Abstract
Patent activity in the field of medical device technology and especially in the area of artificial pancreas development has surged in recent years. According to the search presented in this article, the number of granted U.S. patents in the area of closed-loop glucose control (CLGC) increased from 24 filed in 1991 to 247 filed in 2001. A company active in the area of diabetes technology development will likely need to understand a patent landscape consisting of hundreds of patents. Currently, both in the United States and in Europe, patentability requirements seem to be raised in order to ensure patent quality. However, the current patent landscape reflects the work of the patent offices in the past, as already granted patents are not affected by changes made to the patent grant procedure today.
Regarding the increasing amount of patents and considering the complexity of CLGC systems, the attempt to develop a CLGC system will become more and more venturesome regarding the risk of infringement of already existing patents. The consequence of this situation can be that less innovation takes place.
This article highlights some important general aspects of the patent system, briefly characterizes the current CLGC patent landscape, and illustrates by means of two exemplary patents what one angle of said patent landscape looks like. It is our opinion that, in order to support the rapid development of an artificial pancreas for patients with diabetes, adequate action to lower this hurdle should be undertaken by a consortium of all parties involved (industries, patient organizations, health-care professionals, and institutional payers).
Keywords: artificial pancreas, closed loop glucose control, freedom to operate, patent, patent landscape, patent search
Introduction
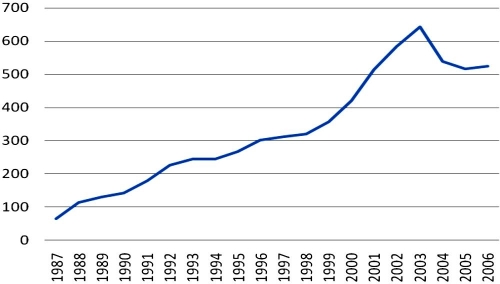
To develop a practically applicable closed-loop glucose control (CLGC) system, also called artificial pancreas, was and is the aim of many activities in the diabetes technology community. Such a system would simplify the treatment of diabetes drastically, if not technically cure diabetes. Clearly this would go along with a tighter metabolic control. As a reflection of the intensified CLGC development activities, patent activity has increased drastically over the past two decades (Figure 1). Starting out with less than 100 applications per year in 1987, the number of applications peaked in 2003 with almost 650 filings. Disregarding the high numbers of international applications in 2001 to 2003, a steady trend with an average increase of plus 23 applications filed each year can be recorded.
Figure 1.
Number of internationally filed patent applications per year in the category of medical injection devices, e.g., insulin pumps and insulin pens (IPC Class A61M 005 “Medical Injection Devices”), from 1987 to 2006.
Figure 1 also illustrates that the patent landscape related to CLGC systems consists of a vast amount of patents. In addition, the numbers in Figure 1 do not include national or regional applications and are limited to only one specific class of technology. In essence, the patent landscape is getting more complex, and presumably no one has a complete overview now or in the future. What is the consequence of this situation? Companies intending to develop CLGC products are affected by patents in two ways. On the one hand, patents are crucial to help protect developments from being exploited by copyists. But on the other hand, patents from other companies may create a complex minefield, making it difficult—if not impossible—for new products to reach the market successfully and remain there successfully. A lawsuit filed in March 2008 demonstrates that patent owners not only sit on their rights, but are also ready to enforce their patents in court,1 which can have the consequence of the respective company going bankrupt. This is especially true for smaller companies, as the risk of being involved in costly court trials benefits larger companies who can afford litigation. Furthermore, larger companies usually dispose of larger patent portfolios, which can be helpful to strike back when sued.
This comment intends to briefly introduce some important basic aspects of the patent system. Then some numbers and an exemplary search related to CLGC systems shall shed light on the volume of the patent minefield in this area. Finally, two patents related to algorithms for CLGC systems will be presented in order to illustrate how generic some patents can be.
General Aspects of the Patent System
Idea Behind the Patent System
The patent system intends to encourage inventive activities in order to foster technological development. The idea is to reward inventors for their contribution to the progress of the art. Accordingly, a patentee and the public engage in a trade-off when a patent is granted. The patentee obtains the right to prevent others from commercially exploiting his invention, whereas the public benefits from know-how disclosed when the patent is published by the respective authorities. In order to ensure that both parties to this trade-off shall profit equally, the invention must fulfill certain criteria, most importantly novelty and nonobviousness. The scope of protection conferred by a patent should be proportional to the contribution to the state of the art. An invention in a totally new field of technology normally leads to a patent with a broad scope of protection, whereas an invention in a field crowded with prior art normally leads to a patent with a narrower scope protection. Patent examination (patent office) and patent litigation (court) are instruments to ensure that an enforceable patent really does enrich the state of the art with new and valuable knowledge in relation to the patent's scope of protection.
During an advanced stage of the grant procedure, patent authorities have to decide whether or not an invention is “nonobvious” (U.S. Patent Office) or involves an “inventive step” (European Patent Office). It seems that recently, both in the United States2 and in Europe,3 the bar of nonobviousness and inventive step, respectively, are heightened in order to raise the quality of granted patents. However, measures taken by the office now will have an effect on the patent landscape in the future. This means that for the next 15 years, the patent quality reflects the patent authorities' granting policies from the past 15 years, which seems today to be considered as too low (the maximum term of a patent is 20 years from the filing date, which is typically approximately 15 years from patent grant).
Freedom to Operate versus Patentability
Regarding the increasingly large number of patents and patent applications in the field of medical device technology, freedom to operate (commercially exploiting a product without infringing third parties patent rights) causes growing concern.
While a patent allows excluding others, it does not necessarily allow its holder to exploit his invention, because a product or process according to the invention might employ another technology that might be protected by someone else. In other words, a patent confers only a negative (excluding others) and not a positive (exploiting invention) right. Consequently, a patented technology may depend on another earlier patent, and the patentee may be prevented from exploiting his newly patented invention. Or two competitors may each have patent rights protecting a similar product such that each party is in a position to prevent the other party from exploiting their product.
Even if an invention is patentable, the inventor is not necessarily free to work his invention due to the previously discussed nature of patents, namely, conferring only negative rights. Nevertheless, a patent application may prevent someone else from patenting the exact same invention at a later stage. To bar others from obtaining patent rights, any publication of the invention can alternatively be used instead of filing a patent if the sole purpose is to destroy patentability of an invention.
Intellectual Property and Complex Products
A high number of patent applications in one specific field of technology results in what is called a “patent thicket.” Walking through the thicket (i.e., developing a new product and bringing it successfully to the market), one might get caught. Due to the vast number of possibly relevant patents, no one can see a clear path leading safely through the thicket to a successful exploitation of innovation.
The complexity of patent landscapes is affect by the complexity of the product at stake. For well-characterized inventions, such as a new molecule, it may be possible to conduct a complete prior art search and to draw an intellectual property landscape with clear defining lines. In contrast, a CLGC system has a level of complexity rendering a sufficient complete prior art search nearly impossible to conduct: first, one would have to search for patents claiming systemic aspects of a CLGC system (components used, interactions between components, modularity of components, and methods of using the system); second, patents claiming each of the components (insulin pumps, glucose sensors, communication means, and algorithms) would have to be considered; and third, even some features of the components might require additional searching.
For instance, when developing a membrane of a glucose sensor for a CLGC system, patent searching in all levels of complexity may be necessary: the use of such a membrane in a CLGC system, the membrane as a feature of the glucose sensor, and the membrane as such, i.e., components of the membrane or production methods thereof.
Patents Related to Closed-Loop Glucose Control Systems
In an attempt to roughly estimate the number of patent documents related to CLGC over time, a patent search aiming at CLGC systems as such (top level of complexity) was performed. The Delphion™ software (The Thomson Corporation) was used to search databases from the United States Patent and Trademark Office (USPTO), the European Patent Office (EPO), and the World Intellectual Property Organization. A combination of (i) terms searched in the full text of the patent documents and (ii) a limitation in the field of technology in which patents were classified was found to result in a reasonable quality and quantity data output. Specifically, the terms “closed loop,” “glucose control,” and “artificial pancreas” (including similar terms such as “close loop” or “analyte control”) were combined with the term “diabetes,” and the resulting set was intersected with the most relevant International Patent Classification (IPC) classes [A61M (injection devices), A61B (diagnosis, surgery, identification), and G01N (investigating or analyzing materials by determining their chemical or physical properties)] without results classified in categories C07 (organic chemistry) and C12 (biochemistry).
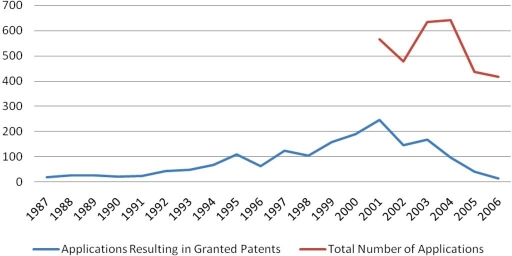
Figure 2 illustrates results obtained using the previously described searching strategy on U.S. patent documents for CGLC. The database for U.S. applications begins only in 2001, as the USPTO did not previously publish patent applications but only granted patents.
Figure 2.
Number of U.S. Patents filed per year related to closed-loop glucose control systems obtained by searching for the terms “closed loop”, “glucose control,” “artificial pancreas,” and “diabetes” combined with the most relevant IPC classes (A61M, A61B, and G01N). Similar terms were considered (e.g., “close” instead of “closed”, or “analyte” instead of “glucose”).
After a more or less constant increase, the number of granted U.S. patents related to CLGC peaks in 2001. Here it must be stated that patents filed during the past years may still be pending, and therefore the number of granted patents from the year 2001 and onward is expected to grow substantially in the future. Expecting that approximately 56% of U.S. applications are granted,4 the number of granted U.S. patents would stabilize at 200 to 350 per year between 2001 and 2006.
The total number of U.S. applications does not follow a clear trend from 2001 to 2006, with around 500 filings per year.
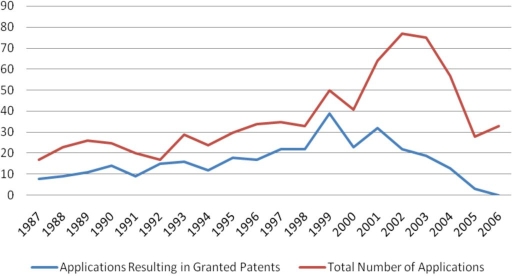
The exact same search strategy was also applied on European patent documents as illustrated in Figure 3. Here the increase in granted patents is less dramatic than in the U.S., peaking in 1999 due to the lag time in the grant procedure (applications filed after 1999 may still be pending and be granted in the future).
Figure 3.
Number of European Patents filed per year related to closed-loop glucose control systems obtained by searching for the terms “closed loop”, “glucose control,” “artificial pancreas,” and “diabetes” combined with the most relevant IPC classes (A61M, A61B, and G01N). Similar terms were considered (e.g., “close” instead of “closed”, or “analyte” instead of “glucose”).
Interestingly, the total number of applications peaks in 2002 and went down to less than half in 2005. This could be due to higher fluctuations with relatively low numbers, but on the other hand, this might indicate a trend reversal, because a similar (but less dramatic) effect can be observed in Figure 1 for international applications.
Comparing absolute numbers between U.S. and European filings per year, it is remarkable that the numbers are six times higher in the United States than in Europe. Here it should be noted that this large difference is at least partly due to differences in the systems. In Europe, it is also possible to apply for a national patent directly, which does not result in EP applications. Furthermore, EP applications are not exclusively published in English, and therefore the search terms defined above do not identify relevant documents published in languages other than English. A further explanation may be that, up to now, the major driving forces behind the development of CLGC systems were U.S. industries who tend to favor U.S. applications only.
Regarding the number of applications filed each year, and considering that the search strategy was specifically directed toward CLGC systems, there are presumably thousands of granted patents with valid claims that are potentially relevant for CLGC systems or parts thereof that we were unable to detect with our search strategy. Nevertheless, these unidentified patents could potentially become relevant regarding freedom to operate for a CLGC system.
To illustrate the patent landscape as roughly characterized earlier, we present two patents with generic claims, both of which claim CLGC systems with one kind of control algorithm each. The two patents mentioned are merely examples in the area of algorithm development, and it is explicitly stated that there are other patents related to the same technology and that there are numerous patents related to other technical aspects of a CLGC system.
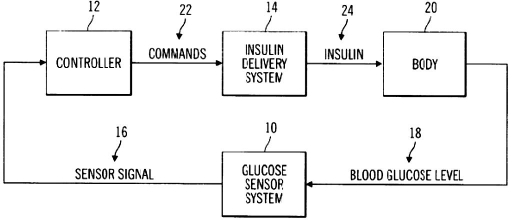
As our first example, U.S. patent 6,558,351 B1 (“Closed-Loop System for Controlling Insulin Infusion”) describes a closed-loop system including a proportional integral derivative (PID) controller. Figure 4 shows a flow diagram of the claimed system from the patent.
Figure 4.
Figure 1 of U.S. Patent 6,558,351 B1
The main claim reads as follows:
A closed-loop infusion system for infusing a fluid into a user, the system comprising
a sensor system that includes a sensor for monitoring glucose concentration of the user and produces a sensor signal, which is representative of the glucose concentration of the user, and wherein the sensor signal is used to generate a controller input;
a proportional, integral, derivative controller that uses the controller input to generate commands; and
a delivery system that infuses a liquid, which includes insulin, into the user, wherein the operation of the delivery system is affected by the commands.
This patent, filed in 2001 (and thus expiring in 2021 at the latest), was granted in the United States in 2003, and three years later, a European patent (EP 1,185,321 B1, filed in 2000 and thus expiring in 2020 at the latest) issued with a slightly modified claim.
The second example, U.S. patent 6,544,212 B2 (“Diabetes Management System”), describes a closed-loop system using a model predictive controller (MPC) as indicated in Figure 5.
Figure 5.
Figure 5 of U.S. Patent 6,544,212 B2
The granted U.S. patent claims the following:
A system for providing glycemic control to a subject, the system comprising
an insulin delivery unit;
a glucose sensor; and
a control unit including a processor unit that receives glucose value readings from the glucose sensor, executes an algorithm that predicts a glucose value at a predetermined time in the future, compares that predicted glucose value to a predetermined glucose value range, and determines a corrective amount of insulin to be administered when the predictive glucose value lies outside the predetermined glucose value range and a communication unit that transmits the corrective amount to the delivery unit.
The patent was filed in 2001 and granted in 2003 by the USPTO. A corresponding patent in Europe, filed in 2002, is presently pending before the EPO.
Proportional integral derivative and MPC algorithms are regarded as the two main approaches for the development of a control algorithm in a CLGC system.5 Accordingly, patents such as the ones mentioned earlier probably have to be taken into consideration when developing CLGC systems.
A more positive comment is that several, in general, very detailed patents have been filed. Such patents may prevent the scenario that the artificial pancreas as such is blocked by a single patent. As an illustrative example of such an early patent, US 509784, which was filed in 1989, describes a very general view of a single-port artificial pancreas system (with glucose sensing and insulin administration through the same port). The said patent will have thus expired by the time CLGC systems are mature enough to enter the market, and it will make it impossible for a later patent to validly claim a single-port artificial pancreas system in general.
In conclusion, patent filing may still be possible in yet unexplored technological niches, while commercial exploitation will require cross licensing of technologies.
Conclusion
The development of a practically usable CLGC system would be a major step forward for patients with diabetes. Such a technological cure would not only ease daily life, but it would probably prevent the development of diabetes-related complications. This in turn would save a substantial amount of money (direct and indirect costs) for the health care systems. Clearly it has to be demonstrated that such savings balance the costs for the daily usage of such a CGLC system.
In view of the high importance of developing a CGLC system sooner than later, one wonders if we have an issue with patents here. One clearly has to acknowledge the need of companies to secure the outcome of research in which they have heavily invested. Their wish to be able to earn money with a given invention is fully understandable. However, it may be possible that, in the end, holders of patents with a broad scope may block further artificial pancreas development that relies on such technologies by enforcing their rights in court. If patent holders block each other, no CGLC system might become available, and a different approach to fulfill all expectations becomes necessary.
To develop such an approach, which in the end is a political and economic decision, sufficient incentives need to be made available to patent owners to dispose of their rights for the benefit of the society. This may involve lobbying by patient organizations who hold substantial power and clearly want the artificial pancreas to be made available soon. For instance, patient organizations may support technologies deemed of high potential to prove themselves in clinical trials prior to commercialization. Such would be a powerful incentive for large industries to back innovative CLGC systems. It may also involve standardization efforts to ensure interoperability of the various components of a CLGC system (pump, sensor, and algorithm) among manufacturers. The question is whether a driving force behind such an initiative can be found.
Abbreviations
- CLGC
closed-loop glucose control
- EPO
European Patent Office
- IPC
international patent classification
- MPC
model predictive controller
- PID
proportional integral derivative
- USPTO
United States Patent and Trademark Office
References
- 1.Orenstein BW. Roche sues for diabetes patent infringement. http://www.devicelink.com/ivdt/archive/08/03/002.html. Accessed 21 April 2008.
- 2.KSR v. Teleflex. http://www.supremecourtus.gov/opinions/06pdf/04-1350.pdf. Accessed 18 April 2008.
- 3.European Patent Office. “EPO grants fewer patents despite rise in applications. http://www.epo.org/topics/news/2008/20080401.html. Accessed 18 April 2008.
- 4.Trilateral Co-Operation. Patent activity at Trilateral Offices. Table 4. http://www.trilateral.net/tsr/tsr_2006/4_tril_off_pat_act_2006.pdf. Accessed 17 April 2008.
- 5.Hovorka R. Continuous glucose monitoring and closed-loop systems. Diabet Med. 2006;23(1):1–12. doi: 10.1111/j.1464-5491.2005.01672.x. [DOI] [PubMed] [Google Scholar]