Abstract
The rewards of promiscuity for males are undisputed. But why should a female mate promiscuously, particularly when her partners offer no resources other than sperm and increase her chances of succumbing to predation or disease? This question has been hotly debated but at present remains largely unresolved [Jennions, M. D. & Petrie, M. (2000) Biol. Rev. 75, 21–64]. One possibility is that females exploit postcopulatory mechanisms, such as sperm competition, to increase both the quality and quantity of their offspring. In this paper, we use the Trinidadian guppy, a species with a resource-free mating system, to test the hypothesis that females gain multiple benefits from multiple mating. Our results indicate that multiply mated females secure substantive advantages: They have shorter gestation times and larger broods, and they produce offspring with better developed schooling abilities and escape responses than their singly mated counterparts.
The guppy (Poecilia reticulata) is a freshwater poeciliid fish with a promiscuous mating system in which female choice plays a central role (1). Like other poeciliids, guppies are livebearers with internal fertilization. Males provide no resources during mating, nor do they defend territories against rival males. Furthermore, females can store sperm for several months and produce a succession of litters from a single insemination (2). In such a mating system, in which females receive no material benefits, polyandry remains an enigma, especially because it leads to a reduction in foraging efficiency (3) and increased vulnerability to predation (4) and parasites (1). Nonetheless, receptive female guppies regularly solicit matings from several males (1). This behavior implies that the costs of multiple mating are offset by substantive benefits.
Potential benefits of multiple mating, which need not be mutually exclusive (5), include an increase in offspring quantity and an improvement in offspring quality. For example, females may mate with more than one male to replenish dwindling sperm stores (6) or to bet-hedge against male infertility (7). The recently documented association between courtship intensity and sperm number in male guppies (8) suggests that females do indeed choose to mate with “sperm-rich” males. In addition, multiple mating may confer an advantage to females because it enhances offspring quality (for example, see ref. 9). In this paper, we test the hypothesis that multiply mated females would benefit from higher fecundity and improved offspring performance. Because fecundity can be increased by reducing the length of the brood cycle (10) and/or by producing more offspring, we measured both variables. Furthermore, as newborn guppies are vulnerable to predation (11) and cannibalism (12), we quantified predator evasion skills, namely schooling behavior and the escape response (13), to evaluate offspring performance.
Our basic protocol was as follows. Virgin female guppies were randomly assigned to one of two experimental treatments, hereafter termed single and multiple. Females allocated to the single treatment were allowed to mate repeatedly with the same male over the course of 4 days. Those guppies assigned to the multiple group were presented with a different male on 4 successive days. Our experimental design ensured that females in both groups were given equal opportunities to mate. We confirmed that females in the multiple group had successfully copulated with more than one male by observing their behavior during mating trials and by using a highly polymorphic microsatellite marker to determine paternity in multiply sired broods.
Methods
Mating Trials.
All fish used in this experiment were descendants of wild-caught fish from the lower Tacarigua River, Trinidad. The lower Tacarigua is a high-predation site where guppies occur sympatrically with several predators, including the pike cichlid, Crenicichla alta. Virgin females, aged between 6 and 9 months and matched for size, were used for the mating trials. Females were placed singly in aquaria (30 × 20 × 20 cm) and allowed to settle overnight. Temperature was maintained at 25 ± 0.5°C and illumination was provided by an 18-W fluorescent bulb on a 12:12 h light:dark cycle. The males used in both treatments were randomly taken from mixed-sex aquaria (≈1:1 sex ratio) and, as with the females, matched for size. Each male was randomly assigned to one of the two experimental treatments. In the single male treatment, a male was placed into each of the female tanks for 4 successive days. In the multiple male treatment, a different male was placed in the female tank on each of the 4 days. The males in the single treatment were added and removed from the tanks in the same way as males were in the multiple treatment; females in both treatments were therefore exposed to the same level of disturbance. Tanks were assigned randomly to each treatment and none of the females had visual access to the other tanks. Each consecutive trial lasted 4 hours, and during this time we counted the number of copulations performed by the pairs of fish. Copulation frequency did not significantly differ between treatments (mean copulations per 30 min: single, 1.50 ± 0.38 SE; multiple, 1.46 ± 0.42; t39 = 0.20; P = 0.84). The females initiated all of the copulations observed during the mating trials.
In total, 76 mating trials were performed (38 for each treatment) and 42 broods were produced (21 for each treatment). After the mating trials, females were isolated until giving birth. Gestation was measured as the sum of the time from insemination to fertilization plus development time from fertilization to birth. After measuring the schooling and escape response behavior of the newborn fish (see Schooling Behavior and Juvenile Escape Behavior below), the multiply sired broods were humanely killed by ice immersion and preserved (along with the putative sires and mothers) in 70% ethanol before DNA extraction.
DNA Fingerprinting.
To determine whether multiply mated females produced multiply sired broods, we genotyped a subsample (n = 10) of broods in the multiple treatment. We extracted genomic DNA from these families (putative sires, mothers, and offspring) by using a rapid one-tube extraction method (14). We used PCR to amplify a polymorphic microsatellite locus with seven alleles for our population (GenBank accession no. AF164205; J. S. Taylor and F. Breden, unpublished data). PCR products were resolved on 6% polyacrylamide gels (15), and paternity was assigned according to allele sharing between putative sires, mother, and offspring. By using this method, we were able to unambiguously assign paternity to 48% of the offspring (total number of offspring genotyped was 101). On average, each of the broods was sired by at least 1.62 (± 0.45 SE) males. Offspring that could not be assigned to one of the four males were excluded from our paternity analyses; our estimate of mean number of sires per brood is therefore likely to be a conservative one.
Schooling Behavior.
Newborn guppies were tested the day after birth; all were fed with baby fish food for livebearers before observations. Pairs of fish were selected randomly from a brood and gently placed in a circular cream-colored arena (diameter, 44 cm; water depth, 2.5 cm; and temperature, 25.5°C). The guppies were given 2 min to settle and explore the arena; schooling behavior was measured for the subsequent 5 min by using a standard method (16). We recorded the length of time that each pair spent schooling; our criterion was that the fish should be no more than 3 cm (approximately 3.5 body lengths) apart and swimming and turning in synchrony. In practice, it was straightforward to assign the measure because the fish tended to either swim synchronously in close proximity or be widely separated in the arena. The procedure was repeated until all of the pairs within a brood had been accounted for. Mean schooling times were calculated for each of the 20 broods (with >1 offspring) per treatment. Data were arcsine transformed before statistical analyses.
Juvenile Escape Behavior.
As noted by Birkhead et al. (17), the time taken to capture an animal represents a measure, by proxy, of the likelihood that it will escape a predator in the wild. We therefore used this parameter as a measure of juvenile quality, employing a similar method to the one used by Birkhead et al. (17). By using a small net, each individual newborn was captured from the schooling arena by an experienced and impartial investigator in a standardized way. The mean time to capture was calculated for broods from n = 19 singly mated and n = 20 multiply mated females. The 1-day-old guppies showed well-developed escape responses and were adept at evading capture. We calculated the mean capture times per brood and correlated this measure with mean schooling times per brood (arcsine-transformed proportions). The work was conducted in accordance with United Kingdom animal welfare legislation (specifically, under Home Office Project License PPL 60/2538).
Results
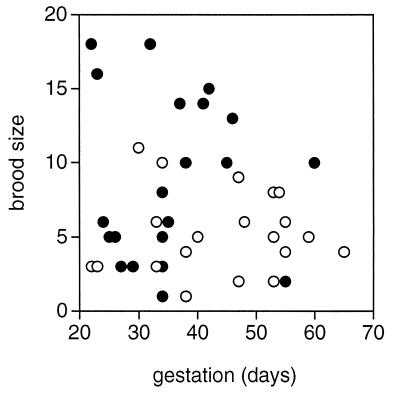
Our data clearly indicate that female guppies gain more from multiple copulations with different partners than from repeated copulations with the same partner. Moreover, our molecular analysis reveals that multiple mating leads to multiple paternity. After confirming that there was no difference in female body size across the treatments (t39 = 0.35; P = 0.73), we found that multiply mated females produced larger broods than their singly mated counterparts [analysis of covariance (ANCOVA) with body length as a covariate: F(1, 39) = 10.10; P = 0.003; Fig. 1]. We also found that gestation was significantly shorter in multiply mated females than in singly mated ones (t40 = 2.57; P = 0.014). On average, multiply mated females produced broods 8.76 days sooner than singly mated females (Fig. 1).
Figure 1.
The relationship between gestation and brood size for singly (○) and multiply (●) mated female guppies. The mean ± SE brood sizes were 5.1 ± 0.61 (single) and 8.8 ± 1.20 (multiple), and the mean gestations in days were 44.1 ± 2.60 (single) and 35.4 ± 2.22 (multiple).
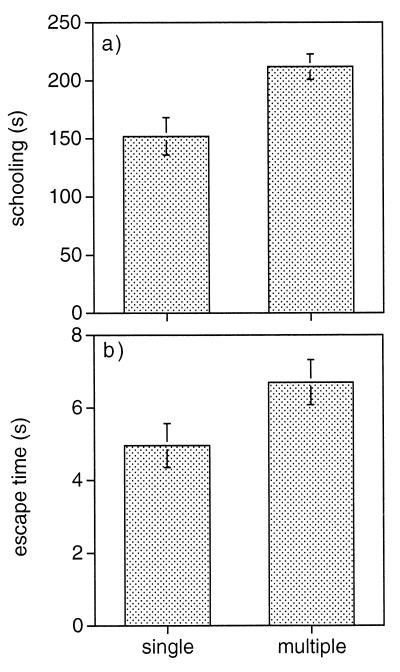
In addition, antipredator skills were better developed in the multiply sired broods. These progeny spent a significantly higher proportion of their time schooling, when tested in pairs, than those born to singly mated females (t33 = 2.8; P = 0.009; Fig. 2a). Furthermore, multiply sired offspring had longer capture times than singly sired fish (t36 = 2.0; P = 0.05; Fig. 2b), although this finding was not significant after a correction for (k = 4) multiple comparisons by using the Dunn–Šidák method (18). All other tests (mentioned above) remained significant after applying this correction factor to our significance levels.
Figure 2.
Mean (±SE) schooling duration (a) and escape times (b) by broods of singly and multiply mated female guppies.
Discussion
Lewontin (10) has noted that even a relatively small reduction in gestation length (in his example, egg-laying time) equates to a large increase in fecundity. Thus, although our conclusions are restricted to the first reproductive cycle of female guppies, the combined effects of brood size and gestation lead us to predict significant gains to the lifetime reproductive success of multiply mated females. Given high mortality rates of adult fish, particularly in high predation populations such as the one from which our fish originated (19), there are clear advantages to females of maximizing fecundity in their early reproductive life.
If reduced female fecundity was attributable to genetic incompatibility or male sterility, a higher proportion of females in the singly mated group would have failed to produce offspring. Instead, we found that the proportion of females producing broods (21 of 38) was identical between the two experimental treatments. An alternative explanation for the reduction in brood size in the singly mated group is that females were sperm limited and therefore would have benefited directly by increasing their sperm stores. Pilastro and Bisazza (20) recently have shown that male guppies inseminate up to 92% of their available sperm during a single copulation. Thus, males that mate repeatedly with a single female may allocate progressively smaller ejaculates per copulation over time. The difference in brood size between the two treatments therefore may have been a result of sperm limitation in singly mated females rather than any fecundity-enhancing effect of multiple mating (21).
These quantity gains are supplemented by improvements in offspring quality. Juvenile fish benefit from the dilution and confusion effects of schooling from the moment they begin to associate (13). Enhanced evasive skills are equally important in reducing the capture success of predators (13). The significant association between capture and schooling times (r = 0.327; n = 35; P = 0.04) indicates that broods with the highest schooling tendency are also better at evading capture and thus are more likely to survive this vulnerable stage. In line with previous work (22), there is no evidence that greater relatedness enhances the schooling behavior of juvenile guppies. If it did, we would have seen higher schooling tendencies in the progeny of singly sired broods.
We conclude, therefore, that offspring born to multiply mated females gain significant benefits as juveniles. Although it is unknown whether these benefits are transmitted genetically or maternally induced (23), a plausible explanation for them is that genetically superior males produce more sperm (24). Thus, “fit” males, which can afford to invest heavily in sperm production and other associated traits, will be at an advantage in sperm competition (25). Previous work on guppies confirms that such a mechanism is possible. Sperm production rate is positively correlated with courtship intensity (8) and body size (20), which in turn are cues used by females during mate choice (1). Because these phenotypic traits are condition-dependent (26, 27), the association between them and ejaculate size will favor viable males in sperm competition. As a consequence, multiple mating will yield more viable offspring. Whether these benefits extend to sneaky mating, to which females are frequently exposed (4), remains to be tested.
A question that arises from our data is why, if multiple mating is costly, do females continue to mate with “poor quality” males? There are two possible answers. First, a female may copulate indiscriminately to increase the likelihood of finding a genetically compatible mate (25). However, as noted above, this idea is not consistent with our finding that the proportion of females producing broods did not differ between the single and multiple treatments. Moreover, because female guppies are provided with multiple cues during mate choice, several of which correlate with female mating preferences and offspring characteristics (26, 28), it seems improbable that the selection of genetically compatible partners is relegated to chance. Second, females may mate with more than one male to “upgrade” previous matings (29). This hypothesis is consistent with our finding that gestation was shorter in multiply mated females. Our design was such that females were given no choice of partner during the mating trials; females were sequentially paired with randomly chosen males. On this basis, females assigned to the single-male treatment may have delayed the process of fertilization in case a more “suitable” male was encountered. This finding also may explain the very long gestation times of some of the singly mated females, in contrast to the normal interbrood interval of 25–35 days (1). Female control of fertilization is clearly an intriguing topic for further study.
Recent work indicates that multiple paternity is widespread in natural populations of guppies in Trinidad (30) as well as being implicated in the evolution of multiple sexual ornaments (31). Until now, it has been unclear why females should seek multiple partners. Our study has uncovered the benefits of this behavior. Promiscuity is evidently rewarding for females as well as for males.
Acknowledgments
We thank Jerry Coyne, Jeff Graves, Mike Ritchie, and the anonymous referees for helpful comments on the manuscript; J. S. Taylor and F. Breden for molecular advice; and the Natural Environment Research Council, U.K., for financial support.
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.180207297.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.180207297
References
- 1.Houde A E. Sex, Color, and Mate Choice in Guppies. Princeton: Princeton Univ. Press; 1997. [Google Scholar]
- 2.Constantz G D. In: Sperm Competition and the Evolution of Animal Mating Systems. Smith R L, editor. Orlando, FL: Academic; 1984. pp. 465–485. [Google Scholar]
- 3.Magurran A E, Seghers B H. Proc R Soc London Ser B. 1994;258:89–92. [Google Scholar]
- 4.Magurran A E, Nowak M A. Proc R Soc London Ser B. 1991;246:31–38. doi: 10.1098/rspb.1991.0121. [DOI] [PubMed] [Google Scholar]
- 5.Eberhard W G. Female Control: Sexual Selection by Cryptic Female Choice. Princeton: Princeton Univ. Press; 1996. [Google Scholar]
- 6.Fjerdingstad E J, Boomsma J J. Behav Ecol Sociobiol. 1998;42:257–261. [Google Scholar]
- 7.Sheldon B C. Proc R Soc London Ser B. 1994;257:25–30. [Google Scholar]
- 8.Matthews I M, Evans J P, Magurran A E. Proc R Soc London Ser B. 1997;264:695–700. [Google Scholar]
- 9.Madsen T, Loman J, Hakansson T. Nature (London) 1992;355:440–441. [Google Scholar]
- 10.Lewontin R C. In: The Genetics of Colonizing Species, First International Union of Biological Sciences Symposium on General Biology. Baker H G, Stebbins G L, editors. New York: Academic; 1965. pp. 77–94. [Google Scholar]
- 11.Magurran A E. Ann Zool Fenn. 1990;27:51–66. [Google Scholar]
- 12.Meffe G K, Snelson J, F F, editors. Ecology and Evolution of Livebearing Fishes. Englewood Cliffs, NJ: Prentice–Hall; 1989. pp. 13–31. [DOI] [PubMed] [Google Scholar]
- 13.Fuiman L A, Magurran A E. Rev Fish Biol Fisheries. 1994;4:145–183. [Google Scholar]
- 14.Estoup A, Largiader C R, Perrot E, Chourrout D. Mol Mar Biol Biotechnol. 1996;5:295–298. [Google Scholar]
- 15.Sambrook J, Fritsch E F, Maniatis T. Molecular Cloning: A Laboratory Manual. Plainview, NY: Cold Spring Harbor Lab. Press; 1989. [Google Scholar]
- 16.Magurran A E, Seghers B H. Ethology. 1990;84:334–342. [Google Scholar]
- 17.Birkhead T R, Fletcher F, Pellatt E J. Behav Ecol Sociobiol. 1998;44:179–191. [Google Scholar]
- 18.Sokal R R, Rohlf F J. Biometry. San Francisco: Freeman; 1995. [Google Scholar]
- 19.Reznick D N, Butler M J, Rodd F H, Ross P. Evolution. 1996;50:1651–1660. doi: 10.1111/j.1558-5646.1996.tb03937.x. [DOI] [PubMed] [Google Scholar]
- 20.Pilastro A, Bisazza A. Proc R Soc London Ser B. 1999;266:1887–1891. [Google Scholar]
- 21.Travis J T, Trexler J C, Mulvey M. Copeia. 1990. , 722–729. [Google Scholar]
- 22.Griffiths S W, Magurran A E. Behav Ecol Sociobiol. 1999;45:437–443. [Google Scholar]
- 23.Gil D, Graves J, Hazon N, Wells A. Science. 1999;286:126–128. doi: 10.1126/science.286.5437.126. [DOI] [PubMed] [Google Scholar]
- 24.Yasui Y. Am Nat. 1997;149:573–584. [Google Scholar]
- 25.Parker G A. Nature (London) 1992;355:395–396. [Google Scholar]
- 26.Reynolds J D, Gross M R. Proc R Soc London Ser B. 1992;264:57–62. [Google Scholar]
- 27.Nicoletto P F. Anim Behav. 1993;46:441–450. [Google Scholar]
- 28.Kodric-Brown A. Behav Ecol Sociobiol. 1993;32:415–420. [Google Scholar]
- 29.Jennions M D, Petrie M. Biol Rev. 2000;75:21–64. doi: 10.1017/s0006323199005423. [DOI] [PubMed] [Google Scholar]
- 30.Kelly C, Godin J-G J, Wright J. Proc R Soc London Ser B. 1999;266:2403–2408. [Google Scholar]
- 31.Brooks R, Couldridge V. Am Nat. 1999;154:37–45. doi: 10.1086/303219. [DOI] [PubMed] [Google Scholar]