Abstract
This article discusses the use of microneedles in automated diabetes therapy systems. Advanced bioengineered systems have the potential to close the loop between diagnostic and therapeutic elements of diabetes treatment, thus constituting a “smart” system. Prevalent insulin therapies, and most glucose sensing techniques, involve the transfer of physical entities through the skin. Micromachined needles (microneedles) can achieve this in a noninvasive or minimally invasive manner while contributing various other technological merits. The dynamics of autonomous diabetes therapy systems include highly complex interdependencies between the various physical and biological entities involved, thus warranting multidisciplinary research initiatives. The iterative development of a noninvasive, bioengineered interface such as microneedles necessitates a better understanding of the human skin, its molecular architecture as a polymer film, and its role as a functional biological unit. This review addresses application-specific requirements of a microneedle-based interface system specifically for autonomous diabetes therapy. Key design issues and related parametric interdependencies specific to this application are discussed.
Keywords: bio-microelectromechanical systems, diabetes therapy, microneedles
Introduction
Currently about 250 million people in the world suffer from diabetes mellitus. Exogenous insulin replacement is the primary form of treatment for type 1 diabetes, and it is also used in cases of advanced untreated type 2 diabetes.1,2 Insulin therapy requires periodic monitoring of blood glucose levels combined with intermittent injections of insulin to optimize the blood glucose levels while minimizing the risk of hypoglycemia. A number of insulin preparations are currently available whose onset and duration of action can vary widely. Combining a short-acting preparation with a longer-acting one is often required to treat patients with insulin resistance or those whose glucose levels gyrate over wide ranges. Unfortunately, manual administration of the insulin is essentially an “approximate therapy.” The dynamics of natural production of insulin are highly complex and nonlinear; the conventional method cannot replicate the optimum insulin levels accurately either quantitatively or temporally. Moreover, an unignorable factor in the manual technique is the possibility of human error or patient noncompliance.
Advanced bioengineered systems have the potential to close the loop between diagnostic and therapeutic elements of diabetes treatment, thus constituting a “smart” system.3 Prevalent insulin therapies, and most glucose sensing techniques, involve the transfer of physical entities through the skin. Traditional approaches used to collect biofluids or delivery drugs include needle puncture, electroporation or vaporization, or removal of the stratum corneum through gels or tapes. Micromachined needles (microneedles) can enable collection of the same information, with significantly less trauma to the tissue, and have the potential of even eliminating it. This review addresses those factors important in the design of such a system. In particular, the design of microneedle systems is reviewed.
Autonomous Diabetes Therapy Systems
Autonomous insulin therapy systems that automatically monitor the glucose levels and intermittently inject the requisite amount of insulin at appropriate times can address many of the problems associated with manual techniques.4–6 At the broadest level, a generic device consists of the following parts: (a) a glucose sensor (diagnostic component), (b) an insulin delivery mechanism (therapeutic component), and (c) a feedback mechanism that bridges between glucose sensing and insulin delivery units (control component). Figure 1 depicts the elements of a prototypical microneedle-based diabetes therapy system.
Figure 1.
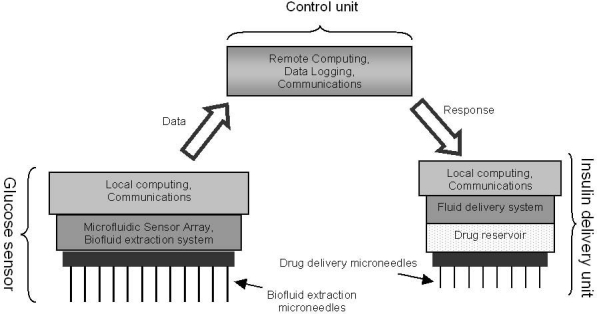
Elements of the prototypical microneedle-based diabetes therapy system.
Currently there is an assortment of stand-alone glucose sensors and insulin delivery mechanisms available or being developed. Not all types of glucose sensors and insulin delivery devices are conducive to use in autonomous systems. The diagnostic and therapeutic units of choice should fulfill the following criteria:
Require minimal intervention on part of the patient
Be extremely reliable
Preferably be minimally invasive or completely noninvasive
Stand-alone glucose sensors vary extensively in their approach, implementation, or complexity. Typical variables are as follow.
Degree of invasiveness—highly invasive (implantable sensors9), moderately invasive (finger stick type10), or noninvasive (extracorporeal watch type11)
Sensing technique (colorimetric,12 electrochemical,13 ultrasound,14 dielectric spectroscopy,15 near infrared16)
Insulin delivery mechanisms vary based on their method of administration, namely intravenous injection,17 subcutaneous injection,18 intraperitoneal injection,19 or nasal delivery.20 Of these, the subcutaneous injection mechanism is the most prevalent due to accurate dosage control and moderate to low levels of invasiveness.
The feedback mechanism, or the control unit, is the most critical component of the automated insulin therapy device. The dynamics of glucose absorption and insulin production are highly complex. Translating empirical data and available knowledge on internal metabolisms into efficient mathematical equations and control algorithms is not an easy task. Ideally, the program should allow for a patient-specific tailoring of the glucose level vs insulin delivery scheme in order to closely match real metabolic activities. In general, two kinds of feedback control algorithms are being used.21
Closed-loop control—only uses feedback from the glucose sensor. No external interaction.
Partially closed-loop control—uses feedback from glucose sensor, as well as the physician's assessment of the patient's requirement. Figure 2 depicts the control diagram of a typical partially closed-loop therapy model.
Figure 2.
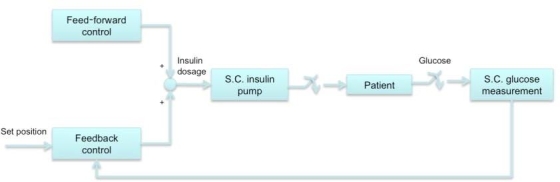
Typical control flow of partially closed-loop insulin therapy models.21 S.C., subcutaneous.
Open-loop control schemes are also being used for preprogrammed therapy.22 These schemes control the insulin delivery mechanism based solely on the physician's input. As they do not provide for any feedback from the glucose sensor, they can only be regarded as partially autonomous systems.
Microneedle-Based Skin Interface Systems
Even though noninvasive glucose monitoring has greatly improved recently,23 the techniques used by these devices still lag in accuracy as compared to direct electrochemical glucose measurements. This is because these devices rely on inexact mathematical algorithms. Accurate calibration of these devices is problematic due to varying lipids, proteins, and water levels in humans. Although noninvasive techniques remain an option for glucose sensing, administration of insulin necessitates at least minimal invasion
Traditional methods of subcutaneous insulin injection or biofluid sampling employ hypodermic needles. Although utilitarian, this method causes undesirable pain and tissue trauma. This is particularly troublesome in the case of diabetes where frequent sampling and drug delivery are required. One solution to these problems is the use of microneedles, a minimally invasive skin interface tool.
Microneedles are microscopic needles capable of piercing the skin and creating micrometer-sized perforations. Perforations of micrometer dimensions are large enough to allow macromolecules such as insulin to pass through. The microneedles are long enough to penetrate the outermost layer of skin. However, depending on the application, either they do not penetrate deep enough to reach the underlying nerves, thus being totally painless,24 or they just graze the tips of nerves, causing sensation but reducing pain nevertheless.24a
System Architecture of Microneedle-Based Diabetes Therapy Device
Microneedles form the generic abiotic front-end interface to the biotic domain. The use of microneedles imposes certain architectural requirements pertaining to the autonomous device. The micrometer size domain of microneedles (∼10 to 500 μm in diameter) necessitates the use of microfluidic components and microelectromechanical systems technologies. At the microscale, effects such as laminar flow, diffusion, fluidic resistance, surface to volume ratio, and surface tension become dominant,25 thus scaling or shrinking of traditional large devices cannot be done. Microfluidic-based versions of components, e.g., chambers, channels, pumps, valves, and mixers, are required. It should be noted that a natural predilection toward smaller device footprints has led to the use of microfluidics in even nonmicroneedle-based autonomous sensors.
An advantage of using microfluidics, apart from smaller device size, is the convenience of adding communication and data logging capabilities. The glucose sensor and the insulin delivery unit need not be physically located at the same position on the patient's body and may interact wirelessly with each other. The unit may record raw and significant data, which can be analyzed later by a physician or researcher. Historical data can also be actively utilized by more complex algorithms such that the insulin delivery scheme is dynamically tailored based on past conditions or responses. Advanced systems in the future might follow a node-based approach for global-level monitoring and incorporate distributed computing algorithms and low-power communication protocols at those nodes.
Anatomy of Skin: Diabetes Therapy-Specific Perspective
To understand the nuances involved in interfacing with skin, one needs to understand its anatomy from an application-specific perspective; in this case, microneedle-based diabetes therapy. The skin hosts simultaneously occurring complex physiological, biomechanical, and biochemical processes. These are brought about by tissues within its two layers: the dermis and the epidermis. The epidermis is the tough and waterproof outer layer of the skin that protects the body's interior from foreign substances. The underlying dermis is a thicker layer and is responsible for imparting strength and elasticity to the skin. Figure 3 illustrates the cross-sectional view of human skin.
Figure 3.
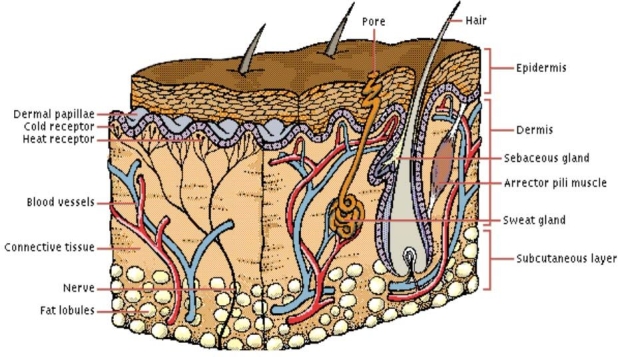
Cross section of skin.26
An estimated 90% of the epidermal cells are “keratinocytes.” They produce keratin, a tough, fibrous, intracellular protein. Keratinocytes are stacked in layers. The youngest cells occupy the lower layers while older cells are present in the upper ones. The lower layer cells multiply continually and migrate to the upper layer. By the time the cells move up to the outermost layer (stratum corneum) of the epidermis, they are dead, completely filled with keratin, and are continually sloughed.
The stratum corneum in particular dominates various design considerations, especially the micromachined interfaces. This layer is a thin, flexible, high impedance bio-polymer composed of interconnected “dead” cells called corneocytes. This complexly organized, anucleate, 15- to 20-μm-thick, biopolymeric structure is essential to life and serves to couple the organism to the environment. This structure is particularly well developed in humans who lack a protective mantle of fur. The stratum corneum precludes passive transdermal delivery of insulin, as its molecules are too large (∼50 Å in diameter) to pass through. Thus, the key requirement to develop the microneedle interface is that the stratum corneum needs to be ruptured for successful insulin delivery.
Glucose (molecular mass 180 daltons, ∼1 Å in diameter) is present in both blood and ISF below the stratum corneum. The epidermal layer, from approximately 40 to 400 μm depth, contains ISF that can be sampled for glucose measurements. Blood capillaries are present just below the epidermis at penetration depths of about 400 μm. Nerve cells tips are also found at the same depth as capillaries.
Microneedle Design for Autonomous Diabetes Therapy Systems
An application-independent optimum design for microneedles does not exist. Design selection is highly application specific and involves simultaneous consideration of multiple parameters. Common design variables include geometric features (length, diameter, shape), choice of material, array layout, and physical architecture (beveled tip, conical, side-opened). There is always a trade-off among the various output characteristics, such as fragility, biocompatibility, penetration force, fluid flow rates, ease of fabrication, and cost. Application-specific requirements of diabetes therapy systems necessitate that certain microneedle characteristics have higher priority. Key design issues and related parametric interdependencies specific to diabetes therapy systems are discussed next.
Microneedles for the Glucose Sensing Component
Glucose sensing can be done by sampling either blood or ISF. The choice of the biofluid sampled is the primary factor determining microneedle design. Numerous studies have been performed on differences between blood glucose levels in blood and ISF.27–29 It has been generally observed that a time lag exists in the distribution of glucose from blood to ISF. Estimates of the lag time range from 0 to 45 minutes.28 However, once equilibrium is reached, blood and ISF glucose levels correlate highly. In order to understand design variations between blood extracting microneedles and ISF extracting microneedles, it is important to understand physiological differences between blood and ISF.
Microneedles for Interstitial Fluid Sampling. The depth of microneedle penetration needs to be in the approximate range of 50–150 μm to extract ISF. At these depths, microneedle insertion is painless.24 Such a low microneedle height requirement translates to a higher latitude in design variations. Microneedles have two possible failure scenarios: fracture or buckling. In general, shorter needles, of the same diameter and material, can withstand higher pressures without failing. Thus, needles composed of relatively lower strength material, e.g., silicon dioxide, can be used for ISF sampling. Silicon dioxide is also highly biocompatible, an additional advantage. Reduced height allows for smaller needle diameters without inducing buckling.30 A smaller tip diameter results in a much higher ratio of fracture force vs insertion force into skin.31 This increases the margin of safety for employing microneedles without failure. Microneedle lumen diameters for ISF sampling can typically be as low as 10 μm.
A small microneedle diameter coupled with the low density of ISF induces extremely high capillary forces. Capillary forces also increase with higher hydrophilicity of the microneedle material. This facilitates extraction of the fluid even without a pumping mechanism. Unfortunately, the flow rate through the microneedle declines with decreasing diameter.32 Thus, an initial latent time exists before the microneedles are filled with ISF.33 Most commercial ISF glucose sensors require around 0.5–2 μl of fluid,34 and this figure is continuously decreasing. In order to increase flow rates, an array of microneedles is used to achieve the required flow. Vacuum pump-assisted ISF sampling using microneedles in humans has been demonstrated and shown to successfully track changing glucose levels following insulin injection with a time lag of less than 20 minutes.35
Microneedles for Blood Sampling. Blood capillaries are present just below the epidermis. Generally, blood microcapillaries are found at penetration depths of about 400 μm. The nerve tips are also present in the same depth vicinity. Thus some microneedles within the array might just graze the topmost nerve cells. However, the extremely small diameters and controlled shank length reduce the odds of encountering a nerve or of stimulating it enough to induce much pain.36,37 Research that explored the effect of microneedle design on pain in humans found that needles ranging from 480 to 1450 μm in length resulted in pain scores 5 to 40% of a 26-gauge hypodermic needle.24a Figure 4 depicts the relative insertion depths of microneedles used for blood or ISF sampling.
Figure 4.
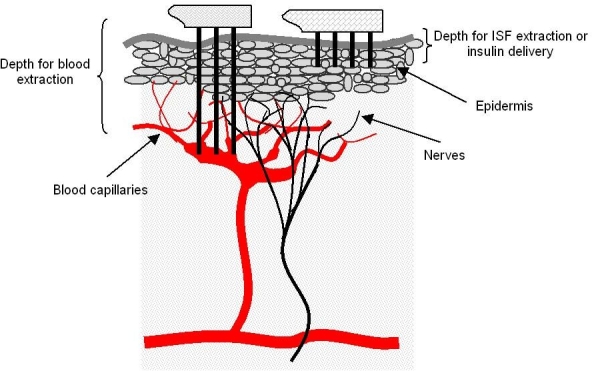
Relative insertion depths of microneedles.
In order to extract blood without significant pain, microneedle shank lengths need to be around 400–900 μm. At these lengths the microneedle needs to be built using higher strength materials such as metal or silicon. A common model used by researchers is the dimension of the female mosquito proboscis.38,39 The microneedle diameter needs to be large enough to allow convenient passage to the largest blood cells. Also, the larger length necessitates larger diameters to preclude needle failure via buckling. Typical microneedle diameters have to be at least 50 μm wide. Even though capillary action alone can be enough to extract blood, factors such as higher fluid density, larger conduit diameter, and material of choice can mitigate the effect. In such a case, a microfluidic pumping device is needed to generate negative pressure.38
Microneedles for the Insulin Delivery Component
Insulin can be delivered into the epidermal region, and thus these microneedles have the same length requirements as those required for ISF sampling. In order to regulate the delivery of insulin, the delivery component would need to incorporate a pumping mechanism controlled via the feedback unit. The active pumping scheme removes the dependence on capillary force. This obviates restriction on material choice based on degree of hydrophilicity. Various kinds of microneedles have been built using polymers,40,41 metals,42,43 silicon dioxide,44,45 and silicon.46–48 An insulin molecule being small in size, any suitable lumen diameter of microneedle can be chosen and typically ranges from 10 to 100 μm at the tip.
For fluid infusion, flow rates of greater than 1 ml/hour for a single microneedle have been demonstrated.49 Even with modest rates, and employing a needle array, the requisite amount of dosage can be transferred easily via microneedles. Reduction of glucose levels by insulin delivery using microneedles has been exhibited successfully in animal models.42,50 Studies have shown a 47–80% drop in glucose levels by 0.05–0.5 units of insulin delivered in this way.
Microneedle Array Design
Deciding the microneedle array specifications (pitch, size of array) is as important as design of the individual microneedle. Microneedles can sometimes get clogged by tissue being trapped in a needle lumen during insertion (beveled-tip51 or side-opened52,53 needle designs minimize these effects). Employing numerous needles minimizes the influence of individual needle failures or passage blockages. Also, as mentioned earlier, use of an array formation increases fluid flow rates. The flow rate increases linearly with the number of microneedles in the array. However, care has to be taken not to place needles too close to each other, as otherwise a “bed-of-nails” effect can result in the skin being pushed down uniformly without penetration.54 Generally, microneedles are placed more than 200 μm apart, and array size can be as small as a few microneedles to hundreds.
Fluid flow through a microneedle is generally assumed to be laminar. It largely depends on the pressure difference across the needle and is set by the microfluidic pump and the capillary forces. Unfortunately, a fluid-mechanical description of the skin has not been established yet, and thus modeling flow through a microneedle is a complex task, which is complicated further by the fact that biofluids generally exhibit non-Newtonian behavior. Various nonlinear in vivo effects, such as liquid absorption in the epidermis, hindrance to fluid motion due to the presence of cells, and saturation, play a role in fluid dynamics. Furthermore, there are present pressure losses due to flow down a microneedle. These can be attributed to entrance losses, drag on the duct walls, and losses as a consequence of specific microneedle geometry (expansions, bends, etc.).55 Nevertheless, simplified modeling of fluid flow can be used to obtain rough estimates of design variables. To model fluid flow in microneedles, the basic modified Bernoulli equation is often used as an approximation. Equation (1) depicts this relation:
| (1) |
where Δp is the pressure drop, q is the flow rate, K1 and K2 are macroscopic values that represent inertial minor losses in piping systems, ρ is the density of liquid, μ is the viscosity, D is the diameter of the needle, and L is the length. To establish rough flow rates in microneedles, the Hagen–Poiseuille relation, depicted in Equation (2), is used. This equation describes slow viscous incompressible flow through a constant circular cross section:
| (2) |
For n microneedles, the net flow rate gets multiplied by a factor of n.
Application-Specific Considerations and Challenges
Microneedle design for autonomous diabetes therapy needs to account for the nature of application. Microneedles should be robust enough to withstand repeated penetration and extended use without failure. Microneedle reliability is highly crucial as their failure can lead to incorrect or even failed dosage. Even though microneedle insertion and failure force studies have been done,31,43 extensive characterization of their durability is needed to accurately ascertain their practical lifetime.
Due to prolonged use requirements, the degree of biocompatibility also becomes an important factor. This necessitates that the microneedles comply with stringent precision requirements and adhere to high quality control. The biocompatibility of materials needs to be established clinically. Although silicon is relatively versatile for microneedle fabrication, its degree of biocompatibility can lag as compared to certain metals. Microneedles made of biodegradable polymers40,41 should also be explored further.
An automated diabetes therapy device constitutes a safety-critical system. The system intends to distance human intervention from its functionality by removing patient and physician from the active loop. The nature of application opens up an assortment of technical, ethical, and legal issues. A discussion of these issues is beyond the scope of this review. However, they would need to be addressed when this technology is put into application.
Technical Merits of Microneedle Use for Diabetes Therapy
Relatively painless insertion and ease of use translate to reduced risk of patient noncompliance and human error. Furthermore, it is speculated that microneedle use would demand minimal medical training. These benefits are especially welcome considering their relevance to diabetic children.
Some researchers believe that the small volumes of insulin passing through the microneedles via auxiliary pumping systems allow for precise quantitative control and continuous delivery. Traditional therapy schemes assume only infrequent administration of insulin and thus need to employ slow-releasing synthetic analogs to replace a basal supply of insulin. Unfortunately, these slow-acting insulins also take longer times to start taking effect. This prevents effective predictions of future blood glucose profiles.56 By allowing controlled injection over an extended period of time, it becomes possible to deliver short half-life insulin more frequently. Thus the insulin concentration can be maintained within the therapeutic window more accurately.
Alternative to Microneedles
A few noninvasive alternatives to microneedle-based interfaces are currently being developed.57 Most promising techniques include iontophoresis,58 electroporation,59 and use of low-frequency ultrasound.60 All of these methods can potentially be used for autonomous diabetes therapy systems and, at times, have been shown to have great value to this application. It is imperative that multiple approaches to the same issue are explored simultaneously to expedite the obsolescing of conventional methods.
Conclusion
The dynamics of autonomous diabetes therapy systems include highly complex interdependencies between the various physical and biological entities involved, thus warranting multidisciplinary research initiatives. Optimum utilization of this technology depends on our capacity to identify and resolve, qualitatively and quantitatively, the various parametric entities involved. Successful clinical implementation would entail overcoming numerous challenges in areas of design, fabrication, electronics integration, packaging, deployment, testing, and data interpretation, among many others. The development will necessitate integration of broad advances in a wide variety of fields, namely microfluidics, clinical medicine, cell and molecular biology, physiology, anatomy, microfabrication, information technology, and signal processing.
The confluence of emerging disciplines promises to bridge the biotic (organism) and abiotic (environment) realms in unanticipated ways. The iterative development of a noninvasive, bioengineered interface such as microneedles necessitates a better understanding of the human skin. Seamless coupling to the body surface demands better knowledge of the molecular architecture of the skin as a polymer film and its role as a functional biological unit.
Abbreviations
- ISF
interstitial fluid
References
- 1.Davidson MB. Starting insulin therapy in type 2 diabetic patients: does it really matter how? Diabetes Care. 2005;28(2):494–495. doi: 10.2337/diacare.28.2.494. [DOI] [PubMed] [Google Scholar]
- 2.Blonde L. Easing the transition to insulin therapy in people with type 2 diabetes. Diabetes Educ. 2007;33(Suppl 7):232S–240S. doi: 10.1177/0145721707305695. [DOI] [PubMed] [Google Scholar]
- 3.Khanna P, Hoath S, Smallwood R, Bhansali S. Smart Biosensor Technology. CRC Press; 2007. The challenge of human skin-engineering the biotic/abiotic interface; pp. 249–268. [Google Scholar]
- 4.Hovorka R. Continuous glucose monitoring and closed-loop systems. Diabet Med. 2006;23(1):1–12. doi: 10.1111/j.1464-5491.2005.01672.x. [DOI] [PubMed] [Google Scholar]
- 5.Renard E. Implantable closed-loop glucose-sensing and insulin delivery: the future for insulin pump therapy. Curr Opin Pharmacol. 2002;2(6):708–716. doi: 10.1016/s1471-4892(02)00216-3. [DOI] [PubMed] [Google Scholar]
- 6.Steil GM, Panteleon AE, Rebrin K. Closed-loop insulin delivery– the path to physiological glucose control. Adv Drug Deliv Rev. 2004;56(2):125–144. doi: 10.1016/j.addr.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 7.Jeffrey D. Newman JD, Turner AP. Home blood glucose biosensors: a commercial perspective. Biosens Bioelectron. 2005;20(12):2435–2453. doi: 10.1016/j.bios.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 8.Bodenlenz M, Schaupp LA, Druml T, Sommer R, Wutte A, Schaller HC, Sinner F, Wach P, Pieber TR. Measurement of interstitial insulin in human adipose and muscle tissue under moderate hyperinsulinemia by means of direct interstitial access. Am J Physiol Endocrinol Metab. 2005;289(2):E296–E300. doi: 10.1152/ajpendo.00431.2004. [DOI] [PubMed] [Google Scholar]
- 9.Renard E. Implantable closed-loop glucose-sensing and insulin delivery: the future for insulin pump therapy. Curr Opin Pharmacol. 2002;2(6):708–716. doi: 10.1016/s1471-4892(02)00216-3. [DOI] [PubMed] [Google Scholar]
- 10.Guerci B, Benichou M, Floriot M, Bohme P, Fougnot S, Franck P, Drouin P. Accuracy of an electrochemical sensor for measuring capillary blood ketones by fingerstick samples during metabolic deterioration after continuous subcutaneous insulin infusion interruption in type 1 diabetic patients. Diabetes Care. 2003;26(4):1137–1141. doi: 10.2337/diacare.26.4.1137. [DOI] [PubMed] [Google Scholar]
- 11.Tierney MJ, Tamada JA, Potts RO, Jovanovic L, Garg S. Cygnus Research Team. Clinical evaluation of the GlucoWatch biographer: a continual, non-invasive glucose monitor for patients with diabetes. Biosens Bioelectron. 2001;16(9-12):621–629. doi: 10.1016/s0956-5663(01)00189-0. [DOI] [PubMed] [Google Scholar]
- 12.Schier GM, Moses RG, Gan IE, Blair SC. An evaluation and comparison of Reflolux II and Glucometer II, two new portable reflectance meters for capillary blood glucose determination. Diabetes Res Clin Pract. 1988;4(3):177–181. doi: 10.1016/s0168-8227(88)80015-9. [DOI] [PubMed] [Google Scholar]
- 13.Heller A. Implanted electrochemical glucose sensors for the management of diabetes. Annu Rev Biomed Eng. 1999;1:153–175. doi: 10.1146/annurev.bioeng.1.1.153. [DOI] [PubMed] [Google Scholar]
- 14.Kost J, Mitragotri S, Gabbay RA, Pishko M, Langer R. Transdermal monitoring of glucose and other analytes using ultrasound. Nat Med. 2000;6(3):347–350. doi: 10.1038/73213. [DOI] [PubMed] [Google Scholar]
- 15.Caduff A, Hirt E, Feldman Y, Ali Z, Heinemann L. First human experiments with a novel non-invasive, non-optical continuous glucose monitoring system. Biosens Bioelectron. 2003;19(3):209–217. doi: 10.1016/s0956-5663(03)00196-9. [DOI] [PubMed] [Google Scholar]
- 16.Burmeister JJ, Arnold MA. Evaluation of measurement sites for noninvasive blood glucose sensing with near-infrared transmission spectroscopy. Clin Chem. 1999;45(9):1621–1627. [PubMed] [Google Scholar]
- 17.Parker RS. Doyle FJ 3rd, Peppas NA. The intravenous route to blood glucose control. IEEE Eng Med Biol Mag. 2001;20(1):65–73. doi: 10.1109/51.897829. [DOI] [PubMed] [Google Scholar]
- 18.Lenhard MJ, Reeves GD. Continuous subcutaneous insulin infusion: a comprehensive review of insulin pump therapy. Arch Intern Med. 2001;161(19):2293–2300. doi: 10.1001/archinte.161.19.2293. [DOI] [PubMed] [Google Scholar]
- 19.Pitt HA, Saudek CD, Zacur HA. Long-term intraperitoneal insulin delivery. Ann Surg. 1992;216(4):483–492. doi: 10.1097/00000658-199210000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gómez-Pérez FJ, Rull JA. Insulin therapy: current alternatives. Arch Med Res. 2005;36(3):258–272. doi: 10.1016/j.arcmed.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 21.Takahashi D, Xiao Y, Hu F. Int J Telemed Appl. 2008. A survey of insulin-dependent diabetes– Part II: Control methods. 739385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spencer WJ. A review of programmed insulin delivery systems. IEEE Trans Biomed Eng. 1983;28(3):237–251. doi: 10.1109/tbme.1981.324696. [DOI] [PubMed] [Google Scholar]
- 23.Caduff A, Hirt E, Feldman Y, Ali Z, Heinemann L. First human experiments with a novel non-invasive, non-optical continuous glucose monitoring system. Biosens Bioelectron. 2003;19(3):209–217. doi: 10.1016/s0956-5663(03)00196-9. [DOI] [PubMed] [Google Scholar]
- 24.Kaushik S, Hord AH, Denson DD, McAllister DV, Smitra S, Allen MG, Prausnitz MR. Lack of pain associated with microfabricated microneedles. Anesth Analg. 2001;92(2):502–504. doi: 10.1097/00000539-200102000-00041. [DOI] [PubMed] [Google Scholar]
- 24a.Gill HS, Denson DD, Burris BA, Prausnitz MR. Effect of microneedle design on pain in human volunteers. Clin J Pain. 2008;24(7):585–594. doi: 10.1097/AJP.0b013e31816778f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beebe DJ, Mensing GA, Walker GM. Physics and applications of microfluidics in biology. Annu Rev Biomed Eng. 2002;4:261–286. doi: 10.1146/annurev.bioeng.4.112601.125916. [DOI] [PubMed] [Google Scholar]
- 26.Structure of the skin, Microsoft Encarta Encylopedia. http://encarta.msn.com. 1993-2005 Microsoft Corporation.
- 27.Kulcu E, Tamada JA, Reach G, Potts RO, Lesho MJ. Physiological differences between interstitial glucose and blood glucose measured in human subjects. Diabetes Care. 2003;26(8):2405–2409. doi: 10.2337/diacare.26.8.2405. [DOI] [PubMed] [Google Scholar]
- 28.Stout PJ, Peled N, Erickson BJ, Hilgers ME, Racchini JR, Hoegh TB. Comparison of glucose levels in dermal interstitial fluid and finger capillary blood. Diabetes Technol Ther. 2001;3(1):81–90. doi: 10.1089/152091501750220046. [DOI] [PubMed] [Google Scholar]
- 29.Boyne MS, Silver DM, Kaplan J, Saudek CD. Timing of changes in interstitial and venous blood glucose measured with a continuous subcutaneous glucose sensor. Diabetes. 2003;52(11):2790–2794. doi: 10.2337/diabetes.52.11.2790. [DOI] [PubMed] [Google Scholar]
- 30.Friedl KE. Analysis: optimizing microneedles for epidermal access. Diabetes Technol Ther. 2005;7(3):546–548. doi: 10.1089/dia.2005.7.546. [DOI] [PubMed] [Google Scholar]
- 31.Davis SP, Landis BJ, Adams ZH, Allen MG, Prausnitz MR. Insertion of microneedles into skmeasurement and prediction of insertion force and needle fracture force. J Biomech. 2004;37(8):1155–1163. doi: 10.1016/j.jbiomech.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 32.Martanto W, Baisch SM, Costner EA, Prausnitz MR, Smith MK. Fluid dynamics in conically tapered microneedles. AIChE J. 2005;51(6):1599–1607. [Google Scholar]
- 33.Mukerjee EV, Collins SD, Isseroff RR, Smith RL. Microneedle array for transdermal biological fluid extraction and in situ analysis. Sens Actuat A Phys. 2004;114(2-3):267–275. [Google Scholar]
- 34.Collison ME, Stout PJ, Glushko TS, Pokela KN, Mullins-Hirte DJ, Racchini JR, Walter MA, Mecca SP, Rundquist J, Allen JJ, Hilgers ME, Hoegh TB. Analytical characterization of electrochemical biosensor test strips for measurement of glucose in low-volume interstitial fluid samples. Clin Chem. 1999;45(9):1665–1673. [PubMed] [Google Scholar]
- 35.Wang PM, Cornwell M, Prausnitz MR. Minimally invasive extraction of dermal interstitial fluid for glucose monitoring using microneedles. Diabetes Technol Ther. 2005;7(1):131–141. doi: 10.1089/dia.2005.7.131. [DOI] [PubMed] [Google Scholar]
- 36.Moon SJ, Lee SS. A novel fabrication method of a microneedle array using inclined deep x-ray exposure. J Micromech Microeng. 2005;15:903–911. [Google Scholar]
- 37.Brazzle J, Papautsky I, Frazier AB. Micromachined needle arrays for drug delivery or fluid extraction. IEEE Eng Med Biol Mag. 1999;18(6):53–58. doi: 10.1109/51.805145. [DOI] [PubMed] [Google Scholar]
- 38.Tsuchiya K, Nakanishi N, Uetsuji Y, Nakamachi E. Development of blood extraction system for health monitoring system. Biomed Microdevices. 2005;7(4):347–353. doi: 10.1007/s10544-005-6077-8. [DOI] [PubMed] [Google Scholar]
- 39.Gattiker GE, Kaler KV, Mintchev MP. Electronic Mosquito: designing a semi-invasive microsystem for blood sampling, analysis and drug delivery applications. Microsystem Technol. 2005;12(1-2):44–51. [Google Scholar]
- 40.Park JH, Allen MG, Prausnitz MR. Polymer microneedles for controlled-release drug delivery. Pharm Res. 2006;23(5):1008–1019. doi: 10.1007/s11095-006-0028-9. [DOI] [PubMed] [Google Scholar]
- 41.Park JH, Allen MG, Prausnitz MR. Biodegradable polymer microneedles: fabrication, mechanics and transdermal drug delivery. J Control Release. 2005;104(1):51–66. doi: 10.1016/j.jconrel.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 42.Davis SP, Martanto W, Allen MG, Prausnitz MR. Hollow metal microneedles for insulin delivery to diabetic rats. IEEE Trans Biomed Eng. 2005;52(5):909–915. doi: 10.1109/TBME.2005.845240. [DOI] [PubMed] [Google Scholar]
- 43.Chandrasekaran S, Frazier AB. Characterization of surface micromachined metallic microneedles. J Microelectromech Syst. 2003;12(3):289–295. [Google Scholar]
- 44.Rodriguez A, Molinero D, Valera E, Trifonov T, Marsal LF, Pallares J, Alcubilla R. Fabrication of silicon oxide microneedles from macroporous silicon. Sens Actuat B Chem. 2005;109(1):135–140. [Google Scholar]
- 45.Rajaraman S, Henderson HT. A unique fabrication approach for microneedles using coherent porous silicon technology. Sens Actuat B Chem. 2005;105(2):443–448. [Google Scholar]
- 46.Paik SJ, Byun S, Lim JM, Park Y, Lee A, Chung S, Junkeun Chang J, Kukjin Chun K, Cho D. In-plane single-crystal-silicon microneedles for minimally invasive microfluid systems. Sens Actuat A Phys. 2004;114(2-3):276–284. [Google Scholar]
- 47.Gardeniers HJ, Luttge R, Berenschot EJ, de Boer MJ, Yeshurun SY, Hefetz M, van't Oever R, van den Berg A. Silicon micromachined hollow microneedles for transdermal liquid transport. J Microelectromech Syst. 2003;12(6):855–862. [Google Scholar]
- 48.Wilke N, Hibert C, O'Brien J, Morrissey A. Silicon microneedle electrode array with temperature monitoring for electroporation. Sens Actuat A Phys. 2005;123-124:319–325. [Google Scholar]
- 49.Martanto W, Moore JS, Kashlan O, Kamath R, Wang PM, O'Neal JM, Prausnitz MR. Microinfusion using hollow microneedles. Pharm Res. 2006;23(1):104–113. doi: 10.1007/s11095-005-8498-8. [DOI] [PubMed] [Google Scholar]
- 50.Martanto W, Davis SP, Holiday NR, Wang J, Gill HS, Prausnitz MR. Transdermal delivery of insulin using microneedles in vivo. Pharm Res. 2004;21(6):947–952. doi: 10.1023/b:pham.0000029282.44140.2e. [DOI] [PubMed] [Google Scholar]
- 51.Perennes F, Marmiroli B, Matteucci M, Tormen M, Vaccari L, Di Fabrizio E. Sharp beveled tip hollow microneedle arrays fabricated by LIGA and 3D soft lithography with polyvinyl alcohol. J Micromech Microeng. 2006;16:473–479. [Google Scholar]
- 52.Griss P, Stemme G. Novel, side opened out-of-plane microneedles for microfluidic transdermal interfacing. Micro Electro Mechanical Systems, 2002. The Fifteenth IEEE International Conference; 2002 Jan 20-24; pp. 467–470. [Google Scholar]
- 53.Zhang P, Jullien GA. Microneedle arrays for drug delivery and fluid extraction. MEMS, NANO and Smart Systems, 2005. Proceedings International Conference; 2005 Jul 24-27; pp. 392–395. [Google Scholar]
- 54.Stoeber B, Liepmann D. Arrays of hollow out-of-plane microneedles for drug delivery. J Microelectromech Syst. 2005;14(3):472–479. [Google Scholar]
- 55.Zahn JD, Talbot NH, Liepmann D, Pisano AP. Microfabricated polysilicon microneedles for minimally invasive biomedical devices. Biomed Microdevices. 2000;2(4):295–303. [Google Scholar]
- 56.Takahashi D, Xiao Y, Hu F, Lewis M. Int J Telemed Appl. 2008. A survey of insulin-dependent diabetes–Part I: Therapies and devices. 405796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Naik A, Kalia YN, Guy RH. Transdermal drug delivery: overcoming the skin's barrier function. Pharm Sci Technol Today. 2000;3(9):318–326. doi: 10.1016/s1461-5347(00)00295-9. [DOI] [PubMed] [Google Scholar]
- 58.Sieg A, Guy RH, Delgado-Charro MB. Reverse iontophoresis for noninvasive glucose monitoring: the internal standard concept. J Pharm Sci. 2003;92(11):2295–2302. doi: 10.1002/jps.10492. [DOI] [PubMed] [Google Scholar]
- 59.Denet AR, Vanbever R, Préat V. Skin electroporation for transdermal and topical delivery. Adv Drug Deliv Rev. 2004;56(5):659–674. doi: 10.1016/j.addr.2003.10.027. [DOI] [PubMed] [Google Scholar]
- 60.Mitragotri S, Kost J. Low-frequency sonophoresis: a noninvasive method of drug delivery and diagnostics. Biotechnol Prog. 2000;16(3):488–492. doi: 10.1021/bp000024+. [DOI] [PubMed] [Google Scholar]


