Abstract
Background
Hemoglobin A1c monitoring is routine care for patients with diabetes and may be obtained as often as every 3 months. Most family practice clinics are not equipped to evaluate a hemoglobin A1c result in the office. Obtaining a hemoglobin A1c result from a central laboratory can result in a delay, added expense, and inconvenience for the patient. To date, there are no published studies on the accuracy of the A1CNow+™, a point-of-care hemoglobin A1c monitoring device.
Methods
Seventy patients having type 1 or type 2 diabetes were enrolled from three pharmacy-managed diabetes clinics. Subjects were required to have a venous blood draw within 1 week of the point-of-care test. The study then evaluated the statistical and clinical significance between both tests.
Results
A good correlation was seen between the A1CNow+ and laboratory values with a correlation coefficient of r = 0.893. The best correlation between the A1CNow+ and the laboratory was seen among hemoglobin A1c values in the range of 7–8.5%.
Conclusion
The access of the A1CNow+ device at point of care makes a hemoglobin A1c evaluation economically and therapeutically beneficial after proving its accuracy in a primary care setting. Advantages of this device may go beyond convenience and economic benefit by allowing patients to acknowledge their level of glucose control at the point of care and to be counseled appropriately.
Keywords: diabetes, hemoglobin A1c, point of care
Introduction
Approximately 21 million children and adults in the United States have diabetes.1 As demonstrated in the Diabetes Control and Complications Trial and in the United Kingdom Prospective Diabetes Study, achieving adequate glycemic control in those with diabetes reduces both micro- and macrovascular complications.2,3 A vital element in attaining adequate glycemic control on the part of health care providers is regular monitoring of patient's glycemic control through regular follow-up.
Although periodic fasting laboratory testing and assessment of self-monitored blood glucose (SMBG) values are essential in the therapeutic management of diabetic patients, obtaining glycosylated hemoglobin A1c values are equally important for long-term evaluation. Glycosylated hemoglobin is formed in a nonenzymatic pathway by the normal exposure of hemoglobin to high plasma levels of glucose. The glucose in the blood binds irreversibly to hemoglobin to form a glycated hemoglobin complex. The normal life span of a red blood cell is approximately 120 days; therefore, the hemoglobin A1c level will change as new red blood cells are made. Hence, the hemoglobin A1c value gives us an estimation of what a person's average blood glucose has been for the past 2 to 3 months. While hemoglobin A1c levels are not used for diagnostic purposes and do not reveal details about daily glycemic fluctuations, they can be useful in verifying reported SMBG values, as well as providing essential information on patients who do not obtain SMBG values. The American Diabetes Association adopts the hemoglobin A1c value of ≥7% as an indicator that warrants intervention in a patient's therapy and establishes a goal of <7% for glycemic control. It is recommended that the hemoglobin A1c be checked quarterly if changes in therapy are made or if the patient is not meeting goals, otherwise it may be routinely checked at least twice a year.1
The most common method by which hemoglobin A1c values are measured is by obtaining a venous blood draw in the office, which is then sent off and evaluated by a laboratory. This can be both time-consuming and costly. In addition, this can be quite inconvenient for the patient in that they must either schedule a return visit or be contacted by phone in order to be given their results along with any necessary intervention or counseling. Studies have shown the benefits of rapid hemoglobin A1c results at the time of the patient encounter by improving glucose control through intensification of therapy and improvement of hemoglobin A1c levels.4–6 Moreover, venous blood draws are widely recognized as an unpleasant experience for many patients. Therefore, having the choice of the finger stick method may be more acceptable to most patients as many will already be familiar with the procedure as a consequence of daily blood glucose testing.
Currently there are several hemoglobin A1c devices available to both patients and health care providers. Some provide results in minutes, whereas most require mailing of the sample collection to a laboratory. We conducted a prospective evaluation to determine the accuracy of the point-of-care device A1CNow+ (Metrika Inc., Sunnyvale, CA) as compared to a standard laboratory. The A1CNow+ is a newer version of the A1cNow Inview and is intended for use by health care professionals only.
Methods
The study was designed as a prospective evaluation and was approved by the University of Florida Institutional Review Board. The study population consisted of type 1 and type 2 diabetes patients obtained from three pharmacist-managed diabetes clinics in the greater Gainesville, Florida area. A total of 70 subjects were enrolled from September 2006 to April 2007 using the following inclusion criteria: current patients at three area family practice clinics who were 18–90 years of age and had type 1 or type 2 diabetes. There were no exclusion criteria.
Patients were seen by their primary care provider and subsequently referred to our pharmacist-run diabetes clinic for further management and counseling. During the clinic visit, patients were asked to participate in the study and sign an informed consent form to confirm their agreement. The finger stick was done according to manufacturer guidelines by one of four trained pharmacists. Patients were required to have had a venous blood draw reporting hemoglobin A1c within 1 week of the point-of-care test in order to be included as a valid measure in the study. Blood draw samples were all analyzed by the same laboratory.
The primary outcome was to evaluate the accuracy of the A1CNow+ device. The correlation between hemoglobin A1c as determined by the point-of-care test and the reference laboratory method was tested by Spearman's correlation. Secondary outcomes included sensitivity and specificity of the point-of-care monitor with hemoglobin A1c levels of 7 and 8.5% as prespecified cutoffs for evaluation of test performance. The hemoglobin A1c level of 7% was chosen because the American Diabetes Association currently uses this as a goal for treatment in diabetes, while a hemoglobin A1c level of 8.5% or greater is consistent with poorly controlled diabetes.1
All subjects in the study were given usual care in regards to their diabetes treatment and all therapeutic changes were based on laboratory values rather than values from the hemoglobin A1c device.
Results
All 70 patients consented to be in our study and had blood samples drawn for both A1CNow+ and laboratory measurements. The mean age of patients in the study was 61.7 ± 12.5 years. Sixty-nine patients had type 2 diabetes. A majority of the patients were female (n = 45). Over half of the patients were Caucasian (51%), followed by African- American and Hispanic at 44 and 5%, respectively (Table 1). The mean hemoglobin A1c value with the A1CNow+ device was 7.8 ± 1.4%. The mean hemoglobin A1c value by the reference laboratory was 7.5 ± 1.4%.
Table 1.
Demographics
| Characteristic | Number (percent) |
|---|---|
| Age | 61.7 (12.5)a |
| Type of diabetes | |
| Type 1 | 1 (1.4) |
| Type 2 | 69 (98.6) |
| Gender | |
| Male | 25 (35) |
| Female | 45 (65) |
| Race | |
| White | 36 (51) |
| Black | 31 (44) |
| Hispanic | 3(5) |
Mean (standard deviation).
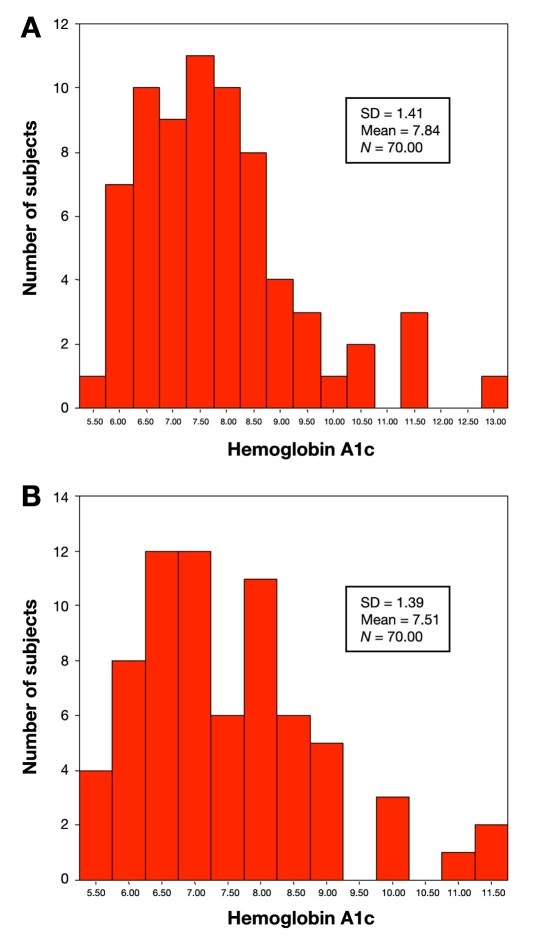
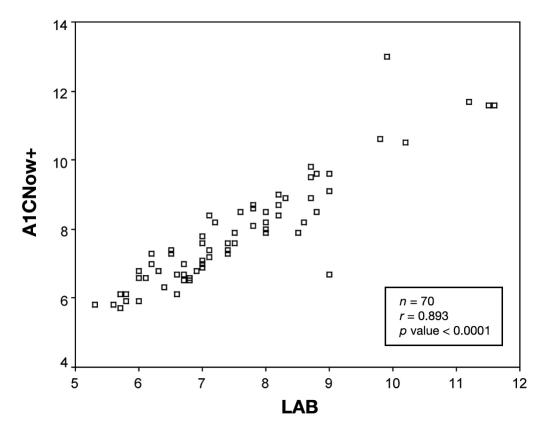
The distribution of hemoglobin A1c values is similar for both the point-of-care device A1CNow+ and the centralized laboratory with the majority of values occurring between 6 and 9% for either test (Figure 1). To assess whether values obtained from the point-of-care test correlated with values obtained by the central laboratory, Spearman's nonparametric correlation was performed. Overall, values obtained from either method were strongly correlated (r = 0.89, p < 0.0001; Figure 2). An r value equal to 1 would represent an exact correlation between the hemoglobin A1c value obtained with the A1CNow+ and the hemoglobin A1c value produced by the central laboratory. Therefore, the correlation coefficient (r = 0.893) represents a good correlation between the A1CNow+ and the laboratory value.
Figure 1.
Hemoglobin A1c distribution using A1CNow+ (a) and a standardized laboratory (b). SD, standard deviation.
Figure 2.
All hemoglobin A1c values obtained.
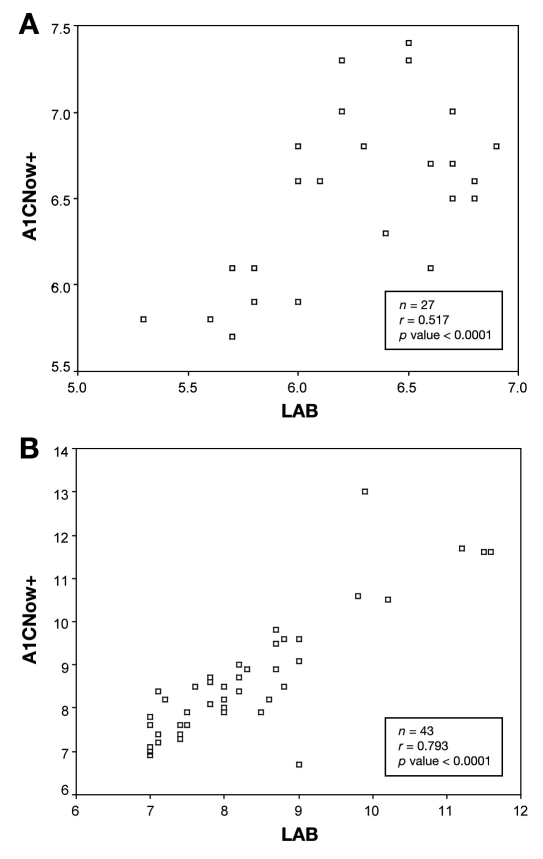
Because of therapeutic implications of using hemoglobin A1c levels of 7 and 8.5% as decision thresholds, we examined the correlations of hemoglobin A1c values between the A1CNow+ and the laboratory using these hemoglobin A1c cutoffs. Figure 3 displays data for all hemoglobin A1c values below and above 7%, as determined by the standardized laboratory. Twenty-seven patients had hemoglobin A1c values determined by the laboratory to be less than 7%, and 43 patients had a hemoglobin A1c value greater than 7%. While the correlations remained significant for values below and above 7% (p < 0.0001), there was a stronger correlation between tests for values >7% (r = 0.79) compared with those below 7% (r = 0.52).
Figure 3.
(a) Hemoglobin A1c values <7% and >7% (b) based on a standardized laboratory with correlating hemoglobin A1CNow+ value obtained.
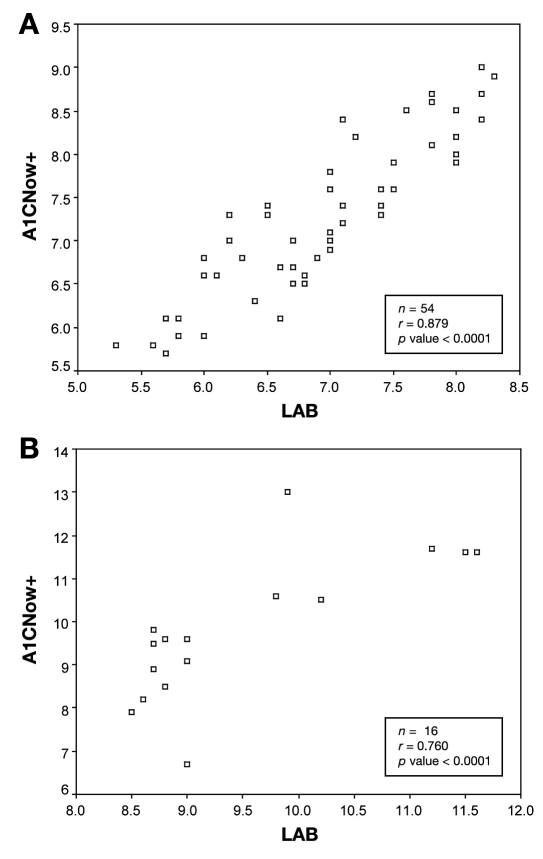
Data were further dichotomized using the hemoglobin A1c value cutoff of 8.5%, as determined by the laboratory measurement. Figure 4 shows analysis hemoglobin A1c values based on this threshold. The correlation coefficients were r = 0.88 and 0.76 for values less than and greater than 8.5%, respectively, and were significant.
Figure 4.
Hemoglobin A1c values <8.5% (a) and >8.5% (b) based on a standardized laboratory with correlating A1CNow+ value obtained.
For the 7% cutoff, the sensitivity, specificity, positive predictive value, and negative predictive value were 0.95, 0.74, 0.85, and 0.91, respectively. For the 8.5% cutoff, the sensitivity, specificity, positive predictive value, and negative predictive value for the 8.5% cutoff were 0.81, 0.87, 0.65, and 0.94, respectively.
Important for the intended use of this device in the primary care setting, a regression model was produced from our data ([expected laboratory hemoglobin A1c] = 1.053 + 0.824[hemoglobin A1c by A1CNow+]) that allows for prediction of the laboratory hemoglobin A1c value given the hemoglobin A1c result from the point-of-care device.
Discussion
In order to improve the management of diabetes, point-of-care devices that measure hemoglobin A1c must be evaluated against gold standard laboratory methods. In our evaluation of the novel A1CNow+ point-of-care device, we saw moderate-strong correlations with the standardized laboratory depending on the hemoglobin A1c cutoff tested. The correlation coefficients in our study were higher than in previous studies with this device, after receiving National Glycohemoglobin Standardization Program (NGSP) certification.7–9 The poorest correlation between the A1CNow+ and the laboratory was seen for hemoglobin A1c <7.5% as reported by the laboratory. However, of these values, only seven were actually >7.5% as reported by the point-of-care test. Therefore, it is unlikely that this diminished correlation would affect therapeutic decision making. Also of note, this study contained little data for extremely high values. Conclusions about the performance of this device at such values cannot be drawn from our sample population. The performance of this meter at extremely high values would have to be studied further in a sample population that contained such values.
After proving a positive correlation between the point-of-care device and the laboratory, clinical decisions can be made from the results of the device. The regression model we developed could be used to estimate the laboratory hemoglobin A1c value based on the results from the A1CNow+. If, for example, the point-of-care device reported a hemoglobin A1c value of 7.5%, we can predict the laboratory value to be 7.2%. The value obtained by the A1CNow+ provides an acceptably accurate result of hemoglobin A1c, as the difference in hemoglobin A1c values between the two methods is not clinically significant. Therefore, therapeutic decisions are unlikely to vary. Further studies with a larger population that also have hemoglobin A1c values across all ranges would be needed to validate our model. In the meantime, the A1CNow+ appears to be a conveniently effective point-of-care hemoglobin A1c evaluation device that provides reliable results in a primary care setting.
Studies have been performed by the manufacturer that compared the A1CNow+ to NGSP-certified laboratories. One study involving 39 patients from a clinic group in Atlanta, Georgia showed a correlation coefficient of 0.95. Further studies at large clinic groups involving 20 patients and 54 patients demonstrated correlation coefficients of 0.98 and 0.97, respectively.10 Results shown by our study and field studies performed by the manufacturer indicate acceptably accurate hemoglobin A1c results with the added advantage of its convenience.
Aside from the convenience the device offers, it also provides a significant cost advantage to a patient who is responsible for fee-for-service and to primary care clinics that use the device for hemoglobin A1c determination. At present, hemoglobin A1c values are assessed by the centralized laboratory from a venous blood sample drawn either at the laboratory or at a clinical practice site, which is subsequently sent to the centralized laboratory for analysis. The cost for evaluation of hemoglobin A1c from the central laboratory can range from $22 to $65 for a patient without insurance coverage. This does not include the additional laboratory draw fee of $10 to $20. The cost for a single test of hemoglobin A1c on average with A1CNow+ is $11.90. Depending on who incurs the cost, this is potentially a cost benefit to a patient who is self-pay or to primary care clinics. However, like most patients with diabetes who have Medicare Part B, there are Current Procedural Terminology codes available for reimbursement to the clinics. The Medicare National Limitation Amount is $21.06 in most states.11 Pharmacists and other health care professionals providing hemoglobin A1c testing to patients would likely need to mark up the cost of the test and include a professional fee, which would increase the cost of using the A1CNow+ and make it more comparable with standard laboratory testing. The access of the A1CNow+ device at point of care makes a hemoglobin A1c evaluation economically and therapeutically beneficial after proving its accuracy in the primary care setting.
One limitation to our study is the fact that relatively few patients had high hemoglobin A1c values (>8.5%). Hemoglobin A1c values from the point-of-care test and standardized laboratory were obtained within 7 days of one another, although it is unclear what effect this would have on hemoglobin A1c. We also did not exclude or screen patients for hemoglobinopathies, which could possibly affect hemoglobin A1c results.
Conclusion
The results of this study suggest that the A1CNow+ is a reliable option for evaluating hemoglobin A1c levels in patients with diabetes when laboratory testing may not be feasible or is too costly. Advantages of this device may go beyond convenience and economic benefit by allowing patients to acknowledge their level of glucose control at the point of care and to be counseled appropriately.
Abbreviations
- NGSP
National Glycohemoglobin Standardization Program
- SMBG
self-monitored blood glucose
References
- 1.American Diabetes Association. [accessed 2008 Mar 12]. Available from: www.diabetes.org.
- 2.The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329(14):977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 3.UK Prospective Diabetes Study (UKPDS) Group. Intensive blood glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes. Lancet. 1998;352(9131):837–853. [PubMed] [Google Scholar]
- 4.Thaler LM, Ziemer DC, Gallina DL, Cook CB, Dunbar VG, Phillips LS, El-Kebbi IM. Diabetes in urban African-Americans. XVII. Availability of rapid HbA1c measurements enhances clinical decision making. Diabetes Care. 1999;22(9):1415–1421. doi: 10.2337/diacare.22.9.1415. [DOI] [PubMed] [Google Scholar]
- 5.Marrero DG, Kraft S, Fineberg N. Effect of immediate feedback of HbA1c on patient glycemic control and physicians' treatment decisions [abstract] Diabetes. 1996;45:7A. [Google Scholar]
- 6.Cagliero E, Levina E, Nathan DM. Immediate feedback of HbA1c levels improves glycemic control in type 1 and insulin-treated type 2 diabetic patients. Diabetes Care. 1999;22(11):1785–1788. doi: 10.2337/diacare.22.11.1785. [DOI] [PubMed] [Google Scholar]
- 7.Sicard DA, Taylor JR. Comparison of point-of-care HbA1c test versus standardized laboratory. Ann Pharmacother. 2005;39(6):1024–1028. doi: 10.1345/aph.1E504. [DOI] [PubMed] [Google Scholar]
- 8.National Glycohemoglobin Standardization Program. [accessed 2007 Sep 12]. Available from: www.ngsp.org.
- 9.Kennedy L, Herman WH GOAL A1C Study Team. Glycated hemoglobin assessment in clinical practice: comparison of the A1cNow point-of-care device with central laboratory testing (GOAL A1C Study) Diabetes Technol Ther. 2005;7(6):907–912. doi: 10.1089/dia.2005.7.907. [DOI] [PubMed] [Google Scholar]
- 10.Metrika Incorporated. A1CNow+ package insert. Sunnyvale, CA: Metrika; 2006. [Google Scholar]
- 11.Metrika Incorporated. [accessed 2008 Mar 7]. Available from: www.metrika.com.