Abstract
The Coalition for Clinical Research—Self-Monitoring of Blood Glucose Scientific Board, a group of nine academic clinicians and scientists from the United States and Europe, convened in San Francisco, California, on June 11–12, 2008, to discuss the appropriate uses of self-monitoring of blood glucose (SMBG) and the measures necessary to accurately assess the potential benefit of this practice in noninsulin-treated type 2 diabetes mellitus (T2DM). Thirteen consultants from the United States, Europe, and Canada from academia, practice, and government also participated and contributed based on their fields of expertise. These experts represent a range of disciplines that include adult endocrinology, pediatric endocrinology, health education, mathematics, statistics, psychology, nutrition, exercise physiology, and nursing. This coalition was organized by Diabetes Technology Management, Inc. Among the participants, there was consensus that:
protocols assessing the performance of SMBG in noninsulin treated T2DM must provide the SMBG intervention subjects with blood glucose (BG) goals and instructions on how to respond to BG data in randomized controlled trials (RCTs);
intervention subjects in clinical trials of SMBG-driven interventions must aggressively titrate their therapeutic responses or lifestyle changes in response to hyperglycemia;
control subjects in clinical trials of SMBG must be isolated from SMBG-driven interventions and not be contaminated by physician experience with study subjects receiving a SMBG intervention;
the best endpoints to measure in a clinical trial of SMBG in T2DM include delta Hemoglobin A1c levels, hyperglycemic events, hypoglycemic events, time to titrate noninsulin therapy to a maximum necessary dosage, and quality of life indices;
either individual randomization or cluster randomization may be appropriate methods for separating control subjects from SMBG intervention subjects, provided that precautions are taken to avoid bias and that the sample size is adequate;
treatment algorithms for assessing SMBG in T2DM may include a dietary, exercise, and/or medication intervention, which are all titratable according to the SMBG values;
the medical literature contains very little information about the performance of SMBG in T2DM from RCTs in which treatment algorithms were used for dysglycemic values; and
research on the performance of SMBG in T2DM based on sound scientific principles and clinical practices is needed at this time.
Keywords: consensus, diabetes, glucose, self monitoring, trial, type 2 diabetes
Introduction
Diabetes is a complex and progressive disease, which often inflicts a serious social and economic burden on the affected person. Collective efforts are needed to improve both the level of knowledge about the disease and the outcomes of patient management. Therefore, the Coalition for Clinical Research—Self-Monitoring of Blood Glucose (CCR-SMBG) was formed, aiming to increase the level of knowledge about diabetes and to determine both appropriate uses of blood glucose (BG) monitoring and appropriate measures required to accurately assess the performance of this practice. The first goal of the CCR-SMBG is to identify critical elements of a proper clinical trial of self-monitoring of blood glucose (SMBG) in type 2 diabetes mellitus (T2DM). The CCR-SMBG Scientific Board convened in San Francisco, California, on June 10–11, 2008, to identify:
the features of an appropriate clinical trial of SMBG in T2DM;
the quality of the evidence in the existing medical literature for randomized controlled trials (RCTs) of SMBG in T2DM;
the best measures to use in a robust clinical trial of SMBG in T2DM;
the features of a treatment algorithm that should be part of a robust clinical trial of SMBG in T2DM;
examples of such treatment algorithms for SMBG; and
how subjects in clinical trials of SMBG should be randomized to prevent control subjects from receiving instruction on SMBG-driven interventions intended only for the intervention subjects.
This consensus report contains a review of the literature of clinical trials of SMBG as well as an analysis of SMBG in T2DM in terms of its purpose, the criteria for its appropriate use, its potential clinical benefits, the appropriate psychosocial assessments of this practice, and statistical issues related to designing appropriate studies. This report also contains detailed examples of dietary, exercise, and medication titration algorithms that can be used in response to dysglycemic SMBG readings. Finally, the report contains the consensus conclusions of the CCR-SMBG's Scientific Board members and consultants on the proper elements of clinical trials for assessing the performance of SMBG in T2DM.
The Purpose of Self-Monitoring of Blood Glucose
The purpose of SMBG is to alert a patient to take appropriate action. The intended outcome of the appropriate use of SMBG is to detect hypoglycemia as well as to generate information for adjusting medication dosages, the dietary regimen, and the exercise regimen. Patients with diabetes might want to know how they are doing each day. Only SMBG provides access to this type of immediate information, which could lead to better lifestyle choices.
Self-monitoring of blood glucose provides a patient with (1) data for detecting high or low BG levels, which can facilitate self-adjustment of medication dosages and behavioral factors that are affecting glycemia; (2) protection by allowing immediate confirmation of acute hypoglycemia or hyperglycemia; (3) education and motivation about the disease to stimulate greater self-care responsibilities; and (4) information for the health care provider to assess and modify the treatment regimen.1
Self-monitoring of blood glucose must be thought of as a tool for data accumulation. When processed, SMBG data become information, and when analyzed, the information becomes knowledge. The types of adjustments that are made in T2DM patients treated with only oral agents are different than those made in T2DM or type 1 DM (T1DM) patients treated with insulin therapy. A protocol testing the benefits of SMBG must specify rational use of the glycemic information to effect improved outcomes.
Criteria for Effective Use of Self-Monitoring of Blood Glucose in Type 2 Diabetes Mellitus
For SMBG to be effective, both the patient and the physician or other caregiver must take responsibility and possess skills for appropriately performing, interpreting, and acting on SMBG information. In order to assess the benefit or lack of benefit of SMBG, activities that are part of each intervention must be clearly stated, and subject adherence to these actions must be determined. Table 1 lists a set of skills that the patient needs in order to perform, interpret, and act appropriately on SMBG information. Table 2 lists a set of skills that the physician or other caregiver needs in order to interpret and act on SMBG information appropriately. It is proposed that these types of lists be used in the design of future trials of SMBG.
Table 1.
Skills of the Patient Needed to Perform, Interpret, and Act on Self-Monitoring of Blood Glucose Information Appropriately
|
Table 2.
Skills of the Physician or Other Caregiver Needed to Interpret and Act on Self-Monitoring of Blood Glucose Information Appropriately
|
Literature Review of Trials of Self-Monitoring of Blood Glucose in Type 2 Diabetes Mellitus
Few trials have combined SMBG with interventions to titrate levels of therapy in response to glucose levels. Whereas SMBG is intended to determine treatment, it turns out that few trials assessing the performance of SMBG in T2DM have specified algorithms for increasing dosages of oral agents, increasing restrictions on dietary intake, or increasing exercise, let alone for performing all three of these interventions simultaneously.
The literature contains seven meta-analyses of the performance of SMBG in T2DM.2–8 Through these meta-analyses and electronic database searches of PubMed, the authors know of 21 RCTs of SMBG in T2DM published between 1986 and 2008.9–32 In approximately one-third of the studies, the patients had an opportunity to use an algorithm when responding to high fasting blood glucose (FBG) or high postprandial BG levels.12,15–17,26,27,29,32 In approximately two-thirds of the studies, the clinicians had the opportunity to use treatment guidelines to support their decision-making process when using SMBG data for FBG12–21,23,24,26–29,31–32 and BG profiles.12–16,21,23,24,26–28,31,32
The length of the interventions ranged from 10 weeks30 to 30 months,14 however, most studies had a duration of 622,23,26,29,31–32 or 12 months.10–13,15–17,28 Only two studies had a different duration of 3 months24 and 4 months.14
The RCTs used a variety of intervention protocols. All 21 RCTs provided SMBG training for the patients assigned to the intervention groups that used SMBG.9–32 While most studies also provided general diabetes counseling or education for patients assigned to the intervention and control groups, a few studies did not provide such counseling or education to any patients.17–22 Some studies also included intervention components on diet,10,14,15,21,23,27,29,30 physical activity,10,14,15,21 medication taking,14,23,32 and stress management.10
Physicians,11–12,14,16–21,24–26,28,29,32 nurses,9–13,16–21,23,24,26–28,30 pharmacists,14,22 dieticians,10,16,21,23,24,27,30 and community health workers provided the interventions.10 Patient contact with clinicians occurred in the form of clinic visits,9,11,12,14,16–21,23–26,28,29,31–32 meetings outside the clinic for education or support,10,13,15,27,30 pharmacy visits,22 home visits,30 telephone contact,11,28,30 internet support,21,24 and mail.11 The number of face-to-face contacts per study ranged from fewer than 59,11,13,16,17,22,24,28,30,31 to 10 or more.12,14,15,21,23,27 Face-to-face contact occurred either weekly,23 biweekly,30 monthly,12,26,29 bimonthly,31 every six weeks,25 every three months,9,11,13,16,17,22,24 or every six months.28 Some studies also included a combination of contact intervals, usually starting with weekly contact and then moving to biweekly or monthly contact.10,14,15,27 The total time of patient–clinician contact is difficult to determine since information on the length of contacts was indicated in only one study.10
Patients in all 21 studies used a glucose monitor.9–32 The number of days per week that patients were advised to perform SMBG was two,14,17,26,32 three,10,25,29 four,16 six,23 or seven.12,13,22,27 The recommended number of SMBG measurements per day was one,13,22 two,10,16,23 three,12,17,29 four,14 five,32 or six.25–27 This diversity of recommended SMBG measurements resulted in a range from 610,17 to 4227 measurements per week. Patients also kept a diary in several studies.9,11,12,14,15,17,21,23,24,26–30,32 The diary was internet based in two of the studies.21,24 One study also provided a handbook on general diabetes information,11 while another provided a calorie book.15
Potential Clinical Benefit of Self-Monitored Blood Glucose in Type 2 Diabetes Mellitus
Treatment of T2DM includes lifestyle interventions, oral glucose-lowering agents, and insulin. Principally, a combined deficiency in insulin secretion and action is responsible for the disease and leads to hyperglycemia in both fasting and postprandial states. Fasting hyperglycemia results from impaired suppression of hepatic glucose production. Postprandial hyperglycemia is due to insufficient meal-induced insulin secretion and lack of glucagon suppression prior to meal-related glucose influx. Hyperglycemia is largely responsible for the manifestation of the late complications of the disease. It is possible, although still controversial, that postprandial glucose peaks have a specific effect on these complications by eliciting an increase in oxidative stress.
Another problem is that some diabetes therapies, namely, sulfonylureas, glinides, and insulin, can induce hypoglycemic events in T2DM. Although such events are less frequent than during insulin therapy in T1DM patients, they contribute to intraday BG variability, resulting from postprandial hyperglycemic peaks and eventual hypoglycemic valleys. Interday BG variability is also present in T2DM, mainly related to the variability in food intake and exercise. When patients are treated with insulin, variability in insulin absorption provides an additional factor of interday variability.
In most cases, patients with T2DM do not self-titrate their treatments like T1DM patients do by adjusting their insulin doses from SMBG data, counting and adjusting meal carbohydrate intake, and adjusting exercise timing, intensity, and duration. Hence, while T1DM patients often play an active role in their treatment, T2DM patients often do not. It is easier to empower T1DM patients than T2DM patients to be highly involved in their disease management. For T2DM patients, this may represent a frustrating situation where they may feel condemned to watch passively from the sidelines while their treatments fail, as evidenced by their gradually increasing Hemoglobin A1c (A1C) levels. Eventually, despite increasingly complex treatment, they usually end up requiring insulin therapy. In summary, the possibility of late complications, the risk of hypoglycemic events, and the frequent reality of having to remain a passive spectator of a disabling disease may profoundly affect the quality of life for patients with T2DM.
For all these reasons, it is clear that the mere assessment of diabetes control through the measurement of A1C, although it provides an essential insight on the quality of metabolic control, is limited. This is because A1C levels do not present patients with a complete picture of their BG status. More specifically, A1C does not provide the patient with a possibility of observing what happens on a daily basis and understanding the very meaning of diabetes as the confluence of fasting hyperglycemia and postprandial hyperglycemic waves. In addition, A1C levels also do not provide insight into the hyperglycemic effect of food, the hypoglycemic effect of therapeutic tools based on lifestyle modifications (such as reducing food intake, ingesting fiber-containing foods, and exercising), or the use of glucose-lowering drugs. As a consequence, the patient cannot adjust the various treatment components. Indeed, without SMBG and with only trimonthly measurements of A1C, the life of a T2DM patient seems like a closed-eye journey, with the eyes being opened only every three months to decide the direction to be taken. This approach is definitely a very inefficient way of tackling the treatment of such a dangerous disease.
Types of Interventions That Use Self-Monitored Blood Glucose in Type 2 Diabetes Mellitus
Appropriate clinical trials of SMBG must include two critical features. First, the results of SMBG testing must drive changes in therapy. This means that investigators must establish SMBG goals with their intervention subjects and must use SMBG to aggressively titrate the intervention specified in the study protocol. Second, control subjects in clinical trials of SMBG must be sequestered from SMBG-driven interventions to avoid contamination by physician experience with study subjects who are receiving an SMBG intervention. This means that investigators must not provide control subjects with any information about SMBG, which could be derived from their experiences with simultaneously administering an SMBG-driven intervention to other study subjects. Self-monitoring of blood glucose is an intervention that has no mode of action or direct effect on glycemic control and does not change any outcomes unless this intervention leads to a particular type of action, such as a change in diet, exercise, or medication. The basic question about the value of SMBG is not whether this practice is useful, but rather whether the algorithms, which must accompany SMBG, are useful. A study of the performance of SMBG is not intended to test the value of algorithms per se, but to compare a treatment algorithm that includes SMBG in decision making to one that is based on A1C alone. Thus proper use of SMBG dictates that the results of this practice must be applied to determine therapy decisions, which in turn will determine glycemic control.
If subjects are randomized within a research site either to receive formal SMBG intervention or not to receive formal SMBG intervention, then there is a risk that the physician and caregiver team might inadvertently apply elements of an SMBG management package to both groups. This practice would constitute an inadvertent upgrade of the “usual care” or control intervention, which may already include SMBG without proper use of the data in many cases. The consequence of this upgrade would be a contamination of the control intervention and a possible devaluation of the benefit of the SMBG intervention (compared to usual care). In that case, the added benefit of the SMBG intervention might be erroneously measured as a low or even undetectable effect. This problem with contamination of usual care with a study intervention does not occur in drug trials where subjects either receive an investigational drug or do not receive an investigational drug. The effects of the drug can be easily deduced from the difference in outcomes between the two groups. In SMBG trials, however, the control subjects are often already partaking of the SMBG intervention but not using it appropriately. The control subjects are then likely to improve their use of this practice with encouragement by an investigator who is interested in the effects of SMBG. Thus both subjects and caregivers must be carefully managed in clinical trials of the performance of SMBG on glycemic control.
Self-monitoring of blood glucose can be used to determine both temporary and permanent modifications of therapy. These two types of therapy modifications can be the basis of two types of trials that can be performed to assess the value of SMBG.
When SMBG values are elevated, then a dietary intervention to reduce calories or an intervention to increase exercise can be immediately initiated, and this intervention can be discontinued when the need is no longer present. A clinical trial of SMBG might measure the effects of a type of dietary or exercise intervention that can be used when needed. There may be an improvement in mean glycemia and in A1C levels if sufficient amounts of these two glucose-lowering interventions are applied when needed because of information provided by SMBG. A solitary dietary intervention, a solitary exercise intervention, or a combination of both, if driven by SMBG values, could make for an appropriate trial of SMBG. At any given physician visit, the A1C might be expected to be better in SMBG subjects than in control subjects, because the intervention subjects are in a position to adjust their regimen between follow-up visits, in addition to increasing drug dosages in response to elevated A1C levels. The measured outcomes in such a temporary intervention trial would be related to improved glycemic control.
A medication intervention with SMBG might specify uptitration of dosage in response to a limited number of elevated BG values early in a study. The higher dose then remains in effect for a long time, usually irrespective of future SMBG values. In such an intervention protocol, future performance of SMBG might no longer influence any modifications in treatment, because the only change in therapy (medication uptitration) has already been performed. The benefits of SMBG in such a study of SMBG-driven medication titration could be questioned. Improved outcomes in an intervention group receiving higher drug doses, regardless of their frequency of SMBG testing, might be attributed solely to the early assignment of higher drug doses and not to the practice of SMBG. The control subjects would presumably have A1C levels measured every three months, and their medications could then be uptitrated on the basis of these levels. It would be difficult to separate the effect of SMBG compared to the effect of using higher medication dosages. These higher dosages could have been prescribed based on A1C levels alone without the aid of SMBG testing. It should be noted, however, that an SMBG medication intervention could result in a subject uptitrating themselves at home with an algorithm that could result in a more rapid uptitration that would occur with trimonthly A1C testing and medication adjustments. Self-monitoring of blood glucose could be thought of as a tool for accelerating uptitration of drug dosages.
An additional factor in the successful management of T2DM is the time to titrate noninsulin medication therapy (usually a combination of oral agents) to reach a goal or a maximum necessary dosage. Currently, patients with T2DM are often maintained on ineffective noninsulin regimens for many years. This is usually because they see their physician only every three months, and it takes years to reach maximal or effective dosages of one or multiple drugs or to transition to insulin. This phenomenon is known as therapeutic inertia. Self-monitoring of blood glucose can facilitate uptitration of noninsulin agents through the use of an algorithm at home. Titration of dosage driven by SMBG can result in more rapid uptitration and initiation of additional drugs. Therefore, SMBG might hasten a patient's arrival at either a point of receiving adequate noninsulin therapy or a point where noninsulin agents must be considered a failure and insulin therapy must be initiated. These treatment modifications can result in moving a patient onto effective therapy faster and saving the cost of several years of ineffective drugs when a patient actually needs higher dosages of noninsulin therapy or initiation of insulin. These outcomes would be beneficial to patients and payers. The measured endpoints in such a permanent medication intervention trial would be related to the time to either maximum dose of effective non-insulin therapy or replacement of noninsulin agents with insulin.
One additional type of protocol that can be designed for an SMBG intervention would specify a fixed frequency of caregiver visits for the control subjects and a variable protocol-driven frequency of caregiver visits for the intervention subjects. The protocol-driven caregiver visit schedule would be based on SMBG results. Greater constancy in achieving SMBG targets (for example, more than 50% of SMBG values above either 120 mg/dl at fasting/premealtime and/or 160 mg/dl at 2 h postmealtime) would result in a lower frequency of visits. In such a study, the SMBG subjects could end up with a greater or lesser number of caregiver visits than the control group during the course of the study. Such a protocol is compatible with measuring outcomes related to glycemic control, time to achieve target therapy, or quality of life. This type of study would generate simultaneous information about outcomes and costs of care, which might be of interest to patients who are seeking to minimize time spent with their caregiver and to payers who are looking to minimize costs of caregiver visits.
There are two potential approaches to test the hypothesis that SMBG is clinically effective in selected patients with T2DM. With both approaches, an optimal frequency of SMBG testing as well as its timing around meals, exercise, and sleep should be specified where appropriate.
The first approach to test this hypothesis would be to add SMBG to a randomly selected proportion of a group of T2DM patients who are not achieving the glycemic goal (most likely as measured by A1C). As the mere act of measuring glucose is unlikely to achieve much benefit, SMBG must be coupled with a straightforward response algorithm to modify lifestyle and/or pharmacologic therapies. As an example, if the goal of the study were primarily to modify dietary behavior, then the rise in glucose postprandially would be emphasized. Subject selection criteria should exclude those patients with T2DM who are receiving therapies that include a risk of hypoglycemia and need SMBG for safety considerations.
The second approach to test this hypothesis would be to remove SMBG from a randomly selected proportion of a group of T2DM patients who are achieving the glycemic goal (most likely as measured by A1C). Again, subject selection would be critical. Glycemic control in patients with early/mild T2DM might not worsen if SMBG is stopped. Likewise, the real benefit of SMBG may be limited to those time periods when A1C is elevated, implying that the therapeutic regimen needs adjusting. Ethical considerations would need to be addressed if an intervention whose benefit is being evaluated were to be removed from a subject's therapeutic regimen.
Both approaches require attention to important features in study design, subject selection, and the interaction between the two (see Table 3).
Table 3.
Important Features of a Clinical Trial of SMBG
|
Endpoints of Interventions That Use Self-Monitoring of Blood Glucose in Type 2 Diabetes Mellitus
The best endpoints to measure in a clinical trial of SMBG in T2DM include A1C levels, hyperglycemic events, hypoglycemic events, time to titrate noninsulin therapy to a maximum necessary dosage, and quality-of-life indices, which could include a composite metric of multiple such indices.33 Glycemic control and glycemic variability can also be assessed by a period of wearing a blinded continuous glucose monitor at the end of the study.
The most widely accepted measure of success of current treatments for T2DM is the A1C level. The delta A1C between the start and the completion of the study in intervention subjects and control subjects can be compared to determine the effect of the intervention on mean glycemic levels. This test should be performed every three months from initial disease diagnosis. In a clinical trial where the A1C level is an endpoint, the investigators might elect to use a central laboratory to make measurements; to measure A1C levels in duplicate; and then to record the mean level for each time point. Most scientific societies agree on a target of 6.5–7% for A1C. At this range, the risk for complications related to chronic hyperglycemia can be minimized according to United Kingdom Prospective Diabetes Study data. Recently, the International Diabetes Federation, following other scientific societies, recommended that postmeal BG levels should not exceed 140 mg/dl, taking into account identified relationships between hyperglycemic peaks, oxidative stress to vessel walls, and cardiovascular outcomes. Such a goal cannot, however, be achieved without SMBG.
Two types of studies could be performed to test the hypothesis that SMBG is a useful intervention in T2DM. First, SMBG could be tested to assess whether this practice contributes to making decisions that result in improved glycemic control in situations where current A1C-driven guidelines do not result in such decisions or improved control. Second, SMBG could be tested to assess whether this practice contributes to better glycemic outcomes than current management of diabetes based only on A1C levels measured at trimonthly clinic visits. A continuous glucose monitor, with the results blinded to the subject, can be worn at the beginning and the end of the study to assess various elements of glycemic control and glycemic variability.
In the first type of study where the performance of SMBG could be tested, the obvious study endpoint is A1C. Subjects who would be recruited in this study should not have an A1C level that leads to augmenting diabetes therapy according to guidelines, e.g., the addition of a second drug, such as metformin, due to failure of first-line therapy. The United Kingdom Prospective Diabetes Study showed a gradual increase of A1C in T2DM patients, mainly related to beta-cell loss. However, in common practice, diabetes therapy remains unchanged, with reinforcement of lifestyle measures until A1C exceeds 7.5%, with confirmation after three months. Hence recruitment in this study could be based on A1C level below 7.5% with an increase of no more than 0.3% during the prior three-month interval. Self-monitoring of blood glucose could be demonstrated as useful if it resulted in the addition of a new drug while an increase in the A1C level did not reach the level to elicit such a change. A six- to seven-point 24 h glucose profile per week, showing two consecutive assessments of elevated fasting or premeal glucose values above 120 mg/dl and/or postmeal values above 160 mg/dl, could promote the addition of a glucose-lowering drug sooner than a trimonthly A1C measurement. As described earlier, patients included in the SMBG-using arm of the study would have to be educated to follow a medication titration algorithm when BG values exceed the mentioned levels. One can reasonably expect that A1C could be significantly lower at two subsequent three-month assessments in SMBG users.
In the second type of study that tests the performance of SMBG, endpoints other than A1C would have to be considered. Among them, high glucose peaks, adverse events related to diabetes treatment (hypoglycemic events and weight gain), and quality-of-life indices could be selected. Measures of mean glycemia and glycemic variability could also be made if subjects wear a blinded, continuous glucose monitor at the end of the study period. This type of study is intended for subjects using at least two oral glucose-lowering drugs, because these people (compared to people using metformin alone) are more prone to (1) inadequate postmeal glucose control, (2) adverse events due to therapy, and (3) impairment of quality of life. The same strategy of using SMBG and following a medication titration algorithm, as previously explained, can be expected to induce self-interventions of lifestyle and uptitrations of drug dosages (according to SMBG-driven algorithms). As a consequence, the recommended endpoints would be expected to be consistent with better glycemic control in the SMBG arm of the study than in the non-SMBG arm. A difference in A1C levels in favor of SMBG users may be less likely to appear in this patient population, because the reduction of high glucose excursions may not have a sufficient effect on A1C lowering, and reduction of hypoglycemic events may even limit A1C lowering. Additional endpoints for assessing the benefit of a SMBG intervention, which allows the intervention subjects to upwardly self-titrate their dosages of medications, would include the time needed to titrate noninsulin therapy to a maximum necessary dosage or the percentage of subjects reaching a maximum necessary dosage of noninsulin therapy during the duration of the study.
In both suggested types of study, ethical considerations could be raised about the non-SMBG or usual care practice in the control arm. A reasonable way to circumvent this issue could be to allow SMBG in these patients according to their own choice, with no specific decision-making instructions. Actually, this situation corresponds to what is being done by most T2DM patients using SMBG in common current practice. Controls could be selected who are historically infrequent users of SMBG. A screening questionnaire could be administered to determine self-reported usage to enroll only infrequent users, but subjects who do not use SMBG properly might not be able to benefit from this intervention. It is important to specify that the usual care intervention for the control subjects (who will not receive the SMBG intervention) would be good clinical practice. This means controls will receive an education program but no special instruction on how often to perform SMBG or what to do about SMBG information except for safety instructions about self-monitoring for acute hypoglycemia symptoms. The control and SMBG intervention groups should be as similar as possible in terms of the amount of clinician contact and attention paid to diabetes management, diet, and exercise. The SMBG intervention cohort would be instructed to use glucose information to modify an aspect of therapy as well as how to organize data for self-review and for review with the medical team. The control group would not receive instruction about how to respond to SMBG data other than in response to hypoglycemic symptoms, and they would be instructed to perform SMBG during the study exactly as they are currently doing. Both groups would be seen by a physician or diabetes nurse at the same frequency (probably every three months) and would have an A1C level drawn every three months. Subjects in both cohorts would have their medication regimen uptitrated during investigator visits based on an algorithm for A1C levels. In the interest of patient safety, a protocol would have to provide for the possibility that the investigator may need to initiate SMBG in a control subject who is not already performing this practice, in which case the subject would be considered a non-SMBG failure, and this action would be an endpoint of the study.
As an overall conclusion of these considerations, it may be difficult to demonstrate a benefit of SMBG in T2DM based on multiple simultaneously measured endpoints in a single study. Several types of SMBG studies could be developed, each to assess a different aspect of glycemic control or quality of life. However, two types of studies, one of subjects receiving single-agent therapy and another of subjects receiving at least two noninsulin agents, could measure separate endpoints that relate to potential benefits of using SMBG.
Research-Based Psychosocial Assessments
Psychosocial outcomes have been measured as part of an assessment of the performance of SMBG. Four major RCTs have been identified that have examined psychosocial outcomes associated with SMBG use in non-insulin-using patients with diabetes.
Muchmore et al.27 used the Diabetes Control and Complications Trial's Diabetes Quality of Life (DQOL) scale in 1994. There were no differences in quality of life change over the study period between control and intervention groups. Of the four DQOL subscales, there was an improvement in treatment satisfaction for the entire group from baseline to Week 24 but not from baseline to the end of the study (Week 44).
Schewedes et al.26 used two of Bradley's measures in 2002: the Diabetes Treatment Satisfaction Questionnaire (DTSQ) and the Patient Well-Being Questionnaire (PWBQ). There were no reported differences in DTSQ scores over the study period between control and intervention groups. While PWBQ scores did not improve in the control group, they did improve in the intervention group, but only for the two items assessing depression and lack of well-being.
Farmer et al.17 and Simon et al.18 (the DIGEM trial) used the EQ-5D in 2008. The more intensive SMBG group evidenced a greater drop in quality of life compared to the control group; this was apparently due to differences in anxiety/depression over the course of the study.
O'Kane et al.16 used the University of Michigan Diabetes Attitude Questionnaire and two of Bradley's measures in 2008: DTSQ and PWBQ. There were no differences reported between intervention and control conditions over the course of the study on any measure except the PWBQ depression subscale, where there was a negative effect in the intervention group compared with the control group, but the statistical analysis was problematic.1
Given these findings, it is recommended that any future RCTs in this population make use of the following measures:
A generic (not diabetes specific) measure such as the EQ-5D. It is brief, allows calculation of health utilities, and is widely used, as in the Diabetes Glycaemic Education and Monitoring trial.
A diabetes-specific measure such as the Diabetes DistressScale (DDS). The DDS is relatively brief (17 items), sensitive to change, and focuses on patients' sense of control over the disease. This appears to be the underlying dimension of distress that is being influenced in a positive direction in one study,26 perhaps due to the more frequent, supportive set of follow-up visits, and in a negative direction in two others,16,18 perhaps due to the frustration of participating in trials where SMBG begins to seem pointless over time, especially when contact with health care providers is infrequent, and there is little sense that one's own actions are making much of a difference.
A patient-reported outcome, which could include (A) overall quality of life (such as the EQ-5D or the Bradley PWBQ); (B) diabetes treatment satisfaction (such as the DQOL short form or the Bradley DTSQ); (C) diabetes-specific distress (such as the DDS); (D) diabetes treatment adherence (such as the Summary of Diabetes Self-Care Activities Measure scale for treatment adherence19 or the Attitudes to Treatment Adherence scale); (E) diabetes symptoms (such as the Diabetes Symptom Checklist20).
A dedicated questionnaire could also be developed for such an RCT, to assess diabetes self-care behavior. The questions would cover (1) frequency of behavior (e.g., how many times a day do you perform SMBG, how many servings of fruits and vegetables do you eat each day, and how many days per week do you exercise >30 min); (2) adherence to treatment recommendations (e.g., how closely, on a scale from completely to not at all, do you follow SMBG (medication, diet, and exercise recommendations provided by your clinician); and (3) frequency of corrective behavior when BG is out of the target range (e.g., how often, on a scale from always to never, do you adjust your medication [eat less, exercise more] when your BG level is high).
Statistical Issues Related to Study Design
The primary outcome, A1C, is typically measured at the baseline and at the end of the study. Because of the nature of the study and its primary outcome measure, randomized block design (RBD)34 is proposed, with three blocks identified by baseline A1C: below 7.5%, 7.5–9%, and above 9%, which can be referred to, respectively, as Block 1, Block 2, and Block 3, which correspond to the severity of the condition.
The basic idea of RBD is to first stratify the participants into relatively homogeneous subpopulations (blocks) and then randomize the subjects within each subpopulation into treatment and control groups. If the blocks are indeed homogeneous, the variance within each block will be lower than the variance of the entire population, which in turn should result in more efficient estimates of the SMBG effect within each block. The two main advantages of an RBD approach are as follows:
When the data is pooled across the RBD blocks, more efficient estimates of the overall treatment effect should be achieved.
Generally, a smaller sample size would be required provided that the initial blocks were indeed homogeneous.
Stratification would allow for a separate assessment of the treatment effect within each RBD block. This is particularly important because it is reasonable to expect that the effect of SMBG would depend on A1C level (e.g., subjects who were in excellent control at baseline would not be able to lower their A1C much further with SMBG).
Intent-to-Treat versus Per Protocol Analyses
The primary analysis should follow the intent-to-treat (ITT) principle, where subjects are analyzed in the treatment group to which they were assigned through randomization. This means that a subject randomized to SMBG should be analyzed in that group even if they did not adhere to the prescribed glucose testing. This approach is necessary to preserve the integrity of the randomization. Subjects who are unwilling or unable to adhere to SMBG may be inherently different from those who do adhere. Such differences could affect the A1C value independently of SMBG and bias the estimated treatment effect. The ITT principle avoids this potential bias by not allowing the analysis groups to be defined by subject adherence.
However, ITT analysis can be conservative and may underestimate the true effect of SMBG. Including patients in the SMBG group who did not actually perform the glucose testing likely dilutes the real impact of SMBG. Secondary “per protocol” analyses can also be performed, where subjects are grouped according to their actual use of SMBG. Subjects randomized to SMBG who did not perform a predetermined amount of glucose testing would be analyzed in the control group, and any subjects randomized to the control group who did perform glucose testing would be analyzed in the SMBG group. Additionally, a “dose response” type analysis may be performed, where the change in A1C is correlated with the amount of SMBG actually performed as a continuous variable. As noted earlier, these analyses based on subject adherence are subject to bias and should be clearly labeled as exploratory or hypothesis generating. Great caution should be taken in interpreting the results if the ITT and per protocol analyses lead to conflicting conclusions.
This potential for bias underscores the need to maximize adherence with SMBG in a well-conducted trial. If there is a high level of adherence, then the ITT and per protocol analyses will lead to the same conclusions. Potential subjects must therefore be well-informed about all aspects of the study and what they will be asked to do. Intervention subjects must receive education on how and when to use their BG monitor. Eligibility should be limited as much as possible to subjects who are likely to adhere to the protocol. It may be helpful to include a “run-in” phase prior to randomization, during which potential subjects are asked to perform glucose testing with a blinded meter. Subjects who do not successfully complete a predetermined minimum number of tests are not eligible for randomization. Once randomized, adherence to SMBG should be carefully monitored throughout the study, and any reasons for not performing SMBG should be elicited from the subject. The use of downloadable glucose meters can improve the accuracy of adherence monitoring.
Sample Size
Here we consider a reduction of at least 0.3% in mean A1C levels occurring from baseline to the end of the study, in the SMBG intervention group compared with the control group, to be a clinically meaningful treatment effect. The distribution of this effect, however, would not be uniform across the three RBD blocks, with the largest effect expected in Block 2 (patients with A1C levels between 7.5% and 9%). This expectation is based on the assumed limited standard deviation (SD) within this block and on the potential for improved A1C levels with self-monitoring. In Block 1, the expected effect size would be lower, and the effect of the SMBG intervention would not be significant within that block. Within Block 3, the potential net effect of the SMBG intervention would be higher, e.g., 0.5%, but this would result in lower effect size because of larger potential variance. Effect size is the basis for sample size estimation and is defined as the expected difference in A1C divided by an estimate of the SD. Thus the effect size increases with larger observed reduction in A1C and decreases with larger variance. These relationships explain the suggestion for RBD—a design that allows maintaining a lower SD within each block, which in turn can result in larger effect size and smaller sample size.
With these assumptions, we calculate the total sample size necessary to achieve 90% power using a two-tailed hypothesis test with a type 1 error rate = 5% (Table 4). The power calculations use the GPOWER software.35 Note that the necessary sample size depends, to a great extent, on both the assumed SD and correlation between pretreatment and posttreatment A1C measurements. Based on previous experience, a correlation of up to 0.3 is assumed. Typically the estimated minimum necessary sample size decreases with increasing correlation between the repeated measurements of the study. This is because a higher correlation implies that poststudy measurements are roughly proportional to baseline, which reduces the variance in the treatment effect and results in a larger effect size.
Table 4.
| SD of A1C at outcome | Correlation with Baseline A1C | |
|---|---|---|
| 0 | 0.3 | |
| 0.75 | 294 | 268 |
| 1.00 | 518 | 472 |
| 1.25 | 806 | 734 |
Total sample size for both treatment groups is combined.
Sample sizes given here need to be increased by 10–20% to account for dropouts and nonadherent subjects.
Interim Analysis to Reestimate Sample Size
As seen in Table 4, the necessary sample size for a clinical trial is very sensitive to the unknown SD and correlation. It may be desirable to incorporate a preplanned interim analysis into the protocol to reevaluate the necessary sample size based on the observed data. After a prespecified number of subjects have completed the study, their A1C data could be used to estimate the overall SD, the SD within each RBD block, and the pre–post correlation. The total sample size can then be increased or decreased depending on whether the SD and the correlation are larger or smaller than originally anticipated. If this interim analysis focuses solely on estimating SD and correlation but not the treatment effect, then there is no “penalty” in terms of need for additional subject recruitment. Alternatively, an interim analysis may also incorporate a plan for stopping the study early if there is a large observed treatment effect. Because this approach would look at treatment effect, a slight increase in the maximum total sample size would typically be needed to preserve the desired statistical power. Either approach to an interim analysis is valid as long as it is explicitly prespecified in the protocol.
Randomization
One of the most important features of a clinical trial of SMBG is sequestration of control subjects to not receive special instruction (besides what they already know at the beginning of the study) from the study caregiver team members on how to practice and use SMBG. Any such instruction would serve to contaminate these control subjects and make them more likely to use SMBG during the study. This additional use of SMBG, inspired by entering the study and receiving inappropriate instructions, would serve to minimize the difference in behaviors between the control and SMBG intervention subjects. The result would be a falsely decreased difference in outcomes between the groups and a greater likelihood of an inappropriate rejection of the hypothesis that the use of SMBG results in improved outcomes.
Studies that seek to assess the benefit of a medical intervention are typically conducted as RCTs at multiple centers. Subjects are randomly assigned to be in control or intervention groups. If a patient characteristic is expected to affect the outcome of a trial, then during the randomization process, the control cohort and the intervention cohort must be assembled to include an equal amount or frequency of that characteristic. The most common form of randomization is referred to as “individual randomization,” in which any individual subject at any site has an equal (or predetermined unequal) chance of being assigned to receive control care or the study intervention. Usually the subjects and investigators are both blinded as to which form of treatment the subject is receiving. It is assumed that in these studies, at any site, the regimen will be exactly the same for controls and study subjects, except for the intervention. This is a widely held assumption, because for most interventions, an investigator is unable to inadvertently influence the outcome by providing additional amounts of intervention to the intervention subjects or by encouraging these subjects to do particularly well. This is because drug doses are generally dispensed in controlled amounts and because investigators are generally unaware of which treatment group any given subject is in.
Trials of behavior are different than drug trials. Behavioral trials are generally not blinded to the investigator, which creates a possibility that an enthusiastic investigator will subconsciously encourage greater adherence with other aspects of disease management, such that the intervention subjects might outperform the controls because of this enthusiasm, which can result in better behavior in areas unrelated to the intervention. In addition to this automatic problem with any unblinded clinical trial, a special problem can arise in a trial of SMBG, namely, contamination of controls, who are not necessarily regular users of SMBG, with education about the benefits of SMBG. It is well-known that individuals allocated to a control treatment may inadvertently receive some aspects of the intervention if they are in proximity to the treated group.36
It might be difficult for physicians or nurses, who are treating both cohorts of subjects, to discuss SMBG only with intervention subjects and to fully ignore the topic with control subjects. The potentially changed habits, renewed awareness of SMBG, and recently acquired knowledge of SMBG-driven treatment algorithms might change the investigator's behavior. This greater enthusiasm for SMBG might serve to introduce the control subjects to additional instruction or discussion about SMBG than would have been the case had their physician not been an investigator conducting a rigorous SMBG protocol. It is likely that with some control subjects in poor control, an investigator will not be able to refrain from using SMBG data to help guide therapy. This exposure of control subjects to a prescriber of SMBG might then degrade the impact of the SMBG intervention through contamination of the controls.
A solution to this potential problem with individual randomization, where both control and intervention subjects receive treatment at the same sites, is to randomize by center or physician.37 This approach, known as cluster randomization, establishes selected centers as control sites and selected sites as intervention sites. An investigator will treat only one type of subject, which eliminates the risk of contamination of control subjects. Both individual randomization and cluster randomization have advantages and disadvantages.
The main advantages of individual randomization are as follows: (1) this method is better known and thought of by many as the “gold standard’ method for randomization; (2) this method avoids the problem of working with poorly motivated investigators who are assigned to provide control care to all their trial subjects but who prefer to work with investigational therapies; (3) this method eliminates the possibility of variability between centers that are all providing control care or intervention care; (4) this method compels the protocol writers to create SMBG algorithms that can be followed by subjects at any doctor's office or clinic and not just at highly academic, specialized centers that might otherwise become the sites selected for administering the intervention; and (5) this method can be powered with fewer total subjects than can cluster randomization, which makes for lower costs in conducting trials.
The main advantages of cluster randomization are as follows: (1) this method avoids introduction of SMBG information or education to controls; (2) this method offers statistical advantages over individualized randomization in terms of decreased variance if the number of sites in the trial of SMBG in T2DM is relatively large (approximately 10 or more) and the number of subjects within each site is relatively small (approximately 25); (3) this method is associated with lower costs of administration with fewer sites than an individually randomized study; (4) the intracluster correlation effect, which characterizes the strength of center effects and may increase the number of subjects, can be estimated with an early interim analysis of data and minimized when RBD trials are conducted; and (5) this method is well regarded in clinical trials of other behavioral interventions. Part of the reason that individual randomization is so well-known is because randomized trials of drug interventions, where individual randomization works well, are conducted more frequently than randomized trials of behavior interventions.
Special safeguards are necessary to avoid recruitment bias in cluster randomized studies. With such trials, it is helpful to identify study subjects before paired study sites are randomly allocated and to use an independent recruiter to locate participants to avoid the problem of selection bias.36 A potential compromise method between individual randomization and cluster randomization is a form of cluster randomization known as pseudo-cluster randomization.38–40 This method is essentially a two-step randomization: the first step is cluster randomization, and the second step is randomization of a small proportion of each cluster (e.g., 20%) to control or intervention in random order. Using this method, the bias is virtually eliminated, and the amount of contamination is minimized.
Individual randomization is the most widely used method for conducting randomized trials, because this method maximizes the probability that confounding variables will be evenly distributed between treatment groups in most cases. The problem of contamination of controls is so serious, however, that careful consideration should be given to cluster randomization in clinical trials of SMBG in T2DM. A cluster randomized study must be powered sufficiently. Safeguards against within-cluster bias can be built in, such as RBD or pseudocluster randomization. Future clinical trials of SMBG might use either individual randomization or cluster randomization, depending on the study populations and the questions that are being asked.
Example of a Dietary Intervention
Because of the wide variation in insulin sensitivity and degree of carbohydrate intolerance, it is most effective to manage postprandial BG with SMBG before and 1 h after each meal and by controlling the carbohydrate load of each meal. Normoglycemia is the goal, and therefore target BG levels are FBG < 100 mg/dl and 60 min postprandial plasma glucose (PPG) < 140 mg/dl. The value of SMBG is to use individual BG readings as well as BG patterns and trends to refine a dietary program specifically designed for the individual. Blood glucose excursions after consuming a meal vary from individual to individual and are highly dependent upon the level of carbohydrate intolerance and insulin sensitivity. This determines the amount of carbohydrate that can be consumed. The postprandial BG at 60 min will provide insight into dietary modifications needed to achieve the target BG in the future. Therefore, SMBG is recommended before and 1 h after each meal every day.
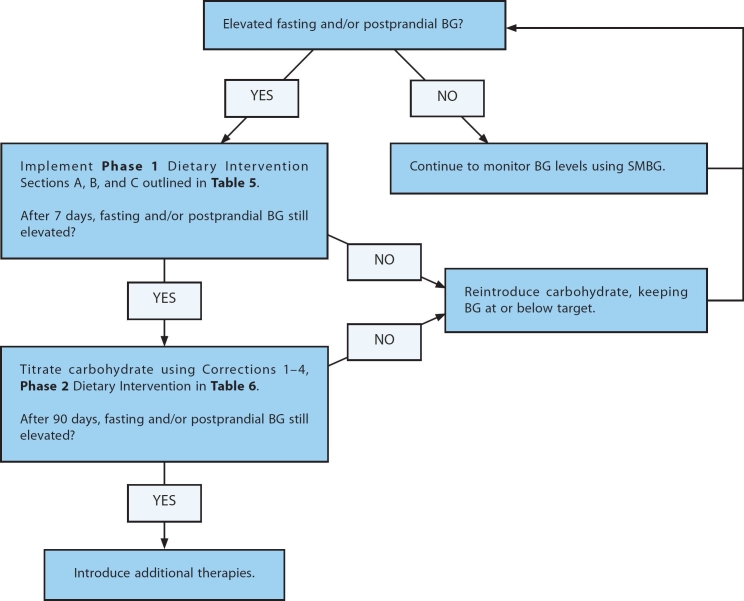
Dietary Intervention is determined on a meal-to-meal basis. An example of a dietary intervention driven by SMBG is illustrated in Figure 1. Dietary manipulations are based on SMBG readings such that the patient learns what meal composition and quantity can be tolerated. Such interventions are intended for individuals that have reached maximum growth and are biologically mature.
Figure 1.
An example of a dietary intervention for persons with T2DM based on SMBG values.
Several factors influence BG levels: time of day, nutrient composition of the meal, quantity of food consumed, stress, physical activity, and class and timing of medications. Although many of these considerations are beyond the scope of this report, FBG and PPG levels can be brought to target by focusing on SMBG data. In cases where BG levels are not within target range, then individuals are encouraged to initiate the Phase 1 Dietary Intervention (Table 5).
Table 5.
Phase 1 Dietary Intervention
| Section A Rapidly absorbed carbohydrates to be avoided | Section B Moderately absorbed carbohydrates to be eaten in moderation | Section C Foods that have minimal direct impact on BG |
|---|---|---|
|
|
|
Phase 1 Dietary Intervention
Individuals are advised to avoid rapidly absorbed carbohydrates (Table 5, Section A), which are typically the main culprit in causing elevated PPG. The majority of these foods have a high glycemic index and cause an immediate spike in BG. It is important to note that carbohydrate foods differ in composition of starch as well as quality according to country and region, which influences the impact on PPG. Carbohydrates, which are more slowly absorbed, (Table 5, Section B) can be eaten in moderation. Foods that have minimal direct impact on BG (Table 5, Section C) can be eaten in usual quantities. Phase 1 provides general guidelines that the individual can implement within their basic eating pattern without the need to count carbohydrate grams at each meal. For patients who are overweight, special attention to portion control should be practiced when choosing foods from Table 5, Section C, as many of these foods are calorically dense. For these patients, there should be, in addition to portion control, an emphasis on encouraging the consumption of lean proteins and using minimal quantities of fat.
If FBG and PPG target levels are achieved with Phase 1 Dietary Intervention, the patient may be able to add small quantities of foods from Section A or B as he or she learns which foods can be tolerated. Self-monitoring of blood glucose will be critical in evaluating the response to the reintroduction of these carbohydrates. If FBG or PPG target levels are not achieved with Phase 1 Dietary Intervention within 7 days, then Phase 2 Dietary Intervention should be initiated.
Phase 2 Dietary Intervention: Composition of Meals
The goal of Phase 2 Dietary Intervention is to improve PPG by controlling carbohydrate intake by counting carbohydrates and managing the composition of each meal. Each meal should be based mostly on nonstarchy vegetables (50% of the meal) complemented with a minimal amount of high-quality carbohydrate, lean protein, and fat, with an emphasis on healthy fats. The baseline intake column in Table 6 shows a starting level of allowed carbohydrates for each meal.
Table 6.
Phase 2 Dietary Intervention
| Carbohydrate Titration | |||||
|---|---|---|---|---|---|
| Meal | Baseline intake | Correction 1 | Correction 2 | Correction 3 | Correction 4 |
| Breakfast | 30 grams | 25 grams | 20 grams | 15 grams | 10 grams |
| Lunch | 45 grams | 40 grams | 35 grams | 30 grams | 25 grams |
| Dinner | 45 grams | 40 grams | 35 grams | 30 grams | 25 grams |
| Snack (optional) | <15 grams | ||||
High quality carbohydrates: Beans, lentils, millet, cracked wheat, and barley.
Lean protein: In adequate amounts, protein foods have little effect on BG levels. Recent research shows that 15–30% of total daily caloric intake should be from protein from either plant or meat sources. Larger quantities of protein can have an extended gradual effect on BG; thus, attention to portion size is recommended. Sources of lean proteins are lean meats, skinless poultry, fish, eggs, tofu, and low-fat cheeses.
All 20 amino acids, with the exceptions of leucine and lysine, are considered gluconeogenic. Some programs recommend estimating the glucose content of protein in order to cover it with a delayed bolus. However, it may be more important to use preprandial medications or, if the patient is on insulin, to adjust the basal infusion rate or the long-acting insulin doses to compensate for the last-arriving glucose formed from metabolized proteins.
Fat: Fat has very little direct effect on BG levels. However, it contributes to delayed gastric emptying and affects the speed at which the carbohydrate source of the meal is absorbed from the intestine. Fat can also cause a temporary state of insulin resistance that can last for many hours, causing a rise in BG several hours after the meal. Main sources of fat in most diets in Europe and the United States are margarine, butter, nuts, oils, avocados, and salad dressings. In recent years, there has been an emphasis on healthy fats, particularly omega-3 fatty acids. Foods rich in omega-3 fatty acids include flaxseeds, flaxseed oil, flaxseed meal, hempseed oil, hempseeds, walnuts, pumpkin seeds, Brazil nuts, sesame seeds, avocados, some dark leafy green vegetables (kale, spinach, purslane, mustard greens, collards), olive oil, canola oil (cold-pressed and unrefined), soybean oil, wheat germ oil, salmon, mackerel, sardines, anchovies, and albacore tuna.
Each meal is managed independently. If PPG is not <140 mg/dl, the offending meal prior to the reading should be analyzed for actual carbohydrate content, and if the carbohydrate level is higher than recommended, the patient should be counseled on how to bring the meal into target range. If the meal was in fact at the recommended carbohydrate level, the level of allowed carbohydrate is then titrated down in 5 g increments until target BG is achieved (Table 6).
Timing of Meals
The timing of meals is important. Meals should be spaced 4–6 h apart. This improves the ability of BG to be at target prior to the next meal.
Snacks
Snacks are not essential for people with T2DM but can be negotiated if BG levels are at target and if the patient is at desired body weight. If BG levels are not at target and/or if the individual is above desired body weight, snacks should be avoided or eliminated. An exception to this would be if the patient has been overeating at meals because of unavoidably long periods between meals, as when such periods are determined by work. If this is the case, snacks should consist of <15 g carbohydrate combined with small amounts of protein and fat.
Hypoglycemia
People with diabetes may experience hypoglycemia (BG <70 mg/dl). If this occurs, 15 g of fast-acting carbohydrate (e.g., 4 oz orange juice, 3 glucose tablets) should be ingested. A hypoglycemic event should be an opportunity to review current medications without necessarily making adjustments to dietary intake (e.g., adding a snack). With T2DM, it is important not to “chase” overeating with increased medications and/or add snacks to avoid hypoglycemia. Meal composition should include sufficient protein and fat with adequate carbohydrate to prevent both elevated PPG and post-prandial hypoglycemia.
Persistent Hyperglycemia
In the event that normoglycemia is not achieved in 90 days with dietary interventions, then other therapies should be initiated.
Example of an Exercise Intervention
Both aerobic and resistance exercise activities are beneficial and suitable for people with diabetes, according to the Canadian Diabetes Association Guidelines on Exercise and Diabetes.41 Examples of aerobic and resistance exercises are provided in Table 7. Walking is a popular and feasible aerobic activity suitable for most overweight, middle-aged, and elderly people with diabetes. Brisk walking on level ground is an example of moderate aerobic exercise, while brisk walking up an incline or jogging would be considered vigorous aerobic exercise. Resistance exercise performed 2 or 3 times/week provides benefits that complement those of aerobic training (e.g., increased lean mass, strength and vigor, reduced body fat, and increased resting metabolic rate).42 Table 8 presents a set of exercise regimens. The magnitude of the drop in BG is dependent upon the intensity and duration of the aerobic exercise as well as the preexercise glycemia. It is important to note that brief intense bouts of anaerobic exercise (intense exercise that lasts from seconds to a couple of minutes, which is associated with dramatic increases in catecholamines) may increase BG concentrations in persons with diabetes.43
Table 7.
Classifications of Different Types of Exercise.
| Aerobic Exercise | ||
|---|---|---|
| Definition | Intensity | Examples |
| Rhythmic, repeated and continuous movements of the same large muscle groups for at least 10 minutes at a time | Moderate Effort: 50-70% of person's maximum heart rate |
|
| Vigorous Effort: >70% of person's maximum heart rate |
|
|
| Resistance Exercise | |
|---|---|
| Definition | Examples |
| Activities that use muscular strength to move a weight or work against a resistant loada |
|
| Start with 1 set of 10-15 repetitions at moderate weight, progress to 2 sets of 10-15 repetitions, then progress to 3 sets of 8 repetitions at heavier weight, 3 times/week. | |
Initial instruction and periodic supervision recommended
Table 8.
Exercise Prescriptiona
| Program stage | Length of program, wk | Frequency, days/wk | % HRmax | % HRR | RPEa | Breathing rate | Time per session, min |
|---|---|---|---|---|---|---|---|
Initial stage
|
1 | 3 | 55-65 | 40-50 | 2-4 | Slightly increased | 15-20 |
| 2 | 3 | 55-65 | 40-50 | 2-4 | Slightly increased | 20-25 | |
| 3 | 3 | 65-70 | 50-60 | 3-5 | Noticeably increased | 20-25 | |
| 4 | 3 | 65-70 | 50-60 | 3-5 | Noticeably increased | 25-30 | |
Improvement
|
5-7 | 4 | 70-75 | 60-70 | 3-5 | Noticeably increased | 25-30 |
| 8-10 | 4 | 70-75 | 60-70 | 3-5 | Noticeably increased | 30-35 | |
| 11-13 | 3-5 | 75-80 | 65-75 | 4-6 | Noticeably increased | 30-35 | |
| 14-16 | 3-5 | 75-80 | 65-75 | 4-6 | Noticeably increased | 30-35 | |
| 17-20 | 3-5 | 75-85 | 70-80 | 4-8 | Noticeably increased | 35-40 | |
| 21-24 | 3-5 | 75-85 | 70-80 | 4-8 | More difficulty talking while exercising | 35-40 | |
Maintenance
|
24-28 | 3-5 | 75-85 | 70-80 | 4-8 | More difficulty talking while exercising | 30-45 |
from Warburton DE, Nicol CW, Bredin SS. Prescribing exercise as preventive therapy. CMAJ. 2006;174:961–74
Dose-Dependent Effect of an Exercise Bout on Blood Glucose Levels
Typically the magnitude of drop in BG concentration during an acute bout of exercise depends on the intensity of the exercise and whether the exercise is performed in a fasted or postprandial state. In general, the greatest drop in glycemia occurs when the activity is initiated after a meal when BG concentration is typically elevated and circulating insulin levels are also high. In addition, the greater the intensity of aerobic exercise, the greater the drop in glucose. For example, in overweight and obese middle-aged persons with T2DM, 40 min of low to moderate intensity cycling (i.e., 50% maximal oxygen uptake) lowers fasting glucose by 10 ± 5 mg/dl, while moderate intensity cycling lowers fasting glucose by 24 ± 5 mg/dl.44 This immediate effect of exercise on glucose concentration appears to be related to the increase in glucose disposal into working muscle as both insulin and muscle contractile activity increases glucose transporter-4 trafficking.
In addition to the timing of exercise, the drop in glycemia associated with exercise also depends on the preexercise glucose concentration, with a greater drop occurring when the preexercise glucose is elevated. In fact, when exercise is performed in the fasting state, when BG is “normal,” glucose concentration may even rise. For example, in men with T2DM exercising for 60 min in the fasting state (60% maximal oxygen uptake), BG had a 27 ± 21% increase when the preexercise BG was ≤108 mg/dl.45 In the same men, BG remained unchanged when the preexercise fasting BG was between 108 and 144 mg/dl and dropped by 12 ± 13% when BG was >144 mg/dl. In the postprandial state, the same exercise task lowered BG between 18 ± 17% and 50 ± 12%, depending on the level of preexercise glycemia.45
The drop in glycemia also depends on the duration of activity, with a greater drop typically observed when the exercise lasts >20 min. For example, short-term intense activities (i.e., 110% of maximal aerobic capacity) lasting 12 min caused an increase in glycemia,46 while longer activities lasting 45 min at 70% of maximal aerobic capacity caused a drop in glycemia from 136 to 86 mg/dl.47
Residual Effects of One Exercise Bout
As mentioned earlier, BG levels can be influenced by one acute exercise bout, even if the exercise lasts only minutes. Increased insulin sensitivity in skeletal muscle persists for up to about 18 h after the end of prolonged, moderately intense exercise,48 and this residual effect of exercise may in fact help blunt the rise in glucose associated with a subsequent meal.49
Exercise Considerations and Contraindications
Before beginning a program of physical activity more vigorous than walking, individuals with diabetes should be assessed for conditions that might be contraindications to certain types of exercise, may predispose them to injury, or be associated with cardiovascular disease.48 Examples of such conditions would include severe autonomic neuropathy, severe peripheral neuropathy, and preproliferative or proliferative retinopathy, all of which require treatment prior to beginning vigorous exercise. An exercise electrocardiogram stress test should be considered for previously sedentary individuals with diabetes at high risk for cardiovascular disease who wish to undertake exercise more vigorous than brisk walking. Previously sedentary individuals may have to gradually increase their exercise, starting with as little as 5 to 10 min per day. Multiple, shorter exercise sessions lasting at least 10 min each in the course of a day should be considered, as this regimen is probably as useful as a single longer session of equivalent length and intensity.50 Patients taking oral hypoglycemic agents or exogenous insulin may experience increased frequency of hypoglycemic events with regular exercise.
An Exercise Intervention
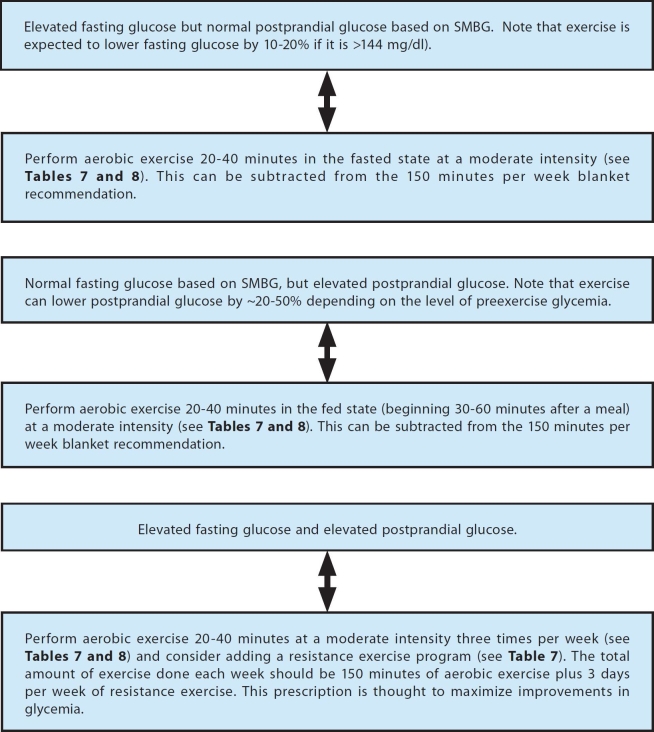
As of this report's publication, there have been no specific recommendations that have been tested to determine what type of exercise should be recommended for dysglycemia in either the fasting or the postprandial state. The recommendations provided here are based on a nonsystematic review of the relevant literature and personal opinions. Figure 2 presents an exercise intervention that can be used in a trial that is intended to assess the performance of SMBG in T2DM.
Figure 2.
An example of an exercise intervention for persons with T2DM based on SMBG values.
Example of a Medication Intervention
Background
The fact that medications for the management of T2DM are generally not titrated or advanced aggressively is one of the most important factors in the failure to achieve glycemic targets (A1C) in many patients. There are several explanations for this so-called clinical inertia. Commonly, clinicians may not be convinced or committed to a specific A1C target. Clinical inertia may also be due to a lack of real understanding and comfort with the medications and their titration schedules or a lack of a systematic roadmap for selecting and advancing medications. In addition, relying solely on A1C to make adjustments can slow the entire process, particularly if there is no point-of-care A1C testing available.
Using SMBG as an additional tool for titrating and advancing medications has the potential to reduce clinical inertia significantly, particularly if both the medical team and the patient agree on SMBG targets and formulate a plan for regular feedback of results and management advice. Some patients are comfortable with following predetermined guidelines for medication titration/adjustment, while others can spot deviations from acceptable values or patterns but prefer to contact the medical team for advice. Methods of staying in contact with a medical team are evolving rapidly and include visits (individual or group), phone calls, faxes, web downloads, and most recently, phone text messaging.
Principles of Medication Intervention
Several basic principles should be considered for incorporating medications into a study of the effectiveness of SMBG in optimizing glycemic control in T2DM patients on noninsulin medications. First, the study design must be very clear on what the glycemic goal is. Will the goal be merely A1C or a combination of endpoints such as A1C, frequency of hypoglycemia that is severe or significant, and glycemic variability? Second, the study must set clear SMBG goals for fasting, premeal, and postmeal measurements. Third, the study should specify clear patterns for testing SMBG according to each glucose-lowering medication used. Fourth, clear boundaries must be established as to which glucose level will constitute a hypoglycemic episode or an excessive glycemic excursion (i.e., the rise from the premeal to the postmeal level). Fifth, the study protocol should clearly specify the circumstances under which a medication adjustment and/or a lifestyle change are necessary to improve glycemic control.
In addition to the aforementioned basic principles of protocol design for a noninsulin medication intervention based on SMBG, a specific algorithm must be used for medication initiation, selection, and dosage titration based on SMBG levels and/or A1C levels. Medication titration or advancement can occur either at the time of a visit with the investigator (based on SMBG and/or A1C levels) or between investigator visits. If there is any modification of the medication regimen per protocol between visits, then the subject will be responsible for applying the algorithm for self-titrating or advancing the medication regimen. For such a protocol, it will be necessary to clearly explain the algorithm to the subject so that modifications can be made appropriately. A subject may be allowed to contact the investigator emergently about how to carry out the algorithm at the time of a medication modification. In that case, the amount of contact must be kept to a minimum to prevent intervention subjects from having access to information, education, and management assistance not available to control subjects. At most what should be provided is clarification about how to accomplish the protocol's Intervention. A protocol might include more than one option for drugs that could be added, as needed, to a noninsulin medication regimen. In that case, the protocol should clearly define the circumstances that would lead to a specific choice of each additional study medication that might be introduced. Finally, it is necessary to specify how A1C and SMBG values will complement each other when it is time to make changes in medication therapy based on both these measures.
Approaches for Using Self-Monitoring of Blood Glucose to Titrate and Adjust Oral Diabetes Medications
There are three basic approaches to SMBG to consider in T2DM for noninsulin users. The first approach is intermittent pattern testing, which tests FPG before the largest meal and PPG after the largest meal, 3 times per week. In the United States, the testing usually starts with the evening meal (dinner). Over time, the schedule can vary as to which meal is bracketed. This approach would total 9 tests per week or approximately 36 tests per month.
The second approach is meal bracket testing, which means rotating which meal is assessed by a premeal SMBG value and a postmeal SMBG value. Testing is done before and after one meal per day (and it is the same meal each day) for three consecutive days. Then a different meal is monitored, with SMBG before and after that meal, for the next three days and so on. Each meal is then revisited every nine days in a rotating schedule. This approach yields 14 tests per week or approximately 56 tests per month.
The third approach is intermittent 7-point testing. This approach specifies that a 7-point set of SMBG tests be performed on three separate days, usually during a 7–14 day period just prior to a visit with the investigator. Alternatively, such a pattern of testing can be performed continuously at a frequency of every 2–4 weeks, unrelated to any investigator visits. The seven tests are performed before each of the three meals, after each of the three meals, and at bedtime on every 7-point testing day. This approach has been very effective in establishing a glucose pattern for a specific timeframe and helps with modifying a medication regimen during a subject visit. This approach yields 7–14 tests per month. Seven-point testing tends to be less helpful than intermittent pattern testing or meal bracketing in supplying ongoing feedback or providing motivation to the patients between medical visits.
An Intervention
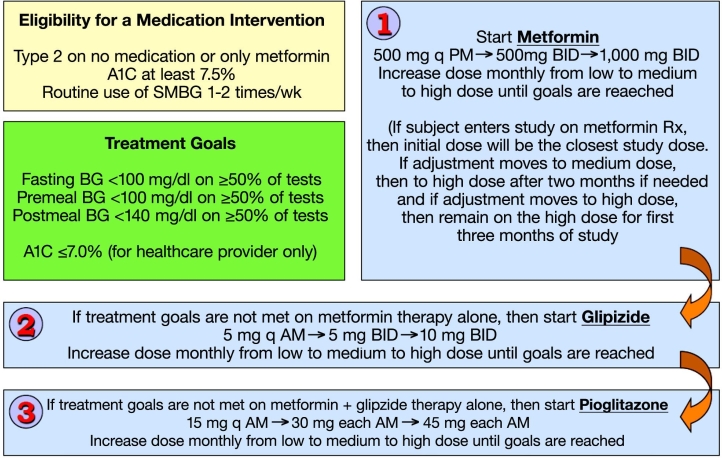
The American Diabetes Association and the European Association for the Study of Diabetes issued a consensus algorithm for the initiation and adjustment of therapy in T2DM this year.51 These guidelines specify the use of various combinations of noninsulin agents in T2DM. These recommended agents are typically initiated in low dosages, and the dosages are then increased as needed. Furthermore, recommended therapy typically involves use of metformin followed by a second noninsulin drug if necessary, and then either insulin or a third noninsulin drug, if necessary. An SMBG intervention to accelerate the time to reach a maximum effective medication can be tested in conjunction with a variety of drug combinations from this algorithm in noninsulin-treated subjects with T2DM.
A trial using a medication intervention can be accomplished by uptitrating doses of two or three oral agents based on SMBG values and by adding medications to the regimen based on the SMBG and A1c values. An example of one such intervention uses three dosages each for metformin, glipizide, and pioglitazone. This particular combination of three noninsulin agents is one of multiple possible drug combinations recommended by the American Diabetes Association and the European Association for the Study of Diabetes as well as other professional organizations for the management of T2DM. Likewise there are many other two- or three-drug combinations of noninsulin drugs that could be assessed for their benefit in the context of an SMBG intervention.
A subject in the intervention arm will perform SMBG at a frequency determined by a protocol and will be instructed to respond to the values according to a protocol. If SMBG values are elevated at least 50% of the time after 1 month, then the medication dose will be increased by one step. The subject will also receive specific instructions on modifying their diet and exercise regimen based on the pattern of SMBG values. The possible dosages of medications in the intervention will be low, medium, and high, so that through monthly dosage increases (if needed), titration can be accomplished over a 3 month period going from the lowest to highest dose. Further increases in medication therapy, consisting of adding an additional oral agent, would be determined by the healthcare provider who will see the subject every 3 months. A decision to add an additional oral agent to the regimen will be based on the pattern of SMBG values and by trimonthly A1C values obtained immediately before each visit. A subject in the control arm of the protocol will be issued SMBG paraphernalia and instructed to monitor themselves as often as they wish and use the information to adjust diet and exercise according to a basic set of instructions. Subjects in both arms of the study will receive instruction on how to recognize hypoglycemic symptoms and respond to them using SMBG.
In each arm, the progression will be from low to medium to high doses based on a monthly assessment of SMBG values by the subject. The targets for adequate control will be fasting BG ≤100 mg/dl, premeal BG ≤100 mg/dl, and post meal BG ≤140 mg/dl on at least 50% of readings, or else a medication uptitration will be needed. The subject and healthcare provider will use these BG targets. The target for A1C will be ≤7.0% or else a medication uptitration will be needed. The healthcare provider will use this target.
In the intervention arm, the metformin dosages will be as follows: low dose, 500 mg each evening; medium dose, 500 mg twice daily; high dose, 1000 mg twice daily. The glipizide dosages will be as follows: low dose, 5 mg each evening; medium dose, 5 mg twice daily; high dose, 10 mg twice daily. The pioglitazone dosages will be as follows: low dose, 15 mg each morning; medium dose, 30 mg each morning; high dose, 45 mg each morning. This intervention is illustrated in Figure 3. Similar trials of interventions using SMBG to accelerate the uptitration process in the presence of combinations of numerous other noninsulin agents could also be developed.
Figure 3.
An example of a medication intervention for persons with T2DM based on SMBG values.
Endpoints
The endpoints for such a medication intervention would be the percentage of subjects achieving target levels of glycemia as well as the percentage of the subjects not achieving target levels of glycemia but reaching the maximum dosages of all three study medications by the end of the study period. Successful oral agent dosage titration would be defined as the sum of both percentages.
Conclusions
Self-monitoring of blood glucose in T2DM is an established practice that has been shown to improve glycemic control in many randomized and epidemiological clinical trials. However, the value of this practice has been recently questioned because of studies with methodological flaws that demonstrated no clinical benefit.1 In order to evaluate the potential benefits or lack of benefits of this practice in noninsulin treated T2DM, the CCR-SMBG Scientific Board has addressed the state of research as of 2008 and put forth recommendations on how to best conduct clinical trials of the performance of SMBG. The authors of this report strongly believe that SMBG is a tool for gathering information, but it is not an intervention in itself. Self-monitoring of blood glucose is intended to beneficially affect patient behavior. Therefore, to assess whether this practice is worthwhile, it is necessary to specify a therapeutic response or lifestyle change to information provided by SMBG. Optimal use of SMBG requires adequate testing frequency, training, and directions for responding to data in the form of specific recommended actions for modifying the amount of food intake, exercise performance, and medication dosages. Such specified actions must be part of any protocol that assesses the performance of SMBG.
Among participants, the consensus was that: (1) protocols assessing the performance of SMBG in noninsulin-treated T2DM must provide the SMBG intervention subjects with BG goals and instructions on how to respond to BG data in RCTs; (2) intervention subjects in clinical trials of SMBG-driven interventions must aggressively titrate their therapeutic responses or lifestyle changes in response to hyperglycemia; (3) control group subjects in clinical trials of SMBG must be isolated from SMBG-driven interventions and not be contaminated by physician experience with study subjects receiving an SMBG intervention; (4) the best endpoints to measure in a clinical trial of SMBG in T2DM include delta A1C levels, hyperglycemic events, hypoglycemic events, time to titrate noninsulin therapy to goal attainment or a maximum necessary dosage, and quality-of-life indices; (5) either individual randomization or cluster randomization may be appropriate methods for separating control subjects from SMBG intervention subjects, provided that precautions are taken to avoid bias and that the sample size is adequate; (6) treatment algorithms for assessing SMBG in T2DM may include a dietary intervention, an exercise intervention, and/or a medication intervention, which are all titratable according to the SMBG values; (7) the medical literature contains very little information about the performance of SMBG in T2DM from RCTs in which treatment algorithms were utilized for dysglycemic values; and (8) research on the performance of SMBG in T2DM based on sound scientific principles and clinical practices is needed at this time.
Acknowledgements
We acknowledge the expert advice of David Horwitz, M.D., Ph.D., Matthias Axel Schweitzer, M.D., M.B.A., David Simmons, M.D., and David Price, M.D.; the helpful suggestions of Jeffrey I. Joseph, D. O., and Serge Jabbour, M.D., FACP, FACE; and the editorial contribution of Jiji Reyes.
Abbreviations
- A1C
Hemoglobin A1c
- BG
blood glucose
- CCR-SMBG
Coalition for Clinical Research—Self-Monitoring of Blood Glucose
- DDS
Diabetes Distress Scale
- DQOL
Diabetes Quality of Life
- DTSQ
Diabetes Treatment Satisfaction Questionnaire
- FBG
fasting blood glucose
- ITT
intent-to-treat
- PWBQ
Patient Well-Being Questionnaire
- PPG
postprandial plasma glucose
- RBD
randomized block design
- RCT
randomized controlled trial
- SMBG
self-monitoring of blood glucose
- SD
standard deviation
- T1DM
type 1 diabetes mellitus
- T2DM
type 2 diabetes mellitus
References
- 1.Klonoff DC. New evidence demonstrates that self-monitoring of blood glucose does not improve outcomes in type 2 diabetes—when this practice is not applied properly. J Diabetes Sci Technol. 2008;2(3):342–348. doi: 10.1177/193229680800200302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McAndrew L, Schneider SH, Burns E, Leventhal H. Does patient blood glucose monitoring improve diabetes control? A systematic review of the literature. Diabetes Educ. 2007;33(6):991–1011. doi: 10.1177/0145721707309807. [DOI] [PubMed] [Google Scholar]
- 3.Sarol JN, Jr, Nicodemus NA, Jr, Tan KM, Grava MB. Self-monitoring of blood glucose as part of a multi-component therapy among non-insulin requiring type 2 diabetes patients: a meta-analysis (1966– 2004) Curr Med Res Opin. 2005;21(2):173–184. doi: 10.1185/030079904X20286. [DOI] [PubMed] [Google Scholar]
- 4.Jansen JP. Self-monitoring of glucose in type 2 diabetes mellitus: a Bayesian meta-analysis of direct and indirect comparisons. Curr Med Res Opin. 2006;22(4):671–681. doi: 10.1185/030079906X96308. [DOI] [PubMed] [Google Scholar]
- 5.Welschen LM, Bloemendal E, Nijpels G, Dekker JM, Heine RJ, Stalman WA, Bouter LM. Self-monitoring of blood glucose in patients with type 2 diabetes who are not using insula systematic review. Diabetes Care. 2005;28(6):1510–1517. doi: 10.2337/diacare.28.6.1510. [DOI] [PubMed] [Google Scholar]
- 6.Kempf K, Neukirchen W, Martin S, Kolb H. Self-monitoring of blood glucose in type 2 diabetes: a new look at published trials. Diabetologia. 2008;51(4):686–688. doi: 10.1007/s00125-008-0946-7. [DOI] [PubMed] [Google Scholar]
- 7.Poolsup N, Suksomboon N, Jiamsathit W. Systematic review of the benefits of self-monitoring of blood glucose on glycemic control in type 2 diabetes patients. Diabetes Technol Ther. 2008;10(Suppl 1):S51–S66. doi: 10.1089/dia.2009.0091. [DOI] [PubMed] [Google Scholar]
- 8.Towfigh A, Romanova M, Weinreb JE, Munjas B, Suttorp MJ, Zhou A, Shekelle PG. Self-monitoring of blood glucose levels in patients with type 2 diabetes mellitus not taking insula meta-analysis. Am J Manag Care. 2008;14(7):468–475. [PubMed] [Google Scholar]
- 9.Moreland EC, Volkening LK, Lawlor MT, Chalmers KA, Anderson BJ, Laffel LM. Use of a blood glucose monitoring manual to enhance monitoring adherence in adults with diabetes: a randomized controlled trial. Arch Intern Med. 2006;166(6):689–695. doi: 10.1001/archinte.166.6.689. [DOI] [PubMed] [Google Scholar]
- 10.Brown SA, Garcia AA, Kouzekanani K, Hanis CL. Culturally competent diabetes self-management education for Mexican Americans: the Starr County border health initiative. Diabetes Care. 2002;25(2):259–268. doi: 10.2337/diacare.25.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jones H, Edwards L, Vallis TM, Ruggiero L, Rossi SR, Rossi JS, Greene G, Prochaska JO, Zinman B. Changes in diabetes self-care behaviors make a difference in glycemic control: the Diabetes Stages of Change (DiSC) study. Diabetes Care. 2003;26(3):732–737. doi: 10.2337/diacare.26.3.732. [DOI] [PubMed] [Google Scholar]
- 12.Kibriya MG, Ali L, Banik NG, Khan AK. Home monitoring of blood glucose (HMBG) in type-2 diabetes mellitus in a developing country. Diabetes Res Clin Pract. 1999;46(3):253–257. doi: 10.1016/s0168-8227(99)00093-5. [DOI] [PubMed] [Google Scholar]
- 13.Miles P, Everett J, Murphy J, Kerr D. Comparison of blood or urine testing by patients with newly diagnosed non-insulin dependent diabetes: patient survey after randomised crossover trial. BMJ. 1997;315(7104):348–349. doi: 10.1136/bmj.315.7104.348. Erratum appears in BMJ 1998;316(7126):195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jaber LA, Halapy H, Fernet M, Tummalapalli S, Diwakaran H. Evaluation of a pharmaceutical care model on diabetes management. Ann Pharmacother. 1996;30(3):238–243. doi: 10.1177/106002809603000305. [DOI] [PubMed] [Google Scholar]
- 15.Wing RR, Epstein LH, Nowalk MP, Scott N, Koeske R, Hagg S. Does self-monitoring of blood glucose levels improve dietary compliance for obese patients with type II diabetes? Am J Med. 1986;81(5):830–836. doi: 10.1016/0002-9343(86)90354-2. [DOI] [PubMed] [Google Scholar]
- 16.O'Kane MJ, Bunting B, Copeland M, Coates VE ESMON study group. Efficacy of self monitoring of blood glucose in patients with newly diagnosed type 2 diabetes (ESMON study): randomised controlled trial. BMJ. 2008;336(7654):1174–1177. doi: 10.1136/bmj.39534.571644.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Farmer A, Wade A, Goyder E, Yudkin P, French D, Craven A, Holman R, Kinmonth AL, Neil A. Impact of self monitoring of blood glucose in the management of patients with non-insulin treated diabetes: open parallel group randomised trial. BMJ. 2007;335(7611):132. doi: 10.1136/bmj.39247.447431.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Simon J, Gray A, Clarke P, Wade A, Neil A, Farmer A Diabetes Glycaemic Education Monitoring Trial Group. Cost effectiveness of self monitoring of blood glucose in patients with non-insulin treated type 2 diabetes: economic evaluation of data from the DiGEM trial. BMJ. 2008;336(7654):1177–1180. doi: 10.1136/bmj.39526.674873.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martínez YV, Prado-Aguilar CA, Rascón-Pacheco RA, Valdivia-Martínez JJ. Quality of life associated with treatment adherence in patients with type 2 diabetes: a cross-sectional study. BMC Health Serv Rese. 2008;8:164. doi: 10.1186/1472-6963-8-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grootenhuis PA, Snoek FJ, Heine RJ, Bouter LM. Development of a type 2 diabetes symptom checklist: a measure of symptom severity. Diabet Med. 1994;11(3):253–261. doi: 10.1111/j.1464-5491.1994.tb00268.x. [DOI] [PubMed] [Google Scholar]
- 21.Cho JH, Chang SA, Kwon HS, Choi YH, Ko SH, Moon SD, Yoo SJ, Song KH, Son HS, Kim HS, Lee WC, Cha BY, Son HY, Yoon KH. Long-term effect of the Internet-based glucose monitoring system on HbA1c reduction and glucose stability: a 30-month follow-up study for diabetes management with a ubiquitous medical care system. Diabetes Care. 2006;29(12):2625–2631. doi: 10.2337/dc05-2371. [DOI] [PubMed] [Google Scholar]
- 22.Johnson JA, Majumdar SR, Bowker SL, Toth EL, Edwards A. Self-monitoring in type 2 diabetes: a randomized trial of reimbursement policy. Diabet Med. 2006;23(11):1247–1251. doi: 10.1111/j.1464-5491.2006.01973.x. [DOI] [PubMed] [Google Scholar]
- 23.Davidson MB, Castellanos M, Kain D, Duran P. The effect of self monitoring of blood glucose concentrations on glycated hemoglobin levels in diabetic patients not taking insula blinded, randomized trial. Am J Med. 2005;118(4):422–425. doi: 10.1016/j.amjmed.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 24.Kwon HS, Cho JH, Kim HS, Song BR, Ko SH, Lee JM, Kim SR, Chang SA, Kim HS, Cha BY, Lee KW, Son HY, Lee JH, Lee WC, Yoon KH. Establishment of blood glucose monitoring system using the internet. Diabetes Care. 2004;27(2):478–483. doi: 10.2337/diacare.27.2.478. [DOI] [PubMed] [Google Scholar]
- 25.Guerci B, Drouin P, Grangé V, Bougnères P, Fontaine P, Kerlan V, Passa P, Thivolet CH, Vialettes B, Charbonnel B ASIA Group. Self-monitoring of blood glucose significantly improves metabolic control in patients with type 2 diabetes mellitus: the Auto-Surveillance Intervention Active (ASIA) study. Diabetes Metab. 2003;29(6):587–594. doi: 10.1016/s1262-3636(07)70073-3. [DOI] [PubMed] [Google Scholar]
- 26.Schwedes U, Siebolds M, Mertes G SMBG Study Group. Meal-related structured self-monitoring of blood glucose: effect on diabetes control in non-insulin-treated type 2 diabetic patients. Diabetes Care. 2002;25(11):1928–1932. doi: 10.2337/diacare.25.11.1928. [DOI] [PubMed] [Google Scholar]
- 27.Muchmore DB, Springer J, Miller M. Self-monitoring of blood glucose in overweight type 2 diabetic patients. Acta Diabetol. 1994;31(4):215–219. doi: 10.1007/BF00571954. [DOI] [PubMed] [Google Scholar]
- 28.Rutten G, van Eijk J, de Nobel E, Beek M, van der Velden H. Feasibility and effects of a diabetes type II protocol with blood glucose self-monitoring in general practice. Fam Pract. 1990;7(4):273–278. doi: 10.1093/fampra/7.4.273. [DOI] [PubMed] [Google Scholar]
- 29.Allen BT, DeLong ER, Feussner JR. Impact of glucose self-monitoring on non-insulin-treated patients with type II diabetes mellitus. Randomized controlled trial comparing blood and urine testing. Diabetes Care. 1990;13(10):1044–1050. doi: 10.2337/diacare.13.10.1044. [DOI] [PubMed] [Google Scholar]
- 30.Estey AL, Tan MH, Mann K. Follow-up intervention: its effect on compliance behavior to a diabetes regimen. Diabetes Educ. 1990;16(4):291–295. doi: 10.1177/014572179001600408. [DOI] [PubMed] [Google Scholar]
- 31.Fontbonne A, Billault B, Acosta M, Percheron C, Varenne P, Besse A, Eschwege E, Monnier L, Slama G, Passa P. Is glucose self-monitoring beneficial in non-insulin-treated diabetic patients? Results of a randomized comparative trial. Diabete Metab. 1989;15(5):255–260. (unnumbered in original) [PubMed] [Google Scholar]
- 32.Barnett AH, Krentz AJ, Strojek K, Sieradzki J, Azizi F, Embong M, Imamoglu S, Perušičová J, Uličiansky V, Winkler G. The efficacy of self-monitoring of blood glucose in the management of patients with type 2 diabetes treated with a gliclazide modified release-based regimen. A multicentre, randomized, parallel-group, 6-month evaluation (DINAMIC 1 study) Diabetes Obes Metab. 2008 doi: 10.1111/j.1463-1326.2008.00894.x. [DOI] [PubMed] [Google Scholar]
- 33.Glasgow RE, Peeples M, Skovlund SE. Where is the patient in diabetes performance measures? The case for including patient-centered and self-management measures. Diabetes Care. 2008;31(5):1046–1050. doi: 10.2337/dc07-1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Trochim W. 2nd ed. Cincinnati: Atomic Dog Publishing; 2001. The research methods knowledge base. [Google Scholar]
- 35.Erdfelder E, Faul F, Buchner A. GPOWER: a general power analysis program. Behav Res Meth Instrum Comput. 1996;28:1–11. [Google Scholar]
- 36.Hahn S, Puffer S, Torgerson DJ, Watson J. Methodological bias in cluster randomised trials. BMC Med Res Methodol. 2005;5(1):10. doi: 10.1186/1471-2288-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eldridge SM, Ashby D, Feder GS, Rudnicka AR, Ukoumunne OC. Lessons for cluster randomized trials in the twenty-first century: a systematic review of trials in primary care. Clin Trials. 2004;1(1):80–90. doi: 10.1191/1740774504cn006rr. [DOI] [PubMed] [Google Scholar]
- 38.Borm GF, Melis RJF, Teerenstra S, Peer PG. Pseudo cluster randomization: a treatment allocation method to minimize contamination and selection bias. Stat Med. 2005;24(23):3535–3547. doi: 10.1002/sim.2200. [DOI] [PubMed] [Google Scholar]
- 39.Teerenstra S, Melis RJ, Peer PG, Borm GF. Pseudo cluster randomization dealt with selection bias and contamination in clinical trials. J Clin Epidemiol. 2006;59(4):381–386. doi: 10.1016/j.jclinepi.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 40.Melis RJ, Teerenstra S, Rikkert MG, Borm GF. Pseudo cluster randomization performed well when used in practice. J Clin Epidemiol. 2008 doi: 10.1016/j.jclinepi.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 41. http://www.diabetes.ca/cpg2003. Accessed August 1, 2008.
- 42.Sigal RJ, Kenny GP, Boulé NG, Wells GA, Prud'homme D, Fortier M, Reid RD, Tulloch H, Coyle D, Phillips P, Jennings A, Jaffey J. Effects of aerobic training, resistance training, or both on glycemic control in type 2 diabetes: a randomized trial. Ann Intern Med. 2007;147(6):357–369. doi: 10.7326/0003-4819-147-6-200709180-00005. Summary for patients appears in Ann Intern Med. 2007;147(6):I16. [DOI] [PubMed] [Google Scholar]
- 43.Marliss EB, Vranic M. Intense exercise has unique effects on both insulin release and its roles in glucoregulation: implications for diabetes. Diabetes. 2002;51(Suppl 1):S271–S283. doi: 10.2337/diabetes.51.2007.s271. [DOI] [PubMed] [Google Scholar]
- 44.Sriwijitkamol A, Coletta DK, Wajcberg E, Balbontin GB, Reyna SM, Barrientes J, Eagan PA, Jenkinson CP, Cersosimo E, DeFronzo RA, Sakamoto K, Musi N. Effect of acute exercise on AMPK signalling in skeletal muscle of subjects with type 2 diabetes: a time-course and dose-response study. Diabetes. 2007;56(3):836–848. doi: 10.2337/db06-1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gaudet-Savard T, Ferland A, Broderick TL, Garneau C, Tremblay A, Nadeau A, Poirier P. Safety and magnitude of changes in blood glucose levels following exercise performed in the fasted and the postprandial state in men with type 2 diabetes. Eur J Cardiovasc Prev Rehabil. 2007;14(6):831–836. doi: 10.1097/HJR.0b013e3282efaf38. [DOI] [PubMed] [Google Scholar]
- 46.Kjaer M, Hollenbeck CB, Frey-Hewitt B, Galbo H, Haskell W, Reaven GM. Glucoregulation and hormonal responses to maximal exercise in non-insulin-dependent diabetes. J Appl Physiol. 1990;68(5):2067–2074. doi: 10.1152/jappl.1990.68.5.2067. [DOI] [PubMed] [Google Scholar]
- 47.Musi N, Fujii N, Hirshman MF, Ekberg I, Fröberg S, Ljungqvist O, Thorell A, Goodyear LJ. AMP-activated protein kinase (AMPK) is activated in muscle of subjects with type 2 diabetes during exercise. Diabetes. 2001;50(5):921–927. doi: 10.2337/diabetes.50.5.921. [DOI] [PubMed] [Google Scholar]
- 48.Sigal RJ, Kenny GP, Wasserman DH, Castaneda-Sceppa C, White RD. Physical activity/exercise and type 2 diabetes: a consensus statement from the American Diabetes Association. Diabetes Care. 2006;29(6):1433–1438. doi: 10.2337/dc06-9910. [DOI] [PubMed] [Google Scholar]
- 49.Galbo H, Tobin L, van Loon LJ. Responses to acute exercise in type 2 diabetes, with an emphasis on metabolism and interaction with oral hypoglycemic agents and food intake. Appl Physiol Nutr Metab. 2007;32(3):567–575. doi: 10.1139/H07-029. [DOI] [PubMed] [Google Scholar]
- 50. http://www.health.gov/PAguidelines. Accessed August 1, 2008.
- 51.Nathan DM, Buse JB, Davidson MB, Ferrannini E, Holman RR, Sherwin R, Zinman B. Management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: update regarding thiazolidinediones: a consensus statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2008;31(1):173–175. doi: 10.2337/dc08-9016. [DOI] [PubMed] [Google Scholar]