Abstract
Background
The variability of the metabolic action of insulin after subcutaneous (sc) injection hampers optimal insulin therapy. Insulin formulations with a reduced tendency to form hexamers might exhibit a reduced variability of absorption from the sc insulin depot into the blood stream.
Methods
We investigated the within-subject variability of pharmacodynamic and pharmacokinetic properties of an ultra-fast insulin (UFI) formulation and regular human insulin (RHI) in patients with type 1 diabetes. Fourteen patients participated in six 10-hour euglycemic glucose clamp experiments. In this double-blind, crossover study, subjects were randomly assigned to a sequence of two experimental blocks: each block consisted of three doses of 0.1 IU/kg UFI or RHI, respectively, administered on separate days by abdominal sc injection.
Results
Ultra-fast insulin has an earlier onset of action and shorter time to maximal plasma insulin concentration when compared to RHI (tGIRmax 99 ± 36 min vs. 154 ± 74 min, p = 0.002; tCmax 33 ± 16 min vs. 97 ± 39 min, p = 0.00001). The within-subject variability of plasma insulin tCmax (p = 0.027) and of tGIRmax (p = 0.022) was less for UFI than for RHI.
Conclusions
In patients with type 1 diabetes, this UFI showed reduced within-subject variability when compared with RHI.
Keywords: human insulin, insulin therapy, prandial insulin, rapid-acting insulin analogs, variability, Viaject
Introduction
Variability of the pharmacokinetic (PK) and pharmaco-dynamic (PD) effects of subcutaneously (sc) injected insulin hampers an optimal insulin therapy.1,2 VIAject™ (Biodel Inc., Danbury, CT) is a novel ultra-fast insulin (UFI U25) formulation.3 The basic concept of this new approach is to pull the zinc ions away from recombinant human insulin, thereby destabilizing the hexamer, preventing reformation of the hexamer upon injection, and simultaneously masking charges on the surface of the insulin molecule.4 This is achieved by the addition of ethylene diamine tetraacetic acid and citric acid; small molecules that are in use in already approved injectable formulations. The aim of this clinical- experimental study was to determine the within-subject variability of PD and PK properties of UFI in comparison to regular human insulin (RHI) in patients with type 1 diabetes.
Research Design and Methods
In this double-blind, crossover study, 14 patients with type 1 diabetes (4 females, 10 males; age 35 ± 11 years [mean ± standard deviation (SD)]; body mass index 25.6 ± 2.3 kg/m2; hemoglobin A1c 7.7 ± 1.2%) were enrolled. Patients using neutral protamine Hagedorn were not injected with basal insulin the evening prior to the study days whereas patients using long-acting insulin analogs received their last injection on the morning prior to the study days. They participated in six 10-hour euglycemic glucose clamp experiments (Biostator; target blood glucose value 90 mg/dl; baseline intravenous insulin infusion to bring blood glucose to target in the 2 h run-in phase; shut off 35 min before dosing, no re-start during the study period). The glucose clamps were continued for 8 hours after dosing. Subjects were randomly assigned to a sequence of 2 experimental blocks. Each block consisted of 3 identical doses of 0.1 IU/kg UFI (25 U/ml) or RHI (U100; Humulin® R, Eli Lilly, IN), respectively, administered by sc injection into the abdominal wall by means of a syringe. The ethics committee approved the study, which was performed according to Good Clinical Practice standards.
Sixth order polynomial functions were fitted to the individual baseline-corrected glucose infusion rate profiles to determine PD summary measures: maximum glucose infusion rate (GIRmax), time to maximum GIR (tGIRmax), time to half-maximal GIRmax [tGIRmax+50% (before) and tGIRmax−50% (after)], and areas under the curve (AUCGIR) for specified time intervals. The following PK parameters were derived from the plasma insulin concentration profiles: maximum insulin concentration (Cmax), time to Cmax (tCmax), time to 50% of Cmax (tCmax+50% and tCmax−50%), and the area under the curve (AUCINS) for specified time intervals. The primary objective of this study was to compare the within-subject variability of PK and PD parameters by using the standard deviation (SD) of the time to reach half maximum plasma insulin concentration (tCmax+50%) and of the time to reach half maximum glucose infusion rate (tGIRmax+50%) after repeated sc administration of UFI and RHI.
UFI and RHI plasma levels were measured by a chemo-luminescence assay. The measured levels were adjusted for baseline levels, and any values <0 µU/ml were set to zero. All PK parameters were calculated from these adjusted data. Cmax and tCmax were reported from the observed adjusted maximum values, tCmax+50% and tCmax−50% were calculated by linear interpolation between the two closest time points, and the AUCINS values were calculated using the linear trapezoidal rule.The magnitude and significance of the within-subject and between-subject variability of PK and PD parameters were estimated by first calculating the SD of the PK and PD parameters for each subject, grouped by insulin type. These values were analyzed using ANOVA, factoring and adjusting for differences between patients (between-subjects) and difference between insulin forms (within-subjects). A p value <.05 was regarded as significant.
Results
Blood glucose levels during the glucose clamps on the 6 study days were comparable on the different study days, as was their variability: 90 ± 5 mg/dl (5.4%; coefficient of variation); 90 ± 4 mg/dl (4.8%); 90 ± 5 mg/dl (5.0%); 90 ± 5 mg/dl (5.5%); 90 ± 4 mg/dl (4.2%), and 90 ± 5 mg/dl (5.5%).
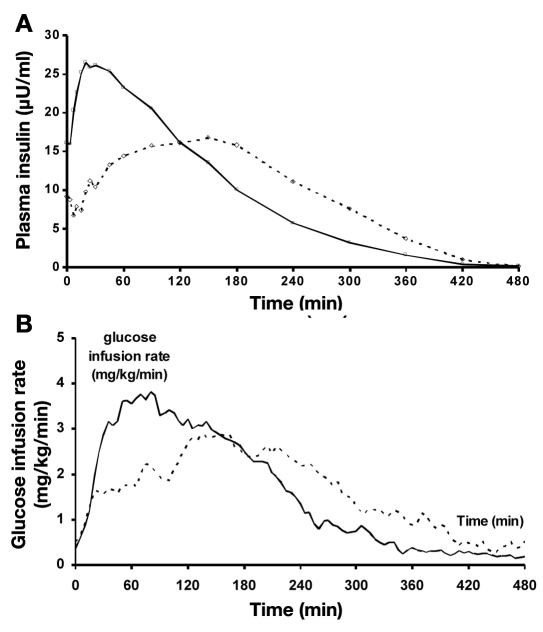
SC injection of UFI resulted in an increase in insulin absorption that is characterized by a more rapid increase in plasma levels than after injection of RHI (tCmax+50%; Table 1; Figure 1a). Maximal plasma insulin levels were also achieved earlier with UFI when compared with RHI. The AUCINS values were different between UFI and RHI for the first three hours after sc injection. The total AUCs were not different.
Table 1.
Within-subject Variability of Pharmacokinetic and Pharmacodynamic Summary Measures After Repeated sc Injection of 0.1 IU/kg Body Weight of a Novel Rapid-acting Insulin in Comparison to Regular Human Insulin in 14 patients with Type 1 Diabetes.
| Pharmacokinetic parameters | Unit | Differences between insulin formulations | Average of within-subject standard deviation (SD) | Difference (95% Confidence Interval) | ||||
|---|---|---|---|---|---|---|---|---|
| p-value | Means | p-value | RHI SD | UFI SD | ||||
| RHI | UFI | |||||||
| Cmax | (mU/liter) | 0.0086 | 23.7 | 32.7 | 0.6240 | 10.0 | 7.6 | 2.4 (−7.9 to 12.7) |
| tCmax | (min) | <0.0001 | 97 | 33 | 0.0270 | 39 | 16 | 24 (3 to 45) |
| tCmax+50% | (min) | <0.0001 | 32 | 8 | 0.0229 | 20 | 6 | 14 (2 to 25) |
| tCmax−50% | (min) | <0.0001 | 215 | 131 | 0.0845 | 65 | 35 | 30 (−5 to 65) |
| AUCINS 0-180 | (mU/liter*min) | <0.0001 | 2552 | 3442 | 0.7647 | 665 | 626 | 38 (−232 to 309) |
| AUCINS 0–480 | (mU/liter*min) | 0.9612 | 4410 | 4395 | 0.5308 | 916 | 823 | 94 (−220 to 407) |
| Pharmacodynamic parameters | ||||||||
| GIRmax | (mg/kg/min) | <0.0001 | 4.1 | 5.6 | 0.4745 | 1.3 | 1.5 | −0.2 (−0.9 to 0.4) |
| tGIRmax | (min) | 0.0015 | 154 | 99 | 0.0221 | 74 | 36 | 38 (6 to 69) |
| tGIRmax+50% | (min) | 0.1317 | 51 | 38 | 0.0601 | 32 | 17 | 15 (−1 to 31) |
| tGIRmax−50% | (min) | 0.0062 | 165 | 119 | 0.0759 | 72 | 39 | 33 (−4 to 70) |
| AUCGIR 0-180 | (mg/kg) | <0.0001 | 276 | 458 | 0.3804 | 89 | 112 | −23 (−79 to 32) |
| AUCGIR 0-480 | (mg/kg) | 0.2197 | 592 | 647 | 0.2889 | 199 | 151 | 47 (−45 to 140) |
| AUCGIR 180-480 | (mg/kg) | 0.0001 | 311 | 193 | 0.0182 | 135 | 67 | 68 (14 to 123) |
Values in bold indicate significant changes in variability.
Figure 1.
Mean plasma insulin levels and glucose infusions rates (baseline corrected) after sc injection of 0.1 IU/kg body weight of a novel rapid-acting insulin (continuous line) in comparison to regular human insulin (broken line) in 14 patients with type 1 diabetes.
The changes in the PD parameters mirror those in the PK parameters (Table 1; Figure 1b). Maximal metabolic activity was achieved earlier with UFI when compared to RHI. The AUCGIR profiles were different between UFI and RHI for the first three hours after sc injection. The total AUCs were not different. It is of interest that the AUCGIR and the variability of the metabolic activity 3 to 6 h after injection were lower with UFI than with RHI. The intra-subject variability of UFI was less for tGIRmax, tCmax, and tCmax+50% (Table 1) when compared to RHI. No serious adverse event occurred during this trial.
Discussion
This study confirms that also in patients with type 1 diabetes, Viaject has faster absorption kinetics and a more rapid onset of insulin action than regular human insulin as previously described in healthy subjects.4 That the total AUCs with both insulin formulations are comparable suggests that the bioavailability is identical. This is not surprising considering that both insulin formulations tested contain recombinant human insulin and not a rapid-acting insulin analog. The major difference between the two insulin formulations is the ingredients added to regular human insulin to produce the UFI formulation. It is well known that a lower insulin concentration is associated with a more rapid insulin absorption/insulin action in comparison to a higher strength. However, in a glucose clamp study with U40 vs. a U100 RHI, no significant differences in insulin absorption/insulin action were observed.5 It cannot be ruled out that with an even higher difference in strength (U25 vs. U100), significant differences could be observed; however, most probably the differences in the PK/PD parameters are not in the range as they were observed in this and other studies.
This study shows that upon repeated administration, the within-subject variability of most of the PK and PD parameters is lower for UFI than for RHI. The reduced variability of UFI can be attributed to the rapid absorption per se. The rapid absorption in turn can be explained by the fact that the UFI formulation is primarily monomeric, and as a result saves the variability and time necessary for the dissociation from hexameric insulin to dimeric to monomeric insulin.
A reduced variability of the onset of action helps to optimize postprandial metabolic control because patients can be confident to induce a reproducible metabolic effect when they inject this prandial insulin. The reduced metabolic effect seen some hours after the sc injection (along with a reduced variability) should reduce the risk of late postprandial hypoglycemic events. It remains to be seen if the variability with higher insulin doses remains the same. The selected dose, however, is often used to cover prandial insulin requirements in this type of patient. It would also be of interest to study in a head-to-head comparison the variability of insulin absorption/insulin action of this UFI against that observed with the different rapid-acting insulin analogs that are on the market. Interestingly, no such comparative studies of the variability of the different rapid-acting insulin analogs have been published.
In conclusion, this novel, rapid-acting and less variable human insulin formulation appears to be a promising candidate for improving postprandial glycemic excursions with a reduced risk of late postprandial hypoglycemia in patients with diabetes.
Abbreviations
- PD
pharmacodynamic
- PK
pharmacokinetic
- RHI
regular human insulin
- sc
subcutaneous
- UFI
ultra fast insulin
Funding
Support by Biodel Inc. is acknowledged.
References
- 1.Heinemann L. Variability of Insulin Action: Does this matter? Insulin. 2008;3:37–45. [Google Scholar]
- 2.Heise T, Nosek L, Stender A, Biilmann Rønn B, Heinemann L, Kapitza C, Draeger E. Lower within-subject variability of insulin detemir in comparison to NPH insulin and insulin glargine in people with type 1 diabetes. Diabetes. 2004 Jun;53(6):1614–1620. doi: 10.2337/diabetes.53.6.1614. [DOI] [PubMed] [Google Scholar]
- 3.Pohl R, Steiner SS. inventors; Biodel, Inc., assignee. Rapid acting drug delivery compositions. United States patent US 7279457. 2007 Oct 9. [Google Scholar]
- 4.Steiner SS, Hompesch M, Pohl R, Simms P, Pfützner A, Heinemann L. A formulation of human insulin with a more rapid onset of action than rapid-acting insulin analogues. Diabetologia. 2008 doi: 10.1007/s00125-008-1095-8. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heinemann L, Weyer C, Rave K, Stiefelhagen O, Rauhaus M, Heise T. Comparison of the time action profiles of U40- and U100-regular human insulin and the rapid-acting insulin analogue B28 Asp. Exp Clin Endocrinol Diabetes. 1997;105:140–144. doi: 10.1055/s-0029-1211742. [DOI] [PubMed] [Google Scholar]