Abstract
The dysglycemia of diabetes includes two components: (1) sustained chronic hyperglycemia that exerts its effects through both excessive protein glycation and activation of oxidative stress and (2) acute glucose fluctuations. Glycemic variability seems to have more deleterious effects than sustained hyperglycemia in the development of diabetic complications as both upward (postprandial glucose increments) and downward (interprandial glucose decrements) changes activate the oxidative stress. For instance, the urinary excretion rate of 8-iso-PGF2α, a reliable marker of oxidative stress, was found to be strongly, positively correlated (r = 0.86, p < .001) with glycemic variability assessed from the mean amplitude of glycemic excursions (MAGE) as estimated by continuous glucose monitoring systems (CGMS). These observations therefore raise the question of whether we have the appropriate tools for assessing glycemic variability in clinical practice. From a statistical point of view, the standard deviation (SD) around the mean glucose value appears as the “gold standard.” By contrast, the MAGE index is probably more appropriate for selecting the major glucose swings that are calculated as the arithmetic mean of differences between consecutive peaks and nadirs, provided that the differences be greater than the SD around the mean values. Furthermore, calculating the MAGE index requires continuous glucose monitoring, which has the advantage to detect all isolated upward and downward acute glucose fluctuations. In conclusion, the increasing use of CGMSs will certainly promote better assessment and management of glycemic variability.
Keywords: glycemic assessment, glycemic importance, glycemic variability
Do We Have to Recommend the Assessment of Glucose Variability in Patients with Type 2 Diabetes?
Today, nobody can deny that vascular complications are mainly or partly dependent on sustained chronic hyperglycemia.1–3 This glycemic disorder can be estimated as a whole from the determination of hemoglobin A1c (HbA1c) level, which integrates both basal and postprandial hyperglycemia.4,5 As a consequence, it is not surprising that the incidence of vascular complications has been identified as depending first on HbA1c and second on fasting and/or postprandial hyperglycemia, whether these parameters were investigated concomitantly or separately. For instance, the UK Prospective Diabetes Study demonstrated that reductions in HbA1c and fasting blood glucose levels were accompanied by substantial decreases in the risk for all diabetes-related endpoints.6 In 1996, Hanefeld et al. depicted postprandial hyperglycemia as a better predictor of subsequent myocardial infarction and cardiovascular mortality than fasting hyperglycemia.7 Further landmark studies have confirmed this finding, suggesting that postprandial hyperglycemia is an independent risk factor for macro-vascular disease.8,9 However, the glycemic disorders in type 2 diabetes are not solely limited to sustained chronic hyperglycemia but can be extended to the glycemic variability that includes both upward and downward acute glucose changes.10 The purpose of the present study is to provide answers to the following questions: (a) Should glycemic variability be considered a component of dysglycemia in diabetes?; (b) Do we have the appropriate tools for assessing glycemic variability?; and finally, (c) Do we have to recommend measurement of glycemic variability in clinical practice?
Should Glycemic Variability Be Considered a Component of Dysglycemia in Diabetes?
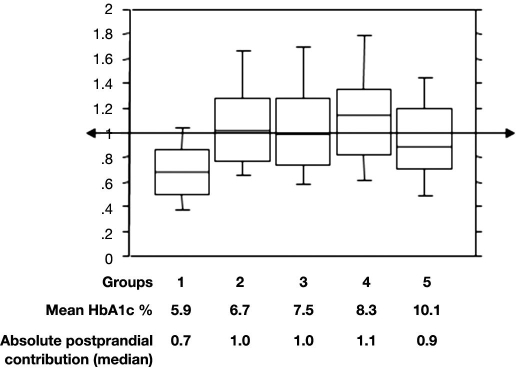
Acute glucose fluctuations from peaks to nadirs include postprandial glucose (PPG) excursions that can be described by two components. The first is the duration of postprandial excursions, and the second is the magnitude of postprandial rise. The first component, the duration of PPG increment, is a major contributor to sustained chronic hyperglycemia, while the magnitude is more a reflection of glucose variability. Calculating the PPG incremental area under curve above the preprandial glucose value can assess the entire phenomenon. By using this mode of calculation, we found that the absolute impact of PPG on hemoglobin A1c (percentage points of A1c) is constant and contributes approximately 1% in all patients with HbA1c levels above 6.5% (Figure 1).11 Although it is difficult to separate the contributions of the two components of the dysglycemia, it seems that both contribute to the two main mechanisms that lead to diabetes complications, namely, the excessive protein glycation and the activation of oxidative stress.12 As PPG excursions result in both acute and sustained hyperglycemia, it seems reasonable to think that PPG excursions and more generally acute glucose fluctuations activate the oxidative stress. For instance, the production of nitrotyrosine, a metabolite derived from nitrosamine stress, was significantly increased at fasting in patients with diabetes, but an additional increase was observed during postmeal periods. A reduction of the postmeal glucose excursions by using a premeal bolus of rapid insulin analog (Aspart) resulted in parallel decreases in glycemic and nitrotyrosine responses.13 Although PPG is usually the major contributor of glucose variability, other fluctuations (especially downward fluctuations) must be taken into account.
Figure 1.
Absolute contributions of postprandial glucose increments to HbA1c (percentage points, median, 95% confidence interval, 10th percentile, and 90th percentile) with worsening diabetes.
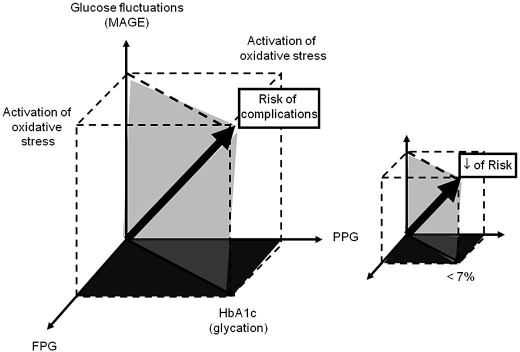
In 2006, we reported that, in type 2 diabetes, the urinary excretion rate of 8-iso-PGF2α, a reliable marker of the activation of oxidative stress, was highly, positively correlated with the glycemic variability assessed from the mean amplitude of glycemic excursions (MAGE).14 A statistically significant correlation was also observed with the PPG increments, but the relationship was less significant. In 2008, Ceriello et al. reported that acute glucose spikes triggered the release of plasma 3-nitrotyrosine, a marker of reactive oxygen species.15 These results are in agreement with our own data and strongly suggest that oscillating glucose can have more deleterious effects than sustained chronic hyperglycemia on endothelial function and oxidative stress, two key players in the development and progression of cardiovascular diseases in diabetes. As a consequence, the concept of “dangerous waves” that was initially limited to PPG “spikes” should be extended to both upward (postprandial) and downward (interprandial) acute fluctuations of glucose around a mean value. However, Kilpatrick et al. did not confirm this hypothesis.16 By using the Diabetes Control and Complications Trial (DCCT) data, these authors reported that the sustained chronic hyperglycemia was predictive of microvascular complications in patients with type 1 diabetes, while the within-day glucose variability was not. These findings are also supported by the data of Wentholt et al. who reported that in type 1 diabetes, the urinary excretion rates of 8-iso-PGF2α did not correlate with glucose fluctuations.17 At first glance, these results seem to be contradictory with the results we found in noninsulin-using type 2 diabetes patients. The discrepancy between patients with type 1 and type 2 diabetes could only be due to the fact that the insulin per se exerts a powerful inhibitory effect on the activation of oxidative stress. This hypothesis, if confirmed, might become a unifying explanation for bridging the divide between the data as observed in insulin-treated type 1 diabetes and noninsulin-treated type 2 diabetes. The consequence of such observations might be an additional argument for implementing earlier insulin treatments in type 2 diabetes when patients are not adequately controlled (HbA1c > 7%) on maximal doses of oral hypoglycemic agents. Despite the findings of Kilpatrick et al. and Wentholt et al. in type 1 diabetes, many studies indicate that diabetes complications, at least in persons with type 2 diabetes, can be considered the result of two major deleterious metabolic alterations (excessive glycation and the generation of oxidative stress) that are activated by three main glycemic disorders: hyper-glycemia, both at fasting and during postprandial periods, and acute glucose fluctuations (Figure 2).10,18 Excessive levels of glucose at fasting and during postprandial periods activate the glycation process, which can be investigated by measuring HbA1c levels. Hyperglycemia at fasting, acute or sustained hyperglycemia over postprandial periods, and more generally acute glucose fluctuations around the mean glucose value activate the oxidative stress. The resulting effect is the risk of complications depicted by the diagonal arrow of a geometric cube whose three-dimensional coordinates on the three axes are fasting plasma glucose (FPG), PPG, and glucose fluctuations (Figure 2).10,18 According to this model, a global antidiabetic therapeutic strategy in type 2 diabetes should be aimed at reducing the values of the three coordinates, i.e., the volume of the cube and therefore the magnitude of the diagonal arrow that illustrates the risk for diabetic complications.
Figure 2.
Model suggested for illustrating the pathophysiological impacts of the excessive glycation of proteins and the activation of oxidative stress on the risk of diabetic complications (diagonal solid arrow). The contributions of the three components of dysglycemia, i.e., hyperglycemia at fasting (FPG), hyperglycemia during postprandial periods (PPG), and acute glucose fluctuations (MAGE), are indicated on the x, y, and z axes, respectively.
Do We Have the Appropriate Tools for Assessing Glycemic Variability?
Several approaches have been proposed that, for two main reasons, all fail to provide a complete representation of glucose oscillations.19 First, glycemic variability is a complex phenomenon that includes both intraday and interday variability. The intraday component corresponds to the within-day vertical glycemic fluctuations. The interday component is defined as day-to-day glucose variations, i.e., glycemic variability along a time-dependent horizontal axis. As a consequence, glycemic variability in patients with diabetes is a composite of the vertical and horizontal components. The second reason explaining why glycemic variability is a complex phenomenon is that this glycemic disorder is a combination of minor and major glucose fluctuations. For instance, it is still difficult to know whether the deleterious effects of glycemic variability, such as the activation of oxidation stress, are only triggered by major glucose swings or by all glucose oscillations, including the minor ones.14,15,17 Therefore, it is not surprising that several approaches have been developed for quantifying glycemic fluctuations.
Assessment of the Intraday Glycemic Variability
From a statistical point of view, the standard deviation (SD) around a mean glucose value measured over a 24 h period using the continuous glucose monitoring system (CGMS) is probably the most appropriate tool for assessing intraday glycemic variability.16,19 Such a method integrates both minor and major fluctuations but does not permit differentiation of the major from the minor ones. The other methods developed for estimating the intraday glycemic variability are more or less based on the determination of differences between maximum and minimum glucose levels.
The MAGE remains certainly the most comprehensive index for assessing the intraday glycemic variability.20 The principle is to estimate the major rises and falls in a glucose profile. The calculation of the MAGE is obtained by measuring the arithmetic mean of the differences between consecutive peaks and nadirs provided that the differences are greater than one SD of the mean glucose value. The measurement can be made either in the peak-to-nadir or nadir-to-peak direction, the direction being selected by the first upward or downward glucose excursion that is greater than one SD. The MAGE index has two main advantages. First, this parameter is not dependent on the mean glucose value, and second, it is designed to quantitate major glucose swings and exclude minor ones.
The M-value of Schlichtkrull et al. is a logarithmic transformation of the deviation of glycemia from an arbitrary assigned “ideal” glucose value.21 The reference value may be different according to the clinical status of the subject. For instance, the glucose value used as reference is usually greater in persons with diabetes than in persons without diabetes. The M-value of Schlichtkrull et al. attempts to provide, in a single numerical value, an expression of both the mean glucose value and the effect of glucose swings. However, the meaning of this parameter is shaded by the complexity of the used formula.
The continuous overall net glycemic action is similar to the SD.22 The determination is based on the assessment of the differences between glucose values measured at regular time intervals, then on the calculation of the SD of these differences.
The average daily risk range23 is computed by using the data from self-monitored blood glucose (SMBG) collected over 1 month at a frequency of 3–5 readings per day. This newly developed method that uses a rather complex formula is aimed at ensuring a better balance between glycemic increments and decrements since most of the methods used are more sensitive to hyperglycemic spikes than to hypoglycemic excursions.
Assessment of Interday Glucose Variability
The mean of daily differences (MODD) currently remains the sole index for estimating interday glycemic variability.24 This parameter is calculated as the mean of the absolute differences between glucose values at the same time on two consecutive days.
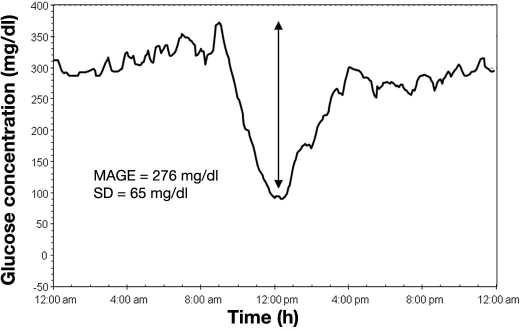
At present, three parameters seem to be preferred: (1) the SD around the mean glucose values, because it remains the “gold standard;” (2) the MAGE, because its calculation is relatively simple, and because it allows the achievement of the major intraday glucose oscillations while the minor ones are not taken into account; and (3) the MODD, because it is the sole parameter to provide an assessment of the interday glycemic variability. Although the estimation of glycemic variability can be made by using a discontinuous SMBG using several daily time points, our opinion is that it is better to use newer technologies based on continuous glucose sensors implanted in the subcutaneous tissue. Although the interstitial glucose concentrations are lower than those measured in the bloodstream, the estimation of these three aforementioned indices using the CGMS remains perfectly reliable since the three methods are applied to measurements of glucose differences and not to absolute glucose levels. This remark explains why the CGMS can be used with the same degree of reliability either in subjects with normal glucose tolerance25 or in diabetes patients exhibiting various degrees of glycemic control. In summary, different tools should be used concomitantly. The following typical example of continuous glucose monitoring in a type 1 diabetes patient treated with insulin can be given to illustrate this statement (Figure 3). This patient had small glucose fluctuations throughout the day but exhibited a rapid and unpredictable glucose drop after breakfast. The nadir was reached at noon with a further glucose increase after lunch and a return to a high stable glucose value at 4:00 pm. In this patient, the calculation showed a profound discrepancy between the SD that remained at a modest level (65 mg/dl, 3.6 mmol/liter), while the MAGE was greatly increased (276 mg/dl, 15.3 mmol/liter). This type of profile, which is encountered in unstable type 1 diabetes, seems to indicate that the SD may minimize the effect of sudden, very large changes in individuals with overall modest fluctuations.
Figure 3.
Example of continuous glucose monitoring in one type 1 diabetes patient treated with a multiple-injection insulin regimen. The standard deviation around the mean glucose value and the MAGE were 65 mg/dl (3.6 mmol/liter) and 276 mg/dl (15.3 mmol/liter), respectively. The discrepancy between the two values was due to the fact that this patient exhibited a single large glucose swing inserted in modest glucose fluctuations over the remainder the day.
Should Glycemic Variability Be Assessed in Clinical Practice?
As PPG increments are the major contributors to glycemic variability in type 2 diabetes, clear statements are needed in terms of the target and management of postprandial excursions.
Postprandial Glucose Recommendations and Targets
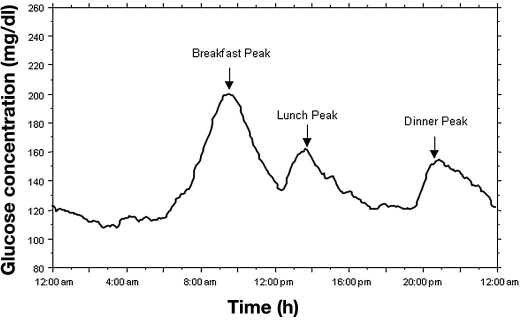
Large discrepancies in PPG threshold values were observed until the guidelines for management of postmeal glucose by the International Diabetes Federation (IDF) were published.26,27 The difficulty to draw clear recommendations for PPG is easily understandable if one admits the following principle: PPG excursions cannot be reflected by one single glucose value but by a multitude of values that are spread over a 3 to 4 h postmeal period. During this period, the magnitude of the elevation of blood glucose concentrations is dependent on several factors.28 In patients with type 2 diabetes, postmeal increments in blood glucose are usually longer and higher than in persons without diabetes. Furthermore, in most patients, the postmeal excursions follow an ordinal decreasing scale that can be described as postbreakfast>postdinner or postlunch excursions (Figure 4).29 On the other hand, postmeal glucose responses are influenced first by the quality and quantity of carbohydrates that are contained in foods and second by physical activity over the postprandial period. By accounting for these factors as a whole, there arises the question of when, i.e., after which meal, PPG testing should be recommended. At present, the question remains unanswered. By contrast, for the 2 h postprandial glycemic target, clear recommendations have been set by different organizations, and large discrepancies persist. For the American Diabetes Association,30 the PPG target is set below 180 mg/dl (10 mmol/liter), a value chosen as the upper limit in the patients who were allocated to the intensively treated group of the DCCT.2 The 140 mg/dl (7.8 mmol/liter) glucose value was selected by the IDF.26,27 The main rationale for this choice is that 140 mg/dl (7.8 mmol/liter) is the limit value that separates the normal range from the state of impaired glucose tolerance when measurements are made at the second hour of an oral glucose tolerance test.
Figure 4.
Mean glucose concentration in 32 noninsulin-using type 2 diabetes patients exhibiting HbA1c levels between 7 and 7.9% (Reproduced from Reference 29, with permission from Diabetes Care.)
Glycemic Variability and Postprandial Hyperglycemia Monitoring and Management
Discontinuous SMBG, and preferably the CGMS, should be performed to assess both glycemic variability and PPG excursions.31,32 The type of monitoring (continuous or discontinuous) and the frequency of glucose testing when the discontinuous monitoring is used depend on both the type of diabetes (1 or 2) and the type of treatment (insulin or tablets). CGMSs are useful in insulin-treated diabetes (either type 1 or type 2) for choosing the best insulin regimen. As CGMS can only be implemented for limited periods of time, the SMBG is routinely used as a surrogate.33,34 In type 1 diabetes treated with basal–bolus regimens, it is usually recommended to use a seven-point glycemic monitoring that includes the following tests: (1) three at the preprandial times, (2) three at the 2 h postprandial time points, and (3) one at bed time.2 The frequency of the SMBG can be reduced in patients who are treated with more conventional insulin regimens: one or two daily injections. In noninsulin-using type 2 diabetes, the SMBG can be further limited according to requirements. One recommendation is the practice of glucose testing by using a circular permutation at different times of the day over a weekly period in order to have a broader picture of glucose fluctuations over daytime. Although the use of both the CGMS and frequent discontinuous SMBG is less crucial in noninsulin-treated patients than in insulin-treated patients, there are many situations in which such assessments can be helpful in type 2 patients who are not on insulin treatment, especially in those who are not sufficiently controlled with maximal doses of oral antidiabetic drugs and who normally require the introduction of insulin treatment.
For instance, the CGMS32 and frequent SMBG33,34 can act to stimulate the acceptance of the need for insulin treatment, especially when measurements are made at time points corresponding to glucose peaks. In this view, the midmorning period is crucial since we have demonstrated that hyperglycemia after breakfast is usually the highest postmeal glucose excursion over daytime.29 The attainment of near-normal glucose values at selected time points, usually at fasting, does not exclude abnormal peaks and troughs in glucose levels over postprandial or postabsorptive periods. The acute variations could remain totally ignored by both patients and health care professionals in the absence of glucose monitoring over periods of unsatisfactory control. For that reason, the expanded use of either the CGMS or frequent SMBG is probably of interest to instruct patients that their blood glucose is beyond acceptable levels and to encourage them to accept insulin therapy or, more generally, to accept a reinforcement of their therapeutic regimens.
An additional example of the usefulness of assessing glycemic variability is given by the problem of the choice of insulin regimen and the adjustment of insulin doses in patients with type 2 diabetes treated with insulin. At first glance, the implementation of insulin treatments in type 2 diabetes could be considered a simple process. Many researchers have proposed to treat the insulin deficiency by using a basal insulin replacement therapy with a single injection of long-acting insulin analog before dinner or at bedtime.35 Such regimens associated with an oral therapy are generally sufficient for controlling the preprandial and interprandial glucose levels. However, some patients persist to exhibit HbA1c higher than 7% even when treat-to-target therapeutic strategies are implemented.35 The use of continuous glucose sensors is one of the methods to solve this problem. In many patients who do not achieve the targets, it appears that PPG excursions, especially after breakfast, remain abnormally high.29 In this case, a better glycemic control can be obtained by injections of prandial insulin before the meal that produces the largest PPG excursions.36 Further injections can be gradually introduced at other premeal times as required. This provides a step-by-step procedure toward an intensive basal–bolus therapy.
In conclusion, glycemic variability is one of the components of glycemic disorders in patients with diabetes. In the near future, the use of CGMS will need to be increased to promote better assessment and management of glycemic variability in both type 1 and type 2 diabetes.
Abbreviations
- CGMS
continuous glucose monitoring system
- DCCT
Diabetes Control and Complications Trial
- FPG
fasting plasma glucose
- HbA1c
hemoglobin A1c
- IDF
International Diabetes Federation
- MAGE
mean amplitude of glycemic excursions
- MODD
mean of daily differences
- PPG
postprandial glucose
- SD
standard deviation
- SMBG
self-monitored blood glucose
References
- 1.Laakso M, Lehto S. Epidemiology of macrovascular disease in diabetes. Diabetes Rev. 1997;5(4):294–315. [Google Scholar]
- 2.Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329(14):977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 3.Stratton IM, Adler AL, Neil HA, Matthews DR, Manley SE, Cull CA, Hadden D, Turner RC, Holman RR. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321(7258):405–412. doi: 10.1136/bmj.321.7258.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sacks DB, Bruns DE, Goldstein DE, Maclaren NK, McDonald JM, Parrott M. Guidelines and recommendations for laboratory analysis in the diagnosis and management of diabetes mellitus. Clin Chem. 2002;48(3):436–472. [PubMed] [Google Scholar]
- 5.Gorus F, Mathieu C, Gerlo E. How should HbA1c measurements be reported? Diabetologia. 2006;49(1):7–10. doi: 10.1007/s00125-005-0073-7. [DOI] [PubMed] [Google Scholar]
- 6.UK Prospective Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352(9131):837–853. [PubMed] [Google Scholar]
- 7.Hanefeld M, Fischer S, Julius U, Schulze J, Schwanebeck U, Schmechel H, Ziegelasch HJ, Lindner J. DIS Group. Risk factors for myocardial infarction and death in newly detected NIDDM: the Diabetes Interventional Study, 11-year follow-up. Diabetologia. 1996;39(12):1577–1583. doi: 10.1007/s001250050617. [DOI] [PubMed] [Google Scholar]
- 8.Ceriello A, Hanefeld M, Leiter L, Monnier L, Moses A, Owens D, Tajima N, Tuomilehto J. Postprandial glucose regulation and diabetic complications. Arch Int Med. 2004;164(19):2090–2095. doi: 10.1001/archinte.164.19.2090. [DOI] [PubMed] [Google Scholar]
- 9.Cavalot F, Petrelli A, Traversa M, Bonomo K, Fiora E, Conti M, Anfossi G, Costa G, Trovati M. Postprandial blood glucose is a stronger predictor of cardiovascular events than fasting blood glucose in type 2 diabetes mellitus, particularly in women: lessons from the San Luigi Gonzaga Diabetes Study. J Clin Endocrinol Metab. 2006;91(3):813–819. doi: 10.1210/jc.2005-1005. [DOI] [PubMed] [Google Scholar]
- 10.Monnier L, Colette C. Glycemic variability: should we and can we prevent it? Diabetes Care. 2008;31(Suppl 2):S150–S154. doi: 10.2337/dc08-s241. [DOI] [PubMed] [Google Scholar]
- 11.Monnier L, Colette C, Owens DR. Type 2 diabetes: a well-characterised but suboptimally controlled disease. Can we bridge the divide? Diabetes Metab. 2008;34(3):207–216. doi: 10.1016/j.diabet.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 12.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414(6865):813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 13.Ceriello A, Quagliaro L, Catone B, Pascon R, Piazzola M, Bais B, Marra G, Tonutti L, Taboga C, Motz E. Role of hyperglycemia in nitrotyrosine postprandial generation. Diabetes Care. 2002;25(8):1439–1443. doi: 10.2337/diacare.25.8.1439. [DOI] [PubMed] [Google Scholar]
- 14.Monnier L, Mas E, Ginet C, Michel F, Villon L, Cristol JP, Colette C. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA. 2006;295(14):1681–1687. doi: 10.1001/jama.295.14.1681. [DOI] [PubMed] [Google Scholar]
- 15.Ceriello A, Esposito K, Piconi L, Ihnat MA, Thorpe JE, Testa R, Boemi M, Giugliano D. Oscillating glucose is more deleterious to endothelial function and oxidative stress than mean glucose in normal and type 2 diabetic patients. Diabetes. 2008;57(5):1349–1354. doi: 10.2337/db08-0063. [DOI] [PubMed] [Google Scholar]
- 16.Kilpatrick ES, Rigby AS, Atkin SL. The effect of glucose variability on the risk of microvascular complications in type 1 diabetes. Diabetes Care. 2006;29(7):1486–1490. doi: 10.2337/dc06-0293. [DOI] [PubMed] [Google Scholar]
- 17.Wentholt IM, Kulik W, Michels RP, Hoekstra JB, DeVries JH. Glucose fluctuations and activation of oxidative stress in patients with type 1 diabetes. Diabetologia. 2008;51(1):183–190. doi: 10.1007/s00125-007-0842-6. [DOI] [PubMed] [Google Scholar]
- 18.Colette C, Monnier L. Acute glucose fluctuations and chronic sustained hyperglycemia as risk factors for cardiovascular diseases in patients with type 2 diabetes. Horm Metab Res. 2007;39(9):683–686. doi: 10.1055/s-2007-985157. [DOI] [PubMed] [Google Scholar]
- 19.Service FJ, O'Brien PC, Rizza RA. Measurements of glucose control. Diabetes Care. 1987;10(2):225–237. doi: 10.2337/diacare.10.2.225. [DOI] [PubMed] [Google Scholar]
- 20.Service FJ, Molnar GD, Rosevear JW, Ackerman E, Gatewood LC, Taylor WF. Mean amplitude of glycemic excursions, a measure of diabetic instability. Diabetes. 1970;19(9):644–655. doi: 10.2337/diab.19.9.644. [DOI] [PubMed] [Google Scholar]
- 21.Schlichtkrull J, Munck O, Jersild M. The M-value, an index of blood sugar control in diabetics. Acta Med Scand. 1965;177:95–102. doi: 10.1111/j.0954-6820.1965.tb01810.x. [DOI] [PubMed] [Google Scholar]
- 22.McDonnell CM, Donath SM, Vidmar SI, Werther GA, Cameron FJ. A novel approach to continuous glucose analysis utilizing glycemic variation. Diabetes Technol Ther. 2005;7(2):253–263. doi: 10.1089/dia.2005.7.253. [DOI] [PubMed] [Google Scholar]
- 23.Kovatchev BP, Otto E, Cox D, Gonder-Frederick L, Clarke W. Evaluation of a new measure of blood glucose variability in diabetes. Diabetes Care. 2006;29(11):2433–2438. doi: 10.2337/dc06-1085. [DOI] [PubMed] [Google Scholar]
- 24.Molnar GD, Taylor WF, Ho MM. Day-to-day variation of continuously monitored glycaemia: a further measure of diabetic instability. Diabetologia. 1972;8(5):342–348. doi: 10.1007/BF01218495. [DOI] [PubMed] [Google Scholar]
- 25.Mazze RS, Strock E, Wesley D, Borgman S, Morgan B, Bergenstal R, Cuddihy R. Characterizing glucose exposure for individuals with normal glucose tolerance using continuous glucose monitoring and ambulatory glucose profile analysis. Diabetes Technol Ther. 2008;10(3):149–159. doi: 10.1089/dia.2007.0293. [DOI] [PubMed] [Google Scholar]
- 26.International Diabetes Federation. Brussels: IDF. 2007. Guideline for management of postmeal glucose; pp. 1–27. [Google Scholar]
- 27.Ceriello A, Colagiuri S, Gerich J, Tuomilehto J. Guideline Development Group. Guideline for management of postmeal glucose. Nutr Metab Cardiovasc Dis. 2008;18(4):S17–S33. doi: 10.1016/j.numecd.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 28.Dinneen S, Gerich J, Rizza R. Carbohydrate metabolism in non-insulin-dependent diabetes mellitus. N Engl J Med. 1992;327(10):707–713. doi: 10.1056/NEJM199209033271007. [DOI] [PubMed] [Google Scholar]
- 29.Monnier L, Colette C, Dunseath GJ, Owens DR. The loss of postprandial glycemic control precedes stepwise degradation of fasting with worsening diabetes. Diabetes Care. 2007;30(2):263–269. doi: 10.2337/dc06-1612. [DOI] [PubMed] [Google Scholar]
- 30.American Diabetes Association. Standards of medical care in diabetes—2008. Diabetes Care. 2008;31(Suppl 1):S12–S54. doi: 10.2337/dc08-S012. [DOI] [PubMed] [Google Scholar]
- 31.Buckingham B, Block J, Wilson DM. Continuous glucose monitoring. Curr Opin Endocrinol Diabetes. 2005;12(4):273–279. doi: 10.1097/MED.0b013e32825a675e. [DOI] [PubMed] [Google Scholar]
- 32.Monnier L, Colette C, Boegner C, Pham TC, Lapinski H, Boniface H. Continuous glucose monitoring in patients with type 2 diabetes: Why? When? Whom? Diabetes Metab. 2007;33(4):247–252. doi: 10.1016/j.diabet.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 33.Monnier L, Colette C, Lapinski H, Boniface H. Self-monitoring of blood glucose in diabetic patients: from the least common denominator to the greatest common multiple. Diabetes Metab. 2004;30(2):113–119. doi: 10.1016/s1262-3636(07)70097-6. [DOI] [PubMed] [Google Scholar]
- 34.Saudek CD, Derr RL, Kalyani RR. Assessing glycemia in diabetes using self-monitoring blood glucose and hemoglobin A1c. JAMA. 2006;295(14):1688–1697. doi: 10.1001/jama.295.14.1688. [DOI] [PubMed] [Google Scholar]
- 35.Riddle MC, Rosenstock J. Gerich J on behalf of the Insulin Glargine 4002 Study Investigators. The treat-to-target trial. Randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetic patients. Diabetes Care. 2003;26(11):3080–3086. doi: 10.2337/diacare.26.11.3080. [DOI] [PubMed] [Google Scholar]
- 36.Monnier L, Colette C. Addition of rapid-acting insulin to basal insulin therapy in type 2 diabetes: indications and modalities. Diabetes Metab. 2006;32(1):7–13. doi: 10.1016/s1262-3636(07)70241-0. [DOI] [PubMed] [Google Scholar]