Abstract
The biological response to implanted biomaterials in mammals is a complex series of events that involves many biochemical pathways. Shortly after implantation, fibrinogen and other proteins bind to the device surface, a process known as biofouling. Macrophages then bind to receptors on the proteins, join into multinucleated giant cells, and release transforming growth factor β and other inflammatory cytokines. In response to these signals, quiescent fibroblasts are transformed into myofibroblasts, which synthesize procollagen via activation of Smad mediators. The procollagen becomes crosslinked after secretion into the extracellular space. Mature crosslinked collagen and other extracellular matrix proteins gradually contribute to formation of a hypocellular dense fibrous capsule that becomes impermeable or hypopermeable to many compounds. Porous substrates and angiogenic growth factors can stimulate formation of microvessels, which to some extent can maintain analyte delivery to implanted sensors. However, stimulation by vascular endothelial growth factor alone may lead to formation of leaky, thin-walled, immature vessels. Other growth factors are most probably needed to act upon these immature structures to create more robust vessels.
During implantation of foreign bodies, the foreign-body response is difficult to overcome, and thousands of biomaterials have been tested. Biomimicry (i.e., creating membranes whose chemical structure mimics natural cellular compounds) may diminish the response, but as of this writing, it has not been possible to create a stealth material that circumvents the ability of the mammalian surveillance systems to distinguish foreign from self.
Keywords: angiogenesis, biosensor, collagen, foreign body response, transforming growth factor beta
Introduction
Over the millennia, mammals have evolved a robust mechanism for dealing with foreign bodies. Thanks in part to the journals of war surgeons, it has been known for over one hundred years that retention of foreign bodies leads to the formation of a dense, hypocellular, collagen-rich capsule. This foreign-body capsule (FBC) is advantageous to the patient in many cases. For example, in a study of patients who retained lead bullet fragments, plumbism (clinical lead poisoning) was very rarely found.1 The development of a capsule that is impermeable to many compounds likely provided an evolutionary advantage. Mutations of wound healing that favored formation of an impermeable capsule would tend to increase the probability of survival after penetrating injuries, by minimizing exposure to toxic compounds, bacterial toxins, or allergenic compounds.
In recent decades, there has been an explosion of bioengineered implantable devices such as joints, blood vessel substitutes, sensors, hernia mesh materials, heart valves, cosmetic and reconstructive implants, and artificial organs. For some of these devices, excessive ingrowth of collagen around the device is a liability. One example is excessive fibrotic scar growth around a cosmetic or reconstructive breast implant. Another example is the biosensor, which typically requires diffusion of an analyte into the sensing membranes for proper function. Sensors are quite sensitive to the effects of an FBC. Although a thin collagen sheath of 60–90 μm surrounding a breast implant would not be clinically discernible, such a structure that developed around a biosensor would retard inward diffusion of key analytes. For details of how an FBC retards entry of key analytes such as glucose, refer to the three-part publication by Sharkawy et al., in which transfer kinetics of compounds through an FBC are carefully quantified.2–4
Although there are a few biosensors that have been approved for short-term use, there remains a scarcity of accurate biosensors, in part because the foreign-body response creates signal drift. If the foreign-body response to implanted sensors could be avoided, many conditions could be better managed. One of the best examples is diabetes mellitus, whose prevalence, according to international monitoring agencies, is rising at an exponential rate. Diabetes is the most common cause of non-traumatic limb amputation, blindness in young and middle-aged adults, and end-stage kidney disease leading to the need for chronic dialysis or kidney transplantation.
Several studies have shown that well-controlled blood glucose levels (which very likely will be easier to achieve with continuous glucose sensors) markedly reduce the rate of these long-term complications in persons with types 1 and 2 diabetes.
Sensors might also be helpful to monitor drug levels, especially in view of the fact that adverse drug reactions are a common cause of morbidity and mortality. Some drugs, such as anticonvulsants and anti-cancer chemotherapeutic agents, have a narrow therapeutic window, i.e., a small increase in concentrations above the therapeutic range leads to toxicity. Other pertinent medications with narrow therapeutic windows include immunosuppressive drugs used for autoimmune conditions and for avoidance of organ rejection. One can imagine a scenario wherein a subcutaneous tacrolimus sensor could be used for the first 3–4 weeks after beginning this medication for a kidney transplant. A tacrolimus data stream during this period would greatly simplify dosing adjustment and enhance patient safety. One might also imagine the situation in which a severely injured person is brought to the emergency department and a combined lactic acid, oxygen, and pH sensor is inserted into the subcutaneous tissue or a vein to monitor whole-body tissue perfusion and oxygenation. If the alarms for rising lactic acid and falling pH are activated, the medical team would know immediately if there is a large drop in tissue perfusion most likely caused by blood loss.
Understanding the biological nature of the foreign-body response is necessary for development of longer-term and more accurate biosensing devices. I believe that solving the riddle of these biological questions will prove to be more difficult than designing and fabricating the sensor per se. This review (which also includes new data) will focus on the foreign-body response to subcutaneously-implanted devices.
Biofouling: the Early Foreign Body Response
Approximately 15 years ago, Tang and Eaton studied the early foreign-body response in mice that could not synthesize complement and mice that could not synthesize immunoglobulin G. They found that such animals nonetheless retained the ability to surround the implanted material with a layer of phagocytes (neutrophils and macrophages). In contrast, they found that animals with very low levels of fibrinogen were unable to mount an inflammatory response to the implanted material unless they were injected with fibrinogen or the material was coated with fibrinogen. Similarly, implants coated with hypofibrinogenemic plasma attracted very few phagocytes. The authors thus concluded that the acute inflammatory response to an implanted polymer was largely triggered by fibrinogen.5 They also provided some data suggesting that other proteins, such as albumin, could “passivate” this foreign-body response by impairing the ability of fibrinogen to bind to the material. The same group later reported that a specific peptide region of fibrinogen, the gamma 190-202 region, was responsible for binding to macrophages via the integrin Mac-1.6 Another group (Kvist et al.) verified that fibrinogen and fibrinogen fragments played an important role in recruiting inflammatory cells to the site of a foreign-body implant. This group also obtained evidence to suggest that tumor necrosis factor α (TNFα) played a role in this early protein fouling.7
The Tang/Eaton group also found that histamine release by mast cells is important to this early-phase foreign-body reaction. Thus, when the mast cell is inhibited in animals and humans by common histamine blockers used for treating gastrointestinal acid disorders, the degree of phagocyte recruitment is decreased.8,9
The concept of passivating the surface of implanted surfaces was also explored by Yung et al. They found that adhesion of neutrophils could be largely blocked by high molecular weight kininogen, whether the substrate was glass10 or polyurethane.11 A group from University of Washington led by Thomas Horbett, working with our research group, also addressed this issue. This team found that retention of the passivating protein was a crucial element in terms of robustly blocking fibrinogen adsorption. For example, blood plasma is capable of displacing many proteins that may initially be capable of preventing the binding of fibrinogen and macrophages to implanted surfaces. Of many potentially passivating proteins tested, hemoglobin had the greatest retention to the polyurethane before and after exposure to blood plasma. However, there were several interesting findings that make the situation less straightforward than originally thought. First, when surfaces exposed to hemoglobin were incubated with human monocytes, rather than with plasma alone, more of the hemoglobin was displaced. This finding suggests that there is an active method (e.g., release of proteases) by which monocytes (which become tissue macrophages) remove potentially passivating proteins. In view of this effect of monocytes, it was not surprising that when our group implanted the hemoglobin-coated materials in rats, the hemoglobin, as measured by radioiodine binding, was rapidly displaced.12 The concept of passivating proteins continues to hold promise, but only if the protein can avoid being displaced or degraded.
There are several caveats that complicate the fibrinogen-macrophage hypothesis of early protein fouling. For example, Horbett and Shen obtained evidence that when it comes to monocyte binding to artificial surfaces, not all surfaces are created equal. For example, monocyte activation (the tendency to form giant cells) was lower upon exposure to tissue-culture polystyrene than to untreated polystyrene.13 There have also been a series of studies using polyethylene oxide-like compounds that are known to be hydrophilic and can be deposited by plasma deposition. These compounds are known to be ultra-low fouling, which means, among other effects, they markedly inhibit the binding of fibrinogen to the implant.14 We and others have shown that when studied in vivo, the presence of PEO-type coatings do in fact reduce the density of the FBC; however, such coatings are not capable of blocking foreign capsule formation altogether.15 It is clear that the biology of the foreign-body response is very complex and that blockade of a single step will probably not be sufficient to block foreign-body encapsulation.
Macrophages, Cytokines, and Fibrosis
In terms of the foreign-body response, the key role of the macrophage is undisputed. Macrophages originate from circulating monocytes, which in turn derive from stem cells in the bone marrow. Current belief suggests that tissue macrophages are on constant surveillance duty to detect foreign materials and foreign invaders. In vitro, macrophages can be observed to engulf microbes and foreign particles. They also have powerful lysosomes that are capable of attacking the engulfed compounds by release of reactive oxygen species, enzymes, and compounds similar to household bleach (hypochlorite).
The macrophage is a complex cell and can secrete a large number of cytokines, which are small protein molecules that mediate immunity, inflammation, and other actions. Macrophages, when activated in the tissue, undergo fusion and form multinucleated giant cells, a process now known to be stimulated by the expression of vitronectin.16 Generally, cytokines act over short distances and short time spans. Specific cytokines include monokines (cytokines made by monocytes), chemokines (cytokines with chemotactic activities), and interleukins (cytokines made by one leukocyte that act on other leukocytes). Other cytokines include interferons, which inhibit viral replication in infected cells.
Over many years, the Anderson group at Case Western Reserve University has performed a series of elegant investigations on the role of the macrophage and its expressed products that pertain to the foreign-body response. They have found that proteomics and protein arrays are helpful in their studies of macrophage products. This group published a study in which the time course of macrophage function after exposure to biomaterials was investigated. Initially, the cells secreted pro-inflammatory interleukin 1 beta (IL-1β) and IL-6, but as time progressed, they expressed more of the anti-inflammatory IL-10. Furthermore, as the inflammatory response diminished, there was a decline in the chemoattractant compound IL-8.17
Transforming growth factor β (TGFβ), especially isoform 1, is expressed by macrophages and many other cells. The fact that macrophages and giant cells are a source of TGFβ was demonstrated convincingly by Hernandez-Pando et al. This group found that injected nitrocellulose particles initially caused the giant cells to express IL-1α, TNFα, and TGFβ. Of these, TGFβ was the most persistently expressed cytokine over time and its expression was associated with extensive chronic fibrosis.18 TGFβ is known to promote fibrosis in many cells and organs, including the lungs,19,20 kidneys,21 liver (cirrhosis),22,23 heart,24 and skin and subcutaneous structures.25
TGFβ is a cytokine that is generated from activation of a latent form of TGF. Once activated, it has a great many effects, including immunologic functions. Many of its effects are mediated by activation (phosphorylation) of the Smad series of intracellular proteins. Smad2 and Smad3 are thought to mediate many of TGFβ's profibrotic effects,19 including those in the skin. For example, Smad3-deficient knockout mice have diminished lung fibrosis after exposure to bleomycin, a toxic compound known to stimulate fibrosis via a TGFβ pathway.20 There are two receptors for TGFβ: the TGFβ receptor II, a transmembrane ligand receptor with serine and threonine phosphorylative activity, and the TGFβ receptor I. The latter receptor is only activated after receptor II is activated.
The key cell that responds to TGFβ and the profibrotic Smads is the myofibroblast. The myofibroblast is an activated fibroblast whose endogenous actin renders it contractile. There are many signals including other growth factors and angiogenic factors that activate myofibroblasts, as reviewed in an excellent article by Wynn.26 Myofibroblasts, which synthesize procollagen, can be identified in tissue by actin staining. The procollagen molecules are formed into a helical structure within the cell, then become crosslinked, to increase strength, after secretion into the extracellular space.
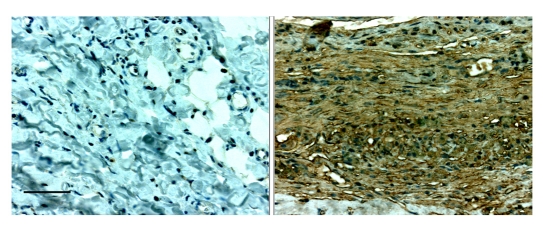
After observing fibrotic encapsulation of subcutaneously-implanted glucose sensors, our group collaborated with Drs. Allen G. Li and Xiao-Jing Wang to explore the role of TGFβ and other cytokines during foreign-body encapsulation. We implanted mock sensors (with polyurethane coats) in rats and excised surrounding tissue samples on day 7, 21, and 48–55 after implantation. The most abundant TGFβ isoform in foreign-body tissue samples was TGFβ1, which was expressed minimally in control tissue. The expression of both TGFβ1 RNA and protein was significantly increased in FBC tissues at all time points, with the highest level on day 7. We also found a very high number of cells that stained for phosphorylated Smad2, an indication of activated TGFβ signaling that paralleled the expression of TGFβ. Type I collagen, which is the most prominent downstream target of TGFβ in fibrotic conditions, was found in abundance in the FBC by Masson's trichrome staining, especially at the later time points. These results strongly suggested that TGFβ plays an important role in the formation of FBCs around subcutaneously implanted devices.27 In a study with results similar to ours, TGFβ1 and TGFβ2 were found in high concentrations in FBCs that surround breast implants, though not in normal breast tissue.28 For a photomicrograph of intense TGFβ1 staining in rat subcutaneous foreign-body capsular tissue using immunohistochemistry, see Figure 1.
Figure 1.
Immunohistochemistry of TGFβ-1 on day 21 of subcutaneous control tissue (left) vs. the foreign-body capsule (right) in a Sprague-Dawley rat. The final stain in the immunochistochemical technique is diaminobenzidine (brown). Hematoxylin was used as a counterstain. Note the much greater presence of the brown TGFβ stain in the foreign capsule tissue. The top margin of the tissue shown in the right panel bordered the implant. The bar in the first panel represents 100 μm for both sections.
It is important to note that many of TGFβ's effects may be mediated by other downstream factors, for example, connective tissue growth factor (CTGF). CTGF is a cysteine-rich, heparin-binding protein that is stimulated by TGFβ. It has been hypothesized that CTGF may be more specific to a profibrotic action as compared to TGFβ, which controls a multitude of biological processes. A strong role of CTGF in foreign-body fibrosis around breast implants in rats was recently suggested by the finding of reduced fibrosis in animals that had been treated with a single injection of a CTGF antisense oligonucleotide or a TGF antisense oligonucleotide.29
Knowing that TGFβ was highly expressed in FBC tissue, our group also became interested in the role of CTGF. Because IL-13, a product of Th2 cells, had been found to stimulate collagen formation in skin fibroblasts30,31 and because its effect can be independent of a TGFβ's effect,32 we also examined IL-13 expression in FBC tissue. As before, we evaluated different stages of the foreign-body response. Using quantitative real-time PCR and immunofluorescence, we found that IL-13 was highly expressed at all time points, with the greatest expression at day 21. The IL-13 expression was accompanied by an increased presence of T cells at all time points. CTGF was also found to be much more highly expressed in foreign-body tissue than in controls. Two extracellular matrix proteins, collagen and decorin, were highly expressed at the middle (3-week) and later (7-week) stages. Given the increased expression of IL-13 and CTGF in foreign-body tissue, and their roles in other fibrotic disorders, we concluded that these cytokines may well contribute to the formation of the FBC.33
Recent Data on the Use of a Neutralizing Antibody to Transforming Growth Factor β
In a previously-unpublished study, we explored the possibility of inhibiting the effect of TGFβ with a neutralizing antibody. Using Yucatan mini pigs, 1 mg of TGFβ neutralizing antibody (AB-101-NA, R&D Systems, Minneapolis, MN) was delivered subcutaneously onto the surface of a planar biosensor over the course of 28 days with the use of miniature osmotic pumps (Alzet, Durect Corp., Cupertino, CA). On day 28, the sensor and surrounding capsule tissue were removed from the animal. Tissue samples were taken near the antibody release point (0 cm), at an intermediate distance (1 cm) and from a distant point (1.7 cm) (positions termed “near”, “intermediate”, and “far”), and fixed in 10% zinc formalin.
Immunohistochemical staining for phosphorylated Smad2 (pSmad2) was conducted on sections of each sample. The number of cells staining for active pSmad2 in an 80 × 80 μm area was counted. Each 80 × 80 μm area was adjacent to the surface of the tissue contacting the biosensor. We hypothesized that the antibody-treated sites would demonstrate a suppression of pSmad2 staining, especially in the “near” tissue samples.
The findings suggested that despite a substantial release rate of the TGF-neutralizing antibody, pSmad2 was not inhibited. The number of cells stained for active pSmad2 in near tissue treated with TGFβ neutralizing antibody did not differ from near tissue treated with saline. Significantly fewer cells with active pSmad2 staining were found in antibody treated tissue from intermediate sensor locations as compared to tissue treated with saline from intermediate locations (20.4 ± 2.6 vs. 34.0 ± 4.1 [mean ± standard error of the mean]; p < .05). This finding is consistent with the TGFβ antibody reducing the effect of the cytokine. However, a greater amount of active pSmad staining was observed in antibody-treated far-tissue samples (26.4 ± 4.2) vs. saline (14.8 ± 4.8; p < .05), and this finding creates some uncertainty in interpreting the data.
These results indicate that treatment with TGFβ neutralizing antibody over 28 days did not have a consistent effect on pSmad2 production in the foreign-body response to a subcutaneously implanted biosensor. Despite the relatively large dose of the neutralizing antibody, these results suggest that the antibody was unable to block the major intracellular effector of TGFβ's action. In such a study, there is always a chance that the antibody is proteolyzed or, because of spatial geometry, does not stay at the intended site long enough to carry out its intended action. Since TGFβ's action was not consistently blocked, we cannot conclude whether blockade of TGFβ can inhibit foreign-body encapsulation. Studies are also underway to use corticosteroids (which have been shown to block the effect of TGFβ34) in an attempt to block foreign-body fibrosis. An alternative method of blocking fibrosis would be to inhibit the more specific growth factor, CTGF.
Angiogenic Growth Factors and Porous Materials
Working in the field of surgical oncology, Judah Folkman pioneered the field of angiogenesis research in the early 1970s.35 Some years later, Brauker and colleagues quantified the microvessels in foreign-capsule tissue surrounding many types of biomaterials implanted in animals. These investigators hypothesized that formation of new capillaries in the region immediately adjacent to biomaterial implants might well be able to sustain the delivery of analytes to the implanted device. More specifically, they found that certain types of porous materials, such as expanded polytetrafluoroethylene, when fabricated with a specific pore size, caused a prolific growth of new vessels into the region adjacent to the implant.36 Sharkawy and colleagues also addressed the relationship of pore structure to angiogenesis and came to a different conclusion: the optimal pore diameter was 60 μm, substantially larger than the optimal pore size according to Brauker et al.3 Using immunohistochemical and standard techniques, our group found that subcutaneously implanted porous membranes of various pore sizes (polyvinyl alcohol sponge and expanded polytetrafluoroethylene) led to increased angiogenesis, as compared to solid, smooth membranes.15
There has been a plethora of studies that have addressed the microgeometry of materials and its effect on the foreign-body response. Though the variety of such studies is great, one can safely draw several conclusions, (1) many cells bind better to porous surfaces than to smooth ones and (2) the number of newly-formed capillaries is greater on most porous or textured surfaces as compared to smooth surfaces. It is difficult to conclude that such effects promote “biocompatibility”, since the meaning of this word is so broad (and is defined differently, depending on one's goals). Nonetheless, one can conclude that cellular and tissue ingrowth tends to be more prolific on porous surfaces. This finding has found its way into the daily practice of clinical medicine. For example, there is a wide usage of porous mesh to close tissue defects, such as those encountered during hernia repairs. These materials facilitate tissue ingrowth into the implanted material. Porous polymer implants are also used to close defects in the head and neck region.37 Recent data suggest that the outcome is best when large pores with sparse lightweight material are used.38 In addition, it now appears that a negative electrical charge facilitates vessel ingrowth into fibro-porous meshes.39 Porous vascular grafts are widely used to create high-flow fistulas required for repeated venous access (e.g., hemodialysis). Over time, such grafts become endothelialized with endogenous endothelial cells.
Stimulated in part by the fields of oncology and cardiology, there has also been a tremendous interest in the use of angiogenic growth factors to maintain communication between implanted devices and the analytes they purport to measure. While most of the interest has centered around vascular endothelial growth factor (VEGF) and other known growth factors, some compounds not previously thought to be angiogenic (e.g., calcitonin) are also now known to stimulate angiogenesis.40 There has been an explosion of research in methods of delivering angiogenic growth factors, which can be accomplished successfully in vivo in several ways, including the use of biodegradable polymer such as poly lactide-glycolic acid (PLGA)41 or related elastomeric compounds.42 In such research, it is critical to minimize release into systemic circulation and to quantify the kinetics of growth factor release, as performed successfully by Ennett et al.43
Our group studied the effect of VEGF release via miniature osmotic pumps in animals with functioning subcutaneously implanted glucose sensor arrays. The fact that different sensing units of the sensing array were different distances from the source of the VEGF allowed us to ascertain the spatial effects of local VEGF release. We found that VEGF caused a definite increase in capillary formation in sensing units that were within 15 mm of the release site; however for units further away, there was no discernible increase in capillaries in the FBC.44 In a separate study, we found that VEGF-induced angiogenesis had beneficial effects on sensor function but that after approximately 6–8 weeks, the sensors eventually failed due to fibrosis.45 Thus, our belief is that though VEGF temporarily improves sensor function, its effect is unlikely to last more than two months.
The role of VEGF in mammalian wound-healing appears to be stimulation of vascular tube formation soon after injury. Other growth factors, such as basic fibroblast growth factor (FGF) and platelet-derived growth factor (PDGF), appear to exert their major effects several days later, and these factors mature the early vascular tubes into more robust vessels. In view of these normal physiologic effects, there has been an interest in releasing combinations of growth factors, such as VEGF and FGF-2.46 In a very compelling study, Mooney and others achieved the initial delivery of a fast-acting angiogenic factor followed by delivery of a vessel-maturation factor in animals who had myocardial infarctions created intentionally.47 The investigators were able to initially release VEGF followed by PDGF from alginate hydrogel drug delivery systems. They found that the sequential release of the factors led to better healing of the myocardial injury, and better preservation of cardiac function than either compound alone.47 A group at University of Connecticut also examined the effects of administering two compounds on wound healing and fibrosis; they found that administration of dexamethasone and VEGF from a composite of polyvinyl alcohol and PLGA was successful in reducing fibrosis and enhancing angiogenesis in rats.48
There are some recent reports in which mimics of VEGF have been found or developed. These compounds can either simulate the effect of VEGF to cause angiogenesis49 or, in the study of Goncalves, can bind to the VEGF receptor and inhibit formation of capillary tubes.50
Not surprisingly, since porous materials and angiogenic growth factors both promote tissue vascularization, some investigators have attempted to combine the two approaches. Elcin and Elcin impregnated biodegradable PLGA sponges with VEGF and observed the angiogenic response to such implants in rats. They found that the VEGF/sponge approach was much more effective than control sponges or VEGF injections alone in creating new vessel formation. They also found a modest increase in vessel formation during week one and a marked increase in formation during weeks two and three.51
Inhibiting the Foreign-body Response with Corticosteroids
It has been known since the 1950's that corticosteroids have a potent effect in blocking immune function and fibrosis. More recently, their specific anti-inflammatory mechanisms have been investigated. Miller et al. found that levels of lung TGFβ-1 were significantly reduced in asthmatic mice treated with systemic corticosteroids, and this finding was associated with a significant decrease in the number of myofibroblasts.34 In view of the fact that myofibroblasts are the cells that synthesize collagen, this finding suggests a mechanism for the well-known effect of corticosteroids to impair collagen formation in postoperative wounds.
Several groups, including ours, have investigated the effect of corticosteroids on prolonging the functional life of implanted biosensors. During release of dexamethasone from a silicone gel in dogs implanted with glucose sensors, we found that there was a strong trend (though not quite statistically significant) for sensors in treated animals to function longer.52 Patil et al. found that PLGA microspheres loaded with dexamethasone were successful in inhibiting the negative tissue reaction at the sensor-tissue interface.53 The groups from Duke University54 and University of South Florida55 also found promising, though not definitive, results suggesting a possible role for corticosteroids to improve the functional life of implanted biosensors.
One caveat to be aware of is the tendency of corticosteroids to inhibit angiogenesis, a finding consistent among several different tissues.56–58 Given the multiple ill effects of systemic corticosteroids, our group's belief is that these compounds hold great promise, though the dosing regimen (and avoidance of systemic spillover) will be extremely important. Given that corticosteroids appear to suppress the effect of TGFβ and the synthesis of collagen by myofibroblasts, this class of hormones holds promise for inhibiting the fibrotic FBC.
Creation of Engineered Biomaterials by Mimicking Nature
Final mention should be made of the use of biomimicry in this field of biomaterials. For example, there has been tremendous interest in the placement of a phospholipid membrane over implanted devices. Such a concept is designed to mimic the mammalian phospholipid cell membrane with the philosophy that mammals will not fight a compound that they “see” continuously in themselves. Most of the work in this area has been with a phospholipid known as phosphorylcholine (PC). This compound has shown some promise in several areas. First, PC-coated vascular devices, in addition to reducing neutrophil binding,59 appear to show less platelet activation and reduced thrombin formation in vitro.60 An in vivo study of the intramuscular implantation of PC-coated polymers in rabbits found a lower degree of fibrosis at 13 weeks, though there was very little difference from controls at 4 weeks.61 A method in which the PC coating is bonded to a device surface using UV-crosslinking appeared to show more robust adhesion than simple physical coating.62 And finally, though sensor response data were not given, a PC-coated glucose sensor was found to be safe in terms of cytotoxicity and biocompatibility tests.63
Other biomimicry concepts include the use of dopamine-like materials that simulate the powerful mussel adhesive.64,65 Such materials appear to be capable of adherently coating many diverse substrates, including polymers and metals, in a conformal fashion. In addition, chitosan, the predominant material from crustacean shells, appears to have many favorable characteristics, including hemostasis,66 antibacterial activity,67 and the ability to serve as a drug delivery material.68
Conclusions
The foreign-body response to implanted biomaterials is a very complex series of biochemical events. Initially, there is biofouling of the implant, characterized by protein sheathing, probably initiated by fibrinogen binding. Macrophages bind to specific sites on the protein coat and initiate a series of steps, including formation of multinucleated giant cells. Macrophages also release TGFβ and other inflammatory cytokines. These cytokines transform quiescent fibroblasts into myofibroblasts, which synthesize procollagen via activation of Smad mediators. After crosslinking, the mature collagen and other extracellular matrix proteins contribute to formation of a hypocellular dense fibrous capsule that is hypopermeable to many compounds. Porous substrates and angiogenic growth factors can stimulate formation of microvessels, which, to some extent, can maintain analyte delivery to implanted sensors. It is probably also necessary for other growth factors to act upon immature vessels to mature the fragile microvessels into more robust vessels.
During implantation of foreign bodies, the foreign-body response is difficult to overcome, and thousands of biomaterials have been tested without discovery of a true stealth material. Biomimicry (e.g., coating a material with phospholipids that mimic cell membranes) may diminish the intensity of the reaction.
Abbreviations
- CTGF
connective tissue growth factor
- FGF
fibroblast growth factor
- FBC
foreign-body capsule
- IL
interleukin
- PC
phosphorylcholine
- PCR
polymerase chain recation
- PDGF
platelet-derived growth factor
- PLGA
poly lactide-glycolic acid
- TGFβ
transforming growth factor β
- TNFα
tumor necrosis factor α
- VEGF
vascular endothelial growth factor
Funding
Supported in part by the Juvenile Diabetes Research Foundation and the Good Samaritan Foundation (Portland, OR).
References
- 1.Scuderi GJ, Vaccaro AR, Fitzhenry LN, Greenberg S, Eismont F. Long-term clinical manifestations of retained bullet fragments within the intervertebral disk space. J Spinal Disord Tech. 2004;17(2):108–111. doi: 10.1097/00024720-200404000-00005. [DOI] [PubMed] [Google Scholar]
- 2.Sharkawy AA, Klitzman B, Truskey GA, Reichert WM. Engineering the tissue which encapsulates subcutaneous implants. I. Diffusion properties. J Biomed Mater Res. 1997;37(3):401–412. doi: 10.1002/(sici)1097-4636(19971205)37:3<401::aid-jbm11>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 3.Sharkawy AA, Klitzman B, Truskey GA, Reichert WM. Engineering the tissue which encapsulates subcutaneous implants. III. Effective tissue response times. J Biomed Mater Res. 1998;40(4):598–605. doi: 10.1002/(sici)1097-4636(19980615)40:4<598::aid-jbm11>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 4.Sharkawy AA, Klitzman B, Truskey GA, Reichert WM. Engineering the tissue which encapsulates subcutaneous implants. II. Plasma-tissue exchange properties. J Biomed Mater Res. 1998;40(4):586–597. doi: 10.1002/(sici)1097-4636(19980615)40:4<586::aid-jbm10>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 5.Tang L, Eaton JW. Fibrin(ogen) mediates acute inflammatory responses to biomaterials. J Exp Med. 1993;178(6):2147–2156. doi: 10.1084/jem.178.6.2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tang L, Ugarova TP, Plow EF, Eaton JW. Molecular determinants of acute inflammatory responses to biomaterials. J Clin Invest. 1996;97(5):1329–1334. doi: 10.1172/JCI118549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kvist PH, Iburg T, Bielecki M, Gerstenberg M, Buch-Rasmussen T, Hasselager E, Jensen HE. Biocompatibility of electrochemical glucose sensors implanted in the subcutis of pigs. Diabetes Technol Ther. 2006;8(4):463–475. doi: 10.1089/dia.2006.8.463. [DOI] [PubMed] [Google Scholar]
- 8.Tang L, Jennings TA, Eaton JW. Mast cells mediate acute inflammatory responses to implanted biomaterials. Proc Natl Acad Sci USA. 1998;95(15):8841–8846. doi: 10.1073/pnas.95.15.8841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zdolsek J, Eaton JW, Tang L. Histamine release and fibrinogen adsorption mediate acute inflammatory responses to biomaterial implants in humans. J Transl Med. 2007;(5):31. doi: 10.1186/1479-5876-5-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yung LY, Lim F, Khan MM, Kunapuli SP, Rick L, Colman RW, Cooper SL. Neutrophil adhesion on surfaces preadsorbed with high molecular weight kininogen under well-defined flow conditions. Immunopharmacology. 1996;32(1–3):19–23. doi: 10.1016/0162-3109(96)00003-3. [DOI] [PubMed] [Google Scholar]
- 11.Yung LY, Colman RW, Cooper SL. Neutrophil adhesion on polyurethanes preadsorbed with high molecular weight kininogen. Blood. 1999;94(8):2716–2724. [PubMed] [Google Scholar]
- 12.Geelhood SJ, Horbett TA, Ward WK, Wood MD, Quinn MJ. Passivating protein coatings for implantable glucose sensors: evaluation of protein retention. J Biomed Mater Res B Appl Biomater. 2007;81(1):251–260. doi: 10.1002/jbm.b.30660. [DOI] [PubMed] [Google Scholar]
- 13.Shen M, Garcia I, Maier RV, Horbett TA. Effects of adsorbed proteins and surface chemistry on foreign body giant cell formation, tumor necrosis factor alpha release and procoagulant activity of monocytes. J Biomed Mater Res A. 2004;70(4):533–541. doi: 10.1002/jbm.a.30069. [DOI] [PubMed] [Google Scholar]
- 14.Shen M, Pan YV, Wagner MS, Hauch KD, Castner DG, Ratner BD, Horbett TA. Inhibition of monocyte adhesion and fibrinogen adsorption on glow discharge plasma deposited tetraethylene glycol dimethyl ether. J Biomater Sci Polym Ed. 2001;12(9):961–978. doi: 10.1163/156856201753252507. [DOI] [PubMed] [Google Scholar]
- 15.Ward WK, Slobodzian EP, Tiekotter KL, Wood MD. The effect of microgeometry, implant thickness and polyurethane chemistry on the foreign body response to subcutaneous implants. Biomaterials. 2002;23(21):4185–4192. doi: 10.1016/s0142-9612(02)00160-6. [DOI] [PubMed] [Google Scholar]
- 16.McNally AK, Jones JA, Macewan SR, Colton E, Anderson JM. Vitronectin is a critical protein adhesion substrate for IL-4-induced foreign body giant cell formation. J Biomed Mater Res A. 2008;86(2):535–543. doi: 10.1002/jbm.a.31658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jones JA, Chang DT, Meyerson H, Colton E, Kwon IK, Matsuda T, Anderson JM. Proteomic analysis and quantification of cytokines and chemokines from biomaterial surface-adherent macrophages and foreign body giant cells. J Biomed Mater Res A. 2007;83(3):585–596. doi: 10.1002/jbm.a.31221. [DOI] [PubMed] [Google Scholar]
- 18.Hernandez-Pando R, Bornstein QL, Aguilar Leon D, Orozco EH, Madrigal VK, Martinez Cordero E. Inflammatory cytokine production by immunological and foreign body multinucleated giant cells. Immunology. 2000;100(3):352–358. doi: 10.1046/j.1365-2567.2000.00025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Flanders KC. Smad3 as a mediator of the fibrotic response. Int J Exp Pathol. 2004;85(2):47–64. doi: 10.1111/j.0959-9673.2004.00377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gu L, Zhu YJ, Yang X, Guo ZJ, Xu WB, Tian XL. Effect of TGF-beta/Smad signaling pathway on lung myofibroblast differentiation. Acta Pharmacol Sin. 2007;28(3):382–391. doi: 10.1111/j.1745-7254.2007.00468.x. [DOI] [PubMed] [Google Scholar]
- 21.Liu Y. Renal fibrosis: new insights into the pathogenesis and therapeutics. Kidney Int. 2006;69(2):213–217. doi: 10.1038/sj.ki.5000054. [DOI] [PubMed] [Google Scholar]
- 22.Gnainsky Y, Kushnirsky Z, Bilu G, Hagai Y, Genina O, Volpin H, Bruck R, Spira G, Nagler A, Kawada N, Yoshizato K, Reinhardt DP, Libermann TA, Pines M. Gene expression during chemically induced liver fibrosis: effect of halofuginone on TGF-beta signaling. Cell Tissue Res. 2007;328(1):153–166. doi: 10.1007/s00441-006-0330-1. [DOI] [PubMed] [Google Scholar]
- 23.de Gouville AC, Huet S. Inhibition of ALK5 as a new approach to treat liver fibrotic diseases. Drug News Perspect. 2006;19(2):85–90. doi: 10.1358/dnp.2006.19.2.977444. [DOI] [PubMed] [Google Scholar]
- 24.Khan R, Sheppard R. Fibrosis in heart disease: understanding the role of transforming growth factor-beta in cardiomyopathy, valvular disease and arrhythmia. Immunology. 2006;118(1):10–24. doi: 10.1111/j.1365-2567.2006.02336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ihn H. Scleroderma, fibroblasts, signaling, and excessive extracellular matrix. Curr Rheumatol Rep. 2005;7(2):156–162. doi: 10.1007/s11926-005-0069-9. [DOI] [PubMed] [Google Scholar]
- 26.Wynn T. Cellular and molecular mechanisms of fibrosis. J Pathol. 2008;214(2):199–210. doi: 10.1002/path.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li AG, Quinn MJ, Siddiqui Y, Wood MD, Federiuk IF, Duman HM, Ward WK. Elevation of transforming growth factor beta (TGFbeta) and its downstream mediators in subcutaneous foreign body capsule tissue. J Biomed Mater Res A. 2007;82(2):498–508. doi: 10.1002/jbm.a.31168. [DOI] [PubMed] [Google Scholar]
- 28.Kuhn A, Singh S, Smith PD, Ko F, Falcone R, Lyle WG, Maggi SP, Wells KE, Robson MC. Periprosthetic breast capsules contain the fibrogenic cytokines TGF-beta1 and TGF-beta2, suggesting possible new treatment approaches. Ann Plast Surg. 2000;44(4):387–391. doi: 10.1097/00000637-200044040-00006. [DOI] [PubMed] [Google Scholar]
- 29.Mazaheri MK, Schultz GS, Blalock TD, Caffee HH, Chin GA. Role of connective tissue growth factor in breast implant elastomer capsular formation. Ann Plast Surg. 2003;50(3):263–268. doi: 10.1097/01.sap.0000046781.75625.69. [DOI] [PubMed] [Google Scholar]
- 30.Oriente A, Fedarko NS, Pacocha SE, Huang SK, Lichtenstein LM, Essayan DM. Interleukin-13 modulates collagen homeostasis in human skin and keloid fibroblasts. J Pharmacol Exp Ther. 2000;292(3):988–994. [PubMed] [Google Scholar]
- 31.Jinnin M, Ihn H, Yamane K, Tamaki K. Interleukin-13 stimulates the transcription of the human alpha2(I) collagen gene in human dermal fibroblasts. J Biol Chem. 2004;279(40):41783–41791. doi: 10.1074/jbc.M406951200. [DOI] [PubMed] [Google Scholar]
- 32.Kaviratne M, Hesse M, Leusink M, Cheever AW, Davies SJ, McKerrow JH, Wakefield LM, Letterio JJ, Wynn TA. IL-13 activates a mechanism of tissue fibrosis that is completely TGF-beta independent. J Immunol. 2004;173(6):4020–4029. doi: 10.4049/jimmunol.173.6.4020. [DOI] [PubMed] [Google Scholar]
- 33.Ward WK, Li AG, Siddiqui Y, Federiuk IF, Wang XJ. Increased expression of interleukin-13 and connective tissue growth factor, and their potential roles during foreign body encapsulation of subcutaneous implants. J Biomaterials Sci Polym Ed. 2008;19(8):1065–1072. doi: 10.1163/156856208784909408. [DOI] [PubMed] [Google Scholar]
- 34.Miller M, Cho JY, McElwain K, McElwain S, Shim JY, Manni M, Baek JS, Broide DH. Corticosteroids prevent myofibroblast accumulation and airway remodeling in mice. Am J Physiol Lung Cell Mol Physiol. 2006;290(1):L162–L169. doi: 10.1152/ajplung.00252.2005. [DOI] [PubMed] [Google Scholar]
- 35.Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285(21):1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 36.Brauker JH, Carr-Brendel VE, Martinson LA, Crudele J, Johnston WD, Johnson RC. Neovascularization of synthetic membranes directed by membrane microarchitecture. J Biomed Mater Res. 1995;29(12):1517–1524. doi: 10.1002/jbm.820291208. [DOI] [PubMed] [Google Scholar]
- 37.Conze J, Rosch R, Klinge U, Weiss C, Anurov M, Titkowa S, Oettinger A, Schumpelick V. Polypropylene in the intra-abdominal position: influence of pore size and surface area. Hernia. 2004;8(4):365–372. doi: 10.1007/s10029-004-0268-8. [DOI] [PubMed] [Google Scholar]
- 38.Klosterhalfen B, Junge K, Klinge U. The lightweight and large porous mesh concept for hernia repair. Expert Rev Med Devices. 2005;2(1):103–117. doi: 10.1586/17434440.2.1.103. [DOI] [PubMed] [Google Scholar]
- 39.Sanders JE, Lamont SE, Karchin A, Golledge SL, Ratner BD. Fibro-porous meshes made from polyurethane micro-fibers: effects of surface charge on tissue response. Biomaterials. 2005;26(7):813–818. doi: 10.1016/j.biomaterials.2004.03.030. [DOI] [PubMed] [Google Scholar]
- 40.Chigurupati S, Kulkarni T, Thomas S, Shah G. Calcitonin stimulates multiple stages of angiogenesis by directly acting on endothelial cells. Cancer Res. 2005;65(18):8519–8529. doi: 10.1158/0008-5472.CAN-05-0848. [DOI] [PubMed] [Google Scholar]
- 41.Sun Q, Chen RR, Shen Y, Mooney DJ, Rajagopalan S, Grossman PM. Sustained vascular endothelial growth factor delivery enhances angiogenesis and perfusion in ischemic hind limb. Pharm Res. 2005;22(7):1110–1116. doi: 10.1007/s11095-005-5644-2. [DOI] [PubMed] [Google Scholar]
- 42.Gu F, Neufeld R, Amsden B. Sustained release of bioactive therapeutic proteins from a biodegradable elastomeric device. J Control Release. 2007;117(1):80–89. doi: 10.1016/j.jconrel.2006.09.077. [DOI] [PubMed] [Google Scholar]
- 43.Ennett AB, Kaigler D, Mooney DJ. Temporally regulated delivery of VEGF in vitro and in vivo. J Biomed Mater Res A. 2006;79(1):176–184. doi: 10.1002/jbm.a.30771. [DOI] [PubMed] [Google Scholar]
- 44.Ward WK, Quinn MJ, Wood MD, Tiekotter KL, Pidikiti S, Gallagher JA. Vascularizing the tissue surrounding a model biosensor: how localized is the effect of a subcutaneous infusion of vascular endothelial growth factor (VEGF)? Biosens Bioelectron. 2003;19(3):155–163. doi: 10.1016/s0956-5663(03)00180-5. [DOI] [PubMed] [Google Scholar]
- 45.Ward WK, Wood MD, Casey HM, Quinn MJ, Federiuk IF. The effect of local subcutaneous delivery of vascular endothelial growth factor on the function of a chronically implanted amperometric glucose sensor. Diabetes Technol Ther. 2004;6(2):137–145. doi: 10.1089/152091504773731320. [DOI] [PubMed] [Google Scholar]
- 46.Nillesen ST, Geutjes PJ, Wismans R, Schalkwijk J, Daamen WF, van Kuppevelt TH. Increased angiogenesis in acellular scaffolds by combined release of FGF2 and VEGF. J Control Release. 2006;116(2):e88–e90. doi: 10.1016/j.jconrel.2006.09.065. [DOI] [PubMed] [Google Scholar]
- 47.Hao X, Silva EA, Månsson-Broberg A, Grinnemo KH, Siddiqui AJ, Dellgren G, Wärdell E, Brodin LA, Mooney DJ, Sylvén C. Angiogenic effects of sequential release of VEGF-A165 and PDGF-BB with alginate hydrogels after myocardial infarction. Cardiovasc Res. 2007;75(1):178–185. doi: 10.1016/j.cardiores.2007.03.028. [DOI] [PubMed] [Google Scholar]
- 48.Patil SD, Papadmitrakopoulos F, Burgess DJ. Concurrent delivery of dexamethasone and VEGF for localized inflammation control and angiogenesis. J Control Release. 2007;117(1):68–79. doi: 10.1016/j.jconrel.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 49.Brown MC, Calvete JJ, Staniszewska I, Walsh EM, Perez-Liz G, Del Valle L, Lazarovici P, Marcinkiewicz C. VEGF-related protein isolated from Vipera palestinae venom, promotes angiogenesis. Growth Factors. 2007;25(2):108–117. doi: 10.1080/08977190701532385. [DOI] [PubMed] [Google Scholar]
- 50.Goncalves V, Gautier B, Coric P, Bouaziz S, Lenoir C, Garbay C, Vidal M, Inguimbert N. Rational design, structure, and biological evaluation of cyclic peptides mimicking the vascular endothelial growth factor. J Med Chem. 2007;50(21):5135–5146. doi: 10.1021/jm0706970. [DOI] [PubMed] [Google Scholar]
- 51.Elcin AE, Elcin YM. Localized angiogenesis induced by human vascular endothelial growth factor-activated PLGA sponge. Tissue Eng. 2006;12(4):959–968. doi: 10.1089/ten.2006.12.959. [DOI] [PubMed] [Google Scholar]
- 52.Ward WK, Troupe JE. Assessment of chronically implanted subcutaneous glucose sensors in dogs: the effect of surrounding fluid masses. ASAIO J. 1999;45(6):555–561. doi: 10.1097/00002480-199911000-00009. [DOI] [PubMed] [Google Scholar]
- 53.Patil SD, Papadimitrakopoulos F, Burgess DJ. Dexamethasone-loaded poly(lactic-co-glycolic) acid microspheres/poly(vinyl alcohol) hydrogel composite coatings for inflammation control. Diabetes Technol Ther. 2004;6(6):887–897. doi: 10.1089/dia.2004.6.887. [DOI] [PubMed] [Google Scholar]
- 54.Norton LW, Koschwanez HE, Wisniewski NA, Klitzman B, Reichert WM. Vascular endothelial growth factor and dexamethasone release from nonfouling sensor coatings affect the foreign body response. J Biomed Mater Res A. 2007;81(4):858–869. doi: 10.1002/jbm.a.31088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ju YM, Yu B, Koob TJ, Moussy Y, Moussy F. A novel porous collagen scaffold around an implantable biosensor for improving biocompatibility. I. In vitro/in vivo stability of the scaffold and in vitro sensitivity of the glucose sensor with scaffold. J Biomed Mater Res A. 2007 Dec 17; doi: 10.1002/jbm.a.31756. Epub. [DOI] [PubMed] [Google Scholar]
- 56.Zuniga J, Fuenzalida M, Guerrero A, Illanes J, Dabancens A, Díaz E, Lemus D. Effects of steroidal and non steroidal drugs on the neovascularization response induced by tumoral TA3 supernatant on CAM from chick embryo. Biol Res. 2003;36(2):233–240. doi: 10.4067/s0716-97602003000200013. [DOI] [PubMed] [Google Scholar]
- 57.McNatt LG, Weimer L, Yanni J, Clark AF. Angiostatic activity of steroids in the chick embryo CAM and rabbit cornea models of neovascularization. J Ocul Pharmacol Ther. 1999;15(5):413–423. doi: 10.1089/jop.1999.15.413. [DOI] [PubMed] [Google Scholar]
- 58.Kasselman LJ, Kintner J, Sideris A, Pasnikowski E, Krellman JW, Shah S, Rudge JS, Yancopoulos GD, Wiegand SJ, Croll SD. Dexamethasone treatment and ICAM-1 deficiency impair VEGF-induced angiogenesis in adult brain. J Vasc Res. 2007;44(4):283–291. doi: 10.1159/000101450. [DOI] [PubMed] [Google Scholar]
- 59.Yung LY, Cooper SL. Neutrophil adhesion on phosphorylcholine-containing polyurethanes. Biomaterials. 1998;19(1–3):31–40. doi: 10.1016/s0142-9612(97)00220-2. [DOI] [PubMed] [Google Scholar]
- 60.van der Heiden AP, Willems GM, Lindhout T, Pijpers AP, Koole LH. Adsorption of proteins onto poly(ether urethane) with a phosphorylcholine moiety and influence of preadsorbed phospholipid. J Biomed Mater Res. 1998;40(2):195–203. doi: 10.1002/(sici)1097-4636(199805)40:2<195::aid-jbm4>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 61.Goreish HH, Lewis AL, Rose S, Lloyd AW. The effect of phosphorylcholine-coated materials on the inflammatory response and fibrous capsule formation: in vitro and in vivo observations. J Biomed Mater Res A. 2004;68(1):1–9. doi: 10.1002/jbm.a.10141. [DOI] [PubMed] [Google Scholar]
- 62.Kim HK, Kim K, Byun Y. Preparation of a chemically anchored phospholipid monolayer on an acrylated polymer substrate. Biomaterials. 2005;26(17):3435–3444. doi: 10.1016/j.biomaterials.2004.09.066. [DOI] [PubMed] [Google Scholar]
- 63.Mang A, Pill J, Gretz N, Kränzlin B, Buck H, Schoemaker M, Petrich W. Biocompatibility of an electrochemical sensor for continuous glucose monitoring in subcutaneous tissue. Diabetes Technol Ther. 2005;7(1):163–173. doi: 10.1089/dia.2005.7.163. [DOI] [PubMed] [Google Scholar]
- 64.Yang M, Yamauchi K, Kurokawa M, Asakura T. Design of silk-like biomaterials inspired by mussel-adhesive protein. Tissue Eng. 2007;13(12):2941–2947. doi: 10.1089/ten.2006.0448. [DOI] [PubMed] [Google Scholar]
- 65.Lee H, Dellatore SM, Miller WM, Messersmith PB. Mussel-inspired surface chemistry for multifunctional coatings. Science. 2007;318(5849):426–430. doi: 10.1126/science.1147241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Xie H, Khajanchee YS, Teach JS, Shaffer BS. Use of a chitosan-based hemostatic dressing in laparoscopic partial nephrectomy. J Biomed Mater Res B Appl Biomater. 2008;85(1):267–271. doi: 10.1002/jbm.b.30946. [DOI] [PubMed] [Google Scholar]
- 67.Belalia R, Grelier S, Benaissa M, Coma V. New bioactive biomaterials based on quaternized chitosan. J Agric Food Chem. 2008;56(5):1582–1588. doi: 10.1021/jf071717+. [DOI] [PubMed] [Google Scholar]
- 68.Kim SE, Park JH, Cho YW, Chung H, Jeong SY, Lee EB, Kwon IC. Porous chitosan scaffold containing microspheres loaded with transforming growth factor-beta1: implications for cartilage tissue engineering. J Control Release. 2003;91(3):365–374. doi: 10.1016/s0168-3659(03)00274-8. [DOI] [PubMed] [Google Scholar]