Abstract
Background and Aims
Skin microvascular assessment has progressed to an important evaluation in patients with diabetes mellitus. This study was done to evaluate a new device using micro-lightguide spectrophotometry in the assessment of skin microvascular function.
Material and Methods
Twenty nondiabetic subjects (age 46.6 ± 14.8 years; mean ± SD) and 20 diabetic patients (age 59.4 ± 8.4 years) participated in repeated microvascular measurements using micro-lightguide spectrophotometry. This technique allows simultaneous, noninvasive measurement of microvascular blood flow and hemoglobin oxygenation (SO2) at the same anatomical area in different tissue layers. A skin probe was placed on nonhairy skin at the thenar eminence of the left hand for the measurement of SO2, and the postischemic reactive hyperemia response (PRH) was measured in skin and underlying muscle tissue.
Results
Repeated measurements in PRH revealed a good correlation at the superficial skin layer (r = 0.97, p < 0.0001) with a coefficient of variation at 9.2 ± 1.7% and at the superficial muscle layer (r = 0.80, p < 0.0002) with a coefficient of variation at 9.7 ± 1.5%. A slightly weaker correlation was observed for the SO2 measurement at the skin layer (r = 0.69 ± p < 0.0001) with a coefficient of variation at 17.5 ± 3.8% and at the muscle layer (r = 0.48; p = 0.0016) with a coefficient of variation at 18.1 ± 10.5%.
Conclusions
Lightguide spectrophotometry is an easy, noninvasive, and reliable method for simultaneous measurement of superficial microvascular blood flow by laser Doppler fluxmetry and skin oxygenation by spectrophotometry. Further studies are required to clarify the validity of these measures in special patient populations such as diabetes mellitus with specified microvascular complications.
Keywords: diabetes mellitus type 2, endothelial function, laser Doppler flux, skin microcirculation, tissue oxygenation
Introduction
The investigation of microvascular skin blood flow has become an important tool for the evaluation of vascular abnormalities in several diseases such as diabetes, hypertension, dyslipidemia, insulin resistance, rheumatoid arthritis, and sclerodermia.1–8 In diabetes mellitus, peripheral vascular dysfunction was shown to predict a wide range of micro- and macrovascular complications, and the assessment of skin microvascular blood flow is widely used for the measurement of microvascular function. In addition to plethysmography, laser Doppler fluxmetry has become an established tool for the investigation of microvascular function in patients with diabetes mellitus, and numerous techniques have been developed to investigate endothelial-dependent or -independent vascular function.8–11 However, questions remain surrounding the validity and reproducibility of data obtained from laser Doppler techniques.
Lightguide tissue spectrophotometry, in combination with simultaneous registration of skin microvascular blood flow using laser Doppler fluxmetry, is a relatively new approach allowing direct conclusions about the relevance of microcirculatory disturbances on tissue nutrition.12,13 We investigated the reproducibility of micro-lightguide spectrophotometry (O2C) at the upper limb in nondiabetic and diabetic subjects during the postischemic reactive hyperemia response (PRH).
Subjects and Methods
Twenty nondiabetic subjects (10 male, 10 female; age 46.6 ± 14.8 years; mean ± SD) and 20 diabetic patients (10 male, 10 female; 2 type 1, 18 type 2; age 59.4 ± 8.4 years, duration of diabetes 9.8 ± 3.0 years; hemoglobin A1c 7.6 ± 0.4%) without major micro- or macrovascular complications participated in the evaluation of a new device for simultaneous measurement of microvascular blood flow and tissue oxygenation in the skin. Studies were performed twice in each patient by two investigators in a quiet and temperature-controlled room at 22–25°C. The subjects rested at least 30 minutes before the investigation and remained in a recumbent position until the end of the investigation.
Micro-lightguide Spectrophotometer (O2C)
The optical method used for measuring relative blood flow by laser Doppler, hemoglobin amount, and hemoglobin oxygenation has been described in detail elsewhere.14 In brief, the micro-lightguide spectrophotometer O2C (Oxygen to See; LEA Medizintechnik, Giessen, Germany) transmits continuous laser light (830 nm and 30 mW) and white light (20 W, 500–800 nm, 1 nm resolution) to the tissue where it is scattered and collected at the skin surface. The collected light is split into its spectral components by a charge-coupled device array and is converted into an electrical signal. The laser Doppler shift is detected, and the product of moving erythrocytes and the time velocity of each erythrocyte are used for the calculation of the relative blood flow (LDF).
White light is used for the detection of oxygen saturation (SO2) and relative amount of hemoglobin (rHb). The tissue hemoglobin value is determined by the amount of light absorbed by the tissue. This measurement represents a hemoglobin concentration per tissue volume and is independent of vessel density, vessel lumen, and hemoglobin quantity in the blood.12 The change in color of the reflected light is due to a wavelength-dependent absorption of the applied white light and can be used for the calculation of the oxygen saturation of hemoglobin. Adjustment of the distance between the application of the light and the detection of the reflected light allows for detection in different tissues.15
In our study, a skin probe (LF 2, LEA Medizintechnik, Giessen, Germany) was placed on the thenar surface of the left hand in between the phalanx of the thumb and the metatarsal of Dig II directly adjacent to the abductor pollicis (Figure 1). LDF, rHb, and SO2 measurements were performed in two different penetration depths at 2 mm as a measure of skin blood flow and oxygenation and at 8 mm as a measure of superficial muscle blood flow and oxygenation. Via a cuff on the upper arm, a suprasystolic occlusion was induced for 4 minutes; after release of the cuff the maximum blood flow was calculated as a measure for the PRH.
Figure 1.
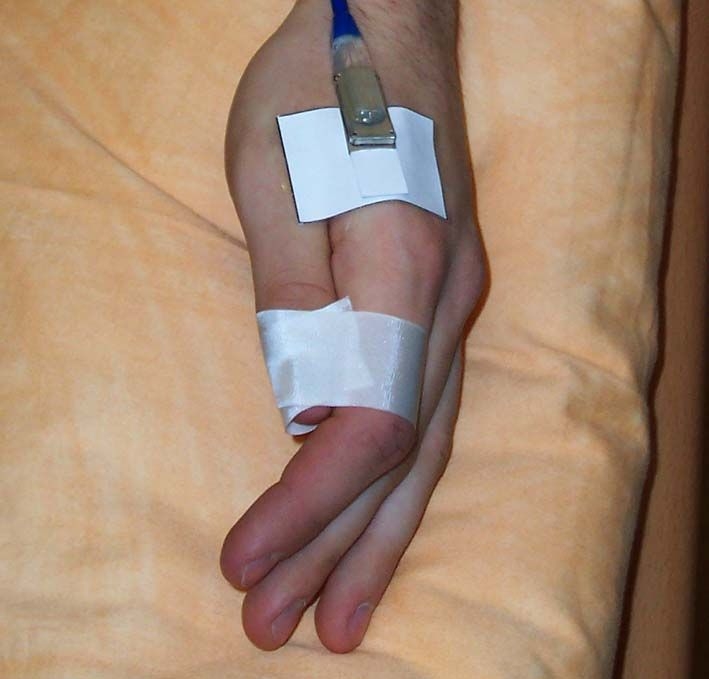
Positioning of the micro-lightguide spectrophotometry probe LF 2 (LEA Medizintechnik, Giessen, Germany) for the measurement of laser Doppler flux and tissue oxygenation at the thenar eminence of the hand.
Statistical Analysis
Results are expressed as mean ± standard error of the mean. Differences between groups were analyzed by the Mann–Whitney U test. A p value < 0.05 was considered statistically significant. Spearman rank correlation and linear regression analysis were performed to assess the consistency of repeated measurements, and the coefficient of variation (CV) was calculated to assess the reliability and reproducibility of the LDF and SO2 measurements.
Results
As shown in Table 1, baseline microvascular blood flow (bLDF), the postischemic response, the relative amount of hemoglobin, and oxygen saturation were significantly higher in the muscle layer compared with the superficial skin layer (p < 0.0001, respectively). Neither at the superficial skin layer nor at the deeper layer of the muscle could a significant difference be observed in bLDF, PRH, rHb, or SO2 between the diabetic patients and the nondiabetic control group.
Table 1.
Mean LDF, PRH, rHb, and SO2 in Nondiabetic and Diabetic Subjects
| Nondiabetic subjects | Diabetic subjects | |
|---|---|---|
| LDF skin baseline (AU a) | 53.4 ± 3.4 | 55.3 ± 2.3 |
| PRH (AU) | 98.8 ± 13.3 | 83.6 ± 7.3 |
| rHb skin (AU) | 57.0 ± 1.9 | 58.2 ± 1.6 |
| SO2 skin (%) | 53.4 ± 3.4 | 55.3 ± 2.3 |
| LDF muscle baseline (AU) | 67.5 ± 9.4 | 77.9 ± 6.3 |
| PRH (AU) | 163.1 ± 11.8 | 165.0 ± 7.2 |
| rHb muscle (AU) | 47.2 ± 2.4 | 47.5 ± 1.4 |
| SO2 muscle (%) | 70.6 ± 2.1 | 70.2 ± 1.7 |
Arbitrary units.
The correlation between repeated measurements for LDF, PRH, and SO2 for both subject groups at the superficial tissue layer is illustrated in Figure 2 and for the measurements in the muscle tissue layer in Figure 3. Although the correlation for SO2 measurements was somewhat weaker compared with microcirculatory measurements (bLDF, PRH), a highly significant correlation could be observed for all measurements at both tissue layers.
Figure 2.
Linear regression of baseline LDF (A), postocclusive reactive hyperemia (B), and tissue oxygenation (C) at the shallow detection layer. AU, arbitrary unit.
Figure 3.
Linear regression of baseline LDF (A), postocclusive reactive hyperemia (B), and tissue oxygenation (C) at the deep detection layer. AU, arbitrary unit.
As shown for the total subject group, a significant correlation for the repeated measurements of bLDF, PRH, and SO2 was also found in the nondiabetic and the diabetic subgroup analysis (Table 2). Again the strongest reproducibility could be found for PRH, while a weaker correlation was observed for bLDF and SO2 readings. Skin and muscle baseline LDF readings were found with a high CV in both detection layers, whereas PRH and SO2 measurements were found with a convenient CV around 10% and SO2 measurements in between 15 and 25%.
Table 2.
CV, Linear Regression of bLDF, PRH, and SO2 in Diabetic and Nondiabetic Subjects
| Nondiabetic control | Diabetic patients | |||||
|---|---|---|---|---|---|---|
| CV (%) | r | p | CV (%) | r | p | |
| bLDF skin | 50.3 ± 16.8 | 0.81 | <0.0001 | 34.1 ± 4.7 | 0.98 | <0.0001 |
| PRH skin | 10.5 ± 1.7 | 0.97 | <0.0001 | 7.8 ± 3.0 | 0.95 | <0.0001 |
| SO2 skin | 16.9 ± 4.6 | 0.74 | 0.0002 | 18.1 ± 6.1 | 0.60 | 0.0051 |
| bLDF muscle | 43.1 ± 11.6 | 0.85 | <0.0001 | 36.7 ± 6.9 | 0.70 | 0.0006 |
| PRH muscle | 8.8 ± 2.0 | 0.93 | <0.0001 | 10.6 ± 2.3 | 0.89 | <0.0001 |
| SO2 muscle | 25.7 ± 20.5 | 0.65 | 0.0025 | 10.5 ± 4.7 | 0.57 | 0.0094 |
Discussion
The measurement of skin microcirculatory blood flow has become a valuable tool for the investigation of microvascular function in patients with diabetes mellitus. In these patients, changes in skin microcirculation have been found to occur many years prior to symptoms of microvascular disease in other tissues.16 Postocclusive reactive hyperemia at the forearm is a commonly used model for studying microvascular reactivity and microvascular endothelial function, and periodic assessment of the postocclusive-reactive hyperemia response across a treatment period has been suggested as an important prognostic marker of microvascular function.17
Lightguide tissue spectrophotometry, a combination of laser Doppler fluxmetry and tissue spectrometry, is a relatively new approach for the simultaneous evaluation of skin microvascular blood flow and skin oxygenation. In previous investigations, this technique was used for the quantification of tissue ischemia in the diabetic foot and to demonstrate the effect of postural changes in patients with chronic venous insufficiency.12,18 The purpose of this study was to establish the reproducibility of the microcirculatory and oxygenation measurements using this technology in the skin of the lower forearm in a diabetic and a nondiabetic population.
Our study revealed a proper intraindividual reproducibility of all measuring parameters observed in our investigation. In accordance with previous studies, baseline LDF readings without stimulation of microvascular blood flow were found with a noticeable inter- and intraindividual variability.19,20 Physiologic regulation of cutaneous microvascular function is a complex process regulated by the influence of environmental temperature, emotional state of the patient, autonomic nerve function, and several local and systemic cofactors. Therefore, several test procedures have been developed for the investigation of skin microvascular blood flow. Postocclusive reactive hyperemia is an established technique for the measurement of microvascular function, which predominately represents endothelial function. In our study the PRH was found with a remarkable strong reproducibility in the superficial skin layer as well as in the subcutaneous muscle layer. This finding is in agreement with a previous study, where the maximal increase in skin microvascular blood flow was the most robust parameter for the measurement of postischemic reactive hyperemia.19 No difference in the reproducibility of this measure could be observed between nondiabetic and diabetic subjects.
Measurement of skin oxygenation is an important tool for the estimation of microvascular tissue nutrition and is often used for the judgement of vascular function in the lower limb. The technique of combined measurement of laser Doppler fluxmetry and lightguide tissue spectrophotometry allows simultaneous measurement of microvascular skin blood flow and skin oxygenation using one skin probe, which can be attached to any conceivable anatomical side of the body. Although measurements using lightguide spectrophotometry as a robust tool for the investigation of tissue nutrition. Parallel measurement of both microvascular blood flow and tissue oxygenation in one detection probe allows for a much more comprehensive judgment of microvascular physiology and might improve our understanding of the importance of microvascular dysfunction in the development of tissue breakdown. In addition, parallel detection in different tissue layers will allow a differentiated judgment of tissue microcirculation in skin tissue and the underlying muscle tissue.
It seems noteworthy that no significant difference in the observed microcirculatory parameters was found between diabetic and nondiabetic subjects. The reason for this unexpected observation might be that our study population was without any history of microvascular complications. Subsequent studies are necessary to evaluate the diagnostic value of this method in diabetic patients with peripheral neuropathy or recent ischemic foot ulcerations or to investigate the effect of different treatment modalities on skin perfusion and tissue nutrition.
In conclusion, the O2C spectrophotometer is a new promising and reliable tool for simultaneous evaluation of microvascular skin blood flow and oxygenation. The use of this new technology will enable new insights into the physiology of tissue nutrition and the pathophysiology of tissue breakdown associated with distinct systemic diseases such as diabetes mellitus.
Abbreviations
- bLDF
baseline microvascular blood flow
- CV
coefficient of variation
- LDF
relative blood flow
- O2C
micro-lightguide spectrophotometry
- PRH
postischemic reactive hyperemia response
- rHb
relative amount of hemoglobin
References
- 1.Pfützner A, Forst T, Engelbach M, Margin T, Goitom K, Löbig M, Beyer J, Kunt T. The influence of isolated small nerve fibre dysfunction on microvascular control in patients with diabetes mellitus. Diabet Med. 2001;18(6):489–494. doi: 10.1046/j.1464-5491.2001.00524.x. [DOI] [PubMed] [Google Scholar]
- 2.Morris SJ, Shore AC, Tooke JE. Responses of the skin microcirculation to acetylcholine and sodium nitroprusside in patients with NIDDM. Diabetologia. 1995;38(11):1337–1344. doi: 10.1007/BF00401767. [DOI] [PubMed] [Google Scholar]
- 3.Forst T, Pfützner A, Bauersachs R, Arin M, Bach B, Biehlmaier H, Küstner E, Beyer J. Comparison of the microvascular response to transcutaneous electrical nerve stimulation and postocclusive ischemia in the diabetic foot. J Diabetes Complications. 1997;11(5):291–297. doi: 10.1016/s1056-8727(96)00078-5. [DOI] [PubMed] [Google Scholar]
- 4.Forst T, Lübben G, Hohberg C, Kann P, Sachara C, Gottschall V, Friedrich C, Rosskopf R, Pfützner A. Influence of glucose control and improvement of insulin resistance on microvascular blood flow and endothelial function in patients with diabetes mellitus type 2. Microcirculation. 2005;12(7):543–550. doi: 10.1080/10739680500253402. [DOI] [PubMed] [Google Scholar]
- 5.La Civita L, Rossi M, Vagheggini G, Storino FA, Credidio L, Pasero G, Giusti C, Ferri C. Microvascular involvement in systemic sclerosis: laser Doppler evaluation of reactivity to acetylcholine and sodium nitroprusside by iontophoresis. Ann Rheum Dis. 1998;57(1):52–55. doi: 10.1136/ard.57.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rossi M, Taddei S, Fabbri A, Tintori G, Credidio L, Virdis A, Ghiadoni L, Salvetti A, Giusti C. Cutaneous vasodilation to acetylcholine in patients with essential hypertension. J Cardiovasc Pharmacol. 1997;29(3):406–411. doi: 10.1097/00005344-199703000-00015. [DOI] [PubMed] [Google Scholar]
- 7.Khan F, Litchfield SJ, Stonebridge PA, Belch JJ. Lipid-lowering and skin vascular responses in patients with hypercholesterolaemia and peripheral arterial obstructive disease. Vasc Med. 1999;4(4):233–238. doi: 10.1177/1358836X9900400405. [DOI] [PubMed] [Google Scholar]
- 8.Brooks BA, McLennan SV, Twigg SM, Yue DK. Detection and characterisation of microcirculatory abnormalities in the skin of diabetic patients with microvascular complications. Diab Vasc Dis Res. 2008;5(1):30–35. doi: 10.3132/dvdr.2008.006. [DOI] [PubMed] [Google Scholar]
- 9.Forst T, Forst S, Strunk K, Löbig M, Welter K, Kazda C, Pfützner A. Impact of insulin on microvascular blood flow and endothelial cell function in the postprandial state in patients with Type 1 diabetes. J Diabetes Complications. 2005;19(3):128–132. doi: 10.1016/j.jdiacomp.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 10.Sarnik S, Hofirek I, Sochor O. Laser Doppler fluxmetry. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2007;151(1):143–146. doi: 10.5507/bp.2007.028. [DOI] [PubMed] [Google Scholar]
- 11.Vinik AI, Erbas T, Park TS, Stansberry KB, Scanelli JA, Pittenger GL. Dermal neurovascular dysfunction in type 2 diabetes. Diabetes Care. 2001;24(8):1468–1475. doi: 10.2337/diacare.24.8.1468. [DOI] [PubMed] [Google Scholar]
- 12.Beckert S, Witte MB, Konigsrainer A, Coerper S. The impact of the Micro-Lightguide O2C for the quantification of tissue ischemia in diabetic foot ulcers. Diabetes Care. 2004;27(2):2863–2867. doi: 10.2337/diacare.27.12.2863. [DOI] [PubMed] [Google Scholar]
- 13.Stirban A, Negrean M, Stratmann B, Gawlowski T, Horstmann T, Götting C, Kleesiek K, Mueller-Roesel M, Koschinsky T, Uribarri J, Vlassara H, Tschoepe D. Benfotiamine prevents macro-and microvascular endothelial dysfunction and oxidative stress following a meal rich in advanced glycation end products in individuals with type 2 diabetes. Diabetes Care. 2006;29(9):2064–2071. doi: 10.2337/dc06-0531. [DOI] [PubMed] [Google Scholar]
- 14.Frank KH, Kessler M, Appelbaum K, Dummler W. The Erlangen micro-lightguide spectrophotometer EMPHO I. Phys Med Biol. 1989;34(14):1883–1900. doi: 10.1088/0031-9155/34/12/011. [DOI] [PubMed] [Google Scholar]
- 15.Jakobsson A, Nilsson GE. Prediction of sampling depth and photon pathlength in laser Doppler flowmetry. Med Biol Eng Comput. 1993;31(3):301–307. doi: 10.1007/BF02458050. [DOI] [PubMed] [Google Scholar]
- 16.Khan F, Elhadd TA, Greene SA, Belch JJ. Impaired skin microvascular function in children, adolescents, and young adults with type 1 diabetes. Diabetes Care. 2000;23(2):215–220. doi: 10.2337/diacare.23.2.215. [DOI] [PubMed] [Google Scholar]
- 17.Minson CT, Wong BJ. Reactive hyperemia as a test of endothelial or microvascular function? J Am Coll Cardiol. 2004;43(11):2147–2148. doi: 10.1016/j.jacc.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 18.Rajbhandari SM, Harris ND, Tesfaye S, Ward JD. Early identification of diabetic foot ulcers that may require intervention using the micro lightguide spectrophotometer. Diabetes Care. 1999;22(8):1292–1295. doi: 10.2337/diacare.22.8.1292. [DOI] [PubMed] [Google Scholar]
- 19.Yvonne-Tee GB, Rasool AH, Halim AS, Rahman AR. Reproducibility of different laser Doppler fluximetry parameters of postocclusive reactive hyperemia in human forearm skin. J Pharmacol Toxicol Methods. 2005;52(2):286–292. doi: 10.1016/j.vascn.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 20.Negrean M, Stirban A, Stratmann B, Gawlowski T, Horstmann T, Götting C, Kleesiek K, Mueller-Roesel M, Koschinsky T, Uribarri J, Vlassara H, Tschoepe D. Effects of low- and high-advanced glycation endproduct meals on macro- and microvascular endothelial function and oxidative stress in patients with type 2 diabetes mellitus. Am J Clin Nutr. 2007;85(5):1236–1243. doi: 10.1093/ajcn/85.5.1236. [DOI] [PubMed] [Google Scholar]




