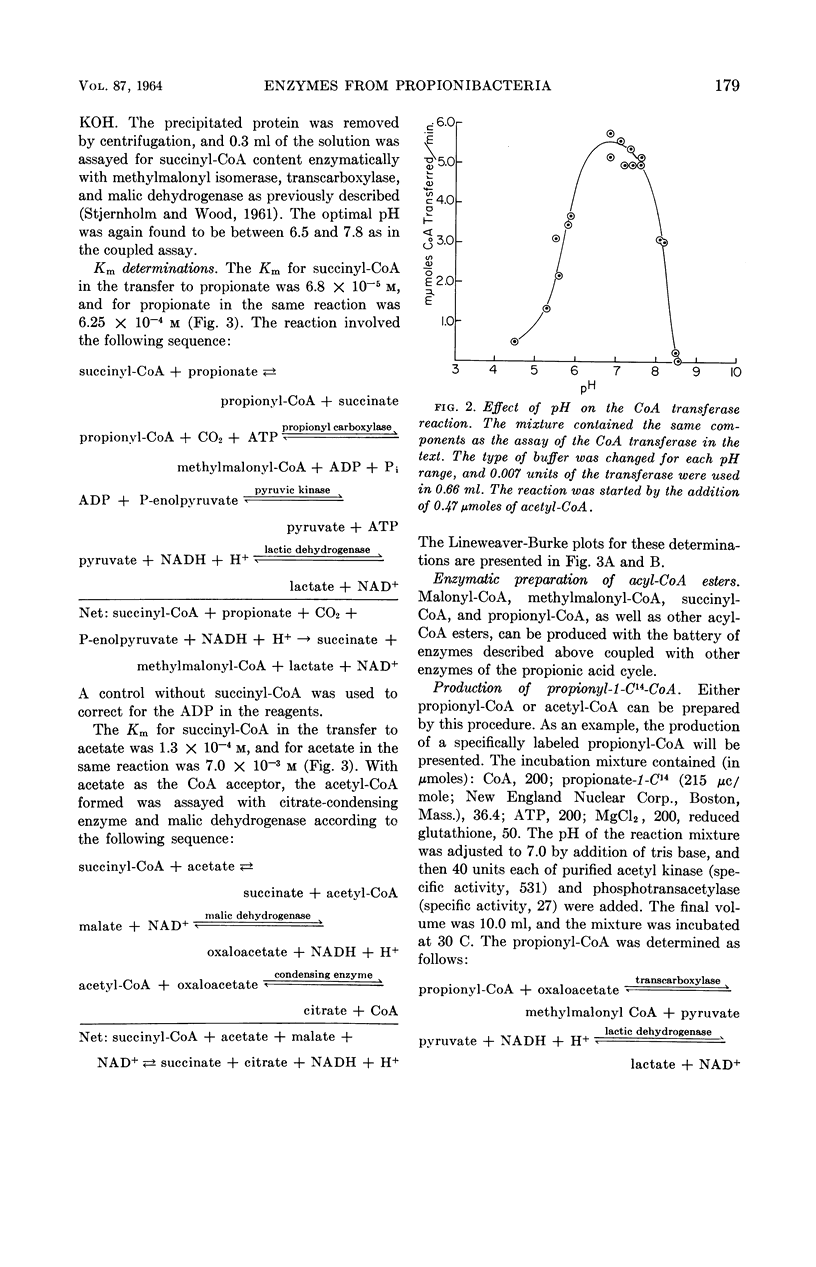
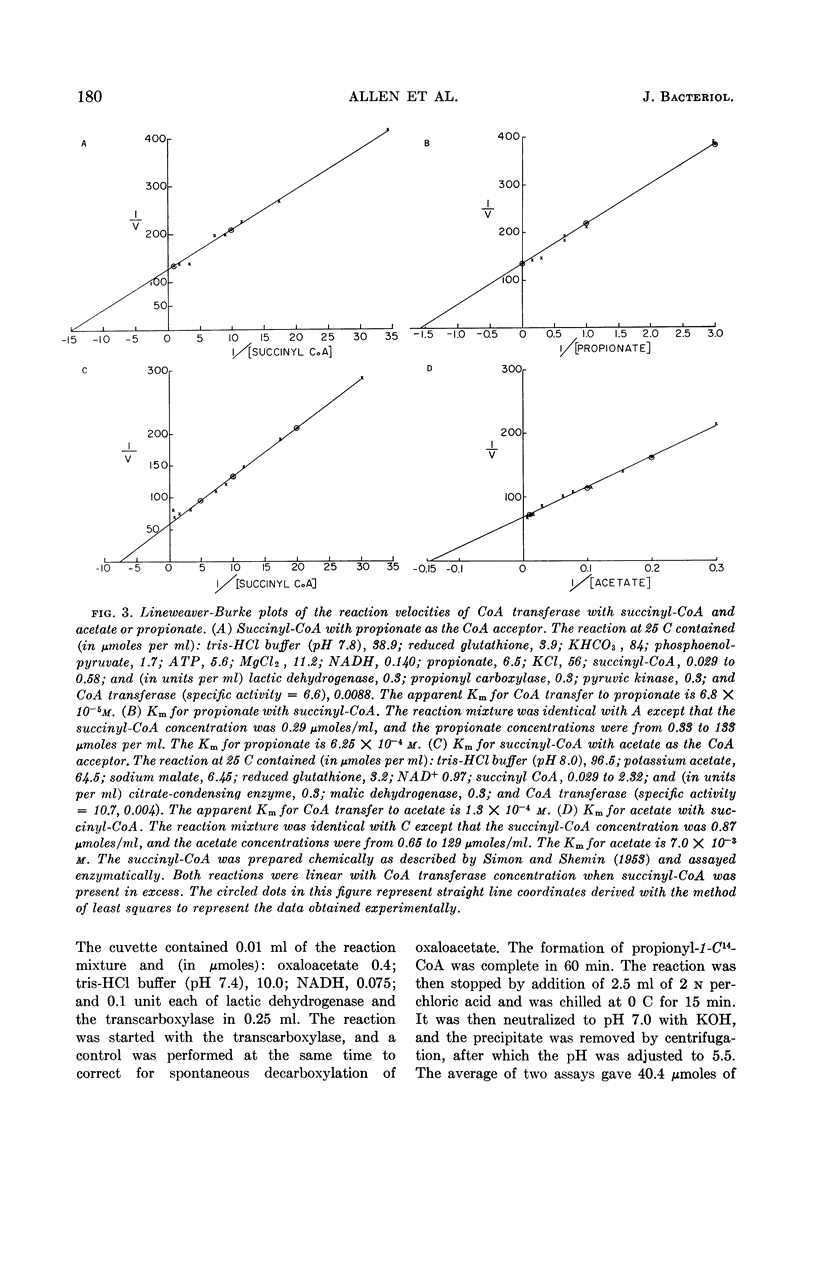
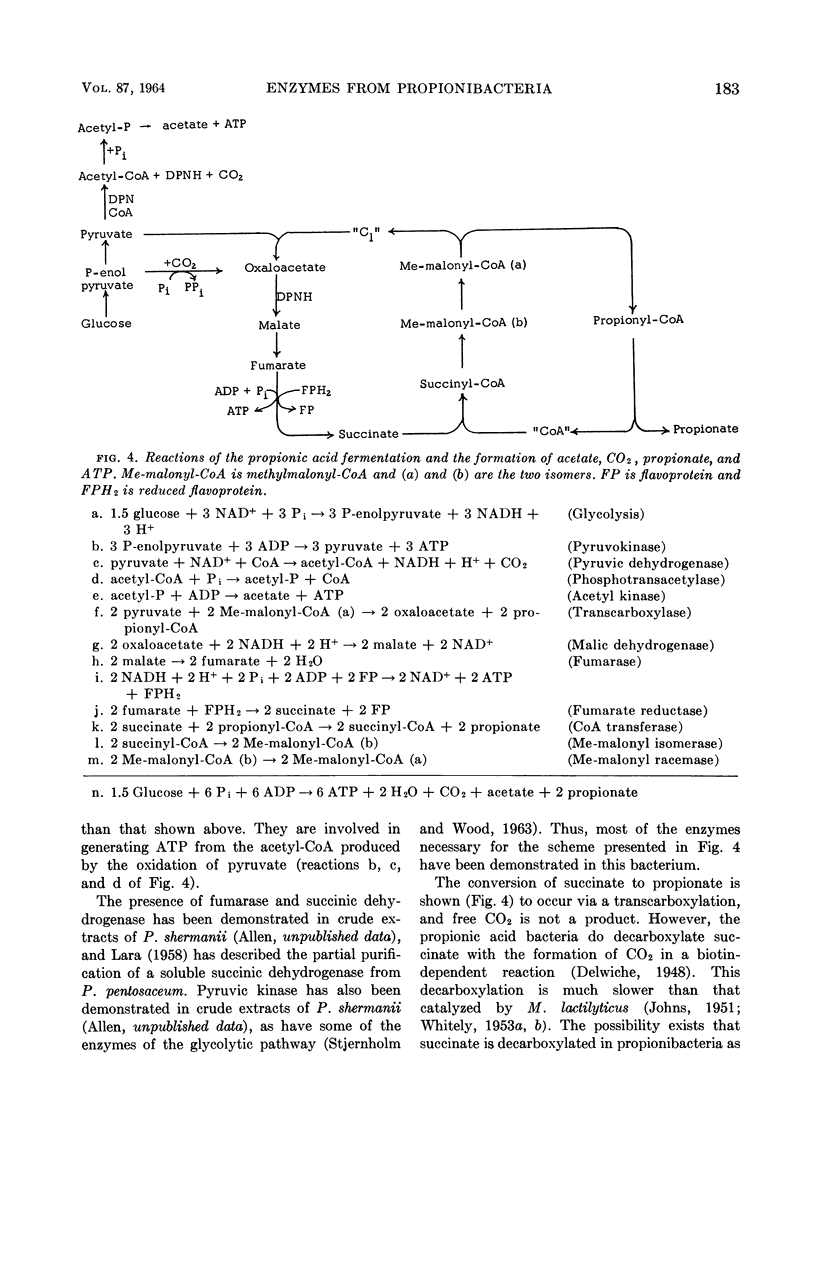
Abstract
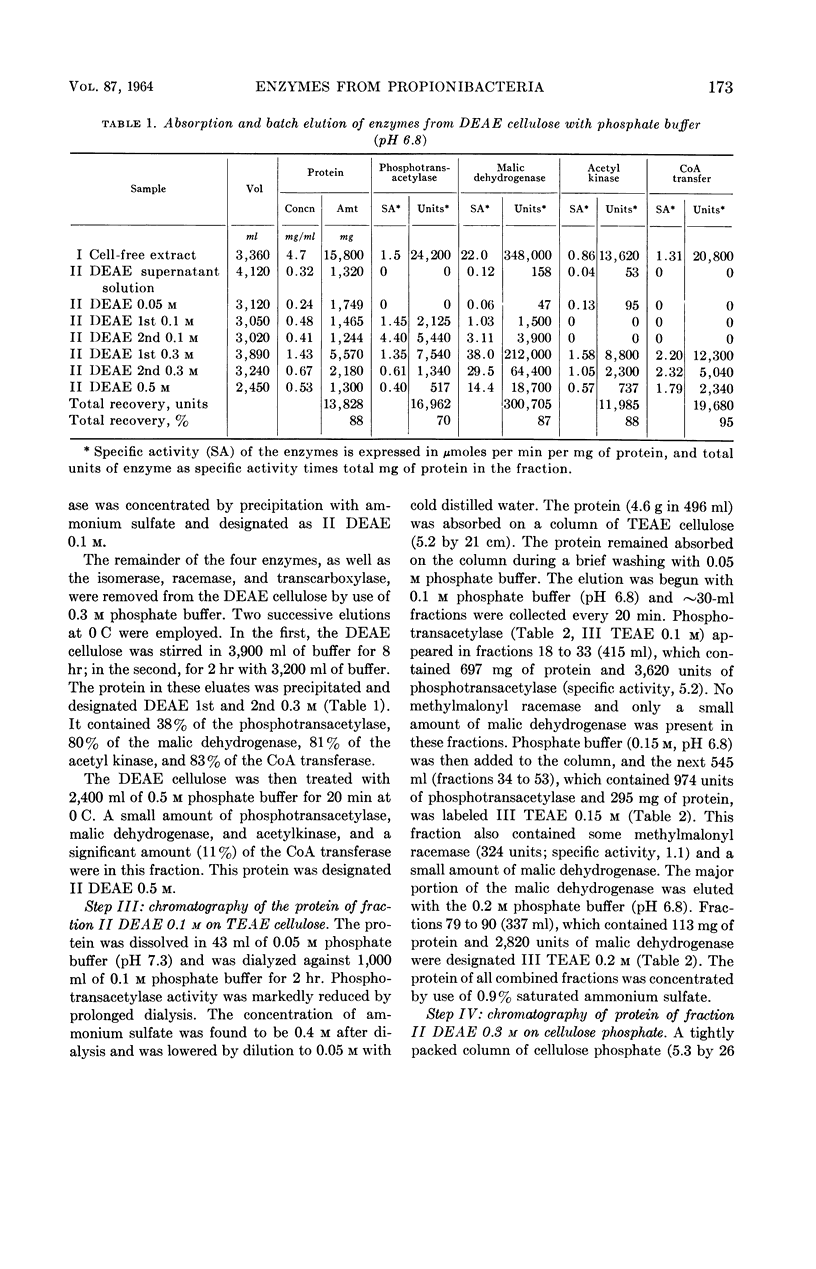
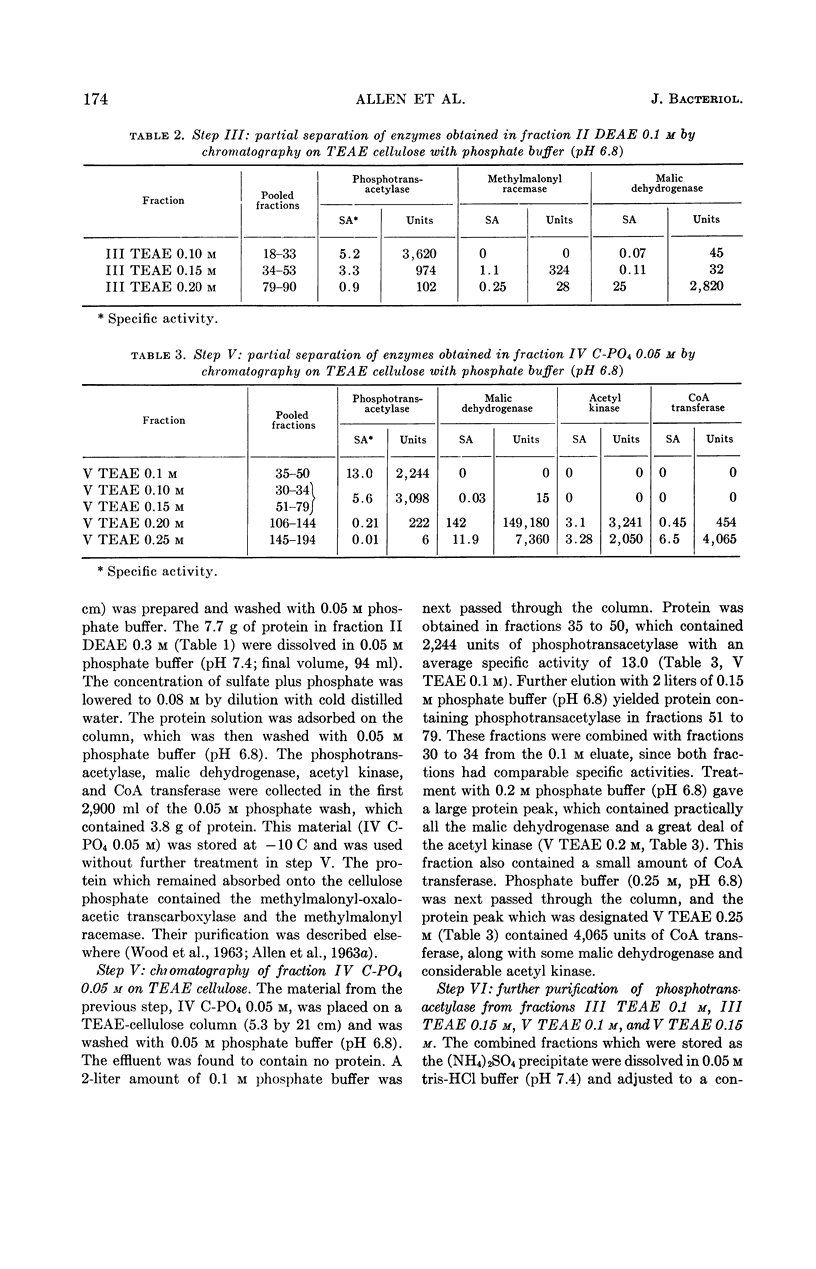
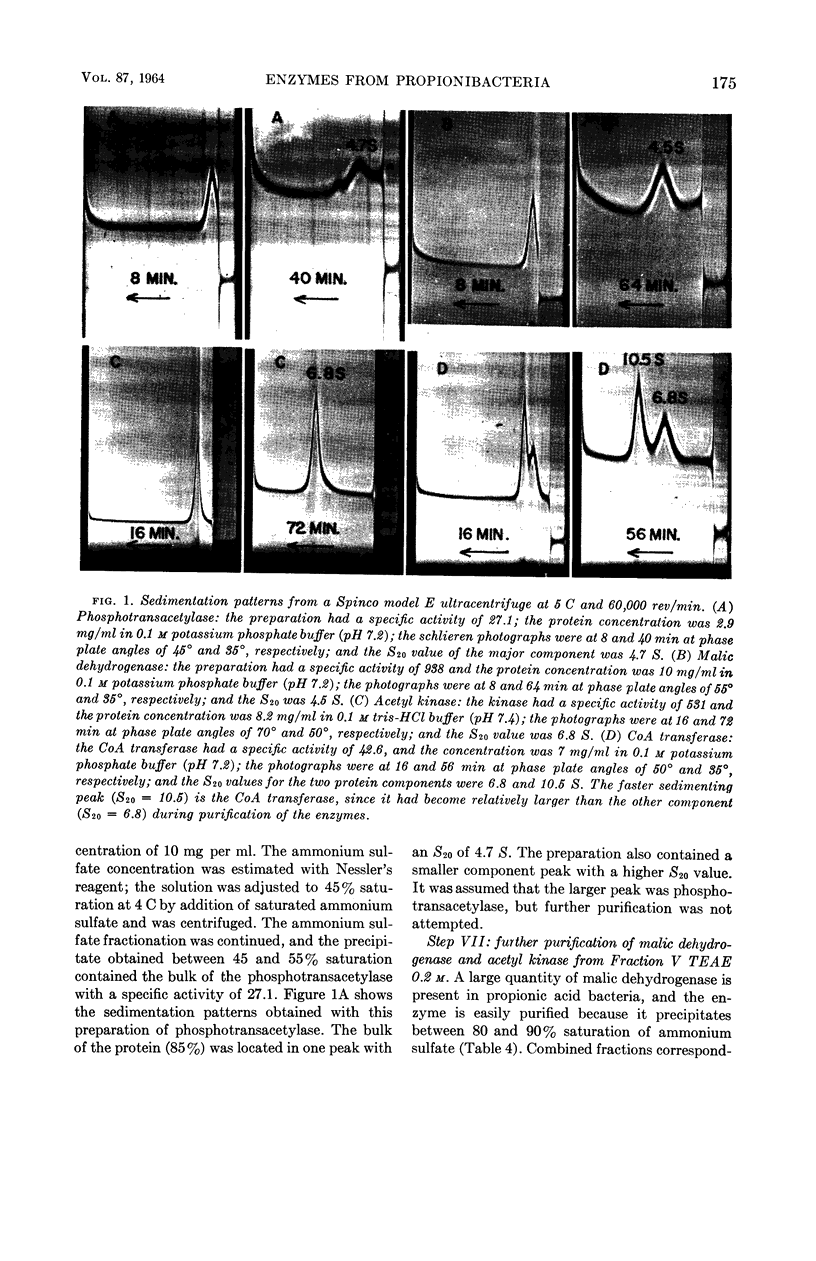
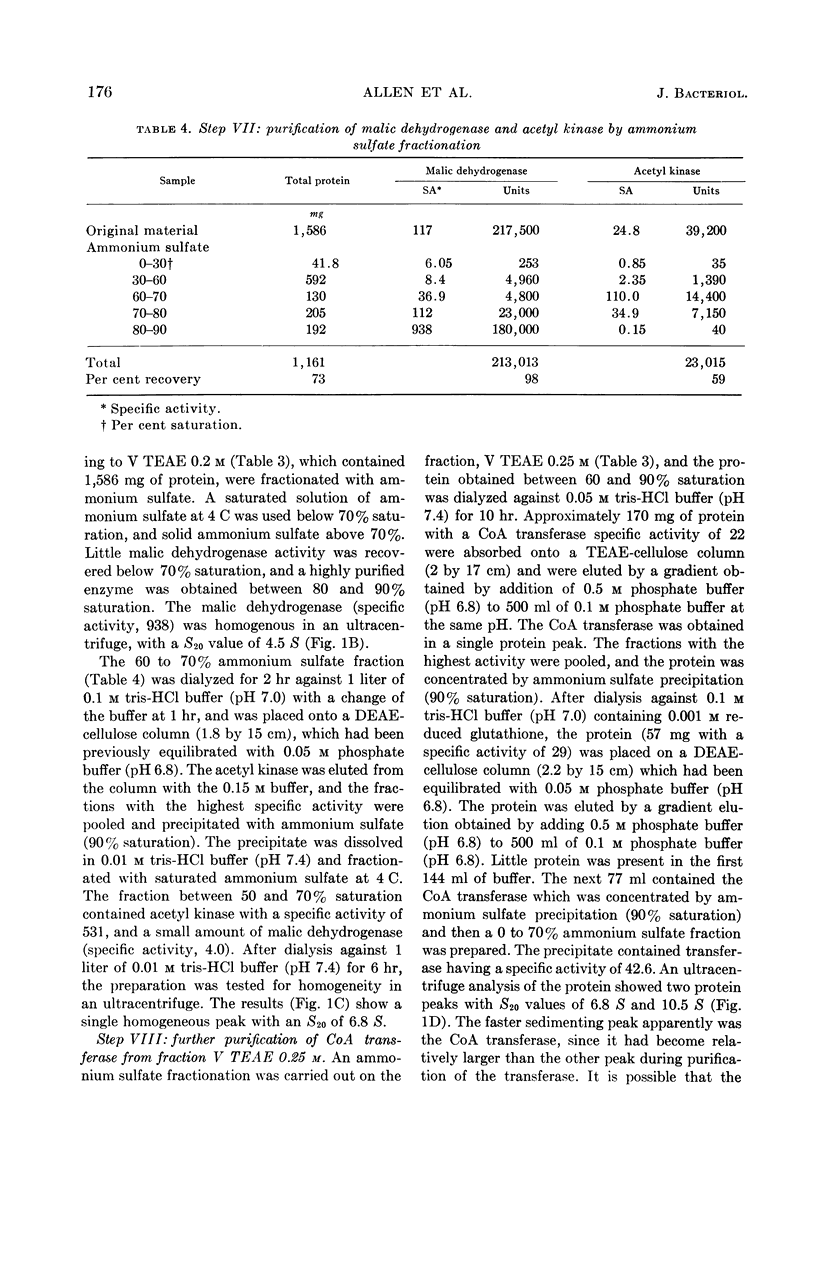
Allen, S. H. G. (Western Reserve University, Cleveland, Ohio), R. W. Kellermeyer, R. L. Stjernholm, and Harland G. Wood. Purification and properties of enzymes involved in the propionic acid fermentation. J. Bacteriol. 87:171–187. 1964.—Chromatographic procedures are described for the separation and purification of phosphotransacetylase, acetyl kinase, malic dehydrogenase and coenzyme A (CoA) transferase. Purity of the enzymes was judged by homogeneity in an ultracentrifuge and by specific activity. Phosphotransacetylase was obtained 85% pure with a specific activity of 27.1. The preparation of acetyl kinase was a homogeneous protein with a specific activity of 531. The malic dehydrogenase likewise was homogeneous with a specific activity of 938. The CoA transferase, which was about 56% pure with a specific activity of 42.6, is the purest preparation of this enzyme yet described. The pH optimum was 6.5 to 7.8, and the Km for succinyl-CoA in the transfer of CoA to acetate was found to be 1.3 × 10−4m; for acetate, in the same transfer, the Km was 7.0 × 10−3m; for succinyl-CoA to propionate it was 6.8 × 10−5m, and for propionate, in the same reaction, 6.2 × 10−4m. Methods are described for the enzymatic production of methyl-malonyl-CoA, malonyl-CoA, propionyl-CoA, acetyl-CoA, and succinyl-CoA. The role of these enzymes in the propionic acid fermentation as well as the possible mechanism responsible for the high yields of adenosine triphosphate from glucose are considered.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLEN S. H., KELLERMEYER R., STJERNHOLM R., JACOBSON B., WOOD H. G. The isolation, purification, and properties of methylmalonyl racemase. J Biol Chem. 1963 May;238:1637–1642. [PubMed] [Google Scholar]
- BAUCHOP T., ELSDEN S. R. The growth of micro-organisms in relation to their energy supply. J Gen Microbiol. 1960 Dec;23:457–469. doi: 10.1099/00221287-23-3-457. [DOI] [PubMed] [Google Scholar]
- DELWICHE E. A., PHARES E. F., CARSON S. F. Succinic acid decarboxylation system in Propionibacterium pentosaceum and Veillonella gazogenes. I. Activation, decarboxylation, and related reactions. J Bacteriol. 1956 May;71(5):598–603. doi: 10.1128/jb.71.5.598-603.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delwiche E. A. Mechanism of Propionic Acid Formation by Propionibacterium pentosaceum. J Bacteriol. 1948 Dec;56(6):811–820. doi: 10.1128/jb.56.6.811-820.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAYAISHI O. Enzymatic decarboxylation of malonic acid. J Biol Chem. 1955 Jul;215(1):125–136. [PubMed] [Google Scholar]
- HEGRE C. S., MILLER S. J., LANE M. D. Studies on methylmalonyl isomerase. Biochim Biophys Acta. 1962 Jan 29;56:538–544. doi: 10.1016/0006-3002(62)90605-4. [DOI] [PubMed] [Google Scholar]
- JOHNS A. T. The mechanism of propionic acid formation by propionibacteria. J Gen Microbiol. 1951 May;5(2):337–345. doi: 10.1099/00221287-5-2-337. [DOI] [PubMed] [Google Scholar]
- KELLERMEYER R. W., WOOD H. G. Methylmalonyl isomerase: a study of the mechanism of isomerization. Biochemistry. 1962 Nov;1:1124–1131. doi: 10.1021/bi00912a025. [DOI] [PubMed] [Google Scholar]
- KMETEC E., BUEDING E. Succinic and reduced diphosphopyridine nucleotide oxidase systems of Ascaris muscle. J Biol Chem. 1961 Feb;236:584–591. [PubMed] [Google Scholar]
- LARA F. J. The succinic dehydrogenase of Propionibacterium pentosaceum. Biochim Biophys Acta. 1959 Jun;33(2):565–567. doi: 10.1016/0006-3002(59)90153-2. [DOI] [PubMed] [Google Scholar]
- MAZUMDER R., SASAKAWA T., KAZIRO Y., OCHOA S. Metabolism of propionic acid in animal tissues. IX. Methylmalonyl coenzyme A racemase. J Biol Chem. 1962 Oct;237:3065–3068. [PubMed] [Google Scholar]
- OVERATH P., KELLERMAN G. M., LYNEN F., FRITZ H. P., KELLER H. J. [On the mechanism of the rearrangement of methylmalonyl-Co A into succinyl-Co A. II. Experiments on the mechanism of action of methylmalonyl-Co A isomerase and methylmalonyl-Co A racemase]. Biochem Z. 1962;335:500–518. [PubMed] [Google Scholar]
- PHARES E. F., DELWICHE E. A., CARSON S. F. Succinic acid decarboxylation system in Propionibacterium pentosaceum and Veillonella gazogenes. II. Evidence for an active C1 complex. J Bacteriol. 1956 May;71(5):604–610. doi: 10.1128/jb.71.5.604-610.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PHARES E. F., LONG M. V., CARSON S. F. An intramolecular rearrangement in the methylmalonyl isomerase reaction as demonstrated by positive and negative ion mass analysis of succinic acid. Biochem Biophys Res Commun. 1962 Jun 19;8:142–146. doi: 10.1016/0006-291x(62)90252-8. [DOI] [PubMed] [Google Scholar]
- ROSE I. A., GRUNBERG-MANAGO M., KOREY S. R., OCHOA S. Enzymatic phosphorylation of acetate. J Biol Chem. 1954 Dec;211(2):737–756. [PubMed] [Google Scholar]
- SANADI D. R., FLUHARTY A. L. ON THE MECHANISM OF OXIDATIVE PHOSPHORYLATION. VII. THE ENERGY-REQUIRING REDUCTION OF PYRIDINE NUCLEOTIDE BY SUCCINATE AND THE ENERGY-YIELDING OXIDATION OF REDUCED PYRIDINE NUCLEOTIDE BY FUMARATE. Biochemistry. 1963 May-Jun;2:523–528. doi: 10.1021/bi00903a023. [DOI] [PubMed] [Google Scholar]
- SEIDMAN I., ENTNER N. Oxidative enzymes and their role in phosphorylation in sarcosomes of adult Ascaris lumbricoides. J Biol Chem. 1961 Mar;236:915–919. [PubMed] [Google Scholar]
- SIEGEL L., ENGLARD S. Beef-heart malic dehydrogenases. I. Properties of the enzyme purified from extracts of acetone-dried powders. Biochim Biophys Acta. 1961 Nov 25;54:67–76. doi: 10.1016/0006-3002(61)90938-6. [DOI] [PubMed] [Google Scholar]
- SIU P. M., WOOD H. G. Phosphoenolpyruvic carboxytransphosphorylase, a CO2 fixation enzyme from propionic acid bacteria. J Biol Chem. 1962 Oct;237:3044–3051. [PubMed] [Google Scholar]
- SRERE P. A., KOSICKI G. W. The purification of citrate-condensing enzyme. J Biol Chem. 1961 Oct;236:2557–2559. [PubMed] [Google Scholar]
- STADTMAN E. R., OVERATH P., EGGERER H., LYNEN F. The role of biotin and vitamin B12 coenzyme in propionate metabolism. Biochem Biophys Res Commun. 1960 Jan;2:1–7. doi: 10.1016/0006-291x(60)90252-7. [DOI] [PubMed] [Google Scholar]
- STADTMAN E. R. The coenzyme A transphorase system in Clostridium kluyveri. J Biol Chem. 1953 Jul;203(1):501–512. [PubMed] [Google Scholar]
- STADTMAN E. R. The purification and properties of phosphotransacetylase. J Biol Chem. 1952 May;196(2):527–534. [PubMed] [Google Scholar]
- STERN J. R., COON M. J., DEL CAMPILLO A., SCHNEIDER M. C. Enzymes of fatty acid metabolism. IV. Preparation and properties of coenzyme A transferase. J Biol Chem. 1956 Jul;221(1):15–31. [PubMed] [Google Scholar]
- STERN J. R. Crystalline beta-hydroxybutyryl dehydrogenase from pig heart. Biochim Biophys Acta. 1957 Nov;26(2):448–449. doi: 10.1016/0006-3002(57)90040-9. [DOI] [PubMed] [Google Scholar]
- Stjernholm R., Wood H. G. METHYLMALONYL ISOMERASE, II. PURIFICATION AND PROPERTIES OF THE ENZYME FROM PROPIONIBACTERIA. Proc Natl Acad Sci U S A. 1961 Mar;47(3):303–313. doi: 10.1073/pnas.47.3.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swick R. W., Wood H. G. THE ROLE OF TRANSCARBOXYLATION IN PROPIONIC ACID FERMENTATION. Proc Natl Acad Sci U S A. 1960 Jan;46(1):28–41. doi: 10.1073/pnas.46.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOLFE J. B., RITTENBERG S. C. Malonate decarboxylation by Pseudomonas fluorescens. III. The rôle of acetyl coenzyme A. J Biol Chem. 1954 Aug;209(2):885–892. [PubMed] [Google Scholar]
- WOOD H. G., ALLEN S. H., STJERNHOLM R., JACOBSON B. Transcarboxylase. III. Purification and properties of methylmalonyl-oxaloacetic transcarboxylase containing tritiated biotin. J Biol Chem. 1963 Feb;238:547–556. [PubMed] [Google Scholar]
- WOOD H. G., STJERNHOLM R. Transcarboxylase. II. Purification and properties of methylmalonyl-oxaloacetic transcarboxylase. Proc Natl Acad Sci U S A. 1961 Mar 15;47:289–303. doi: 10.1073/pnas.47.3.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteley H. R. The Mechanism of Propionic Acid Formation by Succinate Decarboxylation: I. The Activation of Succinate. Proc Natl Acad Sci U S A. 1953 Aug;39(8):772–779. doi: 10.1073/pnas.39.8.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteley H. R. The Mechanism of Propionic Acid Formation by Succinate Decarboxylation: II. The Formation and Decarboxylation of Succinyl-CoA. Proc Natl Acad Sci U S A. 1953 Aug;39(8):779–785. doi: 10.1073/pnas.39.8.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood H. G., Werkman C. H. Mechanism of glucose dissimilation by the propionic acid bacteria. Biochem J. 1936 Apr;30(4):618–623. doi: 10.1042/bj0300618. [DOI] [PMC free article] [PubMed] [Google Scholar]




