Abstract
Background
Using currently available technology, it is possible to apply modern control theory to produce a closed-loop artificial β cell. Novel use of established control techniques would improve glycemic control, thereby reducing the complications of diabetes. Two popular controller structures, proportional–integral–derivative (PID) and model predictive control (MPC), are compared first in a theoretical sense and then in two applications.
Methods
The Bergman model is transformed for use in a PID equivalent model-based controller. The internal model control (IMC) structure, which makes explicit use of the model, is compared with the PID controller structure in the transfer function domain. An MPC controller is then developed as an optimization problem with restrictions on its tuning parameters and is shown to be equivalent to an IMC controller. The controllers are tuned for equivalent performance and evaluated in a simulation study as a closed-loop controller and in an advisory mode scenario on retrospective clinical data.
Results
Theoretical development shows conditions under which PID and MPC controllers produce equivalent output via IMC. The simulation study showed that the single tuning parameter for the equivalent controllers relates directly to the closed-loop speed of response and robustness, an important result considering system uncertainty. The risk metric allowed easy identification of instances of inadequate control. Results of the advisory mode simulation showed that suitable tuning produces consistently appropriate delivery recommendations.
Conclusion
The conditions under which PID and MPC are equivalent have been derived. The MPC framework is more suitable given the extensions necessary for a fully closed-loop artificial β cell, such as consideration of controller constraints. Formulation of the control problem in risk space is attractive, as it explicitly addresses the asymmetry of the problem; this is done easily with MPC.
Keywords: artificial pancreas, artificial pancreatic β cell, model predictive control, physiological modeling, PID control, type 1 diabetes mellitus
Introduction
Poor glycemic control has a strong correlation with micro- and macrovascular diseases, such as retinopathy and stroke.1 Intensive insulin therapy for T1DM improves glycemic control and reduces the complications of the disease.2 Such intensive therapy is burdensome for the afflicted, and thus patient compliance is low. A reduction in the burden would be achieved with an automated closed-loop controller delivering insulin. The benefits of such controllers have been seen throughout the pharmaceutical, chemical, and petroleum industries over the last 50 years.3 A closed-loop artificial pancreatic β cell is therefore proposed with the goal of reducing diabetic complications, without excessive need for user intervention.
An electromechanical closed-loop device for use as an advanced therapeutic method for treating people with type 1 diabetes mellitus (T1DM) is probably 5–10 years away from becoming a reality. The physical components of such a device, namely a continuous glucose monitor and a continuous insulin infusion pump, are currently approved and available. Given the maturity of this hardware, the software, namely a suitable control algorithm, is the bottleneck in the development of an artificial β cell. In ambulatory conditions, delivery of insulin and measurement of glucose must be carried out subcutaneously. However, in an intensive care unit (ICU), these tasks can be carried out through the intravenous (IV) route. Glycemic control in an ICU is an important topic and studies on such control algorithms are under way.4
Although the technology and the precedent for implementation are in place, the system itself is not without complications. In the healthy state, the human body produces two counterregulatory hormones, glucagon and insulin, which raise and lower blood sugar, respectively. However, in treating T1DM, only insulin is administered to decrease blood glucose. In the event that too much insulin is given, a small amount of glucose is usually consumed. Since a first-generation artificial β cell would not have the ability to deliver a counterregulatory agent, a robust controller that can handle uncertainty is required. With the amount of uncertainty inherent to the system, this is a major challenge, as controllers must be detuned to the extent that they might even be considered too sluggish to effectively guard against sustained hyperglycemia.
The reason for this uncertainty is due to both inter-and intrasubject variation, i.e., the dynamics of insulin absorption and the kinetics of glucose-insulin interactions are subject to change. Within an individual subject, sensitivity to insulin can change up to fourfold over timescales ranging from minutes due to physiological stress, e.g., exercise,5 to months due to hormonal changes elicited from psychological stress, e.g., seasonal affective disorder.6 Bearing this in mind, an adequate controller must be capable of adaptation to perform under these conditions.
The following review articles have summarized some of the issues pertinent to choosing controller architecture for controlling glycemia. Parker et al.7 reviewed control algorithms for glycemic control, concluding that model-based controllers had an inherent advantage in blood glucose control, but demanding a certain level of model accuracy for their use to be justified. Hovorka et al.8 published a review article on the state-of-the-art of closed-loop control systems, presenting proportional-integral-derivative (PID) feedback controllers and model predictive control (MPC) algorithms side by side; in this article, however, the relative merits of each control algorithm were not assessed. Bequette9 presented a review of control algorithms and the associated challenges, concluding that in the single-input single-output case (insulin infusion only), MPC would be most appropriate. Each of these reviews has implied that the advantages of model-based control are only evident if sufficiently accurate models are available.
Proportional-integral-derivative control is widely considered the algorithm of choice in the process industries and has become ubiquitous since its inception in the 1930s. Simple tuning rules exist for optimizing its performance, some of which are summarized by Rivera et al.10 However, when tuned aggressively, overshoot of the set point can occur, which is often followed by oscillations. An oscillatory response is highly undesirable in the regulation of blood glucose where the consequence of overshoot is hypoglycemia.
The concept of MPC was developed in the late 1970s in the petrochemical industries by Richalet et al.11 and Cutler and Ramaker.12 It was particularly successful when used in systems exhibiting large time delays and lag times. This is because the algorithm explicitly considers the future effects of control moves and constraints in determining the controller output, unlike traditional feedback methods in which only past outputs are assessed.
The research into suitable control algorithms has produced a divided research community; some groups are in favor of computationally expensive techniques, since the problem itself is inherently complex, with others in favor of simplicity in order to develop a workable solution to this engineering problem. MPC is often perceived as complex and requiring an accurate model, which in this case would mean obtaining a detailed pharmacokinetic-pharmacodynamic model of glucose-insulin interactions in the body. The development of such a model that is consistently accurate in light of the physiological changes of a subject with T1DM would be expensive to develop and burdensome to maintain. Therefore, although accurate modeling is possible, it is not practical.
This article explicitly develops the conditions under which PID and MPC controllers are equivalent and highlights the relevance to closed-loop control of glycemia in T1DM. In order to support the use of such controllers, a series of in silico trials was performed. A simulation study shows that a simple model-based controller can be developed from a rudimentary understanding of the dynamics of intravenously administered insulin. Tuning of such a controller is also handled in a simple fashion with one degree of freedom. Such a controller can then provide satisfactory control of glycemia in a simulated subject with T1DM. As a final precursor to clinical trials in vivo, an advisory mode simulation is run using clinical data, as per the work of Gillis et al.13 thus validating the algorithm on clinical data.
Physiological Modeling
Mathematical representations of the glucose-insulin interactions have been under development since the 1960s14 and continue to be advanced as more thorough investigations take place, giving increasingly complex models.15 Physiological models can be divided into hybrid and fundamental models.16 Fundamental models are constructed by analyzing system behavior at the microscale, e.g., Sedaghat et al.17 examined molecular interactions in the insulin signaling pathway and derived rate laws. Hybrid (semiempirical) models incorporate physiological principles in determining model structure; parameters are then determined from experimental data.
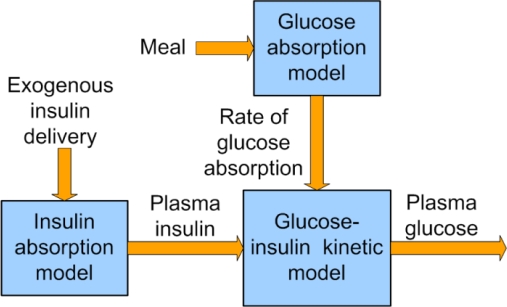
Often, a compartmental approach is taken in developing hybrid models. This is because, as Figure 1 suggests, the interactions between each compartment are limited to a one-way single channel, and hence the effects can be analyzed separately. The modeling work in this article focuses on the glucose-insulin kinetics compartment, i.e., intravenous glucose-insulin interactions and the effect on intravenous glucose.
Figure 1.
Compartmental representation of a complete physiological model of glucose-insulin interactions in the body.
The mathematical model governing the glucose-insulin kinetics compartment is given in Equations (1) and (2),18 including an extension to include glucose absorption from the gut, GM.19 The model also includes IV insulin delivery,20 as shown in Equation (3). This bilinear, third-order model is given in deviation variables, as it considers dynamic behavior around a nominal (basal) steady state. The state variables are defined in Table 1, and the parameters are given in Table 2.
Table 1.
Description of States Used in the Modified Bergman Minimal Model
| Symbol | Description | Unit |
|---|---|---|
| G′(t) | Deviation plasma glucose concentration | mg/dl |
| GM(t) | Rate of glucose absorption from meal | mg/dl/min |
| I′(t) | Deviation plasma insulin concentration | mU/liter |
| I′D(t) | Deviation rate of intravenous insulin delivery | mU/min |
| X(t) | Remote (interstitial) insulin concentration | liter/min |
Table 2.
Model Parameters Used in the Modified Bergman Minimal Model
| (1) |
| (2) |
| (3) |
The total insulin distribution volume is the product of the subject's body weight and the volumetric insulin distribution volume:
| (4) |
The methods dealt with in this article are valid only for linear models, hence the bilinear model [Equation (1)] is linearized about the basal glucose concentration, giving a linear approximation:
| (5) |
According to published model parameters, the characteristic time associated with the remote insulin ) is significantly faster than the characteristic time associated with insulin effects ). This means that the output dynamics are affected primarily by p1 only. Therefore, the next simplifying assumption is that the remote insulin is equilibrating rapidly, as described in Equation (6):
| (6) |
Thus, using Equations (3), (5), and (6) to represent glucose-insulin kinetics gives a second-order linear system. The transfer function model of the effects of IV insulin delivery on plasma glucose is therefore
| (7) |
The utility of a simplified model like this with respect to closed-loop control is shown in the following sections.
Theoretical Development of Model-Based Controllers
Internal Model Control (IMC) Tuning Rules for PID Controllers
The IMC framework is an intuitive structure that allows consideration of robustness and speed of response through one tuning parameter.21 Through this single parameter, systematic consideration can be given to model uncertainty and tradeoffs between performance and robustness. The behavior of the controller depends on the complexity of the internal model used.
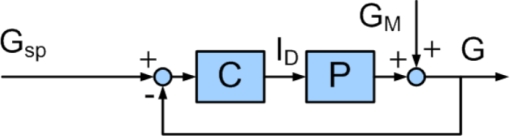
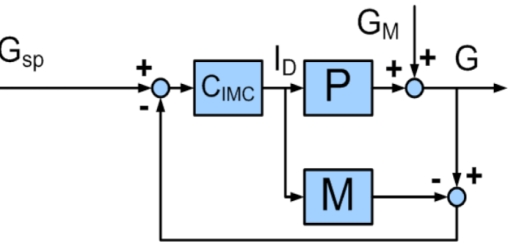
The classical feedback control structure is shown in Figure 2; controller output is calculated based on an error signal, which is the difference between the plant output and a predefined set point. For comparison, the IMC structure is shown in Figure 3; in this case, controller output is calculated based on the difference between plant output and output of the internal model. Elementary loop algebra shows that a standard feedback controller, C, is related to the internal model, M, and the IMC controller, CIMC, by Equation (8):
Figure 2.
Block diagram of classical feedback control. Gsp is the desired glucose concentration, ID is the insulin delivery rate, GM is the disturbance variable, G is the glucose concentration, C is the controller, and P is the process. The controller output depends on the set point error.
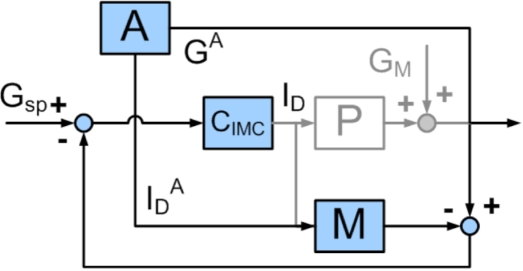
Figure 3.
Block diagram of internal model control feedback structure. Gsp is the desired glucose concentration, ID is the insulin delivery rate, GM is the disturbance variable, G is the glucose concentration, CIMC is the IMC controller, M is the assumed process model, and P is the process. The controller output depends on the set point error and the mismatch between the assumed process model and the process itself.
| (8) |
The IMC controller is constructed as follows. First, an assumed process model is factored into two components, as shown in Equation (9). M+ (an all-pass filter) has a gain of unity and contains all time delays and right half-plane zeros. M− is the stable, realizable part of the model. For the second-order transfer function given in Equation (7), the whole model is stable and realizable, i.e., M+ = 1. Next, a low-pass filter is specified and defined according to Equation (10), where r assumes an integer value. The controller is given by Equation (11). The single parameter τc (the time constant of the filter) relates to the closed-loop speed of response and is therefore used to tune the controller. Large values of τc give more robust control, whereas small values of τc give more aggressive control.
| (9) |
| (10) |
| (11) |
Since the nonminimum phase parts of the model are all included in M+, the controller is guaranteed to be stable and realizable. The system shown in Figure 3 has the closed-loop transfer function given by
| (12) |
For a subset of process models, the IMC controller is equivalent to a PID controller. In this case, r = 1, indicating that the process model is first or second order. Rivera et al.10 first introduced a design method that produced IMC-equivalent PID controllers; this was later expanded to include a larger range of models by Chien and Fruehauf.22 In the case of the model given by Equation (7), the tuning rules are
| (13) |
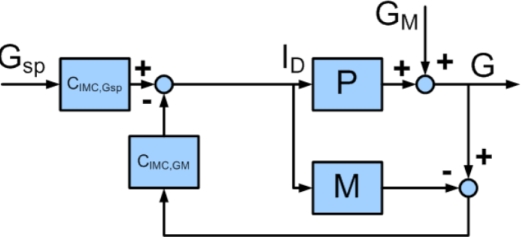
Controller tuning can also depend on the nature of the control problem, whether it be a set point change (a change in the basal insulin infusion rate) or a disturbance rejection (an unannounced meal). A simple modification to the block diagram shown in Figure 4 allows these different challenges to be addressed separately by repositioning the control block, giving two controllers that are tuned separately. The set point change and disturbance rejection are managed by controllers CIMC,Gsp and CIMC,GM, respectively. This architecture is preferable because a controller tuned for set point tracking is sluggish on disturbance rejection, and vice versa.
Figure 4.
Block diagram showing two degree-of-freedom IMC controller. Control block CIMC,GSP deals with set point tracking, and control block IMC,GM handles disturbance rejection. Gsp is the desired glucose concentration, ID is the insulin delivery rate, GM is the disturbance variable, G is the glucose concentration, M is the assumed process model, and P is the process.
The IMC tuning rules therefore reduce the tuning of a three degree-of-freedom PID controller into one parameter. This analysis was carried out in the transfer function domain, as is common in the literature. Providing the process P is stable, the closed-loop system is stable;23 this follows since the controller CIMC is stable by its definition. Therefore, tc determines the degree of robustness in a system with guaranteed stability.
Conditional Equivalence of MPC and IMC
The IMC was first developed by Garcia and Morari21 in order to provide a framework for the comparison of MPC with classical feedback control techniques.23
The comparison is approached as an unconstrained optimization problem in state space, which can then be converted into the transfer function domain for comparison with PID control.
The special case of MPC in question is also known as model algorithmic control (MAC), which was developed by Richalet et al.11 MAC is a one-step ahead unconstrained model predictive controller, operating with no controller output weighting. In modern MPC terminology, this means the move and prediction horizons are equal to unity, and the controller output weighting matrix is equal to zero. The optimization problem to be solved is therefore
| (14) |
where ID(k) is the controller output, Gd(k +1) is the desired glucose value, and G(k + 1|k) is the one-step ahead corrected prediction. The desired glucose value is determined by an exponential trajectory toward the set point from the current state given by
| (15) |
where Gsp(k +1) is the set point, G(k| k) is the corrected prediction, and
| (16) |
where τc is the tuning parameter. The model is defined using impulse response coefficients hj as
| (17) |
and the corrected prediction is given by
| (18) |
where d(k), the bias term, is given by
| (19) |
In the unconstrained, one-step ahead case, an exact solution is possible, as the next control move should correct for the predicted error in one step, i.e.,
| (20) |
where Ê0(k + 1) is the predicted unforced error, given by
| (21) |
Substituting Equations (15) and (17)–(19) into Equation (21), further substituting the result into Equation (20) and rearranging, gives
| (22) |
Taking the z-transform of Equation (22) gives
| (23) |
which is further rearranged to give
| (24) |
where CIMC is defined in Equation (11). The first-order exponential filter used in CIMC is defined in the discrete time transfer function domain as
| (25) |
The control law in Equation (24) is equivalent to that of the system in Figure 3, noting the inherent one-step time delay caused by the feedback loop in discrete time.
The framework of IMC has shown that PID and MPC controllers can be tuned to give identical output. With restrictions placed on the MPC tuning handles, the optimization problem is transformed into the transfer function domain, resulting in an IMC controller. If certain restrictions are placed on the model used, IMC is equivalent to PID control. These controllers are tuned using a single parameter, which can directly account for model uncertainty and hence provide robust control.
Simulation Study
The model-based controller developed in the previous section was tested in silico. This proof-of-concept study was designed to check the adequacy of a controller tuned using these rules. The model used in designing the controller was the second-order linear model defined in Equation (7). Plant-model mismatch was introduced by using a third-order plant, i.e., removing the approximation of Equation (6) with Equation (2), and introducing parameter mismatch. Parameters p1, p3, and Vi were increased by 100% from their nominal values given in Table 2.
As described by Parker et al.25 closed-loop control of T1DM is an asymmetric problem. This is because (i) the immediate dangers from hypoglycemia are much greater than those from hyperglycemia and (ii) the glucose state space is lopsided with regards to the range of acceptable values above and below euglycemia. This issue has been addressed by Kovatchev26 who defined a transform for glucose space into risk space, where state values are normalized around euglycemia, with as much risk associated with 20 mg/dl as 600 mg/dl. In effect, this risk space can be used for the assessment of controller efficacy, as glycemic undershoot is penalized more heavily than in the glucose space. For assessment of controller performance, the output was considered in risk space.
Figure 5 shows the response of the model-based controller to a set point change. For the case of no plant-model mismatch, perfect set point tracking along the exponential trajectory is obtained, as model inversion is possible. However, when the model is not perfect, the resulting control action results in oscillatory control action, which causes an increase in the risk metric. Oscillatory action is highly undesirable, since when there is a hard constraint on the controller action, i.e., because insulin delivery may never go negative, controller actions that would theoretically attenuate the oscillation may not, in practice, be possible. Therefore, more robust tuning is preferred, since although the control is sluggish, the controller output is feasible.
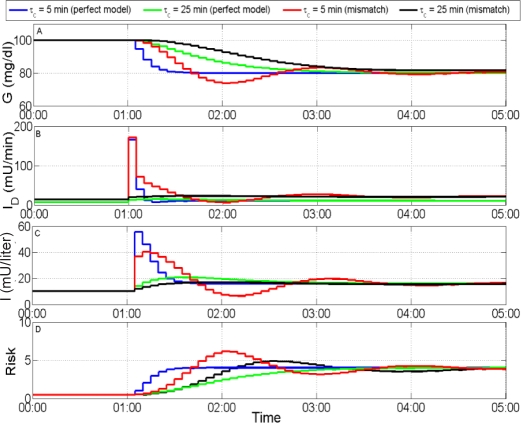
Figure 5.
Simulated response to step change of −20 mg/dl in glucose set point. (A) Plasma glucose concentration, (B) rate of insulin delivery, (C) plasma insulin concentration, and (D) risk metric. The more robust controller (τc = 25 min) produces the best output, even under uncertainty, where controller action is less oscillatory and the maximum risk is lower.
Figure 6 shows the response of the controller to an unannounced meal. The open-loop response obtained through the physiological model shows that unacceptable hyperglycemia occurs if no insulin is delivered. Under these conditions, the best response in terms of risk rating is obtained through an aggressively tuned controller, which performs well even under uncertainty.
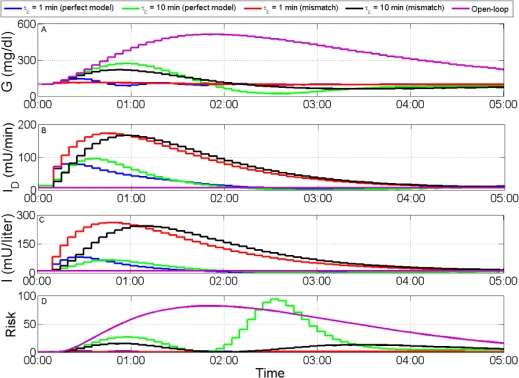
Figure 6.
Simulated responses to an unannounced meal of 100 grams of carbohydrate. (A) Plasma glucose concentration, (B) rate of insulin delivery, (C) plasma insulin concentration, and (D) risk metric. The more aggressive controller (τ = 1 min) produces the best output, even under uncertainty, where controller action is less oscillatory and the maximum risk is lower.
These results show that model uncertainty necessitates robust control. The single tuning parameter τc facilitates this consideration explicitly, thus allowing improved performance to be obtained with the minimum of simulations. With a sufficiently robust controller, the control move is always feasible.
Advisory Mode Control
After a simulation study and before closed-loop implementation of a control algorithm, it is desirable to test the controller on retrospective clinical data. This is to ascertain whether the control algorithm makes quantitatively reasonable suggestions for control moves. This test concept is known as advisory mode control and is common in the petrochemical industry for prototyping. This technique has been implemented by Gillis et al.13 for an MPC controller on clinical data taken from subjects with T1DM. Here advisory mode control is applied to an MPC controller tuned to be equivalent to an IMC controller.
The structure of advisory mode is shown in Figure 7, where a comparison to a standard feedback IMC structure is made through the use of the grayed out blocks, which are not implemented. The sum of plant output and disturbance measurement is replaced with the measurement G. Model output is obtained from historical inputs, thus giving the predicted unforced error, i.e., the effects of the insulin that has already been delivered; this is equivalent to calculating the effects of the insulin on board (IOB). The notion of IOB is used in commercial insulin pumps to prevent insulin overdose; here the effects of insulin on the plasma glucose are calculated explicitly. The bias is the difference between the measurement, G, and the model prediction, Ĝ (from block M), and is used to correct for persistent error. The control action is proportional to the difference between the reference trajectory, Gd(k +1), and the corrected unforced prediction, G(k + 1|k).
Figure 7.
Block diagram showing implementation of advisory control. Gray print shows blocks removed from the closed-loop control block diagram for comparison. A represents an archive of glucose concentrations (GA) and insulin pump delivery rates (IDA). Gsp is the desired glucose concentration, ID is now the advised insulin delivery rate, CIMC is the IMC controller, and M is the assumed process model.
Each dataset consisted of glucose sensor records (CGMS®, Medtronic MiniMed, Northridge, CA), insulin pump records, and subject-reported carbohydrate intake for a 24-hour period. Two controller settings were used for advisory mode control to compare controller output. Figure 8 shows the advice given by a relatively aggressive controller (τC = 10 minutes). The controller advises insulin delivery rates of the same magnitude as would be given by a bolus during hyperglycemic episodes, e.g., at 4:00 a.m. Figure 9 shows the advice given by a relatively robust controller (τC = 50 minutes). The advised insulin delivery rates are relatively small, as would be expected from a more conservative controller. The effects of considering the predicted unforced response are modest because the corrected prediction only considers one step ahead, which is not long enough for the effects of delivered insulin to manifest significantly. In both cases, pump shutdown is advised when hypoglycemia is approached.
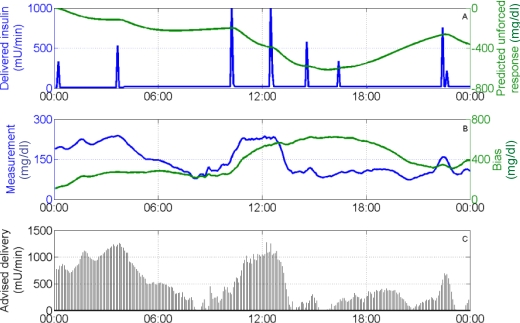
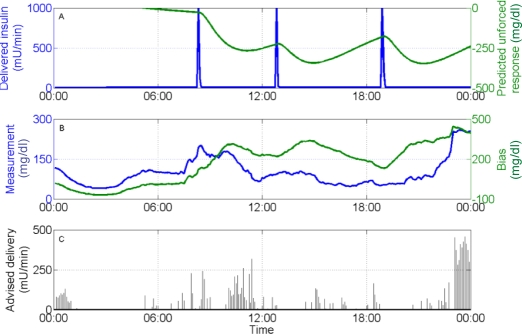
Figure 8.
Advisory results for subject 2, day 6, with τc = 10 min. (A) Insulin delivery rate (left), predicted unforced response (right); (B) glucose sensor measurement (left), controller bias (right); and (C) advised insulin delivery. The advised delivery rate increases with high glucose concentrations and also increases as the predicted unforced response increases (as delivered insulin degrades).
Figure 9.
Advisory results for subject 3, day 10, with τc = 50 min. (A) Insulin delivery rate (left), predicted unforced response (right); (B) glucose sensor measurement (left), controller bias (right); and (C) advised insulin delivery. Pump shutdown is advised as the glucose concentration approaches levels associated with hypoglycemia.
Conclusions
The dictum that MPC is inappropriate for an inherently uncertain system, such as a subject with T1DM, has been disproved by showing conditions under which an appropriately tuned MPC controller will produce identical behavior to that of a PID controller. Furthermore, advantages of the state-space formulation of MPC are retained, as the optimization formulation can be compared easily to measurable properties; also, when tuned as an IMC controller, a single tuning handle relating directly to stability is available.
Although a simple linear model is not an accurate representation of glucose-insulin dynamics, it still has value when designing a model-based controller. This is because a successful controller would, in theory, have such tight control over glycemia that the nonlinearities would be negligible. Development of a model for a subject could consist of obtaining open-loop responses to small insulin step changes and disturbances, such as small, unannounced meals.
The simulation study showed that the model-based tuning rules led to sensible controller output and reasonable system response, although attention must be paid to tuning for robustness due to uncertainty in the subject model. The advisory mode control study also showed feasible output, but because of the retrospective nature of this technique, the issue of validating model predictions cannot be addressed. This highlights the need for a physiologically complex virtual subject population, upon which the degree of controller robustness and efficacy could be assessed properly.
The utility of the risk metric was seen when evaluating controller performance. In order to address the asymmetry of the control problem, future MPC formulations could use output feedback in the risk domain, instead of the glucose domain, thus adding a clinical weighting to the controller cost function.
This prototype study has focused on the Bergman minimal model, which in itself addresses only intravenous insulin administration. Because IV administration and measurement are only suitable for a clinical setting, an extension to subcutaneous administration and measurement is the next logical step for ambulatory closed-loop control.
Finally, given the time-varying nature of glucose-insulin dynamics in the intact human body, a successful controller will doubtless be required to adapt over several timescales to remain efficient. For this purpose, the process could be addressed as a run-to-run problem in which the model inherent to the controller adapts in real time.
Abbreviations
- (ICU)
intensive care unit
- (IMC)
internal model control
- (IOB)
insulin on board
- (IV)
intravenous
- (MAC)
model algorithmic control
- (MPC)
model predictive control
- (PID)
proportional-integral-derivative
- (T1DM)
type 1 diabetes mellitus
Funding
Funding was from Juvenile Diabetes Research Foundation Grants 22-2006-1115 and 22-2006-1108 and National Institutes of Health Grants R21-DK068706, R01-DK068683, and R21-DK069833.
References
- 1.UK Prospective Diabetes Study Group. Intensive blood–glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352:837–853. [PubMed] [Google Scholar]
- 2.Diabetes Control and Complications Trials Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 3.Seborg DE, Edgar TF, Mellichamp DA. Process dynamics and control. 2nd ed. Wiley: Hoboken, NJ; 2004. [Google Scholar]
- 4.Juneja R, Roudebush C, Kumar N, Macy A, Golas A, Wall D, Wolverton C, Nelson D, Carroll J, Flanders SJ. Utilization of a computerized intravenous insulin infusion program to control blood glucose in the intensive care unit. Diabetes Technol Ther. 2007;9(3):232–240. doi: 10.1089/dia.2006.0015. [DOI] [PubMed] [Google Scholar]
- 5.Jovanovic L. Insulin therapy and algorithms for treating type 1 diabetes mellitus. Optimizing insulin therapy in patients with diabetes. CME activity jointly sponsored by Washington Hospital Center and MedStar Research Institute. 2002:13–19. [Google Scholar]
- 6.Allen NHP, Kerr D, Smythe PJ, Martin N, Osola K, Thompson C. Insulin sensitivity after phototherapy for seasonal affective disorder. Lancet. 1992;339(8800):1065–1066. [PubMed] [Google Scholar]
- 7.Parker RS, Doyle III FJ, Peppas NA. The intravenous route to blood glucose control. IEEE Eng Med Biol Mag. 2001;20(1):65–73. doi: 10.1109/51.897829. [DOI] [PubMed] [Google Scholar]
- 8.Hovorka R. Management of diabetes using adaptive control. Int J Adapt Control. 2005;19(5):309–325. [Google Scholar]
- 9.Bequette BW. A critical assessment of algorithms and challenges in the development of a closed-loop artificial pancreas. Diabetes Technol Ther. 2005;7(1):28–47. doi: 10.1089/dia.2005.7.28. [DOI] [PubMed] [Google Scholar]
- 10.Rivera DE, Morari M, Skogestad S. Internal model control 4. PID controller design. Ind Eng Chem Proc DD. 1986;25(1):252–265. [Google Scholar]
- 11.Richalet J, Rault A, Testud JL, Papon J. Model predictive heuristic control: Applications to industrial processes. Automatica. 1978;14(5):413–428. [Google Scholar]
- 12.Cutler CR, Ramaker BL. American Control Conf. CA: San Francisco; 1980. Dynamic matrix control: a computer control algorithm. WP5–B. [Google Scholar]
- 13.Gillis R, Palerm CC, Zisser H, Jovanovic L, Seborg DE, Doyle III FJ. Glucose estimation and prediction through meal responses using ambulatory subject data for advisory mode model predictive control. J Diabetes Sci Technol. 2007;1(6):825–833. doi: 10.1177/193229680700100605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bolie VW. Coefficients of normal blood glucose regulation. J Appl Physiol. 1961;16:783–788. doi: 10.1152/jappl.1961.16.5.783. [DOI] [PubMed] [Google Scholar]
- 15.Dalla Man C, Rizza RA, Cobelli C. Meal simulation model of the glucose-insulin system. IEEE Trans Biomed Eng. 2007;54(10):1740–1749. doi: 10.1109/TBME.2007.893506. [DOI] [PubMed] [Google Scholar]
- 16.Parker RS, Doyle III FJ, Peppas NA. A model-based algorithm for blood glucose control in type I diabetic patients. IEEE Trans Biomed Eng. 1999;46(2):148–157. doi: 10.1109/10.740877. [DOI] [PubMed] [Google Scholar]
- 17.Sedaghat AR, Sherman A, Quon MJ. A mathematical model of metabolic insulin signaling pathways. Am J Physiol Endocrinol Metab. 2002;283(5):E1084–E1101. doi: 10.1152/ajpendo.00571.2001. [DOI] [PubMed] [Google Scholar]
- 18.Bergman RN, Ider YZ, Bowden CR, Cobelli C. Quantitative estimation of insulin sensitivity. Am J Physiol Endocrinol Metab Gastrointest Physiol. 1979;236(6):E667–E677. doi: 10.1152/ajpendo.1979.236.6.E667. [DOI] [PubMed] [Google Scholar]
- 19.Steil G, Clark B, Kanderian S, Rebrin K. Modeling insulin action for development of a closed-loop artificial pancreas. Diabetes Technol Ther. 2005;7(1):94–108. doi: 10.1089/dia.2005.7.94. [DOI] [PubMed] [Google Scholar]
- 20.Bergman RN, Philips LS, Cobelli C. Physiological evaluation of factors controlling glucose tolerance in man. J Clin Invest. 1981;68(6):1456–1467. doi: 10.1172/JCI110398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garcia CE, Morari M. Internal model control 1. A unifying review and some new results. Ind Eng Chem Proc DD. 1982;21(2):308–323. [Google Scholar]
- 22.Chien IL, Fruehauf P. Consider IMC tuning to improve controller performance. Chem Eng Prog. 1990;10:33–41. [Google Scholar]
- 23.Prett DM, Garcia CE. Fundamental process control. Butterworths: Stoneham, MA; 1988. [Google Scholar]
- 24.Hovorka R, Shojaee-Moradie F, Carroll PV, Chassin LJ, Gowrie IJ, Jackson NC, Tudor RS, Umpleby AM, Jones RH. Partitioning glucose distribution/transport, disposal, and endogenous production during IVGTT. Am J Physiol Endocrinol Metab. 2002;282(5):E992–E1007. doi: 10.1152/ajpendo.00304.2001. [DOI] [PubMed] [Google Scholar]
- 25.Parker RS, Gatzke EP, Doyle III FJ. Advanced model predictive control (MPC) for type I diabetic patient blood glucose control. Proceedings of the American Control Conference; Chicago, IL. 2000. pp. 3483–3487. [Google Scholar]
- 26.Kovatchev BP, Cox DJ, Gonder-Frederick LA, Clarke W. Symmetrization of the blood glucose measurement scale and its applications. Diabetes Care. 1997;20(11):1655–1658. doi: 10.2337/diacare.20.11.1655. [DOI] [PubMed] [Google Scholar]