the parasympathetic response ( “ΔΔP,” Table 3) only seen with continuation of vasopressor therapy.
Considering the results of the two methods presented for ANS assessment, the sympathetic measure, LFa, from the ANS method decreased significantly in response to assuming an upright posture in the symptomatic (predosing) state (Figure 1). This correlated with a concurrent (abnormal) drop in systolic BP upon standing. With the introduction of midodrine (Figure 2), LFa increased and was correlated with an increase in systolic BP upon standing (as corrected with vasopressor therapy). With the continuation of vasopressor therapy (Figure 5), the subsequent changes in LFa were smaller and the changes in systolic pressure less significant (Table 3). This may be a result of the plasticity of the nervous system and the normalization of its responses as a consequence of therapy.
In the predosing state (Figure 1), the HRV method LFnu, LF/HF ratio, and rHR measures increased in response to standing and HFnu and TSP decreased. In response to vasopressor introduction, the LF/HF ratio increased, changes in LFnu and rHR were marginal, and HFnu and TSP decreased (Figure 2).
The ANS method continued to model the expected results of the introduction (Figure 3), continuation (Figure 5), and discontinuation (Figure 4) of vasopressor therapy. The HRV method results were not as well correlated. Overall, results of the HRV method did not significantly reflect the expected abnormal or normal responses of the sympathetic and parasympathetic nervous systems in response to a change in posture.
Conclusions
Cardiovascular autonomic neuropathy is a progressive disease. In diabetic patients, an early indicator of CAN is DAN, which in turn can be preceded by OH. A smaller drop in BP along with SW (as diagnosed by the ANS method) may be an earlier indicator of orthostasis and therefore CAN. In this study, patients were treated with low-dose, short-term α1-agonist (vasopressor) therapy, which tended to correct the abnormal trend of SW with an unexpected drop in BP. Included in the findings was a systolic BP trend in response to vasopressor therapy of an (expected) initial increase in BP followed by an eventual decrease in systolic BP. The ANS method seems to model the abnormal and the normal responses to upright posture and changes in vasopressor therapy with greater fidelity than the HRV method.
Independent, simultaneous assessment of progressive parasympathetic and sympathetic dysfunction, autonomic imbalance, and the responses of the two ANS branches to therapy enables early detection and early intervention. Orthostasis, by way of example, illustrates that frequent, sensitive assessments of both ANS branches can improve the negative outcomes associated with CAN.
Acknowledgments
The authors thank Cara Jespersen for her encouragement and support in preparing this document, together with Conni Bergmann Koury for their many edits. We also thank Dr. Aaron Vinik for the inspiration to prepare this manuscript.
Abbreviations
- α1-agonist
α1-adrenergic agonist
- AD
autonomic dysfunction
- ANS
autonomic nervous system
- BP
blood pressure
- CAN
cardiovascular autonomic neuropathy
- CWT
continuous wavelet transform
- DAN
diabetic autonomic neuropathy
- E/I
expiration/ inspiration
- FFT
fast Fourier transform
- FRF
fundamental respiratory frequency
- HF
high frequency
- HFnu
normalized HF
- HR
heart rate
- HRV
HR variability
- LF
low frequency
- LFa
LF area
- LFnu
normalized LF
- mHR
mean HR
- OH
orthostatic hypotension
- RA
respiratory activity
- RFa
respiratory frequency area
- rHR
range HR
- RSA
respiratory sinus arrhythmia
- SB
sympathovagal balance
- SW
sympathetic withdrawal
- TSP
total spectral power
References
- 1.Vinik AI, Freeman R, Erbas T. Diabetic autonomic neuropathy. Semin Neurol. 2003;23(4):365–372. doi: 10.1055/s-2004-817720. [DOI] [PubMed] [Google Scholar]
- 2.Vinik AI, Aysin B, Colombo J. Diabetes Technology Conference. San Francisco: CA; 2005. Dynamic enhanced frequency domain analysis indicates a significant decline in autonomic function before age 50. Nov 10–12. [Google Scholar]
- 3.Vinik AI, Aysin B, Colombo J. Diabetes Technology Conference. San Francisco: CA; 2005. Differentiation of autonomic dysfunction by enhanced frequency domain analysis reveals additional stages in the progression of autonomic decline in diabetics. Nov 10–12. [Google Scholar]
- 4.Freeman R. Assessment of cardiovascular autonomic function. Clin Neurophysiol. 2006;117(4):716–730. doi: 10.1016/j.clinph.2005.09.027. [DOI] [PubMed] [Google Scholar]
- 5.Vinik AI, Freeman R, Erbas T. Diabetic autonomic neuropathy. Semin Neurol. 2003;23(4):365–372. doi: 10.1055/s-2004-817720. [DOI] [PubMed] [Google Scholar]
- 6.Boulton AJM, Vinik AI, Arezzo JC, Bril V, Feldman EI, Freeman R, Malik RA, Maser RE, Sosenko JM, Ziegler D. American Diabetes Association. Diabetic neuropathies: a statement by the American Diabetes Association. Diabetes Care. 28(4):956–962. doi: 10.2337/diacare.28.4.956. [DOI] [PubMed] [Google Scholar]
- 7.Joint Editorial Statement by the American Diabetes Association; the National Heart, Lung, and Blood Institute; the Juvenile Diabetes Foundation International; the National Institute of Diabetes and Digestive and Kidney Diseases; and the American Heart Association. Diabetes mellitus: a major risk factor for cardiovascular disease. Circulation. 1999;100(13):1132–1133. doi: 10.1161/01.cir.100.10.1132. [DOI] [PubMed] [Google Scholar]
- 8.Grundy SM, Benjamin IJ, Burke GL, Chait A, Eckel RH, Howard BV, Mitch W, Smith SC Jr, Sowers JR. Diabetes and cardiovascular disease: a statement for healthcare professionals from the American Heart Association. Circulation. 1999;100(13):1134–1146. doi: 10.1161/01.cir.100.10.1134. [DOI] [PubMed] [Google Scholar]
- 9.Freeman R. Treatment of orthostatic hypotension. Semin Neurol. 2003;23(4):435–442. doi: 10.1055/s-2004-817727. [DOI] [PubMed] [Google Scholar]
- 10.Maser RE, Lenhard MJ. Cardiovascular autonomic neuropathy due to diabetes mellitus: clinical manifestations, consequences, and treatment. J Clin Endocrinol Metab. 2005;90(10):5896–5903. doi: 10.1210/jc.2005-0754. [DOI] [PubMed] [Google Scholar]
- 11.Vinik AI, Ziegler D. Diabetic cardiovascular autonomic neuropathy. Circulation. 2007;115(3):387–397. doi: 10.1161/CIRCULATIONAHA.106.634949. [DOI] [PubMed] [Google Scholar]
- 12.Aysin B, Aysin E, Colombo J, Vinik A. Diabetes may accelerate the onset of orthostasis. 6th Annual Diabetes Technology Meeting; 2006 Nov. 2–6; Atlanta, GA. [Google Scholar]
- 13.Stoupakis G, Colombo J, Rendas-Baum R, Budhwani N, Arora R. AHA Abstr. IL: Chicago; 2002. Postural drop of low frequency component of heart rate variability in the diagnosis of orthostasis. [Google Scholar]
- 14.Arora RR, Aysin E, Aysin B, Colombo J. AHA Scientific Sessions. FL: Orlando; 2007. Therapeutic implications of sympathetic stimulus in orthostatic patients: measured by spectral domain analysis. Nov 4–7. [Google Scholar]
- 15.Borst C, Weiling W, van Brederode JF, Hond A, de Rijk LG, Dunning AJ. Mechanisms of initial heart rate response to postural change. Am J Physiol. 1982;243(5):H676–H681. doi: 10.1152/ajpheart.1982.243.5.H676. [DOI] [PubMed] [Google Scholar]
- 16.Smit AA, Halliwill JR, Low PA, Wieling W. Pathophysiological basis of orthostatic hypotension in autonomic failure. J Physiol. 1999;519(Pt 1):1–10. doi: 10.1111/j.1469-7793.1999.0001o.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vinik AI, Maser RE, Nakave AA. Diabetic cardiovascular autonomic nerve dysfunction. US Endocr Dis. 2007:2–9. [Google Scholar]
- 18.Assessment: Clinical autonomic testing report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 1996;46(3):873–880. [PubMed] [Google Scholar]
- 19.Aring AM, Jones DE, Falko JM. Evaluation and prevention of diabetic neuropathy. Am Fam Physician. 2005;71(11):2123–2130. [PubMed] [Google Scholar]
- 20.Bradley JG, Davis KA. Orthostatic hypotension. Am Fam Physician. 2003;68(12):2393–2398. [PubMed] [Google Scholar]
- 21.Streeten DH, Auchincloss JH Jr, Anderson GH Jr, Richardson RL, Thomas FD, Miller JW. Orthostatic hypertension. Pathogenetic studies. Hypertension. 1985;7(2):196–203. doi: 10.1161/01.hyp.7.2.196. [DOI] [PubMed] [Google Scholar]
- 22.Yoshinari M, Wakisaka M, Nakamura U, Yoshioka M, Uchizono Y, Iwase M. Orthostatic hypertension in patients with type 2 diabetes. Diabetes Care. 2001;24(10):1783–1786. doi: 10.2337/diacare.24.10.1783. [DOI] [PubMed] [Google Scholar]
- 23.Vinik AI, Aysin B, Colombo J. Enhanced frequency domain analysis replaces older heart rate variability methods. Fourth Annual Diabetes Technology Meeting; 2004 Oct 28–30. [Google Scholar]
- 24.Low PA. Testing the autonomic nervous system. Semin Neurol. 2003;23(4):407–421. doi: 10.1055/s-2004-817725. [DOI] [PubMed] [Google Scholar]
- 25.Low PA. Clinical autonomic disorders. 2nd ed. Philadelphia, PA: Lippincott Press; 1997. p. 396. [Google Scholar]
- 26.Bloomfield DM, Kaufman ES, Bigger JT Jr, Fleiss J, Rolnitzky L, Steinman R. Passive head-up tilt and actively standing up produce similar overall changes in autonomic balance. Am Heart J. 1997;134(2 Pt 1):316–320. doi: 10.1016/s0002-8703(97)70140-6. [DOI] [PubMed] [Google Scholar]
- 27.Maule S, Catalfamo E, Del Colle S, Leotta G, Caserta M, Tredici M, Rabbia F, Veglio F. Cardiovascular autonomic function in 422 patients with orthostatic symptoms. Am J Hypertens. 2003;16:505. [Google Scholar]
- 28.Malik M. Clinical guide to cardiac autonomic tests. The Netherlands: Kluwer Academic Publishers; 1998. [Google Scholar]
- 29.Malik M, Camm AJ. Heart rate variability. Armonk, NY: Futura Press; 1995. [Google Scholar]
- 30.Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability, standards of measurement, physiological interpretation, and clinical use. Circulation. 1996;93(5):1043–1065. [PubMed] [Google Scholar]
- 31.Akselrod S, Gordon D, Ubel FA, Shannon DC, Berger AC, Cohen RJ. Power spectrum analysis of heart fluctuations: a quantitative probe of beat to beat cardiovascular control. Science. 1981;213(4504):220–222. doi: 10.1126/science.6166045. [DOI] [PubMed] [Google Scholar]
- 32.Akselrod S, Gordon D, Madwed JB, Snidman NC, Shannon DC, Cohen RJ. Hemodynamic regulation: investigation by spectra analysis. Am J Physiol. 1985;249(4 Pt 2):H867–H875. doi: 10.1152/ajpheart.1985.249.4.H867. [DOI] [PubMed] [Google Scholar]
- 33.Akselrod S, Eliash S, Oz O, Cohen S. Hemodynamic regulation in SHR: investigation by spectral analysis. Am J Physiol. 1987;253(1 Pt 2):H176–H183. doi: 10.1152/ajpheart.1987.253.1.H176. [DOI] [PubMed] [Google Scholar]
- 34.Akselrod S. Spectral analysis of fluctuations in cardiovascular parameters: a quantitative tool for the investigation of autonomic control. Trends Pharmacol Sci. 1988;9(1):6–9. doi: 10.1016/0165-6147(88)90230-1. [DOI] [PubMed] [Google Scholar]
- 35.Uijtdehaage SH, Thayer JF. Accentuated antagonism in the control of human heart rate. Clin Auton Res. 2000;10(3):107–110. doi: 10.1007/BF02278013. [DOI] [PubMed] [Google Scholar]
- 36.Williams CA, Lopes P. The influence of ventilatory control on heart rate variability in children. J Sports Sci. 2002;20(5):407–415. doi: 10.1080/026404102317366663. [DOI] [PubMed] [Google Scholar]
- 37.Cammann H, Michel J. How to avoid misinterpretation of heart rate variability power spectra? Comput Methods Programs Biomed. 2002;68(1):15–23. doi: 10.1016/s0169-2607(01)00154-7. [DOI] [PubMed] [Google Scholar]
- 38.Vinik AI, Arora RR, Maser RE, Ghosh-Dastidar S, Colombo J. β1-adrenergic antagonists change sympathovagal balance in diabetics. US Endocr Disease. Submitted. [Google Scholar]
- 39.Vinik AI, Aysin B, Colombo J. Enhanced frequency domain analysis identifies early autonomic dysfunction that may lead to elevated blood pressure in diabetics. Diabetes Technology Conference; 2005 Nov 10-12; San Francisco, CA.. [Google Scholar]
- 40.Young TM, Mathias CJ. The effects of water ingestion on orthostatic hypotension in two groups of chronic autonomic failure: multiple system atrophy and pure autonomic failure. J Neurol Neurosurg Psychiatry. 2004;75(12):1737–1741. doi: 10.1136/jnnp.2004.038471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gibbons CH, Freeman R. Treatment options for autonomic neuropathies. Curr Treat Options Neurol. 2006;8(2):119–132. doi: 10.1007/s11940-006-0003-0. [DOI] [PubMed] [Google Scholar]
- 42.Sandroni P, Opfer-Gehrking Singer W, Low PA. Pyridostigmine for treatment of neurogenic orthostatic hypotension [correction of hypertension]--a follow-up survey study. Clin Auton Res. 2005;15(1):51–53. doi: 10.1007/s10286-005-0225-3. [DOI] [PubMed] [Google Scholar]
- 43.Aysin B, Aysin E, Colombo J, Vinik A. Comparison of HRV analysis methods during orthostatic challenge: HRV with respiration or without?. IEEE Engineering in Medicine and Biology Conference; Lyons, France. 2007. [DOI] [PubMed] [Google Scholar]