Abstract
Feary, Thomas W. (Tulane University School of Medicine, New Orleans, La.), Earl Fisher, Jr., and Thelma N. Fisher. Isolation and preliminary characteristics of three bacteriophages associated with a lysogenic strain of Pseudomonas aeruginosa. J. Bacteriol. 87:196–208. 1964.—Three bacteriophages designated 7v, 7m, and 7s were isolated from a lysogenic strain of Pseudomonas aeruginosa designated Ps-7. The three viruses were found to be completely unrelated on the basis of plaque morphology, host range, serology, ultraviolet induction, sensitivity to heat, and particle morphology as revealed by electron microscopy. In addition, it was shown that the three phages were incapable of plaque formation on bacteria other than various strains of P. aeruginosa. Of the three phages, only phage 7v was capable of plaque formation on strain Ps-7. The growth of phage 7v on strain Ps-7 exhibited properties which suggest that this virus arises as the result of mutation in a temperate phage for which strain Ps-7 is lysogenic. Phages 7m and 7s are incapable of plaque formation on strain Ps-7, but are adsorbed at characteristic rates to cell suspensions of strain Ps-7. The relationship between phage 7m and strain Ps-7 was shown to meet the classical criteria for lysogeny. Because phage 7s contains ribonucleic acid as its nucleic acid component, it was concluded that its production by strain Ps-7 and the demonstration of immunity of strain Ps-7 to infection by phage 7s were not sufficient evidence to define the nature of the relationship between phage 7s and P. aeruginosa strain Ps-7. It was observed that under certain conditions the infectious titer of phage 7s preparations are markedly reduced in the presence of ribonuclease.
Full text
PDF



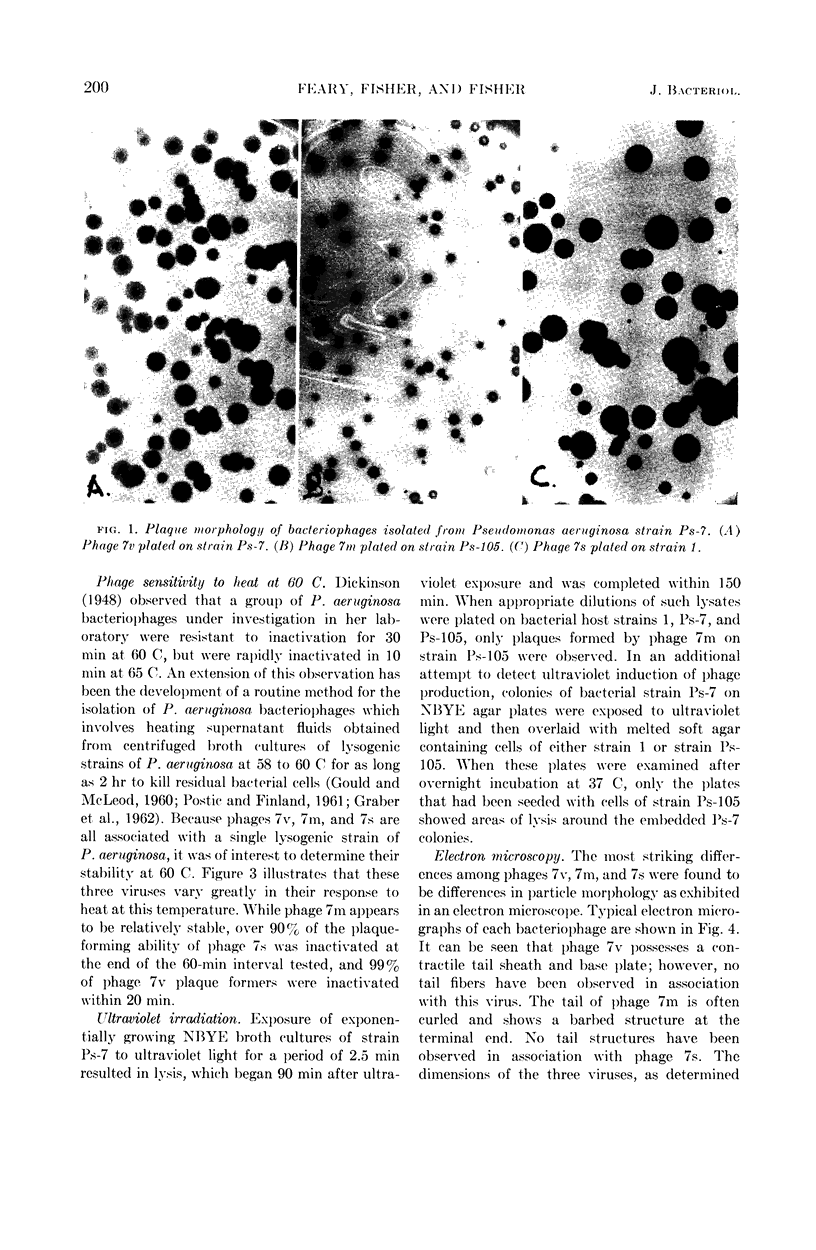
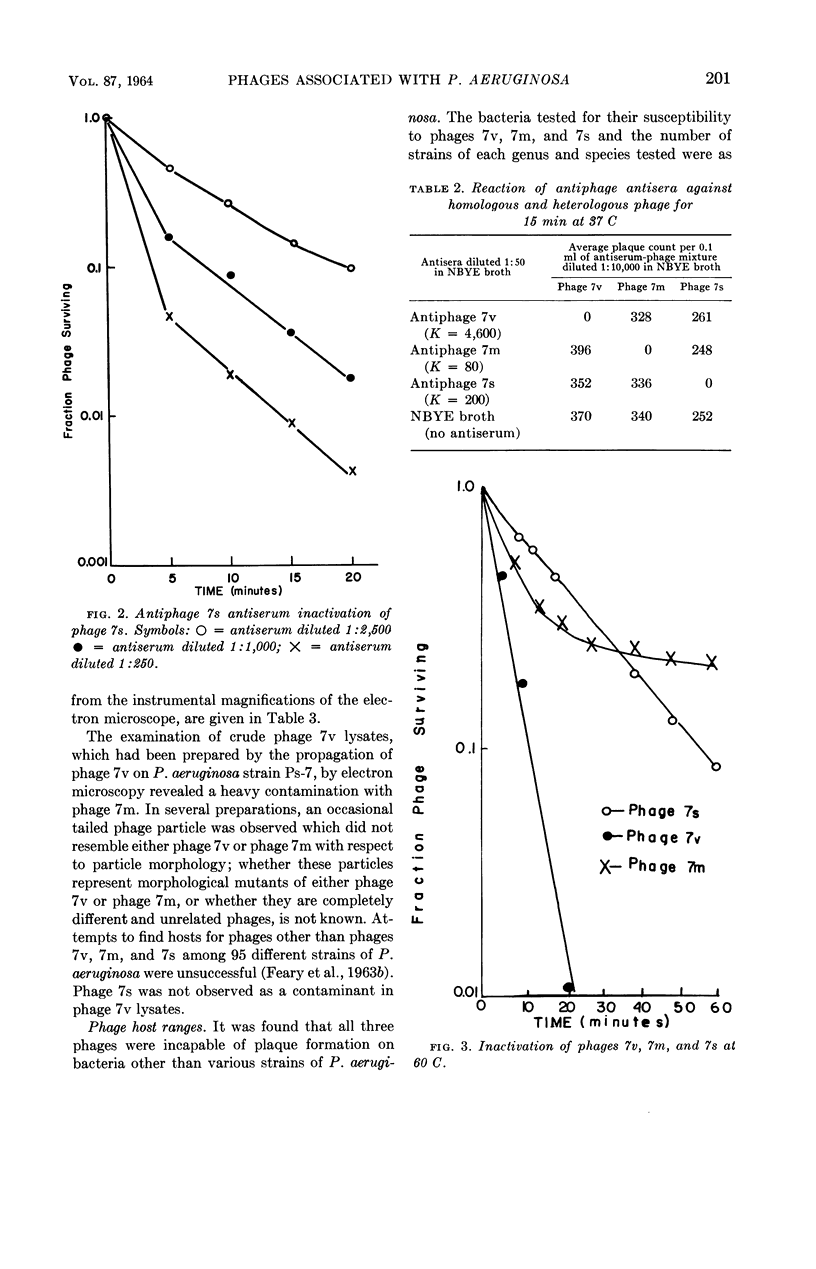
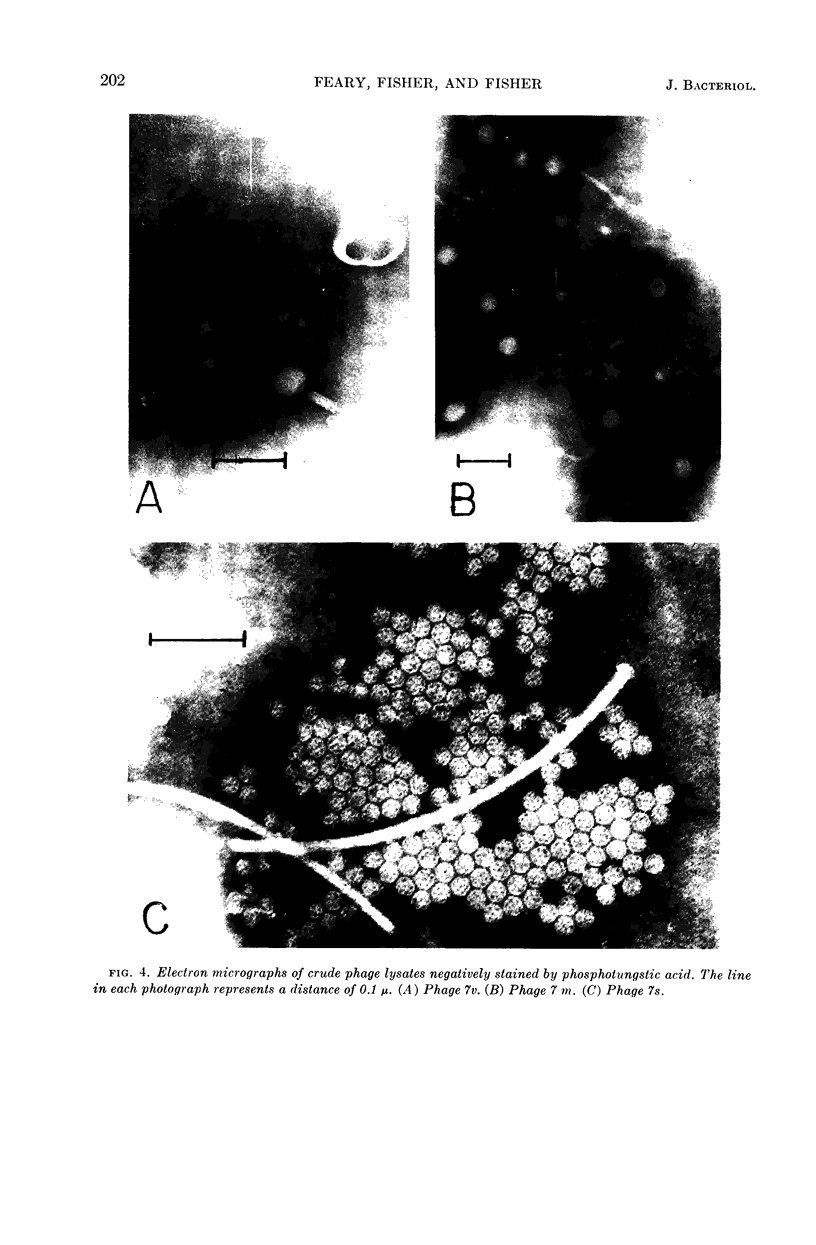



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRADLEY D. E. A study of the negative staining process. J Gen Microbiol. 1962 Nov;29:503–516. doi: 10.1099/00221287-29-3-503. [DOI] [PubMed] [Google Scholar]
- BRENNER S., HORNE R. W. A negative staining method for high resolution electron microscopy of viruses. Biochim Biophys Acta. 1959 Jul;34:103–110. doi: 10.1016/0006-3002(59)90237-9. [DOI] [PubMed] [Google Scholar]
- DOI R. H., SPIEGELMAN S. Homology test between the nucleic acid of an RNA virus and the DNA in the host cell. Science. 1962 Dec 14;138(3546):1270–1272. doi: 10.1126/science.138.3546.1270. [DOI] [PubMed] [Google Scholar]
- Doermann A. H. Lysis and Lysis Inhibition with Escherichia coli Bacteriophage. J Bacteriol. 1948 Feb;55(2):257–276. doi: 10.1128/jb.55.2.257-276.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FEARY T. W., FISHER E., Jr, FISHER T. N. A small RNA containing Pseudomonas aeruginosa bacteriophage. Biochem Biophys Res Commun. 1963 Mar 5;10:359–365. doi: 10.1016/0006-291x(63)90538-2. [DOI] [PubMed] [Google Scholar]
- FEARY T. W., FISHER E., Jr, FISHER T. N. Lysogeny and phage resistance in Pseudomonas aeruginosa. Proc Soc Exp Biol Med. 1963 Jun;113:426–430. doi: 10.3181/00379727-113-28386. [DOI] [PubMed] [Google Scholar]
- FRY B. A., GROS F. The metabolic activities of Escherichia coli during the establishment of lysogeny. J Gen Microbiol. 1959 Dec;21:685–692. doi: 10.1099/00221287-21-3-685. [DOI] [PubMed] [Google Scholar]
- GOULD J. C., McLEOD J. W. A study of the use of agglutinating sera and phage lysis in the classification of strains of Pseudomonas aeruginosa. J Pathol Bacteriol. 1960 Apr;79:295–311. [PubMed] [Google Scholar]
- GRABER C. D., LATTA R., VOGEL E. H., Jr, BRAME R. Bacteriophage grouping of Pseudomonas aeruginosa, with special emphasis on lysotypes occurring in infected burns. Am J Clin Pathol. 1962 Jan;37:54–62. doi: 10.1093/ajcp/37.1.54. [DOI] [PubMed] [Google Scholar]
- HALL B. D., SPIEGELMAN S. Sequence complementarity of T2-DNA and T2-specific RNA. Proc Natl Acad Sci U S A. 1961 Feb 15;47:137–163. doi: 10.1073/pnas.47.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLLOWAY B. W., EGAN J. B., MONK M. Lysogeny in Pseudomonas aeruginosa. Aust J Exp Biol Med Sci. 1960 Aug;38:321–329. doi: 10.1038/icb.1960.34. [DOI] [PubMed] [Google Scholar]
- HOLLOWAY B. W., FARGIE B. Fertility factors and genetic linkage in Pseudomonas aeruginosa. J Bacteriol. 1960 Sep;80:362–368. doi: 10.1128/jb.80.3.362-367.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLLOWAY B. W. Genetic recombination in Pseudomonas aeruginosa. J Gen Microbiol. 1955 Dec;13(3):572–581. doi: 10.1099/00221287-13-3-572. [DOI] [PubMed] [Google Scholar]
- HOLLOWAY B. W., JENNINGS P. A. An infectious fertility factor for Pseudomonas aeruginosa. Nature. 1958 Mar 22;181(4612):855–856. doi: 10.1038/181855b0. [DOI] [PubMed] [Google Scholar]
- JACOB F., WOLLMAN E. L. Induction of phage development in lysogenic bacteria. Cold Spring Harb Symp Quant Biol. 1953;18:101–121. doi: 10.1101/sqb.1953.018.01.019. [DOI] [PubMed] [Google Scholar]
- LWOFF A. Lysogeny. Bacteriol Rev. 1953 Dec;17(4):269–337. doi: 10.1128/br.17.4.269-337.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MEYNELL E. W. Characters of a group of bacillus phages. J Gen Microbiol. 1962 Apr;28:103–117. doi: 10.1099/00221287-28-1-103. [DOI] [PubMed] [Google Scholar]
- McCLOY E. W. Lysogenicity and immunity to Bacillus phage W. J Gen Microbiol. 1958 Feb;18(1):198–220. doi: 10.1099/00221287-18-1-198. [DOI] [PubMed] [Google Scholar]
- POSTIC B., FINLAND M. Observations on bacteriophage typing of Pseudomonas aeruginosa. J Clin Invest. 1961 Nov;40:2064–2075. doi: 10.1172/JCI104432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATSON J. D. The properties of x-ray inactivated bacteriophage. I. Inactivation by direct effect. J Bacteriol. 1950 Dec;60(6):697–718. doi: 10.1128/jb.60.6.697-718.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]