Abstract
Background
Automatic eating detection (AED) can potentially support treatments that need to be synchronized with food intake. This article analyzes an implantable AED device working in conjunction with gastric stimulation intended to treat type 2 diabetes (T2DM). The device continuously senses for changes in tissue impedance and electrical activity induced by food intake and initiates treatment sessions upon detection. This article reviews AED performance as well as its relevance to treatment outcomes.
Methods
Obese T2DM (n = 12) were implanted with gastric leads and the TANTALUS® device. An AED algorithm was embedded in the device and was used to initiate periods of electrical stimulation during food intake. AED performance was assessed using patients' food diaries. The treatment outcome at 37 weeks postimplants was correlated with the rates of stimulation during large meals vs stimulation during periods of no caloric intake.
Results
The algorithm was able to detect 73% of meals consumed while sensing. The rate of false stimulations was 28%. Stimulation during meals was significantly correlated (R2 = 0.45, p < 0.05) with hemoglobin A1c change (average drop in hemoglobin A1c was −1 ± 0.4%) but not with changes in body weight (average drop −4.7 ± 2.8 kg). Stimulation during periods with no caloric intake was negatively correlated with hemoglobin A1c reduction (R2 = 0.27, p < 0.05).
Conclusions
Sensing of gastric activity can be used for detection of food intake. The synchronization of gastric stimulation to periods of food intake is correlated with metabolic outcomes. AED may also benefit other applications such as drug delivery and control of food restriction devices.
Keywords: automatic eating detection, gastric stimulation, type 2 diabetes
Introduction
Early detection of food intake can be a useful tool for facilitating meal-related treatments with reduced dependence on patient adherence. The goal of developing a medical device capable of automatic eating detection (AED) has therefore been the subject of growing research.1,2
It is known that ingested intake having contact with the gut can generate detectable changes in muscle function.2–4 This was the hypothesis underlying earlier animal research utilizing implanted gastric leads to detect small electromechanical changes in the gastric muscle and to relate these to initiation of food intake and satiety.2,4 This method was tested in dogs and showed encouraging results with a detection sensitivity of 86% for meals weighing over 15 grams.2 The correlation between the two signals used for detection (fundus tissue impedance and the rate of antral slow waves) and the size of the meal in grams was statistically significant (p < 0.05, R2 > 0.9). The system was then used in obese nondiabetic patients in conjunction with gastric stimulation, but no systematic testing of detection performance has been reported so far in humans. In this study we report the performance of AED in 12 type 2 diabetes mellitus (T2DM) patients. The AED system was embedded in an implantable device that used the intake detection as a trigger for sessions of synchronized gastric stimulation, a new treatment paradigm for T2DM.
This new type of gastric stimulation aims at enhancing gastric mechanical activity in the early phases of the meal in order to enhance the metabolic response to the caloric load and to improve postprandial glucose levels. This new approach to electrical stimulation of muscles uses synchronized long pulses5 and was shown to enhance gastric contractility and satiety-related vagal afferents7 when applied to the antrum as well as to enhance contractility in cardiac muscles of heart failure patients when applied to the heart.6 Unlike continuous pacing of the gastric muscle,9 synchronized stimulation does not interfere or entrain gastric slow waves.
The device was tested for safety and functionality in large animal studies2 but no comprehensive efficacy study in animals was performed due to the difficulty in developing an overweight type 2 diabetes model, uncontrolled on oral medications in large animals. First efficacy tests were therefore performed in patients following the completion of safety and functionality studies in animals. Clinical trials involving a surgical procedure for treating diabetes were considered justified for obese patients who were unable to control their diabetes on medication and therefore in risk of developing serious diabetes complications. The other surgical alternatives for these patients were the more invasive bariatric procedures such as gastric bypass and gastric banding.
Initial clinical studies5,10 using synchronized gastric stimulation used AED to apply treatment sessions during prandial and postprandial periods, but avoid the period of fasting or noncaloric intake. These clinical studies demonstrated the treatment to results with a combination of improved glycemic control and weight loss in obese and T2DM patients.
The research hypothesis of the current study was that the timing of treatment sessions to periods with caloric intake is related to clinical outcome. We report the design details of the AED system, the detection performance achieved using AED, and the correlation of actual treatment timing to clinical outcomes in terms of changes in glycemic control and body weight. We then use these results to evaluate the required timing accuracy of treatment to meals for achieving a clinically significant outcome using synchronized gastric stimulation.
Materials and Methods
System Configuration and Experimental Setting
Twelve patients with T2DM (5 males, 7 females) from two centers (A.K.H, Vienna, Austria, and Krankenhaus Sachsenhausen, Frankfurt, Germany) were enrolled in a nonrandomized, open-label study approved by the corresponding local ethical committees.10 A set of three bipolar stitch electrodes [TIZER SA™, MetaCure Inc., USA] was implanted in the gastric muscle and was used for measuring gastric electromechanical activity. The same implanted electrode set used in prior animal studies2 was also used in humans. The measurements were fed in real time into an algorithm that applied signal processing methods on the different signals for detecting the onset of food intake.
Each such intake detection triggered a gastric stimulation session that had a fixed duration and was shut off automatically. The stimulation was applied using the same set of electrodes. As a result, the sensing algorithm was disabled during the gastric stimulation session plus an additional period of 15 minutes poststimulation.
For each patient the effect of gastric stimulation on hemoglobin A1c (HbA1c) and weight was measured at 37 weeks postimplant and compared to baseline (calculated as an average of all measurements prior to implant). The change in clinical outcomes was then correlated with the percentage of meals stimulated and the amount of false stimulation (stimulation applied outside meal times as a result of a false positive detection by the AED algorithm).
The results were used to examine the importance of synchronizing the stimulation to meals in achieving optimal improvement in HbA1c and body weight. The results were also used to evaluate the performance of the AED system in humans in terms of sensitivity (S)— the rate of caloric intakes detected by the system—and positive predictive value (PPV)—the rate of stimulations that were triggered by actual intake by the patient. A definition of each performance parameter is given in the AED performance analysis section.
Device and Electrodes Implantation Procedure
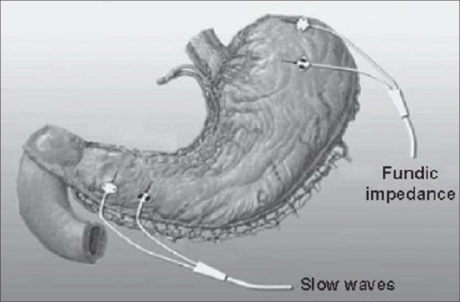
Three bipolar electrodes were implanted: one in the fundus, one in the anterior antrum, and one in the posterior antrum (see Figure 1 for electrode configuration). Each electrode was made of an 18-mm-long, 0.5-mm-diameter platinum-iridium coil coated with titanium nitride. In the clinical trial the electrodes were connected to the implantable device, which performed both the detection and the stimulation tasks [TANTALUS® by MetaCure Inc., USA]. The device was implanted subcutaneously on the left side of the abdomen.
Figure 1.
Electrode configuration.
Sensed Electrical Signals
Two sources for electrical signals were used as inputs for the detection algorithm:
A signal derived from sampling the propagating action potentials in the antrum. These signals are generally referred to as “slow waves” and their rate in humans is typically around three events/minute.11,12 The AED algorithm used the known slowing effect of gastric distention and food intake on the rate of slow waves.13,14 The signals used for detection were measured directly by one of the bipolar electrodes of the antrum, filtered, and then sampled at 50 samples/second. The result was then compared to voltage thresholds to determine the detection time of each slow wave. A moving average slow wave rate (SWR) was calculated for each new detection based on the time intervals between the last six events.
A signal derived from sampling the estimated tissue impedance in the fundus. This signal was used as an indicator of the mechanical distension and relaxation of the fundus. The measurement was performed by applying a short voltage pulse and measuring the resulting current. This was performed 10 times every second and generated a time series of impedance values. The series was filtered using a digital single pole infinite impulse response high pass in order to remove baseline wandering and a low pass for noise reduction. The resulting filtered signal was reflective of changes in the fundus impedance (FI).
Intake Detection Events
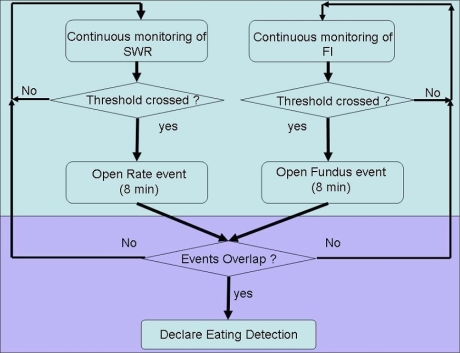
Real-time changes in the values of SWR and FI were used to determine that intake occurred. A rate event was declared by the algorithm when SWR crossed a preprogrammed threshold. Likewise, an impedance event was declared when FI crossed a preprogrammed threshold. In order for the algorithm to declare an eating detection (ED) event, both a rate event and an impedance event had to occur within a preset time window of 8 minutes. The order of occurrence was not relevant for the decision. A graphical representation of the algorithm appears in Figure 2.
Figure 2.
Graphical representation of the algorithm.
Most algorithm parameters were programmed per patient based on a review of previous patient data. This parameter setting was always done in a causal way so that parameters were first set and then the test was performed. In some cases, adjustment of parameters was done during the course of the study. Such adjustments were typically necessary in the early weeks postimplant, as tissue encapsulation of electrodes is known to affect electrode impedance values during the first several weeks.
Since ED was used to trigger sessions of electrical stimulation, the algorithm was programmed to be blinded for 90 minutes following each ED (75 minutes of stimulation window + 15 minutes poststimulation). The use of this blinding period resulted in potential actual intake events that could not be detected by the algorithm. These blinding periods during a stimulation session were deemed acceptable since the AED algorithm has already performed its function, i.e., trigger a stimulation session following a detection of caloric intake.
Human Protocol
All 12 patients were obese type 2 diabetic subjects (five males, seven females) treated with oral antidiabetic medications for at least 3 months prior to enrollment and had HbA1c levels between 7 and 9.4%. Study exclusion criteria were alcohol or drug abuse, psychopathological diseases, eating disorders, and those deemed unable or unwilling to comply with study requirements. Patients analyzed for this article were a subset of a larger group of 24 that were originally enrolled in the study. The other 12 patients were not included in this analysis for one or more of the following reasons: (1) baseline HbA1c <7%, (2) changes in the type or dose of diabetes medication just prior to the study that made it impossible to analyze their diabetes improvement, (3) insulin-treated patients, and (4) drop out prior to 37 weeks.
Subjects were not required to follow a particular diet, but all received a standard dietary counsel in the beginning of the study. The study protocol included a 4-week observation period prior to implant and a 6-week recovery and parameter customization period postimplant. All patients included in this analysis were followed for at least 37 weeks postimplant.
During the first 6 weeks postimplant stimulation was inactive. Stimulation was then activated, triggered only via AED, for the remaining 31 weeks.
Meal Diary Classification
On several occasions during the study subjects were asked to record the times and contents of all intakes of a specific day while ED data were recorded. The data recording was done by the patients using an external hand-held data logger that was wirelessly communicating with the implantable device. Recordings took place in the patients own environment so that compliance and accuracy were not controlled. The patients were asked to make occasional entries to the diaries during the first 37 weeks of the study; therefore, the effect of the diaries themselves on their weight was assumed to be negligible.
All meal diaries text was classified by a nutritionist who was blinded to algorithm detection data. The predetermined classification rule defined intakes with a caloric value of at least 250 calories as “meals,” caloric intakes that did not qualify as meals were defined as “other caloric intake,” and the third classification was “noncaloric intake,” which was typically drinks such as water or diet soft drinks.
Automatic Eating Detection Performance Analysis
Patient meal diaries classifications were compared to ED data for evaluating the AED algorithm performance. The recorded stimulation periods were also compared against intake times for evaluating the correlation between meal stimulation and medical outcome.
True positive (TP) ED was defined as an event that occurred between 20 minutes prior to an initiation of a caloric intake (as recorded in the meal table) and 20 minutes postinitiation of the intake. This range was prospectively selected and was chosen to account for subject meal table entry time variability/inaccuracy, as well as differences between subject and device times.
In addition, S and PPV were defined as follows:
S = TP/total number of caloric intakes
PPV = TP/total number of stimulation sessions
Intakes occurring within the algorithm blinding period were not analyzed for detection performance, as the AED was not actively sensing food intake during these periods. AED performance analysis was only analyzed for “detectable intakes” (i.e., occurring during times when the algorithm was not blinded).
In addition, we defined the parameter of “stimulated meals” as the rate of meals for which gastric stimulation was on for at least 10 minutes during the first 30 minutes of intake. This parameter was defined for the purpose of evaluating the importance of applying gastric stimulation specifically during meals. For that analysis the question of algorithm detection performance was secondary to the question of whether there was any meaningful stimulation time applied during the meals (such stimulation may be, for example, a result of a false detection that occurred before the intake). In parallel, we defined the parameter of “false stimulation” as the rate of stimulations that included less than 10 minutes of stimulation over caloric intake.
The reason for the separate analyses was because the first one (calculated S and PPV parameters) gives the expected performance of the “stand-alone” algorithm and the second (rate of “stimulated meals”) gives the expected performance of the algorithm in the special setting of gastric stimulation and is beneficial in correlating the treatment timing to clinical outcomes.
Because of the variability of recording times (resulting from differences in patient compliance using the diaries and the data logger) the performance results were calculated both as an average per patient giving all patients the same weight and as a weighted average based on the length of recording time per patient.
Clinical Outcomes and Correlation with ED
Changes in HbA1c (indicating changes in glycemic control) and weight from baseline to 37 weeks postimplant were plotted per patient and compared with the rate of stimulated meals and false stimulation.
Since there is a well-known relation between overweight and T2DM we also calculated the correlation between changes in HbA1c and body weight (in kilograms).
Results
Recording and Classification of Intakes
Food diaries from all 12 subjects were classified by the nutritionist. A total of 116 patient diaries with reports on 736 intakes were analyzed. The intakes were classified to 263 meals, 175 noncaloric drinks, and 298 other caloric intakes. Approximately 1032 hours were recorded from the device during the times covered by patient diaries with an average recording time of 86 hours per patient (range 18 to 158 hours). Note that the total recording time and diary use per patient was negligible compared to the total study duration (37 weeks). As such, the effect of diary writing itself on weight was assumed negligible.
Algorithm Performance
From all intakes classified as caloric intakes, 69% occurred during the AED active (nonblinded) time and were therefore considered detectable. Out of the meals (total calories >250), 73% were detectable.
Out of these detectable intakes, 66% were actually detected (range was 45 to 100%), giving an expected stand-alone sensitivity of S = 0.66. For meals, this value was higher (S = 0.73). The time-weighted average detection results gave very similar performance across all analyses; therefore, we will not repeat this observation further. PPV for all caloric intakes was 0.54.
Seventy-three percent of all meals were stimulated for at least 10 minutes during the first 30 minutes of intake. A lower stimulation rate of 67% was calculated for caloric intakes that did not qualify as meals. The rate of false stimulation was 28% (range 9 to 52%).
Clinical Effects of Stimulation during Food Intake
Baseline data collected prior to implant (week 0) were as follows (mean ± standard error of the mean): age was 50.8 ± 2.2 years (range 37–59), HbA1c was 8.2 ± 0.2% (range 7.0–9.4), and weight was 130.0 ± 6.5 kg (range 97–168).
Electrodes and device implant procedure and recovery were without any serious or unanticipated adverse events. The majority of events was related to the postoperative period and was resolved within 2 weeks of the procedure. The change in HbA1c from baseline to 37 weeks was −1.0 ± 0.4. During that period, average patient body weight was reduced by 4.7 ± 2.8 kg, and correlation between weight changes and HbA1c changes was statistically significant (p < 0.05, R2 = 0.38).
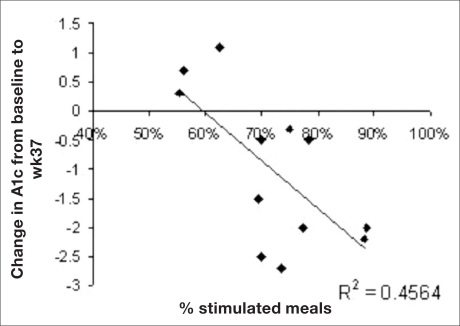
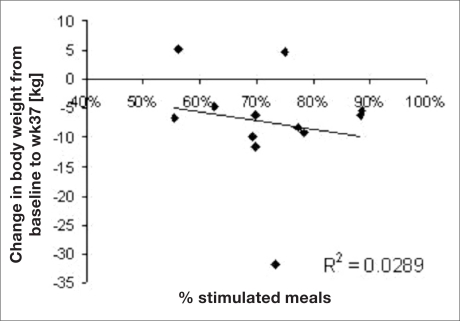
The values of HbA1c changes and the corresponding rate of stimulated meals per patient are depicted in Figure 3. The regression shows a statistically significant correlation (p < 0.01) between the rate of stimulated meals and the drop in HbA1c and suggests that 46% of the variability in HbA1c drop can be explained by the variability in the rate of stimulated meals. No similar correlation was found between the rate of stimulated meals and weight loss (see Figure 4).
Figure 3.
Values of HbA1c changes and the corresponding rate of stimulated meals per patient.
Figure 4.
Rate of stimulated meals and weight loss.
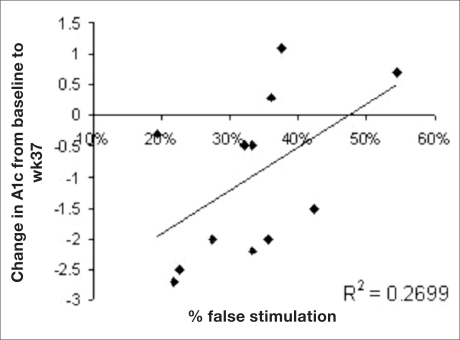
The relationship between the rate of false stimulation per patient and the change in HbA1c is depicted in Figure 5. There was negative correlation (p < 0.05, R2 = 0.27) between the rate of false positive stimulation and the drop in HbA1c. Again, a weaker and nonstatistically significant correlation was found between false positive stimulation and weight loss (p = 0.18, R2 = 0.17).
Figure 5.
Relationship between the rate of false stimulation per patient and the change in HbA1c.
Stimulation of other caloric intakes that did not qualify as meals also tended to affect HbA1c in the same direction. However, this effect was also not statistically significant.
Discussion
This article reviewed the design and clinical use of a device for automatic eating detection and gastric stimulation, a potential new tool in treating T2DM using electrical stimulation timed to food intake. The feasibility for automatic detection of meals can be utilized in a variety of ways for supplying patient-independent treatment and helping overcome some of the barriers to treatment success resulting from poor patient compliance to complicated therapy regimes. The performance of the automatic system in applying treatment on over 70% of all intakes offers an advantage over many patients with poor compliance patterns.
The combination with gastric stimulation provides a new direction for exploring the way in which the gastrointestinal tract modulates glucose metabolism in the early stages of food intake and can potentially provide a new interventional treatment paradigm for diabetes using a less invasive surgical approach.
The evident correlation between treatment parameters and glycemic control versus the weaker correlation to weight loss suggests a direct effect on glucose control that may be generating an indirect effect on weight loss. Such an effect may be the result of reduced demand for insulin or through modulation of different gastric hormones such as ghrelin and glucagon-like pep tide 1 (GLP-1). Such modulation may be related to activation or enhancement of neural pathways conveying the message of early intake from the foregut to distal areas associated with GLP-1 secretion.
Since the trial did not include a control group, the combined effect of the weight and glucose improvement can also be related to the placebo effect of participating in the study, especially since the patients agreed to a surgical procedure. Several steps were taken to minimize this effect: Patients were not required to keep any specific diet or exercise regime and results were reported following a long time period (37 weeks—the longest follow-up period available at the time of writing) by which a large portion of the placebo effect was expected to disappear. Also, the rate of follow-ups was gradually reduced down to once a month at the period of last weight loss and HbA1c measurement. At the time of writing there were no longer term data.
Another aspect of the study weakening the result is the fact that it was planned as a feasibility study and the outcomes are retrospective. The time point selected for reporting was the one with the longest follow-up period available.
Conclusion
Further research is currently underway to study AED and changes in incretin and hormone levels. Future research will evaluate whether AED therapy leads to increased satiety. In addition, fine-tuning of the algorithms is sought to further enhance intake detection sensitivity and specificity.
As in many other cases of novel medical treatment, it may take a significant amount of research to understand the cascade of actions that occur neurally and hormonally. The evaluation of outcome in terms of overall improvement in metabolic and other clinical parameters compared to the surgical risks should be sought when considering the clinical use of the device.
Acknowledgments
The authors thank Michal Kedem for her help in classifying patient food diaries.
Abbreviations
- AED
automatic eating detection
- ED
eating detection
- FI
fundus impedance
- GLP-1
glucagon-like peptide 1
- HbA1c
hemoglobin A1c
- PPV
positive predictive value
- S
sensitivity
- SWR
slow wave rate
- T2DM
type 2 diabetes mellitus
- TP
true positive
Funding
Funding provided by MetaCure Inc. (USA), Bird Foundation.
References
- 1.Dassau E, Bequette BW, Buckingham BA, Doyle FJ., 3rd Detection of a meal using continuous glucose monitoring: implications for an artificial beta-cell. Diabetes Care. 2008;31(2):295–300. doi: 10.2337/dc07-1293. [DOI] [PubMed] [Google Scholar]
- 2.Aviv R, Sanmiguel CP, Kliger A, Policker S, Haddad W, Hagiike M, Soffer EE. The use of gastric electrical signals for algorithm for automatic eating detection in dogs. Neurogastroenterol Motil. 2008;20(4):369–376. doi: 10.1111/j.1365-2982.2007.01044.x. [DOI] [PubMed] [Google Scholar]
- 3.Deutsch JA. Dietary control and the stomach. Prog Neurobiol. 1983;20(3–4):313–332. doi: 10.1016/0301-0082(83)90007-2. [DOI] [PubMed] [Google Scholar]
- 4.Aviv R, Policker S, Brody F, Bitton O, Haddad W, Kliger A, Sanmiguel CP, Soffer EE. Circadian patterns of gastric electrical and mechanical activity in dogs. Neurogastroenterol Motil. 2008;20(1):63–68. doi: 10.1111/j.1365-2982.2007.00992.x. [DOI] [PubMed] [Google Scholar]
- 5.Bohdjalian A, Prager G, Aviv R, Policker S, Schindler K, Kretschmer S, Riener R, Zacherl J, Ludvik B. One-year experience with Tantalus: a new surgical approach to treat morbid obesity. Obes Surg. 2006;16(5):627–634. doi: 10.1381/096089206776945101. [DOI] [PubMed] [Google Scholar]
- 6.Pappone C, Vicedomini G, Salvati A, Meloni C, Haddad W, Aviv R, Mika Y, Darvish N, Kimchy Y, Shemer I, Snir Y, Pruchi D, Ben-Haim SA, Kronzon I. Electrical modulation of cardiac contractility: clinical aspects in congestive heart failure. Heart Fail Rev. 2001;6(1):55–60. doi: 10.1023/a:1009807309006. [DOI] [PubMed] [Google Scholar]
- 7.Peles S, Petersen J, Aviv R, Policker S, Abu-Hatoum O, Ben-Haim SA, Gutterman DD, Sengupta JN. Enhancement of antral contractions and vagal afferent signaling with synchronized electrical stimulation. Am J Physiol Gastrointest Liver Physiol. 2003;285(3):G577–G585. doi: 10.1152/ajpgi.00109.2003. [DOI] [PubMed] [Google Scholar]
- 8.Gonzalez MF, Deutsch JA. Vagotomy abolishes cues of satiety produced by gastric distension. Science. 1981;212(4500):1283–1284. doi: 10.1126/science.7233218. [DOI] [PubMed] [Google Scholar]
- 9.Shikora SA. Implantable gastric stimulation for the treatment of severe obesity. Obes Surg. 2004;14(4):545–548. doi: 10.1381/096089204323013596. [DOI] [PubMed] [Google Scholar]
- 10.Rosak R, et al. Poster presented at the European Association for the Study of Diabetes (EASD) Amsterdam; 2007. Pilot study on the effects of gastric electrical stimulation (TANTALUS) on glycemic control in obese patients with type 2 diabetes (T2DM) Sep 17–21. [Google Scholar]
- 11.Reybould HE, Pandol SJ. The integrated response of the gastrointestinal tract to a meal. In: Yamada T, editor. Gastroenterology. New York: 1999. pp. 1–10. [Google Scholar]
- 12.Mayer EA. The physiology of gastric storage and emptying. In: Johson LR, editor. Physiology of the gastrointestinal tract. New York: Raven Press; 1994. pp. 929–976. [Google Scholar]
- 13.Lin HC, Zhao XT, Chung B, Gu YG, Elashoff JD. Frequency of gastric pacesetter potential depends on volume and site of distension. Am J Physiol. 1996;270(3 Pt 1):G470–G475. doi: 10.1152/ajpgi.1996.270.3.G470. [DOI] [PubMed] [Google Scholar]
- 14.Zhu H, Chen JD. Gastric distension alters frequency and regularity but not amplitude of the gastric slow wave. Neurogastroenterol Motil. 2004;16(6):745–752. doi: 10.1111/j.1365-2982.2004.00571.x. [DOI] [PubMed] [Google Scholar]