Abstract
With the inception of the Edmonton Protocol, intraportal islet transplantation (IPIT) has re-emerged as a promising cell-based therapy for type 1 diabetes. However, current clinical islet transplantation remains limited, in part, by the need to transplant islets from 2–4 donor organs, often through several separate infusions, to reverse diabetes in a single patient. Results from clinical islet transplantation and experimental animal models now indicate that the majority of transplanted islets are destroyed in the immediate post-transplant period, a process largely facilitated by deleterious inflammatory responses triggered by islet-derived procoagulant and proinflammatory mediators. Herein, mechanisms that underlie the pathophysiology of thrombosis and inflammation in IPIT are reviewed, and emerging approaches to improve islet engraftment through attenuation of inflammatory responses are discussed.
Keywords: anticoagulant, anti-inflammatory, cell surface modification, conformal coating, instantaneous blood-mediated inflammatory reaction, intraportal islet transplantation, islet encapsulation, poly(ethylene glycol), type 1 diabetes mellitus
Introduction
Islet Transplantation Has Emerged as a Promising Treatment for Type 1 Diabetes
Islet transplantation has long been conceived as a promising treatment for type 1 diabetes.1–5 Despite advantages over whole pancreas transplantation,6–12 more than half of the islet allografts performed between 1990 and 1998 failed within two months and only 8% of patients remained insulin independent beyond one year.13 In 2000, Shapiro and colleagues introduced the Edmonton Protocol, which combined transplantation of freshly isolated islets with a steroid-free immunosuppressive regimen.14
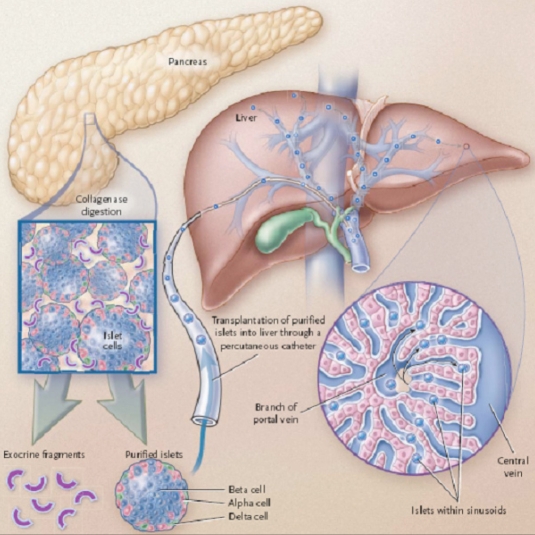
During this procedure, islets are infused percutaneously into the hepatic portal vein (intraportally) where they travel to and ultimately lodge within the liver sinusoids (Figure 1). In their seminal report, 7 of 7 patients remained insulin independent one year post-transplantation.14 This success has reinvigorated widespread interest in islet allotransplantation, and since 2000 more than 500 patients worldwide have received islet transplantation using the Edmonton Protocol and slight modifications thereof.13 Importantly, at the three leading islet transplant centers, 90% of patients receiving islet transplants remained insulin independent at one year15 and Shapiro and colleagues have reported 60% insulin independence at three years,13 rates comparable to, albeit lower than, those observed in whole pancreas transplantation.12
Figure 1.
Intraportal islet transplantation. Under the Edmonton Protocol, islets are isolated from donor pancreases, purified, and infused percutaneously into the portal vein of the liver, where they travel to and ultimately lodge within the distal portal sinusoids. Consequently, islets come into direct contact with blood cells and coagulation proteins and are in immediate proximity to portal vein endothelial cells, resident macrophages of the liver (Kupffer cells), and hepatocytes (Reproduced with permission from the Massachusetts Medical Society.6)
Islet Transplantation Is Compromised by Early Islet Destruction and Primary Non-function
Despite marked improvements, islet transplantation remains limited, in part, by the need to transplant islets from 2–4 donor organs, often in separate infusions, to reverse diabetes in a single patient,8,14,16–19 further burdening a limited donor islet source,20 increasing health care costs,12 and the incidence of procedural complications. Though single-donor islet transplantation has been reported,21 in a recent international trial of the Edmonton Protocol, 44% of patients required three islet infusions, and less than half of them remained insulin independent at one year.22 It has been estimated that a normal human pancreas contains approximately 500,000 islet equivalents (IEQ),19 only 10–20% of which appear to be necessary to maintain euglycemia.23 Currently, patients receive ∼10,000–12,000 IEQ/kg (∼700,000–850,000 IEQ for a 70 kg person), nearly twice the number in a normal pancreas and substantially more than should be required to maintain insulin independence.14,19,23 This discrepancy suggests that transplanted islets are functionally impaired and/or fail to engraft. Indeed, metabolic challenges after transplantation indicate that the functional capacity of transplanted islets is only 20–40% of that of a non-diabetic person even in insulin-independent islet recipients,24 and it has been estimated that as few as 10–20% of infused islets survive clinical transplantation.19 This is supported by animal models, in which 50–70% of transplanted islets are lost in the immediate post-transplant period.25–27 Importantly, rates of insulin independence drop to ∼10% five years post-transplant,28 and it has been suggested that early islet destruction results in engraftment of a limited islet mass that becomes exhausted with long-term metabolic demands.12,27,28
Early Islet Destruction and Primary Non-function Are Mediated by Innate Inflammatory Responses
Despite being transplanted across identical auto- and allo-immune barriers, the extent of graft destruction is significantly greater in islet transplantation than in whole pancreas transplantation. This is perhaps most clearly illustrated in experimental models of syngeneic islet transplantation into non-autoimmune diabetic mice.27,29,30 Even under such ideal transplantation conditions, islet insulin content and function are significantly compromised,30,31 and an estimated 60% of transplanted islet tissue is lost within 3 days post transplantation27 by both necrotic and apoptotic mechanisms,27,29 demonstrating that early islet destruction is not allo- or autoantigen-specific. In contrast, in the absence of immunosuppression, allografted islets that survive such initial inflammatory insults are destroyed by specific immune responses ∼7–22 days later (i.e., allorejection).32–36 While a number of factors likely contribute to early islet destruction in the immediate post-transplant period, including delayed and insufficient revascularization of the graft,37 ischemia-reperfusion injury,38 and glucose and lipotoxicity,39,40 compelling evidence has emerged that early islet destruction is largely mediated by innate inflammatory responses. Animal models of islet transplantation have demonstrated significant inflammation at the graft site, characterized by activation of portal vein endothelial cells (ECs),41 intense infiltration of leukocytes into and around islets,33,41–43 and elevated levels of proinflammatory mediators41,43–46 that adversely effect β-cell viability and function.45,47,48 Unlike conventional implantable materials, which are largely passive bystanders of inflammatory responses and subsequent device failure,49 islets directly contribute to their own destruction via expression and secretion of bioactive mediators that initiate and propagate inflammatory and procoagulant pathways. This is perhaps best illustrated by Bottino et al. who demonstrated that intraportal infusion of islets, but not equivalently sized glass microspheres, triggered increased cytokine production in the immediate post transplant period.44 Therefore, while attenuation of immune responses to transplanted islets remains a critical area of investigation, outcomes of islet transplantation may be dramatically improved through prevention of inflammatory responses largely responsible for early islet destruction and primary non-function. Herein, we describe possible mechanisms through which islets trigger inflammatory responses after IPIT and discuss current areas of investigation that hold particular promise for abrogating such responses.
Pathophysiology of Thrombosis and Inflammation in Intraportal Islet Transplantation
Islets Initiate Activation of Coagulation Cascades
Recent evidence indicates that deleterious inflammatory responses may be generated, in large part, by an instantaneous blood-mediated inflammatory reaction triggered by islets in direct contact with blood (Figure 2).50–53 Korsgren and colleagues have demonstrated that tissue factor (TF), the primary physiological initiator of the coagulation system,54 is expressed by and released from β and α cells of isolated islets.51 TF initiates the extrinsic arm of the coagulation pathway by interacting with factor VIIa, catalyzing the conversion of factor X to its active form, fXa, resulting in conversion of prothrombin to thrombin. Indeed, islets incubated in non-anticoagulated blood in vitro induced a significant thrombotic response, as evidenced by fibrin clots surrounding islets and increased levels of thrombin-antithrombin complex (TAT), prothrombin fragments 1 and 2, and fXIa-antithrombin complex.50,51 Platelets were also activated, as evidenced by reduced platelet counts and release of β-thromboglobulin from alpha granules,51 further amplifying thrombin generation and promoting aggregation of platelets on the islet surface, presumably through interactions between platelet adhesion molecules and islet-derived extracellular matrix proteins.55 Interestingly, Lamblin et al. observed, in a porcine allograft model, elevated TAT upon IPIT, but found no such effect when a similar volume of polystyrene beads was infused, demonstrating the cell-specific nature of the thrombotic response.56 Perhaps more compelling, in nine patients undergoing clinical IPIT, serum levels of prothrombotic markers (TAT, fVIIa-antithrombin, and D-dimer) were significantly elevated 15 minutes to 24 hours post-transplantation,53 and patient serum levels of cross-linked fibrin degradation products have been shown to correlate with pre-transplant levels of TF expression by islets.57
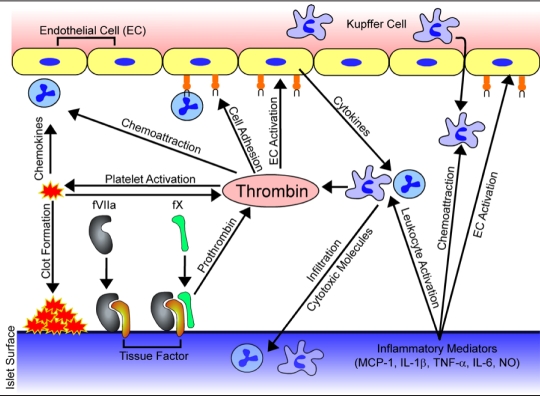
Figure 2.
Mechanisms of thrombosis and inflammation in intraportal islet transplantation. Tissue factor expressed on islets interacts with factor VIIa (fVIIa) resulting in activation of factor X (fX), which converts prothrombin to thrombin, a key mediator of thrombotic and inflammatory events. Local thrombin generation triggers platelet activation and adhesion, further amplifying coagulation cascades, and ultimately entrapping islets within fibrin clots. Furthermore, thrombin acts as a chemoattractant and can trigger expression of endothelial cell adhesion molecules, promoting migration of neutrophils, monocytes, and Kupffer cells to the portal bed. Additionally, islets release a number of inflammatory mediators including MCP-1, IL-1β, TNF-α, IL-6, and NO, which may trigger or exacerbate thrombotic and inflammatory responses post-transplantation through activation of endothelial cells and attraction and activation of leukocytes.
Islet-initiated Coagulation Contributes to Inflammatory Responses
Though perhaps better known for its role in coagulation, thrombin also acts as a conductor of cellular responses during inflammation (Figure 2).58 Thrombin can trigger expression of EC adhesion molecules,58–60 and stimulate EC production of the proinflammatory cytokines interleukin (IL)-6 and IL-8, as well as platelet-activating factor, a potent neutrophil activator.59 Furthermore, thrombin acts as a chemoattractant61 and directly triggers platelet activation, resulting in the release of alpha-granule chemokines and the expression of P-selectin, thereby attracting neutrophils and monocytes to the portal bed and promoting their arrest and activation.59,62,63
In accord with the known effector functions of thrombin, EC activation (expression of intracellular adhesion molecule-1, E-selectin, and P-selectin), neutrophil infiltration, and increased production of cytokines and inflammatory mediators [IL-1β, tumor necrosis factor-α, IL-6, interferon (IFN)-γ, nitric oxide (NO)] are observed 6–12 hours after transplantation in syngeneic animal models of IPIT, resulting in significant islet apoptosis within 24 hours.41,42,64 Though monocytes, Kupffer cells, portal vein ECs, and hepatocytes likely participate in generation of this cytotoxic inflammatory milieu,48 evidence is emerging that neutrophilic granulocytes act as the principle effector cell in early islet destruction.42,65 Yasunami et al. have recently demonstrated that IFN-γ produced by neutrophils plays a crucial role in early islet destruction and that injection of antibodies against neutrophil surface markers Gr-1 and CD11b dramatically attenuates this effect.42 Interestingly, despite use of a simplified in vitro model of islet-blood contact, Moberg et al. have demonstrated that neutrophils begin to infiltrate islets within 15 minutes, and are the predominant cellular infiltrate.65 Significantly, addition of melagatran, a low molecular weight thrombin inhibitor, has been shown to reduce neutrophil infiltration while preserving islet morphology.66 Hence, islet-initiated thrombin generation appears to contribute significantly to the initiation and/or elaboration of inflammatory responses implicated in islet destruction and primary non-function.
Islet-derived Inflammatory Mediators Contribute to Thrombotic and Inflammatory Responses
While blood-mediated responses play a critical role in islet destruction, evidence of inflammation and islet death in syngeneic animal models of islet transplantation into the kidney capsule suggest that direct islet-blood contact is not a prerequisite for initiation of inflammatory responses.27,29,43,45,66 As a result of the metabolic and mechanical stress associated with isolation and culture, isolated islets express and/or release an array of inflammatory mediators32,48,67–79 that may trigger or exacerbate thrombotic and inflammatory responses post-transplantation (Figure 2). Indeed, an inverse correlation between pre-transplant expression levels of inflammatory mediators and islet engraftment has been observed in both animal models72,76 as well as clinical islet transplantation.57,77 Significantly, Piemonti et al. have demonstrated increased rates of insulin independence and significant reduction in insulin requirements in patients who received islet grafts expressing low levels of monocyte chemoattractant protein-1 (MCP-1)77; similar results have been reported in syngeneic murine models.76 Soluble factors released from islets have been shown to activate portal vein ECs41,80 and Kupffer cells,48,81 further contributing to the elaboration of inflammatory responses. Indeed, in an animal model of IPIT, transient inhibition of Kupffer cells has been found to reduce levels of proinflammatory mediators (TNF-α, IL-β, NO) 3–6 hours post transplantation, resulting in improved islet engraftment.44 While the contribution of islet-derived inflammatory mediators in early islet destruction has yet to be fully elucidated, particularly in IPIT where coagulation-mediated inflammatory events are presumed to dominate, their role in potentiating the inflammatory response must be considered. Moreover, consideration has recently been given to islet transplantation to alternative sites82–86 or pre-vascularized supports,87–91 and islet-derived inflammatory mediators will likely play a significant role in the fate of these grafts as well.
Emerging Strategies to Inhibit Thrombotic and Inflammatory Responses
Pre-transplant Manipulation of Islet Inflammatory Pathways
Through appropriate culture conditions and additives, cell signaling processes may be manipulated to downregulate expression of islet-derived prothrombotic and inflammatory mediators.73,75,92–95 Use of specially formulated culture media93 or supplementation with the vitamin nicotinamide92 has been shown to downregulate TF and MCP-1 production by islets. Matsuda et al. have recently demonstrated that incubation of islets with the p38 pathway inhibitor SB203580 for one hour prior to transplantation suppressed IL-1β, TNF-α, and inducible nitric oxide synthase (iNOS) expression by islets, markedly increasing the diabetes reversal rate after transplantation of a marginal islet mass.73 Additionally, signaling pathways may be modulated to reduce islet susceptibility to cytokine or nitric oxide mediated damage.32,96–99 Pre-transplant overnight culture with the anti-inflammatory agent lisofylline has been shown to reduce proinflammatory cytokine-induced islet apoptosis, thereby allowing insulin independence to be achieved using 30% fewer islets.98 As islet culture and shipping are being used more frequently in clinical islet transplantation,8,17,21,100 supplementation of media with modulators of inflammatory pathways should provide a facile approach for abrogating islet-initiated thrombosis and inflammation.
Systemic Administration of Anticoagulant and Anti-inflammatory Agents
While the immunosuppressive agents administered under the Edmonton Protocol are effective T- and B cell inhibitors,14,101 they appear to have minimal impact on innate inflammatory responses against islets mediated principally by neutrophils and macrophages. Therefore, adjunctive administration of anticoagulant and/or anti-inflammatory agents presents a rational strategy for improving islet engraftment. Table 1 summarizes notable systemic anticoagulant and anti-inflammatory therapies which have improved early outcomes in animal models of islet transplantation. Renal subcapsule transplantations have also been included as the efficacy of such therapies may translate to intraportal transplantation, despite potential differences in the pathophysiology of early graft destruction. For example, pravastatin (Pravachol)102,103 and 15-deoxyspergualin104,105 have proven effective in both kidney and intraportal transplant models. Nonetheless, a need exists to evaluate the efficacy of anti-inflammatory agents in the proper clinical context.
Table 1.
Anti-inflammatory and Anticoagulant Agents for Improving Islet Engraftment in Vivo
| Therapeutic agent | Animal model | Transplant site | Treatment regimen | Proposed mechanism(s) | Ref. |
|---|---|---|---|---|---|
| Anti-IFN-γ mAb | Mouse iso & allo | Liver | IP (d 0,2,4) | IFN-γ blockade | 46 |
| Anti-IL-1β mAb | Mouse iso & allo | Liver | IP (d 0,2,4) | IL-1β blockade | 46 |
| Anti-TNF-α mAb | Mouse iso & allo | Liver | IP (d 0,2,4) | TNF-α blockade | 46 |
| Acetylsalicylic acid | Rat to mouse | Kidney | Oral (daily) | Inhibition of COX-2 and NF-κB, ↑ anti-inflammatory cytokine production | 160 |
| IL-1ra(Anakinra) | Rat to mouse | Kidney | IP (d −1, 0, 4h) | Inhibition of IL-1 action | 160 |
| Activated protein C | Mouse iso | Liver | IV (−1 h) | Inactivation of fVa and fVIIIa, fibrinolysis, anti-apoptotic, NF-κB inhibition | 41 |
| Pravastatin (Pravachol) | Canine auto | Liver | Oral (d −2 to 13) | Inhibition of Ras production, suppression of macrophages, neutrophils, NK cells | 110 |
| Pravastatin (Pravachol) | Mouse iso | Kidney | Oral (d 0−14) | Inhibition of Ras production, suppression of macrophages, neutrophils, NK cells | 103 |
| Low MW dextran sulfate | Porcine to mousea | Liver | IV (−10 m, d 1−6) | Inhibition of complement and coagulation | 107 |
| α1-antitrypsin | Mouse allo | Kidney | IP(d −1, 1×/3 d) | Serine protease inhibition, inhibition of neutrophil elastase, inhibition of cytokines | 32 |
| S-methyl-isothiourea | Porcine to rata | Liver | SC (7 d continuous) | Inhibition of iNOS, hepatic NO generation | 165 |
| S-(2-aminoethyl)-isourea | Porcine to rat | Liver | SC (7 d continuous) | Inhibition of iNOS, hepatic NO generation | 165 |
| 4-phenylbutyrate | Mouse iso | Kidney | Oral (d −2 to 7, 2×/d) | Inhibition of IL-1β production | 166 |
| α-galactosylceramide | Mouse iso | Liver | IP (d −15, −11, −7) | Inhibition of IFN-γ by NKT cells | 42 |
| Anti-tissue factor mAb | Primate allo | Liver | IV (−10 to 20 m) | Tissue factor blockade | 117 |
| Nicotinamide | Rat iso | Liver | IP (daily) | Inhibition of NO-mediated toxicity | 167 |
| 15-deoxyspergualin | Mouse iso | Kidney | IP (d 0−4) | Inhibition of macrophage function, inhibition of NF-κB dependent cytokine production | 104 |
| 15-deoxyspergualin | Primate allo | Liver | IV (−4 h to d 14) | Inhibition of macrophage function, inhibition of NF-κB dependent cytokine production | 105 |
iso, isograft; allo, allograft; IP, intraperitoneal; IV, intravenous; Auto, autograft; SC, subcutaneous.
athymic animals
While anticoagulants such as melagatran,66 heparin,50 and N-acetyl-L-cysteine106 have demonstrated efficacy in vitro, few investigations have adequately explored the efficacy of systemic anticoagulant therapies in vivo.41,107,108 Contreras et al. have recently demonstrated that intra-venous administration of activated protein C (APC) dramatically inhibits intrahepatic fibrin deposition, portal vein EC activation, cytokine production, and leukocyte infiltration, consequently reducing the incidence of islet apoptosis and increasing the rate of conversion to euglycemia after transplantation of a marginal islet mass.41 Interestingly, single-dose administration of APC one hour prior to transplantation dramatically attenuated inflammatory events 6–12 hours later. This is particularly compelling given the relatively short half-life of APC (10–20 minutes),109 suggesting that the portal bed may be “primed” to receive islets. Yasunami et al. have demonstrated this phenomenon through repeated administration of the glycolipid α-galactosylceramide prior to transplantation, a process that dramatically reduces early islet loss through inhibition of Vα14 natural killer T cell-dependent IFN-γ production by neutrophils.42
In contrast to immunosuppression,101 effective inhibition of deleterious early inflammatory responses may be achieved with short-course therapy. In a murine model of IPIT, Satoh et al. have recently shown that islet dose may be reduced four-fold through simultaneous blockade of IL-1β, TNF-α, and IFN-γ in the four days post-transplant.46 Similarly, short-course oral administration of pravastatin, a cholesterol-lowering drug approved by the Food and Drug Administration, has been shown to reduce the number of islets required to reverse diabetes in a canine autograft model of IPIT.110 While single dose or short-term therapy holds considerable promise for improving the outcome of IPIT, challenges remain in finding therapeutics and treatment regimens that minimize adverse complications.
Localized Protection of Islets Through Re-engineering the Islet-host Interface
As adverse side effects of systemic anticoagulant and anti-inflammatory therapy may limit their potential therapeutic impact, recent efforts have focused on developing strategies to locally attenuate thrombosis and inflammation (Figure 3). Under normal physiological conditions, ECs lining the extensive microvasculature of pancreatic islets provide both physical and biochemical barriers to thrombosis and inflammation.68 During islet isolation and culture, however, this barrier is disrupted,68,111 exposing procoagulant and inflammatory mediators while simultaneously stripping away EC-derived regulators of inflammation. In this regard, the native endothelium has emerged as a structural and biochemical model for re-engineering the islet-host interface.112–114 Indeed, Johansson et al. have recently lined human islets with aortic endothelial cells via co-culture, and found that the presence of a peripheral EC layer prolonged clotting times, increased platelet counts, and decreased TAT levels in vitro.115
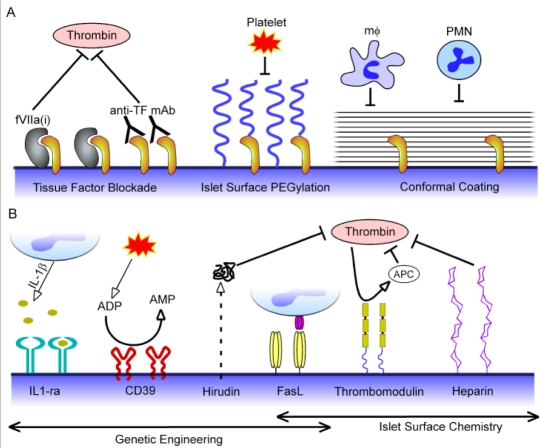
Figure 3.
Emerging strategies to locally attenuate thrombosis and inflammation through re-engineering of the islet-host interface. (A) A physical barrier to thrombosis and inflammation may be achieved through blockade of tissue factor (TF) expressed on the islet surface by incubating islets with site inactivated factor VIIa (fVIIa(i)) or anti-TF antibodies prior to transplantation. Though more commonly explored as strategies for protecting islets from host immune responses, islet surface PEGylation and conformal coatings may also provide physical barriers to thrombosis and inflammation by masking tissue factor and/or inhibiting platelet adhesion and infiltration of leukocytes (monocytes [mϕ] and polymorphonuclear leukocytes [PMNs]). (B) Genetic engineering and tissue-targeted chemistry may be used to actively modulate the islet surface to display or express anticoagulant and anti-inflammatory molecules. Expression of IL-1 receptor antagonist (IL1-ra) may sequester cytotoxic IL-1β produced by activated leukocytes, while the ectonucleotidase CD39 degrades proinflammatory and procoagulant adenine nucleotides (ATP and ADP) released from activated endothelium and platelets to AMP. Thrombin, a key mediator of thrombosis and inflammation in islet transplantation, may be locally inhibited through genetic engineering of islets to secrete hirudin and by chemical modification of islet surfaces with heparin and thrombomodulin. Though presentation of Fas ligand (FasL) on islet surfaces through genetic engineering and tissue-targeted chemistry has been primarily explored to confer local immunoprotection to islet grafts, such an approach might also improve early islet engraftment by induction of neutrophil and macrophage apoptosis via the Fas-FasL pathway.
Alternatively, several investigators have begun to explore biomaterial-based or genetic engineering approaches to mimic the native endothelium. Within the intact pancreas, ECs provide a physical barrier between islet-expressed TF and coagulation factors and also prevent adhesion and/or infiltration of platelets and leukocytes.116 In this regard, generation of barriers between islets and the intraportal environment offers a rational approach for passively inhibiting islet-mediated thrombotic and inflammatory events (Figure 3A). Indeed, blockade of TF through pre-incubation of islets with site-inactivated fVIIa51 or anti-TF antibody51,117 has been shown to inhibit thrombotic responses and improve islet survival both in vitro51 and in vivo.117 Microencapsulation devices prevent cell-cell contact and dramatically impede diffusion of antibodies and other macromolecules to their respective targets on the islet surface,118–121 and, therefore, may provide a strategy to shield islet-associated TF and prevent leukocyte infiltration into the graft. However, most microcapsules developed for islet encapsulation (Figure 4A) are not suitable for transplantation into the liver microvasculature due to their large diameter (400–800 μm).122–124 Therefore, several investigators have deposited coatings of defined thickness that conform to the islet surface, thereby reducing void volume while retaining the presence of a polymer barrier125–130 (Figure 4B–D). An emerging approach to generating conformal barriers has been through immobilization of poly(ethylene glycol) (PEG) to the islet surface, creating a steric barrier to prevent molecular recognition between cell surface receptors and soluble ligands.9,131–137 In vitro, covalent coupling of PEG to islets has been shown to inhibit islet-mediated activation of lymphocytes in co-culture,138 and protect islets from complement137 and TNF-α.139 Moreover, PEG has been shown to inhibit graft infiltration by host immune cells in models of renal subcapsular transplantation,140 a protective mechanism that may be operative in intraportal transplantation as well. Importantly, PEGylation of islets has been shown to improve early islet engraftment in a xenogenic model of IPIT.9 This effect was attributed to shielding of islets from complement and xenoreactive antibodies,9,137 however it is conceivable that TF expressed on the islet surface was also masked. Indeed, Chen et al. have reported similar results in an allograft model.141 Despite promising preliminary results, the efficacy of islet PEGylation may be limited by dependence on purely steric barriers. Therefore, several groups have began to explore the possibility of constructing permselective membranes of nanoscale thickness directly on the surface of individual islets.142–144 Krol et al.142 and Miura et al.143 have reported the coating of islets with polyelectrolyte multilayer films, while Teramura et al. have recently coated islets with a multilayered poly(vinyl alcohol) film covalently assembled via thiol/disulfide exchange.144 While the efficacy of these films has yet to be evaluated in vivo, they offer promising approaches for generating conformal barriers for IPIT.
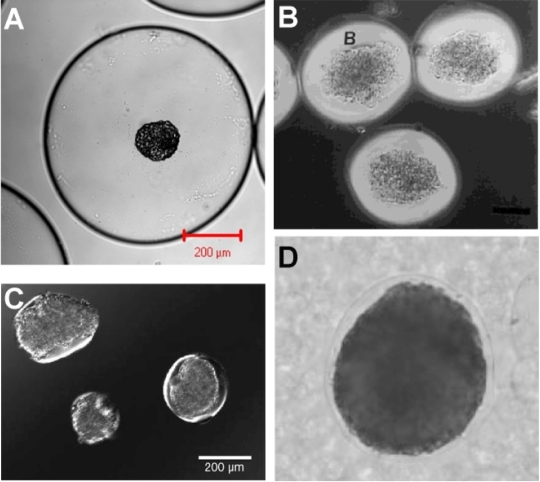
Figure 4.
Conformal coatings significantly reduce void volume relative to conventional microcapsules and offer a potential approach to protecting islets during intraportal islet transplantation. (A) An islet encapsulated in a conventional alginate microcapsule. (B) Conformal PEG hydrogel coatings fabricated on porcine islets via interfacial polymerization. (Reprinted with permission from Wiley-Liss, Inc., a subsidiary of John Wiley & Sons, Inc.130) (C) Islets coated with PEG hydrogel via selective withdrawal, (Adapted with permission from Wyman JL et al.128). (D) An alginate hydrogel coating formed on a HEK 293 cell spheroid using an aqueous two phase emulsion. (Reprinted with permission from Wiley-Liss, Inc., a subsidary of John Wiley & Sons, Inc.168)
Arguably more critical than establishment of a physical barrier, however, is the ability of the native endothelium to actively regulate coagulation and inflammation (Figure 3B). Heparin, an EC surface glycosaminoglycan, provides one such biochemical barrier through its ability to enhance the capacity of cofactor II and antithrombin to inactivate thrombin. Moreover, heparin can inhibit the formation of NO through its capacity to bind superoxide disumtase145 and has been shown to limit complement activity.146–148 Korsgren and colleagues have recently employed biotin/avidin interactions to immobilize macromolecular heparin complexes to the surface of islets.149 Significantly, surface heparinization of intraportal islet grafts reduced TAT production and early islet damage in an allogeneic porcine model. In light of the significant thrombotic response observed after clinical islet transplantation,51,53,57 where heparin is delivered systemically during islet infusion,14,21 these findings potentially illustrate the increased therapeutic efficacy achieved through local delivery of anticoagulants to the portal bed. Direct comparison between delivery of islet-grafted and systemic heparin will be necessary to unequivocally demonstrate this concept.
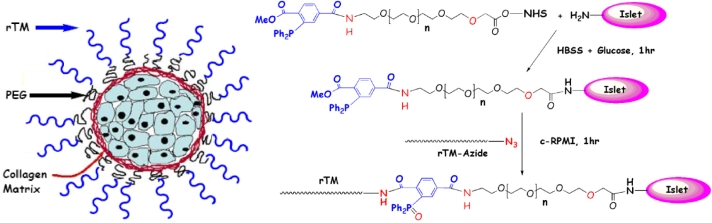
Perhaps of greater physiological significance than heparin, thrombomodulin (TM), expressed constitutively by endothelial cells, binds thrombin, sequestering it from participating in thrombotic and inflammatory processes, while simultaneously redirecting its catalytic activity towards the generation of the potent anticoagulant and anti-inflammatory APC.150,151 Our lab has recently developed a novel two-step process for covalently conjugating recombinant human TM to the islet surface in a chemo- and bio-orthogonal manner (Figure 5).152 Using a biosynthetic approach, we have generated a TM construct containing a C-terminal azido (N3) group, which can be covalently tethered to the islet surface via Staudinger ligation153 through the use of a heterobifunctional N-Hydroxysuccinimide-PEG-triphenylphosphine linker (Figure 5). Conjugation of TM to islets does not influence islet viability or function and increases APC production with an attendant inhibition of islet-mediated coagulation. Moreover, local presentation of TM may act in synergy with the physical barrier provided by surface-grafted PEG chains.
Figure 5.
Site-specific immobilization of thrombomodulin (TM) on pancreatic islets through use of Staudinger ligation. Using a biosynthetic approach, human TM was genetically engineered to contain a C-terminal azido (N3) group (rTM-azide), which can react chemoselectively with phosphine via Staudinger ligation. Phosphine groups were generated on the islet surface via active ester coupling between cell surface amines and a heterobifunctional phosphine-poly(ethylene glycol)- N-Hydroxysuccinimide linker, thereby allowing TM to be covalently tethered to islets in a chemo- and bio-orthogonal manner (Reprinted with permission from the American Chemical Society.152).
In addition to thrombin generation, local release of adenine nucleotides, including adenosine triphosphate (ATP) and adenosine diphosphate (ADP), from activated endothelium and platelets further potentiate proinflammatory and prothrombotic events. CD39, a transmembrane protein expressed on endothelial cells, regulates these events through its capacity to catalyze the degradation of ATP and ADP to adenosine monophosphate (AMP).154,155 Dwyer et al. have recently generated transgenic mice that express human CD39 on pancreatic islets. These islets were found to have increased ATPase activity compared to wild-type controls and a consequent capacity to inhibit islet-mediated coagulation.156 Similarly, genetic engineering approaches have been used to induce expression of the potent anticoagulant hirudin157 as well as the anti-inflammatory IL-1ra,158,159 an inhibitor of IL-1β action that has improved islet engraftment when administered systemically.160 Both genetic engineering161 and cell surface chemistry approaches162 have been used to display Fas ligand (FasL) on the islet surface, a strategy that could improve the outcome of IPIT by local induction of neutrophil and macrophage apoptosis via the Fas-FasL pathway.163,164 Hence, resurfacing the biochemical landscape of islet surfaces through genetic engineering and tissue-targeted chemistry holds considerable promise for locally attenuating thrombotic and inflammatory responses in IPIT.
Conclusions
Concomitant with continuing advancements in islet isolation and immunosuppressive therapy is an emerging need to address the innate inflammatory responses that underlie early islet destruction and primary non-function. Mitigation of thrombotic and inflammatory responses through pre-transplant downregulation of inflammatory mediators expressed by islet grafts, systemic administration of anti-inflammatory and anticoagulant agents, and re-engineering of the islet surface holds considerable promise for improving islet engraftment. While recent investigations, many of which have been described herein, have provided a framework for understanding mechanisms of thrombosis and inflammation in IPIT, continued exploration of this process, particularly in larger animal models, will be necessary to further identify key therapeutic targets. Use of adjunctive anti-inflammatory therapy may also attenuate immune responses through inhibition of inflammatory cell recruitment and antigen presentation, potentially facilitating use of modified immunosuppressive therapies with important clinical benefits. Furthermore, even as xenogenic tissue and insulin producing cell lines emerge as viable alternatives to allogeneic islet grafts, pernicious inflammatory responses are likely to persist and, in this regard, anti-inflammatory therapeutics will play an important role in the efficacy of the next generation of cell-based therapies for diabetes.
Abbreviations
- APC
activated protein C
- EC
endothelial cell
- FasL
Fas ligand
- IEQ
islet equivalents
- IFN
interferon
- IL
interleukin
- iNOS
inducible nitric oxide synthase
- IPIT
intraportal islet transplantation
- MCP-1
monocyte chemoattractant protein-1
- NO
nitric oxide
- PEG
poly(ethylene glycol)
- TAT
thrombin-antithrombin complex
- TF
tissue factor
- TM
thrombomodulin
References
- 1.Tzakis AG, Ricordi C, Alejandro R, Zeng Y, Fung JJ, Todo S, Demetris AJ, Mintz DH, Starzl TE. Pancreatic islet transplantation after upper abdominal exenteration and liver replacement. Lancet. 1990;336(8712):402–405. doi: 10.1016/0140-6736(90)91946-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Najarian JS, Sutherland DE, Matas AJ, Steffes MW, Simmons RL, Goetz FC. Human islet transplantation: a preliminary report. Transplant Proc. 1977;9(1):233–236. [PubMed] [Google Scholar]
- 3.Kemp CB, Knight MJ, Scharp DW, Ballinger WF, Lacy PE. Effect of transplantation site on the results of pancreatic islet isografts in diabetic rats. Diabetologia. 1973;9(6):486–491. doi: 10.1007/BF00461694. [DOI] [PubMed] [Google Scholar]
- 4.Ballinger WF, Lacy PE. Transplantation of intact pancreatic islets in rats. Surgery. 1972;72(2):175–186. [PubMed] [Google Scholar]
- 5.Williams P. Notes on diabetes treated with extract and by grafts of sheep's pancreas. BMJ. 1894;2:1303–1304. [Google Scholar]
- 6.Robertson RP. Islet transplantation as a treatment for diabetes - a work in progress. N Engl J Med. 2004;350(7):694–705. doi: 10.1056/NEJMra032425. [DOI] [PubMed] [Google Scholar]
- 7.Markmann JF, Deng S, Desai NM, Huang X, Velidedeoglu E, Frank A, Liu C, Brayman KL, Lian MM, Wof B, Bell E, Vitamaniuk M, Doliba N, Matschinsky F, Markmann E, Barker CF, Naji A. The use of non-heart-beating donors for isolated pancreatic islet transplantation. Transplantation. 2003;75(9):1423–1429. doi: 10.1097/01.TP.0000061119.32575.F4. [DOI] [PubMed] [Google Scholar]
- 8.Froud T, Ricordi C, Baidal DA, Hafiz MM, Ponte G, Cure P, Pileggi A, Poggioli R, Ichii H, Khan A, Ferreira JV, Pugliese A, Esquenazi VV, Kenyon NS, Alejandro R. Islet transplantation in type 1 diabetes mellitus using cultured islets and steroid-free immunosuppression: Miami experience. Am J Transplant. 2005;5(8):2037–2046. doi: 10.1111/j.1600-6143.2005.00957.x. [DOI] [PubMed] [Google Scholar]
- 9.Contreras JL, Xie D, Mays J, Smyth CA, Eckstein C, Rahemtulla FG, Young CJ, Anthony Thompson J, Bilbao G, Curiel DT, Eckhoff DE. A novel approach to xenotransplantation combining surface engineering and genetic modification of isolated adult porcine islets. Surgery. 2004;136(3):537–547. doi: 10.1016/j.surg.2004.05.031. [DOI] [PubMed] [Google Scholar]
- 10.de Vos P, Marchetti P. Encapsulation of pancreatic islets for transplantation in diabetes: the untouchable islets. Trends Mol Med. 2002;8(8):363–366. doi: 10.1016/s1471-4914(02)02381-x. [DOI] [PubMed] [Google Scholar]
- 11.Rajotte RV. Islet cryopreservation protocols. Ann N Y Acad Sci. 1999;875:200–207. doi: 10.1111/j.1749-6632.1999.tb08504.x. [DOI] [PubMed] [Google Scholar]
- 12.Frank A, Deng SP, Huang XL, Velidedeoglu E, Bae YS, Liu CY, Abt P, Stephenson R, Mohiuddin M, Thambipillai T, Markmann E, Palanjian M, Sellers M, Naji A, Barker CF, Markmann JF. Transplantation for type 1 diabetes: Comparison of vascularized whole-organ pancreas with isolated pancreatic islets. Ann Surg. 2004;240(4):631–640. doi: 10.1097/01.sla.0000140754.26575.2a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gaglia JL, Shapiro AM, Weir GC. Islet transplantation: progress and challenge. Arch Med Res. 2005;36(3):273–280. doi: 10.1016/j.arcmed.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 14.Shapiro AM, Lakey JR, Ryan EA, Korbutt GS, Toth E, Warnock GL, Kneteman NM, Rajotte RV. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343(4):230–238. doi: 10.1056/NEJM200007273430401. [DOI] [PubMed] [Google Scholar]
- 15.Shapiro AM, Ricordi C, Hering B. Edmonton's islet success has indeed been replicated elsewhere. Lancet. 2003;362(9391):1242. doi: 10.1016/S0140-6736(03)14526-6. [DOI] [PubMed] [Google Scholar]
- 16.Hirshberg B, Rother KI, Digon BJ, 3rd, Lee J, Gaglia JL, Hines K, Read EJ, Chang R, Wood BJ, Harlan DM. Benefits and risks of solitary islet transplantation for type 1 diabetes using steroid-sparing immunosuppression: the National Institutes of Health experience. Diabetes Care. 2003;26(12):3288–3295. doi: 10.2337/diacare.26.12.3288. [DOI] [PubMed] [Google Scholar]
- 17.Goss JA, Schock AP, Brunicardi FC, Goodpastor SE, Garber AJ, Soltes G, Barth M, Froud T, Alejandro R, Ricordi C. Achievement of insulin independence in three consecutive type-1 diabetic patients via pancreatic islet transplantation using islets isolated at a remote islet isolation center. Transplantation. 2002;74(12):1761–1766. doi: 10.1097/00007890-200212270-00020. [DOI] [PubMed] [Google Scholar]
- 18.Ricordi C. Islet transplantation: a brave new world. Diabetes. 2003;52(7):1595–1603. doi: 10.2337/diabetes.52.7.1595. [DOI] [PubMed] [Google Scholar]
- 19.Korsgren O, Nilsson B, Berne C, Felldin M, Foss A, Kallen R, Lundgren T, Salmela K, Tibell A, Tufveson G. Current status of clinical islet transplantation. Transplantation. 2005;79(10):1289–1293. doi: 10.1097/01.tp.0000157273.60147.7c. [DOI] [PubMed] [Google Scholar]
- 20.Shapiro AM, Nanji SA, Lakey JR. Clinical islet transplant: current and future directions towards tolerance. Immunol Rev. 2003;196:219–236. doi: 10.1046/j.1600-065x.2003.00085.x. [DOI] [PubMed] [Google Scholar]
- 21.Hering BJ, Kandaswamy R, Ansite JD, Eckman PM, Nakano M, Sawada T, Matsumoto I, Ihm SH, Zhang HJ, Parkey J, Hunter DW, Sutherland DE. Single-donor, marginal-dose islet transplantation in patients with type 1 diabetes. JAMA. 2005;293(7):830–835. doi: 10.1001/jama.293.7.830. [DOI] [PubMed] [Google Scholar]
- 22.Shapiro AM, Ricordi C, Hering BJ, Auchincloss H, Lindblad R, Robertson RP, Secchi A, Brendel MD, Berney T, Brennan DC, Cagliero E, Alejandro R, Ryan EA, DiMercurio B, Morel P, Polonsky KS, Reems JA, Bretzel RG, Bertuzzi F, Froud T, Kandaswamy R, Sutherland DE, Eisenbarth G, Segal M, Preiksaitis J, Korbutt GS, Barton FB, Viviano L, Seyfert-Margolis V, Bluestone J, Lakey JR. International trial of the Edmonton protocol for islet transplantation. N Engl J Med. 2006;355(13):1318–1330. doi: 10.1056/NEJMoa061267. [DOI] [PubMed] [Google Scholar]
- 23.Emamaullee JA, Shapiro AM. Factors influencing the loss of beta- cell mass in islet transplantation. Cell Transplant. 2007;16(1):1–8. doi: 10.3727/000000007783464461. [DOI] [PubMed] [Google Scholar]
- 24.Ryan EA, Lakey JR, Rajotte RV, Korbutt GS, Kin T, Imes S, Rabinovitch A, Elliott JF, Bigam D, Kneteman NM, Warnock GL, Larsen I, Shapiro AM. Clinical outcomes and insulin secretion after islet transplantation with the Edmonton protocol. Diabetes. 2001;50(4):710–719. doi: 10.2337/diabetes.50.4.710. [DOI] [PubMed] [Google Scholar]
- 25.Davalli AM, Ogawa Y, Ricordi C, Scharp DW, Bonner-Weir S, Weir GC. A selective decrease in the beta cell mass of human islets transplanted into diabetic nude mice. Transplantation. 1995;59(6):817–820. [PubMed] [Google Scholar]
- 26.Davalli AM, Ogawa Y, Scaglia L, Wu YJ, Hollister J, Bonner-Weir S, Weir GC. Function, mass, and replication of porcine and rat islets transplanted into diabetic nude mice. Diabetes. 1995;44(1):104–111. doi: 10.2337/diab.44.1.104. [DOI] [PubMed] [Google Scholar]
- 27.Biarnes M, Montolio M, Nacher V, Raurell M, Soler J, Montanya E. Beta-cell death and mass in syngeneically transplanted islets exposed to short- and long-term hyperglycemia. Diabetes. 2002;51(1):66–72. doi: 10.2337/diabetes.51.1.66. [DOI] [PubMed] [Google Scholar]
- 28.Ryan EA, Paty BW, Senior PA, Bigam D, Alfadhli E, Kneteman NM, Lakey JR, Shapiro AM. Five-year follow-up after clinical islet transplantation. Diabetes. 2005;54(7):2060–2069. doi: 10.2337/diabetes.54.7.2060. [DOI] [PubMed] [Google Scholar]
- 29.Davalli AM, Scaglia L, Zangen DH, Hollister J, Bonner-Weir S, Weir GC. Vulnerability of islets in the immediate posttransplantation period. Dynamic changes in structure and function. Diabetes. 1996;45(9):1161–1167. doi: 10.2337/diab.45.9.1161. [DOI] [PubMed] [Google Scholar]
- 30.Mattsson G, Jansson L, Nordin A, Andersson A, Carlsson PO. Evidence of functional impairment of syngeneically transplanted mouse pancreatic islets retrieved from the liver. Diabetes. 2004;53(4):948–954. doi: 10.2337/diabetes.53.4.948. [DOI] [PubMed] [Google Scholar]
- 31.Ahn YB, Xu G, Marselli L, Toschi E, Sharma A, Bonner-Weir S, Sgroi DC, Weir GC. Changes in gene expression in beta cells after islet isolation and transplantation using laser-capture microdissection. Diabetologia. 2007;50(2):334–342. doi: 10.1007/s00125-006-0536-5. [DOI] [PubMed] [Google Scholar]
- 32.Lewis EC, Shapiro L, Bowers OJ, Dinarello CA. Alpha1-antitrypsin monotherapy prolongs islet allograft survival in mice. Proc Natl Acad Sci USA. 2005;102(34):12153–12158. doi: 10.1073/pnas.0505579102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaufman DB, Platt JL, Rabe FL, Dunn DL, Bach FH, Sutherland DE. Differential roles of Mac-1+ cells, and CD4+ and CD8+ T lymphocytes in primary nonfunction and classic rejection of islet allografts. J Exp Med. 1990;172(1):291–302. doi: 10.1084/jem.172.1.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Katz SM, Bennett F, Stecker K, Clark JH, Pham T, Wang ME, Kahan BD, Stepkowski SM. ICAM-1 antisense oligodeoxynucleotide improves islet allograft survival and function. Cell Transplant. 2000;9(6):817–828. doi: 10.1177/096368970000900608. [DOI] [PubMed] [Google Scholar]
- 35.Toyofuku A, Yasunami Y, Nabeyama K, Nakano M, Satoh M, Matsuoka N, Ono J, Nakayama T, Taniguchi M, Tanaka M, Ikeda S. Natural killer T-cells participate in rejection of islet allografts in the liver of mice. Diabetes. 2006;55(1):34–39. [PubMed] [Google Scholar]
- 36.Gores PF, Sutherland DE, Platt JL, Bach FH. Depletion of donor Ia+ cells before transplantation does not prolong islet allograft survival. J Immunol. 1986;137(5):1482–1485. [PubMed] [Google Scholar]
- 37.Jansson L, Carlsson PO. Graft vascular function after transplantation of pancreatic islets. Diabetologia. 2002;45(6):749–763. doi: 10.1007/s00125-002-0827-4. [DOI] [PubMed] [Google Scholar]
- 38.Emamaullee JA, Rajotte RV, Liston P, Korneluk RG, Lakey JR, Shapiro AM, Elliott JF. XIAP overexpression in human islets prevents early posttransplant apoptosis and reduces the islet mass needed to treat diabetes. Diabetes. 2005;54(9):2541–2548. doi: 10.2337/diabetes.54.9.2541. [DOI] [PubMed] [Google Scholar]
- 39.Lee Y, Ravazzola M, Park BH, Bashmakov YK, Orci L, Unger RH. Metabolic mechanisms of failure of intraportally transplanted pancreatic beta-cells in rats: role of lipotoxicity and prevention by leptin. Diabetes. 2007;56(9):2295–2301. doi: 10.2337/db07-0460. [DOI] [PubMed] [Google Scholar]
- 40.Robertson RP, Harmon JS. Diabetes, glucose toxicity, and oxidative stress: A case of double jeopardy for the pancreatic islet beta cell. Free Radic Biol Med. 2006;41(2):177–184. doi: 10.1016/j.freeradbiomed.2005.04.030. [DOI] [PubMed] [Google Scholar]
- 41.Contreras JL, Eckstein C, Smyth CA, Bilbao G, Vilatoba M, Ringland SE, Young C, Thompson JA, Fernández JA, Griffin JH, Eckhoff DE. Activated protein C preserves functional islet mass after intraportal transplantation: a novel link between endothelial cell activation, thrombosis, inflammation, and islet cell death. Diabetes. 2004;53(11):2804–2814. doi: 10.2337/diabetes.53.11.2804. [DOI] [PubMed] [Google Scholar]
- 42.Yasunami Y, Kojo S, Kitamura H, Toyofuku A, Satoh M, Nakano M, Nabeyama K, Nakamura Y, Matsuoka N, Ikeda S, Tanaka M, Ono J, Nagata N, Ohara O, Taniguchi M. Valpha14 NK T cell-triggered IFN-gamma production by Gr-1+CD11b+ cells mediates early graft loss of syngeneic transplanted islets. J Exp Med. 2005;202(7):913–918. doi: 10.1084/jem.20050448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Montolio M, Tellez N, Soler J, Montanya E. Role of blood glucose in cytokine gene expression in early syngeneic islet transplantation. Cell Transplant. 2007;16(5):517–525. doi: 10.3727/000000007783464920. [DOI] [PubMed] [Google Scholar]
- 44.Bottino R, Fernandez LA, Ricordi C, Lehmann R, Tsan MF, Oliver R, Inverardi L. Transplantation of allogeneic islets of Langerhans in the rat liver: effects of macrophage depletion on graft survival and microenvironment activation. Diabetes. 1998;47(3):316–323. doi: 10.2337/diabetes.47.3.316. [DOI] [PubMed] [Google Scholar]
- 45.Montolio M, Biarnes M, Tellez N, Escoriza J, Soler J, Montanya E. Interleukin-1beta and inducible form of nitric oxide synthase expression in early syngeneic islet transplantation. J Endocrinol. 2007;192(1):169–177. doi: 10.1677/joe.1.06968. [DOI] [PubMed] [Google Scholar]
- 46.Satoh M, Yasunami Y, Matsuoka N, Nakano M, Itoh T, Nitta T, Anzai K, Ono J, Taniguchi M, Ikeda S. Successful islet transplantation to two recipients from a single donor by targeting proinflammatory cytokines in mice. Transplantation. 2007;83(8):1085–1092. doi: 10.1097/01.tp.0000260161.81775.58. [DOI] [PubMed] [Google Scholar]
- 47.Rabinovitch A, Suarez-Pinzon WL. Cytokines and their roles in pancreatic islet beta-cell destruction and insulin-dependent diabetes mellitus. Biochem Pharmacol. 1998;55(8):1139–1149. doi: 10.1016/s0006-2952(97)00492-9. [DOI] [PubMed] [Google Scholar]
- 48.Barshes NR, Wyllie S, Goss JA. Inflammation-mediated dysfunction and apoptosis in pancreatic islet transplantation: implications for intrahepatic grafts. J Leukoc Biol. 2005;77(5):587–597. doi: 10.1189/jlb.1104649. [DOI] [PubMed] [Google Scholar]
- 49.Ziats NP, Miller KM, Anderson JM. In vitro and in vivo interactions of cells with biomaterials. Biomaterials. 1988;9(1):5–13. doi: 10.1016/0142-9612(88)90063-4. [DOI] [PubMed] [Google Scholar]
- 50.Bennet W, Sundberg B, Groth CG, Brendel MD, Brandhorst D, Brandhorst H, Bretzel RG, Elgue G, Larsson R, Nilsson B, Korsgren O. Incompatibility between human blood and isolated islets of Langerhans: a finding with implications for clinical intraportal islet transplantation? Diabetes. 1999;48(10):1907–1914. doi: 10.2337/diabetes.48.10.1907. [DOI] [PubMed] [Google Scholar]
- 51.Moberg L, Johansson H, Lukinius A, Berne C, Foss A, Källen R, Østraat Ø, Salmela K, Tibell A, Tufveson G, Elgue G, Nilsson Ekdahl K, Korsgren O, Nilsson B. Production of tissue factor by pancreatic islet cells as a trigger of detrimental thrombotic reactions in clinical islet transplantation. Lancet. 2002;360(9350):2039–2045. doi: 10.1016/s0140-6736(02)12020-4. [DOI] [PubMed] [Google Scholar]
- 52.Moberg L. The role of the innate immunity in islet transplantation. Ups J Med Sci. 2005;110(1):17–55. doi: 10.3109/2000-1967-181. [DOI] [PubMed] [Google Scholar]
- 53.Johansson H, Lukinius A, Moberg L, Lundgren T, Berne C, Foss A, Felldin M, Källen R, Salmela K, Tibell A, Tufveson G, Ekdahl KN, Elgue G, Korsgren O, Nilsson B. Tissue factor produced by the endocrine cells of the islets of Langerhans is associated with a negative outcome of clinical islet transplantation. Diabetes. 2005;54(6):1755–1762. doi: 10.2337/diabetes.54.6.1755. [DOI] [PubMed] [Google Scholar]
- 54.Giesen PL, Nemerson Y. Tissue factor on the loose. Semin Thromb Hemost. 2000;26(4):379–384. doi: 10.1055/s-2000-8456. [DOI] [PubMed] [Google Scholar]
- 55.van Deijnen JH, Hulstaert CE, Wolters GH, van Schilfgaarde R. Significance of the peri-insular extracellular matrix for islet isolation from the pancreas of rat, dog, pig, and man. Cell Tissue Res. 1992;267(1):139–146. doi: 10.1007/BF00318700. [DOI] [PubMed] [Google Scholar]
- 56.Lamblin A, Tournoys A, Gmyr V, Jourdain M, Lefebvre J, Kerr-Conte J, Proye C, Pattou F. Blood mediated reaction following intraportal islet allograft in pigs. Ann Chir. 2001;126(8):743–750. doi: 10.1016/s0003-3944(01)00594-6. [DOI] [PubMed] [Google Scholar]
- 57.Bertuzzi F, Marzorati S, Maffi P, Piemonti L, Melzi R, de Taddeo F, Valtolina V, D'Angelo A, di Carlo V, Bonifacio E, Secchi A. Tissue factor and CCL2/monocyte chemoattractant protein-1 released by human islets affect islet engraftment in type 1 diabetic recipients. J Clin Endocrinol Metab. 2004;89(11):5724–5728. doi: 10.1210/jc.2004-0659. [DOI] [PubMed] [Google Scholar]
- 58.Dugina TN, Kiseleva EV, Chistov IV, Umarova BA, Strukova SM. Receptors of the PAR family as a link between blood coagulation and inflammation. Biochemistry (Mosc) 2002;67(1):65–74. doi: 10.1023/a:1013952114485. [DOI] [PubMed] [Google Scholar]
- 59.Coughlin SR. Thrombin signalling and protease-activated receptors. Nature. 2000;407(6801):258–264. doi: 10.1038/35025229. [DOI] [PubMed] [Google Scholar]
- 60.Rabiet MJ, Plantier JL, Dejana E. Thrombin-induced endothelial cell dysfunction. Br Med Bull. 1994;50(4):936–945. doi: 10.1093/oxfordjournals.bmb.a072935. [DOI] [PubMed] [Google Scholar]
- 61.Esmon CT. Interactions between the innate immune and blood coagulation systems. Trends Immunol. 2004;25(10):536–542. doi: 10.1016/j.it.2004.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zarbock A, Polanowska-Grabowska RK, Ley K. Platelet-neutrophil-interactions: linking hemostasis and inflammation. Blood Rev. 2007;21(2):99–111. doi: 10.1016/j.blre.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 63.Gawaz M, Langer H, May AE. Platelets in inflammation and atherogenesis. J Clin Invest. 2005;115(12):3378–3384. doi: 10.1172/JCI27196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yin D, Ding JW, Shen J, Ma L, Hara M, Chong AS. Liver ischemia contributes to early islet failure following intraportal transplantation: benefits of liver ischemic-preconditioning. Am J Transplant. 2006;6(1):60–68. doi: 10.1111/j.1600-6143.2005.01157.x. [DOI] [PubMed] [Google Scholar]
- 65.Moberg L, Korsgren O, Nilsson B. Neutrophilic granulocytes are the predominant cell type infiltrating pancreatic islets in contact with ABO-compatible blood. Clin Exp Immunol. 2005;142(1):125–131. doi: 10.1111/j.1365-2249.2005.02883.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ozmen L, Ekdahl KN, Elgue G, Larsson R, Korsgren O, Nilsson B. Inhibition of thrombin abrogates the instant blood-mediated inflammatory reaction triggered by isolated human islets: possible application of the thrombin inhibitor melagatran in clinical islet transplantation. Diabetes. 2002;51(6):1779–1784. doi: 10.2337/diabetes.51.6.1779. [DOI] [PubMed] [Google Scholar]
- 67.de Groot M, Schuurs TA, Keizer PP, Fekken S, Leuvenink HG, van Schilfgaarde R. Response of encapsulated rat pancreatic islets to hypoxia. Cell Transplant. 2003;12(8):867–875. [PubMed] [Google Scholar]
- 68.Linn T, Schmitz J, Hauck-Schmalenberger I, Lai Y, Bretzel RG, Brandhorst H, Brandhorst D. Ischaemia is linked to inflammation and induction of angiogenesis in pancreatic islets. Clin Exp Immunol. 2006;144(2):179–187. doi: 10.1111/j.1365-2249.2006.03066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gysemans CA, Waer M, Valckx D, Laureys JM, Mihkalsky D, Bouillon R, Mathieu C. Early graft failure of xenogeneic islets in NOD mice is accompanied by high levels of interleukin-1 and low levels of transforming growth factor-beta mRNA in the grafts. Diabetes. 2000;49(12):1992–1997. doi: 10.2337/diabetes.49.12.1992. [DOI] [PubMed] [Google Scholar]
- 70.Ozasa T, Newton MR, Dallman MJ, Shimizu S, Gray DW, Morris PJ. Cytokine gene expression in pancreatic islet grafts in the rat. Transplantation. 1997;64(8):1152–1159. doi: 10.1097/00007890-199710270-00013. [DOI] [PubMed] [Google Scholar]
- 71.Johansson U, Olsson A, Gabrielsson S, Nilsson B, Korsgren O. Inflammatory mediators expressed in human islets of Langerhans: implications for islet transplantation. Biochem Biophys Res Commun. 2003;308(3):474–479. doi: 10.1016/s0006-291x(03)01392-5. [DOI] [PubMed] [Google Scholar]
- 72.Berney T, Molano RD, Cattan P, Pileggi A, Vizzardelli C, Oliver R, Ricordi C, Inverardi L. Endotoxin-mediated delayed islet graft function is associated with increased intra-islet cytokine production and islet cell apoptosis. Transplantation. 2001;71(1):125–132. doi: 10.1097/00007890-200101150-00020. [DOI] [PubMed] [Google Scholar]
- 73.Matsuda T, Omori K, Vuong T, Pascual M, Valiente L, Ferreri K, Todorov I, Kuroda Y, Smith CV, Kandeel F, Mullen Y. Inhibition of p38 pathway suppresses human islet production of pro-inflammatory cytokines and improves islet graft function. Am J Transplant. 2005;5(3):484–493. doi: 10.1046/j.1600-6143.2004.00716.x. [DOI] [PubMed] [Google Scholar]
- 74.Ehrnfelt C, Kumagai-Braesch M, Uzunel M, Holgersson J. Adult porcine islets produce MCP-1 and recruit human monocytes in vitro. Xenotransplantation. 2004;11(2):184–194. doi: 10.1046/j.1399-3089.2003.00104.x. [DOI] [PubMed] [Google Scholar]
- 75.Bottino R, Balamurugan AN, Tse H, Thirunavukkarasu C, Ge X, Profozich J, Milton M, Ziegenfuss A, Trucco M, Piganelli JD. Response of human islets to isolation stress and the effect of antioxidant treatment. Diabetes. 2004;53(10):2559–2568. doi: 10.2337/diabetes.53.10.2559. [DOI] [PubMed] [Google Scholar]
- 76.Schroppel B, Zhang N, Chen P, Chen D, Bromberg JS, Murphy B. Role of donor-derived monocyte chemoattractant protein-1 in murine islet transplantation. J Am Soc Nephrol. 2005;16(2):444–451. doi: 10.1681/ASN.2004090743. [DOI] [PubMed] [Google Scholar]
- 77.Piemonti L, Leone BE, Nano R, Saccani A, Monti P, Maffi P, Bianchi G, Sica A, Peri G, Melzi R, Aldrighetti L, Secchi A, Di Carlo V, Allavena P, Bertuzzi F. Human pancreatic islets produce and secrete MCP-1/CCL2: relevance in human islet transplantation. Diabetes. 2002;51(1):55–65. doi: 10.2337/diabetes.51.1.55. [DOI] [PubMed] [Google Scholar]
- 78.Chen MC, Proost P, Gysemans C, Mathieu C, Eizirik DL. Monocyte chemoattractant protein-1 is expressed in pancreatic islets from prediabetic NOD mice and in interleukin-1 beta-exposed human and rat islet cells. Diabetologia. 2001;44(3):325–332. doi: 10.1007/s001250051622. [DOI] [PubMed] [Google Scholar]
- 79.Thomas HE, Darwiche R, Corbett JA, Kay TW. Interleukin-1 plus gamma-interferon-induced pancreatic beta-cell dysfunction is mediated by beta-cell nitric oxide production. Diabetes. 2002;51(2):311–316. doi: 10.2337/diabetes.51.2.311. [DOI] [PubMed] [Google Scholar]
- 80.Fontaine MJ, Blanchard J, Rastellini C, Lazda V, Herold KC, Pollak R. Pancreatic islets activate portal vein endothelial cells in vitro. Ann Clin Lab Sci. 2002;32(4):352–361. [PubMed] [Google Scholar]
- 81.Clayton HA, Davies JE, Sutton CD, Bell PR, Dennison AR. A coculture model of intrahepatic islet transplantation: activation of Kupffer cells by islets and acinar tissue. Cell Transplant. 2001;10(1):101–108. [PubMed] [Google Scholar]
- 82.Andrades P, Asiedu C, Rodriguez C, Goodwin KJ, McCarn J, Thomas JM. Subcutaneous pancreatic islet transplantation using fibrin glue as a carrier. Transplant Proc. 2007;39(1):191–192. doi: 10.1016/j.transproceed.2006.10.019. [DOI] [PubMed] [Google Scholar]
- 83.Caiazzo R, Gmyr V, Hubert T, Delalleau N, Lamberts R, Moerman E, Kerr-Conte J, Pattou F. Evaluation of alternative sites for islet transplantation in the minipig: interest and limits of the gastric submucosa. Transplant Proc. 2007;39(8):2620–2623. doi: 10.1016/j.transproceed.2007.08.015. [DOI] [PubMed] [Google Scholar]
- 84.Chen X, Zhang X, Larson C, Chen F, Kissler H, Kaufman DB. The epididymal fat pad as a transplant site for minimal islet mass. Transplantation. 2007;84(1):122–125. doi: 10.1097/01.tp.0000266909.58117.e3. [DOI] [PubMed] [Google Scholar]
- 85.Lau J, Mattsson G, Carlsson C, Nyqvist D, Kohler M, Berggren PO, Jansson L, Carlsson PO. Implantation site-dependent dysfunction of transplanted pancreatic islets. Diabetes. 2007;56(6):1544–1550. doi: 10.2337/db06-1258. [DOI] [PubMed] [Google Scholar]
- 86.Stagner JI, Rilo HL, White KK. The pancreas as an islet transplantation site. Confirmation in a syngeneic rodent and canine autotransplant model. JOP. 2007;8(5):628–636. [PubMed] [Google Scholar]
- 87.De Vos P, Hillebrands JL, De Haan BJ, Strubbe JH, Van Schilfgaarde R. Efficacy of a prevascularized expanded polytetrafluoroethylene solid support system as a transplantation site for pancreatic islets. Transplantation. 1997;63(6):824–830. doi: 10.1097/00007890-199703270-00006. [DOI] [PubMed] [Google Scholar]
- 88.Pileggi A, Molano RD, Ricordi C, Zahr E, Collins J, Valdes R, Inverardi L. Reversal of diabetes by pancreatic islet transplantation into a subcutaneous, neovascularized device. Transplantation. 2006;81(9):1318–1324. doi: 10.1097/01.tp.0000203858.41105.88. [DOI] [PubMed] [Google Scholar]
- 89.Blomeier H, Zhang X, Rives C, Brissova M, Hughes E, Baker M, Powers AC, Kaufman DB, Shea LD, Lowe WL., Jr Polymer scaffolds as synthetic microenvironments for extrahepatic islet transplantation. Transplantation. 2006;82(4):452–459. doi: 10.1097/01.tp.0000231708.19937.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dufour JM, Rajotte RV, Zimmerman M, Rezania A, Kin T, Dixon DE, Korbutt GS. Development of an ectopic site for islet transplantation, using biodegradable scaffolds. Tissue Eng. 2005;11(9–10):1323–1331. doi: 10.1089/ten.2005.11.1323. [DOI] [PubMed] [Google Scholar]
- 91.Hiscox AM, Stone AL, Limesand S, Hoying JB, Williams SK. An islet-stabilizing implant constructed using a preformed vasculature. Tissue Eng Part A. 2008;14(3):433–440. doi: 10.1089/tea.2007.0099. [DOI] [PubMed] [Google Scholar]
- 92.Moberg L, Olsson A, Berne C, Felldin M, Foss A, Källen R, Salmela K, Tibell A, Tufveson G, Nilsson B, Korsgren O. Nicotinamide inhibits tissue factor expression in isolated human pancreatic islets: implications for clinical islet transplantation. Transplantation. 2003;76(9):1285–1288. doi: 10.1097/01.TP.0000098905.86445.0F. [DOI] [PubMed] [Google Scholar]
- 93.Marzorati S, Antonioli B, Nano R, Maffi P, Piemonti L, Giliola C, Secchi A, Lakey JR, Bertuzzi F. Culture medium modulates proinflammatory conditions of human pancreatic islets before transplantation. Am J Transplant. 2006;6(11):2791–2795. doi: 10.1111/j.1600-6143.2006.01512.x. [DOI] [PubMed] [Google Scholar]
- 94.Marzorati S, Melzi R, Nano R, Antonioli B, Di Carlo V, Piemonti L, Bertuzzi F. In vitro modulation of monocyte chemoattractant protein-1 release in human pancreatic islets. Transplant Proc. 2004;36(3):607–608. doi: 10.1016/j.transproceed.2004.02.048. [DOI] [PubMed] [Google Scholar]
- 95.Amoli MM, Mousavizadeh R, Sorouri R, Rahmani M, Larijani B. Curcumin inhibits in vitro MCP-1 release from mouse pancreatic islets. Transplant Proc. 2006;38(9):3035–3038. doi: 10.1016/j.transproceed.2006.08.172. [DOI] [PubMed] [Google Scholar]
- 96.Eckhoff DE, Smyth CA, Eckstein C, Bilbao G, Young CJ, Thompson JA, Contreras JL. Suppression of the c-Jun N-terminal kinase pathway by 17beta-estradiol can preserve human islet functional mass from proinflammatory cytokine-induced destruction. Surgery. 2003;134(2):169–179. doi: 10.1067/msy.2003.219. [DOI] [PubMed] [Google Scholar]
- 97.Zeender E, Maedler K, Bosco D, Berney T, Donath MY, Halban PA. Pioglitazone and sodium salicylate protect human beta-cells against apoptosis and impaired function induced by glucose and interleukin-1beta. J Clin Endocrinol Metab. 2004;89(10):5059–5066. doi: 10.1210/jc.2004-0446. [DOI] [PubMed] [Google Scholar]
- 98.Yang ZD, Chen M, Ellett JD, Carter JD, Brayman KL, Nadler JL. Inflammatory blockade improves human pancreatic islet function and viability. Am J Transplant. 2005;5(3):475–483. doi: 10.1111/j.1600-6143.2005.00707.x. [DOI] [PubMed] [Google Scholar]
- 99.Riachy R, Vandewalle B, Moerman E, Belaich S, Lukowiak B, Gmyr V, Muharram G, Kerr Conte J, Pattou F. 1,25-Dihydroxyvitamin D3 protects human pancreatic islets against cytokine-induced apoptosis via down-regulation of the Fas receptor. Apoptosis. 2006;11(2):151–159. doi: 10.1007/s10495-006-3558-z. [DOI] [PubMed] [Google Scholar]
- 100.Goss JA, Goodpastor SE, Brunicardi FC, Barth MH, Soltes GD, Garber AJ, Hamilton DJ, Alejandro R, Ricordi C. Development of a human pancreatic islet-transplant program through a collaborative relationship with a remote islet-isolation center. Transplantation. 2004;77(3):462–466. doi: 10.1097/01.TP.0000100397.86756.A3. [DOI] [PubMed] [Google Scholar]
- 101.Nanji SA, Shapiro AM. Islet transplantation in patients with diabetes mellitus: choice of immunosuppression. BioDrugs. 2004;18(5):315–328. doi: 10.2165/00063030-200418050-00004. [DOI] [PubMed] [Google Scholar]
- 102.Arita S, Nagai T, Ochiai M, Sakamoto Y, Smith CV, Shevlin L, Smith CV, Mullen Y. Pravastatin prevents primary nonfunction of canine islet autografts. Transplant Proc. 1998;30(2):411. doi: 10.1016/s0041-1345(97)01331-6. [DOI] [PubMed] [Google Scholar]
- 103.Arita S, Une S, Ohtsuka S, Atiya A, Kasraie A, Shevlin L, Mullen Y. Prevention of primary islet isograft nonfunction in mice with pravastatin. Transplantation. 1998;65(11):1429–1433. doi: 10.1097/00007890-199806150-00003. [DOI] [PubMed] [Google Scholar]
- 104.Kenmochi T, Miyamoto M, Mullen Y. Protection of mouse islet isografts from nonspecific inflammatory damage by recipient treatment with nicotinamide and 15-deoxyspergualin. Cell Transplant. 1996;5(1):41–47. doi: 10.1177/096368979600500108. [DOI] [PubMed] [Google Scholar]
- 105.Thomas JM, Contreras JL, Smyth CA, Lobashevsky A, Jenkins S, Hubbard WJ, Eckhoff DE, Stavrou S, Neville DM, Jr, Thomas FT. Successful reversal of streptozotocin-induced diabetes with stable allogeneic islet function in a preclinical model of type 1 diabetes. Diabetes. 2001;50(6):1227–1236. doi: 10.2337/diabetes.50.6.1227. [DOI] [PubMed] [Google Scholar]
- 106.Beuneu C, Vosters O, Ling Z, Pipeleers D, Pradier O, Goldman M, Verhasselt V. N-Acetylcysteine derivative inhibits procoagulant activity of human islet cells. Diabetologia. 2007;50(2):343–347. doi: 10.1007/s00125-006-0529-4. [DOI] [PubMed] [Google Scholar]
- 107.Goto M, Johansson H, Maeda A, Elgue G, Korsgren O, Nilsson B. Low molecular weight dextran sulfate prevents the instant blood-mediated inflammatory reaction induced by adult porcine islets. Transplantation. 2004;77(5):741–747. doi: 10.1097/01.tp.0000114872.26990.4f. [DOI] [PubMed] [Google Scholar]
- 108.Johansson H, Goto M, Dufrane D, Siegbahn A, Elgue G, Gianello P, Korsgren O, Nilsson B. Low molecular weight dextran sulfate: a strong candidate drug to block IBMIR in clinical islet transplantation. Am J Transplant. 2006;6(6):305–312. doi: 10.1111/j.1600-6143.2005.01186.x. [DOI] [PubMed] [Google Scholar]
- 109.Berg DT, Gerlitz B, Shang J, Smith T, Santa P, Richardson MA, Kurz KD, Grinnell BW, Mace K, Jones BE. Engineering the proteolytic specificity of activated protein C improves its pharmacological properties. Proc Natl Acad Sci USA. 2003;100(8):4423–4428. doi: 10.1073/pnas.0736918100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Arita S, Nagai T, Ochiai M, Sakamoto Y, Shevlin LA, Smith CV, Mullen Y. Prevention of primary nonfunction of canine islet autografts by treatment with pravastatin. Transplantation. 2002;73(1):7–12. doi: 10.1097/00007890-200201150-00003. [DOI] [PubMed] [Google Scholar]
- 111.Lukinius A, Jansson L, Korsgren O. Ultrastructural evidence for blood microvessels devoid of an endothelial cell lining in transplanted pancreatic islets. Am J Pathol. 1995;146(2):429–435. [PMC free article] [PubMed] [Google Scholar]
- 112.Cui W, Barr G, Faucher KM, Sun XL, Safley SA, Weber CJ, Chaikof EL. A membrane-mimetic barrier for islet encapsulation. Transplant Proc. 2004;36(4):1206–1208. doi: 10.1016/j.transproceed.2004.04.059. [DOI] [PubMed] [Google Scholar]
- 113.Liu H, Faucher KM, Sun XL, Feng J, Johnson TL, Orban JM, Apkarian RP, Dluhy RA, Chaikof EL. A membrane-mimetic barrier for cell encapsulation. Langmuir. 2002;18(4):1332–1339. [Google Scholar]
- 114.Wilson JT, Cui W, Sun XL, Tucker-Burden C, Weber CJ, Chaikof EL. In vivo biocompatibility and stability of a substrate-supported polymerizable membrane-mimetic film. Biomaterials. 2007;28(4):609–617. doi: 10.1016/j.biomaterials.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 115.Johansson U, Elgue G, Nilsson B, Korsgren O. Composite islet-endothelial cell grafts: a novel approach to counteract innate immunity in islet transplantation. Am J Transplant. 2005;5(11):2632–2639. doi: 10.1111/j.1600-6143.2005.01076.x. [DOI] [PubMed] [Google Scholar]
- 116.Olsson R, Carlsson PO. The pancreatic islet endothelial cell: emerging roles in islet function and disease. Int J Biochem Cell Biol. 2006;38(4):492–497. doi: 10.1016/j.biocel.2005.06.021. [DOI] [PubMed] [Google Scholar]
- 117.Berman DM, Cabrera O, Kenyon NM, Miller J, Tam SH, Khandekar VS, Picha KM, Soderman AR, Jordan RE, Bugelski PJ, Horninger D, Lark M, Davis JE, Alejandro R, Berggren PO, Zimmerman M, O'Neil JJ, Ricordi C, Kenyon NS. Interference with tissue factor prolongs intrahepatic islet allograft survival in a nonhuman primate marginal mass model. Transplantation. 2007;84(3):308–315. doi: 10.1097/01.tp.0000275401.80187.1e. [DOI] [PubMed] [Google Scholar]
- 118.Chaikof EL. Engineering and material considerations in islet cell transplantation. Annu Rev Biomed Eng. 1999;1:103–127. doi: 10.1146/annurev.bioeng.1.1.103. [DOI] [PubMed] [Google Scholar]
- 119.Wilson JT, Chaikof EL. Challenges and emerging technologies in the immunoisolation of cells and tissues. Adv Drug Deliv Rev. 2008;60(2):124–145. doi: 10.1016/j.addr.2007.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lim F, Sun AM. Microencapsulated islets as bioartificial endocrine pancreas. Science. 1980;210(4472):908–910. doi: 10.1126/science.6776628. [DOI] [PubMed] [Google Scholar]
- 121.Lanza RP, Hayes JL, Chick WL. Encapsulated cell technology. Nat Biotechnol. 1996;14(9):1107–1111. doi: 10.1038/nbt0996-1107. [DOI] [PubMed] [Google Scholar]
- 122.Calafiore R, Basta G, Luca G, Boselli C, Bufalari A, Giustozzi GM, Moggi L, Brunetti P. Alginate/polyaminoacidic coherent micro-capsules for pancreatic islet graft immunoisolation in diabetic recipients. Ann N Y Acad Sci. 1997;831:313–322. doi: 10.1111/j.1749-6632.1997.tb52206.x. [DOI] [PubMed] [Google Scholar]
- 123.Leblond FA, Simard G, Henley N, Rocheleau B, Huet PM, Halle JP. Studies on smaller (approximately 315 microM) microcapsules: IV. Feasibility and safety of intrahepatic implantations of small alginate poly-L-lysine microcapsules. Cell Transplant. 1999;8(3):327–337. doi: 10.1177/096368979900800303. [DOI] [PubMed] [Google Scholar]
- 124.Schneider S, von Mach MA, Kraus O, Kann P, Feilen PJ. Intraportal transplantation of allogenic pancreatic islets encapsulated in barium alginate beads in diabetic rats. Artif Organs. 2003;27(11):1053–1056. doi: 10.1046/j.1525-1594.2003.07159.x. [DOI] [PubMed] [Google Scholar]
- 125.Basta G, Osticioli L, Rossodivita ME, Sarchielli P, Tortoioli C, Brunetti P, Calafiore R. Method for Fabrication of Coherent Microcapsules – a New, Potential Immunoisolatory Barrier for Pancreatic-Islet Transplantation. Diabetes Nutr Metab. 1995;8:105–112. [Google Scholar]
- 126.Cruise GM, Hegre OD, Lamberti FV, Hager SR, Hill R, Scharp DS, Hubbell JA. In vitro and in vivo performance of porcine islets encapsulated in interfacially photopolymerized poly(ethylene glycol) diacrylate membranes. Cell Transplant. 1999;8(3):293–306. doi: 10.1177/096368979900800310. [DOI] [PubMed] [Google Scholar]
- 127.May MH, Sefton MV. Conformal coating of small particles and cell aggregates at a liquid-liquid interface. Ann N Y Acad Sci. 1999;875:126–134. doi: 10.1111/j.1749-6632.1999.tb08498.x. [DOI] [PubMed] [Google Scholar]
- 128.Wyman JL, Kizilel S, Skarbek R, Zhao X, Connors M, Dillmore WS, Murphy WL, Mrksich M, Nagel SR, Garfinkel MR. Immunoisolating pancreatic islets by encapsulation with selective withdrawal. Small. 2007;3(4):683–690. doi: 10.1002/smll.200600231. [DOI] [PubMed] [Google Scholar]
- 129.Zekorn T, Siebers U, Horcher A, Schnettler R, Zimmermann U, Bretzel RG, Federlin K. Alginate coating of islets of Langerhans: in vitro studies on a new method for microencapsulation for immuno-isolated transplantation. Acta Diabetol. 1992;29(1):41–45. doi: 10.1007/BF00572829. [DOI] [PubMed] [Google Scholar]
- 130.Cruise GM, Hegre OD, Scharp DS, Hubbell JA. A sensitivity study of the key parameters in the interfacial photopolymerization of poly(ethylene glycol) diacrylate upon porcine islets. Biotechnol Bioeng. 1998;57(6):655–665. doi: 10.1002/(sici)1097-0290(19980320)57:6<655::aid-bit3>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 131.Chen AM, Scott MD. Immunocamouflage: prevention of transfusion-induced graft-versus-host disease via polymer grafting of donor cells. J Biomed Mater Res A. 2003;67(2):626–636. doi: 10.1002/jbm.a.10146. [DOI] [PubMed] [Google Scholar]
- 132.Murad KL, Gosselin EJ, Eaton JW, Scott MD. Stealth cells: prevention of major histocompatibility complex class II-mediated T-cell activation by cell surface modification. Blood. 1999;94(6):2135–2141. [PubMed] [Google Scholar]
- 133.Scott MD, Murad KL, Koumpouras F, Talbot M, Eaton JW. Chemical camouflage of antigenic determinants: stealth erythrocytes. Proc Natl Acad Sci USA. 1997;94(14):7566–7571. doi: 10.1073/pnas.94.14.7566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lee DY, Yang K, Lee S, Chae SY, Kim KW, Lee MK, Han DJ, Byun Y. Optimization of monomethoxy-polyethylene glycol grafting on the pancreatic islet capsules. J Biomed Mater Res. 2002;62(3):372–377. doi: 10.1002/jbm.10246. [DOI] [PubMed] [Google Scholar]
- 135.Panza JL, Wagner WR, Role HLR, Rao RH, Beckman EJ, Russell AJ. Treatment of rat pancreatic islets with reactive PEG. Biomaterials. 2000;21(11):1155–1164. doi: 10.1016/s0142-9612(99)00283-5. [DOI] [PubMed] [Google Scholar]
- 136.Kellam B, De Bank PA, Shakesheff KM. Chemical modification of mammalian cell surfaces. Chem Soc Rev. 2003;32(6):327–337. doi: 10.1039/b211643j. [DOI] [PubMed] [Google Scholar]
- 137.Xie D, Smyth CA, Eckstein C, Bilbao G, Mays J, Eckhoff DE, Contreras JL. Cytoprotection of PEG-modified adult porcine pancreatic islets for improved xenotransplantation. Biomaterials. 2005;26(4):403–412. doi: 10.1016/j.biomaterials.2004.02.048. [DOI] [PubMed] [Google Scholar]
- 138.Jang JY, Lee DY, Park SJ, Byun Y. Immune reactions of lymphocytes and macrophages against PEG-grafted pancreatic islets. Biomaterials. 2004;25(17):3663–3669. doi: 10.1016/j.biomaterials.2003.10.062. [DOI] [PubMed] [Google Scholar]
- 139.Lee DY, Nam JH, Byun Y. Effect of polyethylene glycol grafted onto islet capsules on prevention of splenocyte and cytokine attacks. J Biomater Sci Polym Ed. 2004;15(6):753–766. doi: 10.1163/156856204774196144. [DOI] [PubMed] [Google Scholar]
- 140.Lee DY, Park SJ, Lee S, Nam JH, Byun Y. Highly poly(ethylene) glycolylated islets improve long-term islet allograft survival without immunosuppressive medication. Tissue Eng. 2007;13(8):2133–2141. doi: 10.1089/ten.2006.0009. [DOI] [PubMed] [Google Scholar]
- 141.Chen AM, Scott MD. Current and future applications of immunological attenuation via pegylation of cells and tissue. BioDrugs. 2001;15(12):833–847. doi: 10.2165/00063030-200115120-00005. [DOI] [PubMed] [Google Scholar]
- 142.Krol S, del Guerra S, Grupillo M, Diaspro A, Gliozzi A, Marchetti P. Multilayer nanoencapsulation. New approach for immune protection of human pancreatic islets. Nano Lett. 2006;6(9):1933–1939. doi: 10.1021/nl061049r. [DOI] [PubMed] [Google Scholar]
- 143.Miura S, Teramura Y, Iwata H. Encapsulation of islets with ultra-thin polyion complex membrane through poly(ethylene glycol)-phospholipids anchored to cell membrane. Biomaterials. 2006;27(34):5828–5835. doi: 10.1016/j.biomaterials.2006.07.039. [DOI] [PubMed] [Google Scholar]
- 144.Teramura Y, Kaneda Y, Iwata H. Islet-encapsulation in ultra-thin layer-by-layer membranes of poly(vinyl alcohol) anchored to poly(ethylene glycol)-lipids in the cell membrane. Biomaterials. 2007;28(32):4818–4825. doi: 10.1016/j.biomaterials.2007.07.050. [DOI] [PubMed] [Google Scholar]
- 145.Tibell LA, Sethson I, Buevich AV. Characterization of the heparin- binding domain of human extracellular superoxide dismutase. Biochim Biophys Acta. 1997;1340(1):21–32. doi: 10.1016/s0167-4838(97)00024-1. [DOI] [PubMed] [Google Scholar]
- 146.Edens RE, Linhardt RJ, Bell CS, Weiler JM. Heparin and derivatized heparin inhibit zymosan and cobra venom factor activation of complement in serum. Immunopharmacology. 1994;27(2):145–153. doi: 10.1016/0162-3109(94)90049-3. [DOI] [PubMed] [Google Scholar]
- 147.Maillet F, Petitou M, Choay J, Kazatchkine MD. Structure-function relationships in the inhibitory effect of heparin on complement activation: independency of the anti-coagulant and anti-complementary sites on the heparin molecule. Mol Immunol. 1988;25(9):917–923. doi: 10.1016/0161-5890(88)90130-7. [DOI] [PubMed] [Google Scholar]
- 148.Sahu A, Pangburn MK. Identification of multiple sites of interaction between heparin and the complement system. Mol Immunol. 1993;30(7):679–684. doi: 10.1016/0161-5890(93)90079-q. [DOI] [PubMed] [Google Scholar]
- 149.Cabric S, Sanchez J, Lundgren T, Foss A, Felldin M, Källen R, Salmela K, Tibell A, Tufveson G, Larsson R, Korsgren O, Nilsson B. Islet surface heparinization prevents the instant blood-mediated inflammatory reaction in islet transplantation. Diabetes. 2007;56(8):2008–2015. doi: 10.2337/db07-0358. [DOI] [PubMed] [Google Scholar]
- 150.Esmon CT. Crosstalk between inflammation and thrombosis. Maturitas. 2004;47(4):305–314. doi: 10.1016/j.maturitas.2003.10.015. [DOI] [PubMed] [Google Scholar]
- 151.Esmon CT. Coagulation inhibitors in inflammation. Biochem Soc Trans. 2005;33(Pt 2):401–405. doi: 10.1042/BST0330401. [DOI] [PubMed] [Google Scholar]
- 152.Stabler CL, Sun XL, Cui W, Wilson JT, Haller CA, Chaikof EL. Surface re-engineering of pancreatic islets with recombinant azido-thrombomodulin. Bioconjug Chem. 2007;18(6):1713–1715. doi: 10.1021/bc7002814. [DOI] [PubMed] [Google Scholar]
- 153.Saxon E, Bertozzi CR. Cell surface engineering by a modified Staudinger reaction. Science. 2000;287(5460):2007–2010. doi: 10.1126/science.287.5460.2007. [DOI] [PubMed] [Google Scholar]
- 154.Atkinson B, Dwyer K, Enjyoji K, Robson SC. Ecto-nucleotidases of the CD39/NTPDase family modulate platelet activation and thrombus formation: Potential as therapeutic targets. Blood Cells Mol Dis. 2006;36(2):217–222. doi: 10.1016/j.bcmd.2005.12.025. [DOI] [PubMed] [Google Scholar]
- 155.Robson SC, Wu Y, Sun X, Knosalla C, Dwyer K, Enjyoji K. Ectonucleotidases of CD39 family modulate vascular inflammation and thrombosis in transplantation. Semin Thromb Hemost. 2005;31(2):217–233. doi: 10.1055/s-2005-869527. [DOI] [PubMed] [Google Scholar]
- 156.Dwyer KM, Mysore TB, Crikis S, Robson SC, Nandurkar H, Cowan PJ, D'Apice AJ. The transgenic expression of human CD39 on murine islets inhibits clotting of human blood. Transplantation. 2006;82(3):428–432. doi: 10.1097/01.tp.0000229023.38873.c0. [DOI] [PubMed] [Google Scholar]
- 157.Cabric S, Elgue G, Nilsson B, Korsgren O, Schmidt P. Adenovirus-mediated expression of the anticoagulant hirudin in human islets: a tool to make the islets biocompatible to blood. Cell Transplant. 2006;15(8–9):759–767. doi: 10.3727/000000006783464390. [DOI] [PubMed] [Google Scholar]
- 158.Giannoukakis N, Rudert WA, Ghivizzani SC, Gambotto A, Ricordi C, Trucco M, Robbins PD. Adenoviral gene transfer of the interleukin-1 receptor antagonist protein to human islets prevents IL-1beta-induced beta-cell impairment and activation of islet cell apoptosis in vitro. Diabetes. 1999;48(9):1730–1736. doi: 10.2337/diabetes.48.9.1730. [DOI] [PubMed] [Google Scholar]
- 159.Téllez N, Montolio M, Biarnés M, Castaño E, Soler J, Montanya E. Adenoviral overexpression of interleukin-1 receptor antagonist protein increases beta-cell replication in rat pancreatic islets. Gene Ther. 2005;12(2):120–128. doi: 10.1038/sj.gt.3302351. [DOI] [PubMed] [Google Scholar]
- 160.Gysemans C, Stoffels K, Giulietti A, Overbergh L, Waer M, Lannoo M, Feige U, Mathieu C. Prevention of primary non-function of islet xenografts in autoimmune diabetic NOD mice by anti-inflammatory agents. Diabetologia. 2003;46(8):1115–1123. doi: 10.1007/s00125-003-1154-0. [DOI] [PubMed] [Google Scholar]
- 161.Lau HT, Yu M, Fontana A, Stoeckert CJ., Jr Prevention of islet allograft rejection with engineered myoblasts expressing FasL in mice. Science. 1996;273(5271):109–112. doi: 10.1126/science.273.5271.109. [DOI] [PubMed] [Google Scholar]
- 162.Yolcu ES, Askenasy N, Singh NP, Cherradi SE, Shirwan H. Cell membrane modification for rapid display of proteins as a novel means of immunomodulation: FasL-decorated cells prevent islet graft rejection. Immunity. 2002;17(6):795–808. doi: 10.1016/s1074-7613(02)00482-x. [DOI] [PubMed] [Google Scholar]
- 163.Akgul C, Edwards SW. Regulation of neutrophil apoptosis via death receptors. Cell Mol Life Sci. 2003;60(11):2402–2408. doi: 10.1007/s00018-003-3110-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Grage-Griebenow E, Durrbaum-Landmann I, Pryjma J, Loppnow H, Flad HD, Ernst M. Apoptosis in monocytes. Eur Cytokine Netw. 1998;9(4):699–700. [PubMed] [Google Scholar]
- 165.Brandhorst D, Brandhorst H, Zwolinski A, Nahidi F, Bretzel RG. Prevention of early islet graft failure by selective inducible nitric oxide synthase inhibitors after pig to nude rat intraportal islet transplantation. Transplantation. 2001;71(2):179–184. doi: 10.1097/00007890-200101270-00002. [DOI] [PubMed] [Google Scholar]
- 166.Hsu BR, Chen ST, Fu SH. Enhancing engraftment of islets using perioperative sodium 4-phenylbutyrate. Int Immunopharmacol. 2006;6(13–14):1952–1959. doi: 10.1016/j.intimp.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 167.Ueki M, Yasunami Y, Motoyama K, Funakoshi A, Ikeda S, Tanaka M. The amelioration of hyperglycemia in streptozotocin-induced diabetic rats after the intraportal transplantation of an insufficient number of islets by nicotinamide treatment. Transplantation. 1995;60(4):313–317. doi: 10.1097/00007890-199508270-00001. [DOI] [PubMed] [Google Scholar]
- 168.Leung A, Ramaswamy Y, Munro P, Lawrie G, Nielsen L, Trau M. Emulsion strategies in the microencapsulation of cells: pathways to thin coherent membranes. Biotechnol Bioeng. 2005;92(1):45–53. doi: 10.1002/bit.20597. [DOI] [PubMed] [Google Scholar]