Abstract
In recent years, a variety of devices (drug-eluting stents, artificial organs, biosensors, catheters, scaffolds for tissue engineering, heart valves, etc.) have been developed for implantation into patients. However, when such devices are implanted into the body, the body can react to these in a number of different ways. These reactions can result in an unexpected risk for patients. Therefore, it is important to assess and optimize the biocompatibility of implantable devices. To date, numerous strategies have been investigated to overcome body reactions induced by the implantation of devices. This review focuses on the foreign body response and the approaches that have been taken to overcome this. The biological response following device implantation and the methods for biocompatibility evaluation are summarized. Then the risks of implantable devices and the challenges to overcome these problems are introduced. Specifically, the challenges used to overcome the functional loss of glucose sensors, restenosis after stent implantation, and calcification induced by implantable devices are discussed.
Keywords: biocompatibility, calcification, foreign body reaction, glucose sensor, implantable device, stent
Introduction
The progress made in recent years in the areas of biotechnology, tissue engineering, biomaterials, cell and molecular biology, polymer science, and other related fields has resulted in numerous medical and pharmaceutical advances. Of particular significance is the progress that has been made in the areas of implantable devices and drug/device combination products, such as drug-eluting stents,1–5 artificial organs,6–9 biosensors,10,11 catheters,12 scaffolds for tissue engineering,13–15 and heart valves.16,17 Nevertheless, the biocompatibility of implantable devices remains a critical issue in limiting device longevity and functionality, particularly in the case of biosensors. The foreign body reaction to implantable devices also presents a significant risk for patients.
Various natural, synthetic, and semisynthetic materials are currently utilized in the fabrication of implantable devices. Naturally occurring materials include collagen,18,19 chitosan,20–22 alginate,23 hyaluronan,24 and dextran.25,26 Commonly used synthetic polymers include poly(lactic acid)(PLA) and poly(lactic-co-glycolic acid)(PLGA),27–33 poly(ethylene glycol)(PEG),34–36 2-hydroxy ethyl methacrylate,33 and poly(vinyl alcohol)(PVA).37–39 Although these materials are commonly used and considered to be relatively biocompatible, a number of studies have shown that devices made from these materials have biocompatibility issues.40 In addition, it has been shown that biological reactions that are adverse for a material in one application may not be adverse for the same material in a different application.41 Similarly, a material found to be safe in one application may not be safe in another application.41
This review focuses on the foreign body reaction induced following the implantation of devices. The biological response following device implantation and methods for biocompatibility evaluation are summarized. Then the risks associated with implantable devices and challenges used to overcome these problems are introduced.
Biological Responses to Implantable Devices
Inflammatory Reaction
The inflammatory response is caused by the tissue injury that results from implantation of the device as well as the continual presence of the device in the body. When a tissue is injured by device implantation, a wound healing response is initiated through a series of complex events.42,43 The main stages in this process include acute inflammation, chronic inflammation, and the formation of granulomatous tissue.
The acute inflammatory response is of relatively short duration (up to a few days).42 This phase is mostly responsible for provisional matrix formation and cleaning of the wound site.42,43 To begin with, vessels dilate and excess blood flows into the injury site and then a blood clot, which is composed mostly of fibrin, is formed to close the wound.42,44 The permeation of salts, proteins, and water through the endothelial tight junctions of the capillary walls is increased, resulting in edema.45 This response results principally from hemodynamic forces and is presumably an attempt by the host to dilute the insulting agent, reducing the concentration of harmful molecules. Numerous blood and tissue proteins such as cytokines and growth factors are released, and leukocytes adhere to the endothelium of the blood vessels and infiltrate the injured site.46–48 Cells are recruited rapidly to the injury site; during the acute inflammatory phase, these are mainly neutrophils, which are the main component of white blood cells. Monocytes are then called into the site and these differentiate into macrophages. These phagocytic cells act to remove foreign material (such as bacteria and dead cells) to clean up the wound site.
Persistent inflammatory stimuli, such as the continual presence of a foreign object, lead to chronic inflammation. This phase is generally characterized by the presence of macrophages, monocytes, and lymphocytes, as well as the proliferation of blood vessels and connective tissue to restructure the affected area.49,50 The restructuring phase proceeds as follows. The fibrin clot formed during the acute inflammatory phase is converted into a highly vascularized granulation tissue by the proliferation of fibroblasts and vascular endothelial cells. Growth factors such as platelet-derived growth factor, fibroblast growth factor, transforming growth factor-β (TGF-β), TGF-α/epidermal growth factor, interleukin-1, and tumor necrosis factor are important for the generation of fibrous tissue and blood vessels, as well as for the regeneration of epithelial cells.51–55 The formation of blood vessels is essential to wound healing, supplying necessary nutrients. The new blood vessels are formed by budding or sprouting of preexisting vessels in a process known as neovascularization or angiogenesis.56,57 Fibroblasts are active in synthesizing collagen and proteoglycans. Eventually, the granulation tissue is replaced by an extracellular matrix (ECM). ECM is a structural network of macromolecules surrounding stromal cells that underlies most endothelia and epithelia. The ECM consists of four major macromolecules—collagen, elastin, structural glycoproteins, and proteoglycans—and acts not only as a physical scaffold, but also as a crucial modulator of biologic processes, including differentiation, development regeneration, tumor progression, and repair.58,59 Chronic inflammation is less uniform histologically compared to acute inflammation, and the wound healing response is generally dependent on the extent or degree of injury.
Figure 1 shows representative micrographs of subcutaneous tissue following implantation of PVA hydrogel/PLGA microsphere composites (containing no drug) into the subcutaneous tissue of rats. Figure 1A is typical of the acute inflammatory response (day 3), with a large infiltration of neutrophils into the local site. Figure 1B is representative of chronic inflammation (day 30), multinucleate giant cells, fibrosis, and mixed inflammatory cells are present. In Figure 1C, mineralization (yellow box) can be seen around the implant at 60 days. Figure 1D shows fibrosis around the implant, and collagen is stained blue using Masson's trichrome stain at day 30.
Figure 1.
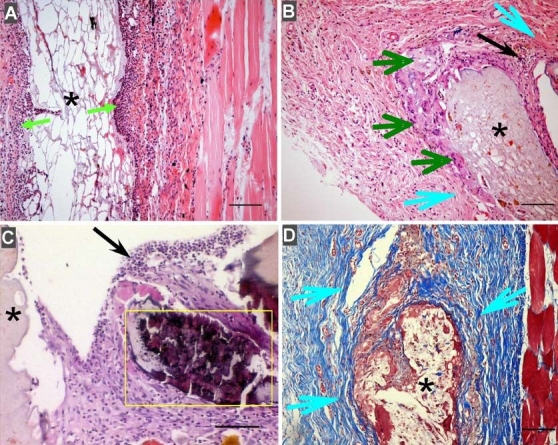
Foreign body response to PVA hydrogel/PLGA microsphere composites (containing no drug) implanted into the subcutaneous tissue of rats. (A) Acute inflammation at day 3 after implantation (green arrow: neutrophils). (B) Chronic inflammation at day 30 after implantation (dark green arrow: multinucleate giant cells; light blue arrow: fibrosis; and black arrow: mixed inflammatory cells). (C) Mineralization (yellow box) around the implant at day 60. (D) Fibrosis around the implant (yellow arrows) stained with Masson's trichrome stain (collagen is stained blue). Bar: 100 µm.
Foreign Body Reaction
A biomaterial implanted into the body induces a foreign body reaction.44,60 According to review articles, the degree of this reaction depends on the properties of the device, such as (1) shape, (2) size, (3) surface chemistry and roughness, (4) design, (5) morphology and porosity, (6) composition, (7) sterility issues, (8) contact duration, and (9) degradation.44,61–64 Following implantation, a polymer–blood interface is immediately created and nonspecific absorption of blood and tissue fluid proteins onto the surface of the device is induced.65 In fact, the extent of nonspecific protein absorption can be used to evaluate the degree of biocompatibility of the device. Following nonspecific protein absorption, immune and inflammatory cells such as monocytes, leukocytes, and platelets intervene in order to protect the body from the foreign object. The end stage of the foreign body reaction involves walling off the device by a vascular, collagenous fibrous capsule that is typically 50–200 mm in thickness.43 This fibrous wall confines the implanted device and prevents it from interacting with surrounding tissues.
Biocompatibility Testing
It is generally accepted that the term “biocompatibility” is defined not only by the lack of cytotoxicity of a bio-material, but also by the biofunctionality of the material, which enables it to support cell–biomaterial interactions according to the local and organ-specific situation where the biomaterial is applied.66 Biocompatibility studies on an implantable device require complex experiments both in vitro and in vivo in order to test the local and systemic effects of the material on culture cells, tissue sections, and the whole body. In vitro cell culture tests are often used to screen the biocompatibility of implantable devices. Such tests are sensitive, reliable, convenient, and reproducible screening methods.40,67,68 Permanent cell lines are generally employed for such in vitro testing. For instance, a rat primary culture of osteoblasts is a well-established model used to investigate biocompatibility by evaluating cellular viability.69–72 Osteoblasts support the formation, secretion, and mineralization of the extracellular bone matrix and thus provide important parameters in the study of biomaterial/cell interactions.
International Organization for Standardization (ISO) 10993 provides a series of standards for evaluating the biocompatibility of a medical device prior to clinical testing. In vitro testing is covered by ISO 10993-573 and includes positive and negative control materials, extraction conditions, and choice of cell lines and cell media, as well as important aspects of the test procedures, including tests on extracts and tests on direct and indirect contents. The standard also allows for qualitative or quantitative evaluation. Short- and long-term medium (modified eagle's medium, MEM) extraction tests defined in ISO 10993-5 are widely accepted. The typical method of the MEM extraction test is described as follows. The test material is extracted in Dulbecco's modified eagle's medium. In the short-term test, the extraction period is generally 24 hours, whereas in the long-term test it takes weeks to months for the extraction. Before initiating the test, the culture medium is replaced by the same amount of extraction fluid and the response of the cells is evaluated.
Evaluation of the biocompatibility and biofunctionality of materials is conducted by methods based on the assessment of cytotoxicity, mutagenesis/carcinogenesis, and cell biofunction.40,67,68,74–76 Regarding cytotoxicity testing, the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay is the most popular. MTT is a standard colorimetric assay for measuring cellular growth. MTT is a soluble compound by nature, which when reduced by the mitochondrial enzyme present in living cells, it turns into formazon, which is a purple-colored, water-insoluble compound. The amount of living cells in the sample can be estimated by measuring the amount of formazon spectrophotometrically following dissolution in a suitable organic solvent. The Ames test is the most widely accepted method used to evaluate mutagenesis/carcinogenesis. This method involves the detection of mutations in the metabolic function of bacteria and uses a strain of Salmonella typhimurium that carries a defective (mutant) gene. This bacterium cannot synthesize the amino acid histidine from the ingredients in the culture medium. However, some types of mutations (including this one) can be reversed, by a back mutation, where the gene regains its function following exposure to the mutagenic substance. These revertants are able to grow in a medium lacking histidine. By comparing the number of colonies of revertants, mutagenesis and carcinogenesis induced by materials can be estimated. To evaluate the biofunctionality of cells, various parameters, such as cell adhesion, spreading, and biosynthetic function, are employed.
Koschwanez and colleagues77 introduced a method of in vivo biocompatibility testing of implantable glucose sensors to be used for diabetes management. The cage implant system is an in vivo method used to assess the inflammatory response to a biomaterial78–80 or biosensor.81 Briefly, the specimen of interest is inserted into a stainless steel mesh cage, which is implanted into animals. The exudates from the cage are collected over the course of the experiment and examined for the presence of inflammatory cells, enzyme secretions, and cell–material interactions, including fibrous encapsulation and the presence of infection.81 This method allows for serial examination of the fluid surrounding the sensor, without sacrifice of the animal until the end of the experiment. However, this method is limited as only fluid, not tissue, is examined. The tissue chamber system provides an alternative method. A lightweight aluminum or titanium frame is fastened chronically to the back of the animal (e.g., rat or hamster82,83). A skin fold is secured between the two plates using stainless steel bolts, and the implanted device, such as a biosensor, is inserted into the skin fold. This system is convenient for histological evaluation of the sensor. Real-time visualization of the device is possible by removing the skin and covering the site with glass. This system is also used to visualize tumor growth and microvascularization. Although these methods are helpful, a serial sacrifice study involving explantation of tissue samples and pathohistological examination is often required to assess inflammation, fibrous encapsulation, and calcification of the device and surrounding tissue.
Challenges to Overcome Problems Induced by Implantable Devices
Functional Loss of Glucose Sensor Due to Fibrosis Encapsulation
Monitoring blood glucose concentrations is important for an intensive insulin regimen for patients with diabetes. Currently, metabolic monitoring depends on finger pricking and external monitoring several times per day to determine glucose levels. However, the pain and inconvenience of such testing reduce patient compliance. Implantable glucose sensors are a promising solution to this problem and, recently, numerous researchers are attempting to develop implantable glucose sensors.77 These sensors offer a simplistic and minimally invasive means for routine monitoring of blood glucose levels. The subcutaneous tissue is regarded as an appropriate site for sensor implantation since it is easily accessible for insertion and removal. In addition, it has been reported that glucose concentrations in the subcutaneous fluid are directly related to blood glucose concentrations.77 Despite outstanding advances in the in vitro functionality of such sensors, it is difficult to achieve reliable long-term continuous glucose monitoring in vivo due to the gradual loss of sensor functionality following implantation. Rebrin and associates84 investigated the stability of glucose sensors following subcutaneous implantation into dogs. Their experiments were terminated as a result of loss of sensitivity of implanted sensors and/or instability of measurement. A similar functional loss of glucose sensors has been reported by numerous researchers.85–89 Surgical implantation of glucose sensors is accompanied by tissue injury, and the surface of the device interacts with the body for a long time, both initiating and maintaining the foreign body reaction. Most functional loss of biosensor activity is assumed to be caused by histological changes that occur in the tissue surrounding the implant (specifically, inflammatory reaction and/or fibrous encapsulation). There are many implications in the literature that glucose diffusion is influenced negatively by nonspecific protein absorption from the tissue fluid to the sensor surface,85,90 and the fibrous capsule that forms around implanted sensors restricts the transport of even low molecular weight analytes such as glucose to the sensor surface.91–98 Consequently, maintaining glucose sensor function in an in vivo environment remains a key challenge.
To overcome this issue, a wide range of approaches has been reported. Numerous materials have been employed as coatings. In order to minimize tissue reactions around the sensors, Ju and colleagues99 developed a three-dimensional porous collagen scaffold, which was prepared by cross-linking collagen with nordihydroguaiaretic acid. This scaffold is stable to incubation with collagenase solution for 4 weeks, and the sensitivity of the coated sensor was not different from that of the uncoated sensor. Koschwanez et al.100 investigated porous PLA coatings to reduce fibrosis and promote blood microvessel formation in tissue adjacent to the sensor surface. Three-week subcutaneous rat studies showed the anticipated effect of porous PLA coatings enhancing vascularity and decreasing collagen deposition. Several researchers have used Nafion™ membranes as a coating material for the protection of biosensors.101–103 Nafion is an ionomer made from a sulfonated tetrafluorethylene copolymer. Results from these studies indicated that the coatings did not impair sensor function, suggesting the usefulness of these materials to enhance the function and lifetime of implantable biosensors by providing a controlled local environment around the sensors. In addition, hydrogel coatings have been applied in a broad range of biomaterial and pharmaceutical applications.104,105 These include poly(hydroxyethyl methacrylate),106,107 PEG,108 polyurethane,109,110 and PVA.111 Coatings made from these polar, uncharged, water-swellable, flexible aqueous layers can mask the surface of the device and possibly improve sensor functionality and lifetime. PVA,111 as well as other hydrogel materials, is an attractive outer membrane coating for sensors because water-soluble analytes such as glucose can diffuse readily through the water-swollen polymer gel layer. The degree of analyte diffusion is modulated readily by controlling the cross-link density of the gel, which in turn controls the water content of the gel and the openness of the polymer network.111
To suppress inflammation, anti-inflammatory agents can be applied at the site. For example, our group has been investigating the effect of coadministration of dexamethasone with biosensors.111–115 Dexamethasone is a strong anti-inflammatory agent and has an influence on platelet function, smooth muscle cell proliferation, and collagen synthesis.116–118 Our group developed a dexamethasone-loaded PLGA microsphere/PVA hydrogel coating.119,120 PVA is biocompatible and can be fabricated easily using the freeze–thaw technique,121 which is a mild cross-linking method and therefore avoids degradation of the electrochemical-sensing enzyme and of the drug-releasing polymer matrices (microspheres) entrapped within the hydrogel. PVA hydrogels possess high stability under a range of temperature and pH conditions and can be constructed to have mechanical strength similar to that of human soft tissues.111,122 PLGA microspheres containing dexamethasone were embedded in the PVA hydrogel to create a “smart” coating that allows rapid diffusion of the analytes with slow and controlled long-term release of the dexamethasone via the microspheres. These “smart” coatings were able to control the inflammatory reaction for 1 month.112–114 The same hydrogel coatings without drug are shown in Figure 1 to induce inflammation and fibrosis. This can be compared to Figure 2 where subcutaneous tissue samples taken from rats implanted with dexamethasone containing “smart” coatings (at days 7 and 30) are similar to that of normal tissue.
Figure 2.
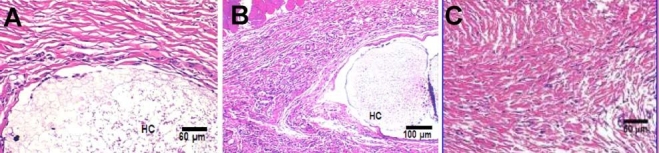
Pharmacodynamic changes in representative tissue sections after subcutaneous implantation of PLGA microsphere/PVA hydrogel composites (HC) containing dexamethasone. (A) Day 7 after implantation. (B) Day 30 after implantation. (C) Untreated normal tissue. Hematoxylin and eosin stains inflammation-mediating cells basophilic (purple) and subcutaneous connective tissue eosinophilic (pink).
In addition to suppressing the inflammatory reaction, enhancement of neovascularization at the implant site may improve sensor lifetime. Since fibrosis-associated vessel regression is assumed to be a major factor in the loss of biosensor function in vivo, researchers have hypothesized that neovascularization at the site of sensor implantation would enhance biosensor function directly. To induce neovascularization at specific sites, vascular endothelial cell growth factor (VEGF) has been used with biosensors.123–127 Ward et al.125 infused VEGF) via an osmotic pump at the glucose sensor implant site. Glucose sensor function was found to be favored in the presence of a VEGF infusion port. They concluded that this was due to increased neovascularization in the surrounding foreign body capsule. Our laboratory has developed a VEGF- and dexamethasone-loaded PLGA microsphere/PVA hydrogel coating as a means to modify the environment around implanted sensors.127 This coating succeeded in suppressing inflammation and fibrosis for the 4-week experimental period, as well as facilitating neoangiogenesis (Figure 3). This result suggested the possibility of markedly increasing sensor lifetime. A similar hydrogel coating system was reported by Norton and associates.126
Figure 3.
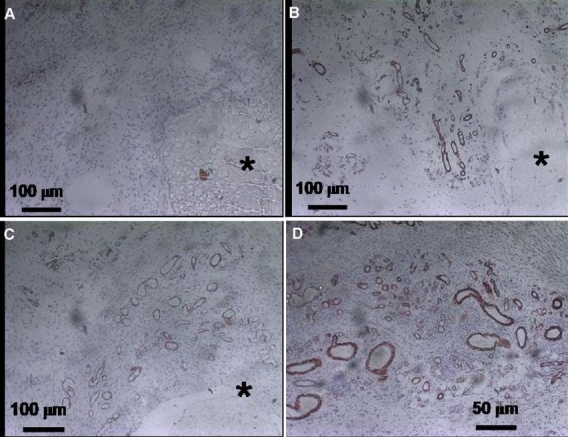
Pharmacodynamic changes in representative subcutaneous tissue sections of rats implanted with PLGA microsphere/PVA hydrogel composites (HC) containing dexamethasone and VEGF combination over (A) week 1, (B) week 2, (C) week 3, and (D) week 4 postimplantation. Cells containing markers for angiogenesis are stained (smooth muscle actin staining).127
Restenosis after Stent Implantation
Coronary stents are used for patients undergoing percutaneous coronary revascularization. Although the restenosis rate after coronary stent implantation is lower compared to balloon angioplasty, stent restenosis remains a major problem. The implanted stent contacts the vessel wall directly and protrudes into the endovascular tissue. This can harm the endothelial vascular tissue, resulting in an inflammatory reaction, which may play a key role in the process of neointimal proliferation, leading to restenosis.128,129 The risk of restenosis depends on the magnitude and persistence of the local inflammation.
Drug-eluting stents provide one solution to help prevent restenosis and these have been reviewed by Wu et al.130 Sustained release of drugs incorporated in the stents provides the drug directly to the local tissue. Thus, a high drug concentration can be maintained at the stent site with only a minimal amount of drug reaching the systemic circulation. Thus systemic side effects can be avoided. To date, various drugs have been investigated, including immunosuppressive drugs (sirolimus, everolimus, tacrolimus, ABT-578), antiproliferative (paclitaxel, antinomycin, angiopeptin) and antimigratory (batimastat) drugs, and gene therapeutic reagents (antisense and siRNA, vascular endothelial growth factor, and endothelial nitric oxide synthetase).131–134
Among the available stent products, sirolimus is the most successful drug-eluting stent. Sirolimus-eluting stents have demonstrated dramatically reduced rates of restenosis compared to conventional bare metal stents in several clinical trials. The Cordis Cypher™ sirolimus-eluting stent has been approved in the United States and in Europe135 and is deployed routinely in millions of percutaneous coronary intervention cases. The U.S. Food and Drug Administration approved Boston Scientific's TAXUS Express2™ paclitaxel-eluting coronary stent.135 Further improvements and novel innovations will be continued in an effort to reduce the risk of restenosis and to prolong and enhance stent functionality.
Calcification of Implantable Devices
Calcification is the process in which mineral calcium builds up in implantable devices. Calcification can dramatically compromise device function in vivo. For example, it is well known that calcification contributes to the failure of bioprosthetic heart valves. Although glutaraldehyde-pretreated porcine aortic valves are widely used to replace diseased heat valves, the functional durability of bioprosthetic heart valves is limited (lifetime of ∼15 years). The mechanism of calcification is assumed to be associated with cell devitalization and accumulation of cellular debris following implantation.136 To begin with, connective tissue cells are devitalized by several risk factors, such as mechanical damage and extraction of chemical cross-linking agents from the implantable device. Once tissue cells are devitalized, membrane phosphorus cannot work, which reacts with the calcium-containing extracellular fluid, resulting in the formation of calcium phosphate crystals. Figure 1C is an example of granulomatous tissue showing mineralization. The development of techniques to mitigate calcification is crucial in maintaining heart valve functionality. Much research has been conducted into the prevention of calcification of biomaterial implants. This research can be divided into two strategies. One is the combination of an implantable device and a drug delivery system137 where drugs are used to assist in device biocompatibility. Applied drugs include ethanehydroxydiphophonate,138–143 FeCl3 and AlCl3 (inhibitors of the growth and dissolution rate of hydroxyapatite crystals),142,144,145 levamisole (alkaline phosphatase inhibitor) and protamine sulfate (charge modifier),144 and dexamethasone (anti-inflammatory agent).114 The other strategy is modification of the preparation procedure and of the components of implantable devices. For instance, ethanol preincubation is known to be effective in preventing the calcification of glutaraldehyde cross-linked heart valves.146,147 Since some surfactants such as sodium dodecyl sulfate reduce mineralization, these are used in commercial products, such as commercial porcine valves.148,149 To prevent calcification induced by glutaraldehyde, chemical modification of the device surface has been investigated [e.g., using hyaluronic acid,152 sulfonated poly(ethylene oxide) and heparin,151,152 protamine,153,154 bovine pericardium,155–157 and L-arginine156,157].
In addition to glutaraldehyde products, a number of researchers have reported the possibility that some materials regarded as being biocompatible induce calcification. These include collagen sponges,158 polyurethane,159,160 and PVA.161,162 For instance, others have investigated the calcification properties of collagen sponges incubated in cell-free media and indicated that a cellular calcification of collagen-based biomaterials can occur under the culture conditions currently used in tissue engineering.158,163,164 Levy et al.164 implanted collagen sponges that were cross-linked with glutaraldehyde and formaldehyde subcutaneously into rats for 21 days and observed calcification. Golomb and associates reported the formation of calcium phosphate deposits in a polyurethane matrix in both in vitro159 and in vivo studies.137 Based on observations in human primary fibroblasts from hollow fibers, Schwenter et al.161 indicated the possibility that the PVA matrix induces calcification in vivo. It is probable that other biomaterials induce calcification, resulting in adverse effects.
Conclusions
Implantation of devices such as glucose biosensors result in inflammation and fibrosis unless care is taken to overcome the foreign body response. The selection of materials for use with these sensors can minimize the body's response, and there have been considerable advances in the development of biocompatible materials in recent years. In particular, use of a biocompatible outer coating that mimics the body tissue has been shown to minimize these negative responses while maintaining sensor functionality. This can be achieved through the use of a hydrogel coating. Anti-inflammatory agents released at the local site have been the most successful in preventing inflammation and fibrosis. A hydrogel coating has been developed that provides a slow release of anti-inflammatory drugs and other agents while allowing rapid diffusion of analytes through the hydrogel for sensing.114,115,127 Research has also focused on the release of growth factors at the sensor site for the purpose of inducing blood vessel growth to ensure an adequate analyte supply. It is anticipated that these efforts to develop biocompatible materials for glucose biosensors will assist in the realization of totally implantable long-term biosensors in the near future.
Acknowledgements
This work was supported by the U.S. Army Medical Research TATRC (W81XWH-07-1-0688) and the National Institutes of Health (1R01RR14171).
Abbreviations
- ECM
extracellular matrix
- ISO
International Organization for Standardization
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- MEM
modified eagle's medium
- PEG
poly(ethylene glycol)
- PLA
poly(lactic acid)
- PLGA
poly(lactic-co-glycolic acid)
- PVA
poly(vinyl alcohol)
- TGF
transforming growth factor
- VEGF
vascular endothelial cell growth factor
References
- 1.Kipshidze NN, Tsapenko MV, Leon MB, Stone GW, Moses JW. Update on drug-eluting coronary stents. Expert Rev Cardiovasc Ther. 2005;3(5):953–968. doi: 10.1586/14779072.3.5.953. [DOI] [PubMed] [Google Scholar]
- 2.Moses JW, Kipshidze N, Leon MB. Perspectives of drug-eluting stents: the next revolution. Am J Cardiovasc Drugs. 2002;2(3):163–172. doi: 10.2165/00129784-200202030-00004. [DOI] [PubMed] [Google Scholar]
- 3.Klonoff DC. Technological advances in the treatment of diabetes mellitus: better bioengineering begets benefits in glucose measurement, the artificial pancreas, and insulin delivery. Pediatr Endocrinol Rev. 2003;1(2):94–100. [PubMed] [Google Scholar]
- 4.Frazier OH, Jacob LP. Small pumps for ventricular assistance: progress in mechanical circulatory support. Cardiol Clin. 2007;25(4):553–564. doi: 10.1016/j.ccl.2007.09.001. vi. [DOI] [PubMed] [Google Scholar]
- 5.Belverud S, Mogilner A, Schulder M. Intrathecal pumps. Neurotherapeutics. 2008;5(1):114–122. doi: 10.1016/j.nurt.2007.10.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kizilel S, Garfinkel M, Opara E. The bioartificial pancreas: progress and challenges. Diabetes Technol Ther. 2005;7(6):968–985. doi: 10.1089/dia.2005.7.968. [DOI] [PubMed] [Google Scholar]
- 7.O'Connor RC, Lyon MB, Guralnick ML, Bales GT. Long-term follow-up of single versus double cuff artificial urinary sphincter insertion for the treatment of severe postprostatectomy stress urinary incontinence. Urology. 2008;71(1):90–93. doi: 10.1016/j.urology.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 8.Fine DM, Tobias AH. Cardiovascular device infections in dogs: report of 8 cases and review of the literature. J Vet Intern Med. 2007;21(6):1265–1271. doi: 10.1892/07-019.1. [DOI] [PubMed] [Google Scholar]
- 9.Prokop A. Bioartificial organs in the twenty-first century: nanobiological devices. Ann N Y Acad Sci. 2001;944:472–490. doi: 10.1111/j.1749-6632.2001.tb03856.x. [DOI] [PubMed] [Google Scholar]
- 10.Wilson GS, Zhang Y, Reach G, Moatti-Sirat D, Poitout V, Thévenot DR, Lemonnier F, Klein JC. Progress toward the development of an implantable sensor for glucose. Clin Chem. 1992;38(9):1613–1617. [PubMed] [Google Scholar]
- 11.Koschwanez HE, Reichert WM. In vitro, in vivo and post explantation testing of glucose-detecting biosensors: current methods and recommendations. Biomaterials. 2007;28(25):3687–3703. doi: 10.1016/j.biomaterials.2007.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Callahan TD, 4th, Natale A. Catheter ablation of atrial fibrillation. Med Clin North Am. 2008;92(1):179–201. doi: 10.1016/j.mcna.2007.09.001. xii. [DOI] [PubMed] [Google Scholar]
- 13.Matsumoto T, Okazaki M, Nakahira A, Sasaki J, Egusa H, Sohmura T. Modification of apatite materials for bone tissue engineering and drug delivery carriers. Curr Med Chem. 2007;14(25):2726–2733. doi: 10.2174/092986707782023208. [DOI] [PubMed] [Google Scholar]
- 14.Salgado AJ, Coutinho OP, Reis RL. Bone tissue engineering: state of the art and future trends. Macromol Biosci. 2004;4(8):743–765. doi: 10.1002/mabi.200400026. [DOI] [PubMed] [Google Scholar]
- 15.Abukawa H, Papadaki M, Abulikemu M, Leaf J, Vacanti JP, Kaban LB, Troulis MJ. The engineering of craniofacial tissues in the laboratory: a review of biomaterials for scaffolds and implant coatings. Dent Clin North Am. 2006;50(2):205–216. doi: 10.1016/j.cden.2005.11.006. viii. [DOI] [PubMed] [Google Scholar]
- 16.Nozyński JK, Religa Z, Wszołek J, Zembala-Nozyńska E, Rozentryt P. Biological heart valve–an alternative to mechanical valve. Med Sci Monit. 2001;7(3):550–562. [PubMed] [Google Scholar]
- 17.Black MM, Drury PJ. Mechanical and other problems of artificial valves. Curr Top Pathol. 1994;86:127–159. doi: 10.1007/978-3-642-76846-0_4. [DOI] [PubMed] [Google Scholar]
- 18.Sano A, Hojo T, Maeda M, Fujioka K. Protein release from collagen matrices. Adv Drug Deliv Rev. 1998;31(3):247–266. doi: 10.1016/s0169-409x(97)00119-1. [DOI] [PubMed] [Google Scholar]
- 19.Geiger M, Li RH, Friess W. Collagen sponges for bone regeneration with rhBMP-2. Adv Drug Deliv Rev. 2003;55(12):1613–1629. doi: 10.1016/j.addr.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 20.Uchegbu IF, Schätzlein AG, Tetley L, Gray AI, Sludden J, Siddique S, Mosha E. Polymeric chitosan-based vesicles for drug delivery. J Pharm Pharmacol. 1998;50(5):453–458. doi: 10.1111/j.2042-7158.1998.tb06185.x. [DOI] [PubMed] [Google Scholar]
- 21.Khor E, Lim LY. Implantable applications of chitin and chitosan. Biomaterials. 2003;24(13):2339–2349. doi: 10.1016/s0142-9612(03)00026-7. [DOI] [PubMed] [Google Scholar]
- 22.Borchard G, Junginger HE. Modern drug delivery applications of chitosan. Adv Drug Deliv Rev. 2001;52(2):103. doi: 10.1016/s0169-409x(01)00188-0. [DOI] [PubMed] [Google Scholar]
- 23.de Vos P, Hoogmoed CG, Busscher HJ. Chemistry and biocompatibility of alginate-PLL capsules for immunoprotection of mammalian cells. J Biomed Mater Res. 2002;60(2):252–259. doi: 10.1002/jbm.10060. [DOI] [PubMed] [Google Scholar]
- 24.Vercruysse KP, Prestwich GD. Hyaluronate derivatives in drug delivery. Crit Rev Ther Drug Carrier Syst. 1998;15(5):513–555. [PubMed] [Google Scholar]
- 25.Cadée JA, Brouwer LA, den Otter W, Hennink WE, van Luyn MJ. A comparative biocompatibility study of microspheres based on crosslinked dextran or poly(lactic-co-glycolic)acid after subcutaneous injection in rats. J Biomed Mater Res. 2001;56(4):600–609. doi: 10.1002/1097-4636(20010915)56:4<600::aid-jbm1133>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 26.Draye JP, Delaey B, Van de Voorde A, Van Den Bulcke A, De Reu B, Schacht E. In vitro and in vivo biocompatibility of dextran dialdehyde cross-linked gelatin hydrogel films. Biomaterials. 1998;19(18):1677–1687. doi: 10.1016/s0142-9612(98)00049-0. [DOI] [PubMed] [Google Scholar]
- 27.Shive MS, Anderson JM. Biodegradation and biocompatibility of PLA and PLGA microspheres. Adv Drug Delivery Rev. 1997;28(1):5–24. doi: 10.1016/s0169-409x(97)00048-3. [DOI] [PubMed] [Google Scholar]
- 28.Athanasiou KA, Niederauer GG, Agrawal CM. Sterilization, toxicity, biocompatibility and clinical applications of polylactic acid/polyglycolic acid copolymers. Biomaterials. 1996;17(2):93–102. doi: 10.1016/0142-9612(96)85754-1. [DOI] [PubMed] [Google Scholar]
- 29.Daugherty AL, Cleland JL, Duenas EM, Mrsny RJ. Pharmacological modulation of the tissue response to implanted polylactic-co-glycolic acid microspheres. Eur J Pharm Biopharm. 1997;44(1):89–102. [Google Scholar]
- 30.Lunsford L, McKeever U, Eckstein V, Hedley ML. Tissue distribution and persistence in mice of plasmid DNA encapsulated in a PLGA-based microsphere delivery vehicle. J Drug Target. 2000;8(1):39–50. doi: 10.3109/10611860009009208. [DOI] [PubMed] [Google Scholar]
- 31.Ronneberger B, Kissel T, Anderson JM. Biocompatibility of ABA triblock copolymer microparticles consisting of poly(L-lactic-co-glycolic-acid) A-blocks attached to central poly(oxyethylene) B-blocks in rats after intramuscular injection. Eur J Pharm Biopharm. 1997;43:19–28. [Google Scholar]
- 32.Ronneberger B, Kao WJ, Anderson JM, Kissel T. In vivo biocompatibility study of ABA triblock copolymers consisting of poly(L-lactic-co-glycolic acid) A blocks attached to central poly(oxyethylene) B blocks. J Biomed Mater Res. 1996;30(1):31–40. doi: 10.1002/(SICI)1097-4636(199601)30:1<31::AID-JBM5>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 33.Royals MA, Fujita SM, Yewey GL, Rodriguez J, Schultheiss PC, Dunn RL. Biocompatibility of a biodegradable in situ forming implant system in rhesus monkeys. J Biomed Mater Res. 1999;45(3):231–239. doi: 10.1002/(sici)1097-4636(19990605)45:3<231::aid-jbm11>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 34.Dalsin JL, Hu BH, Lee BP, Messersmith PB. Mussel adhesive protein mimetic polymers for the preparation of nonfouling surfaces. J Am Chem Soc. 2003;125(14):4253–4258. doi: 10.1021/ja0284963. [DOI] [PubMed] [Google Scholar]
- 35.Tsai WB, Grunkemeier JM, McFarland CD, Horbett TA. Platelet adhesion to polystyrene-based surfaces preadsorbed with plasmas selectively depleted in fibrinogen, fibronectin, vitronectin, or von Willebrand's factor. J Biomed Mater Res. 2002;60(3):348–359. doi: 10.1002/jbm.10048. [DOI] [PubMed] [Google Scholar]
- 36.Shen M, Horbett TA. The effects of surface chemistry and adsorbed proteins on monocyte/macrophage adhesion to chemically modified polystyrene surfaces. J Biomed Mater Res. 2001;57(3):336–345. doi: 10.1002/1097-4636(20011205)57:3<336::aid-jbm1176>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 37.Paradossi G, Cavalieri F, Chiessi E, Spagnoli C, Cowman MK. Poly(vinyl alcohol) as versatile biomaterial for potential biomedical applications. J Mater Sci Mater Med. 2003;14(8):687–691. doi: 10.1023/a:1024907615244. [DOI] [PubMed] [Google Scholar]
- 38.Oka M, Ushio K, Kumar P, Ikeuchi K, Hyon SH, Nakamura T, Fujita H. Development of artificial articular cartilage. Proc Inst Mech Eng [H] 2000;214(1):59–68. doi: 10.1243/0954411001535246. [DOI] [PubMed] [Google Scholar]
- 39.Maruoka S, Matsuura T, Kawasaki K, Okamoto M, Yoshiaki H, Kodama M, Sugiyama M, Annaka M. Biocompatibility of polyvinylalcohol gel as a vitreous substitute. Curr Eye Res. 2006;31(7-8):599–606. doi: 10.1080/02713680600813854. [DOI] [PubMed] [Google Scholar]
- 40.Mendes SC, Reis RL, Bovell YP, Cunha AM, van Blitterswijk CA, de Bruijn JD. Biocompatibility testing of novel starch-based materials with potential application in orthopaedic surgery: a preliminary study. Biomaterials. 2001;22(14):2057–2064. doi: 10.1016/s0142-9612(00)00395-1. [DOI] [PubMed] [Google Scholar]
- 41.Anderson JM, Langone JJ. Issues and perspectives on the biocompatibility and immunotoxicity evaluation of implanted controlled release systems. J Control Release. 1999;57(2):107–113. doi: 10.1016/s0168-3659(98)00178-3. [DOI] [PubMed] [Google Scholar]
- 42.Anderson JM. Biological responses to materials. Annu Rev Mater Res. 2001;31:81–110. [Google Scholar]
- 43.Ratner BD, Bryant SJ. Biomaterials: where we have been and where we are going. Annu Rev Biomed Eng. 2004;6:41–75. doi: 10.1146/annurev.bioeng.6.040803.140027. [DOI] [PubMed] [Google Scholar]
- 44.Fournier E, Passirani C, Montero-Menei CN, Benoit JP. Biocompatibility of implantable synthetic polymeric drug carriers: focus on brain biocompatibility. Biomaterials. 2003;24(19):3311–3331. doi: 10.1016/s0142-9612(03)00161-3. [DOI] [PubMed] [Google Scholar]
- 45.Anderson JM. Inflammatory response to implants. ASAIO Trans. 1988;34(2):101–107. doi: 10.1097/00002480-198804000-00005. [DOI] [PubMed] [Google Scholar]
- 46.Jutila MA. Leukocyte traffic to sites of inflammation. APMIS. 1992;100(3):191–201. doi: 10.1111/j.1699-0463.1992.tb00861.x. [DOI] [PubMed] [Google Scholar]
- 47.Pober JS, Cotran RS. The role of endothelial cells in inflammation. Transplantation. 1990;50(4):537–544. doi: 10.1097/00007890-199010000-00001. [DOI] [PubMed] [Google Scholar]
- 48.Cotran RS, Pober JS. Cytokine-endothelial interactions in inflammation, immunity, and vascular injury. J Am Soc Nephrol. 1990;1(3):225–235. doi: 10.1681/ASN.V13225. [DOI] [PubMed] [Google Scholar]
- 49.Johnston RB., Jr Current concepts: immunology. Monocytes and macrophages. N Engl J Med. 1988;318(12):747–752. doi: 10.1056/NEJM198803243181205. [DOI] [PubMed] [Google Scholar]
- 50.Williams GT, Williams WJ. Granulomatous inflammation–a review. J Clin Pathol. 1983;36(7):723–733. doi: 10.1136/jcp.36.7.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wahl SM, Wong H, McCartney-Francis N. Role of growth factors in inflammation and repair. J Cell Biochem. 1989;40(2):193–199. doi: 10.1002/jcb.240400208. [DOI] [PubMed] [Google Scholar]
- 52.Fong Y, Moldawer LL, Shires GT, Lowry SF. The biologic characteristics of cytokines and their implication in surgical injury. Surg Gynecol Obstet. 1990;170(4):363–378. [PubMed] [Google Scholar]
- 53.Kovacs EJ. Fibrogenic cytokines: the role of immune mediators in the development of scar tissue. Immunol Today. 1991;12(1):17–23. doi: 10.1016/0167-5699(91)90107-5. [DOI] [PubMed] [Google Scholar]
- 54.Golden MA, Au YP, Kirkman TR, Wilcox JN, Raines EW, Ross R, Clowes AW. Platelet-derived growth factor activity and mRNA expression in healing vascular grafts in baboons. Association in vivo of platelet-derived growth factor mRNA and protein with cellular proliferation. J Clin Invest. 1991;87(2):406–414. doi: 10.1172/JCI115011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pierce GF, Mustoe TA, Altrock BW, Deuel TF, Thomason A. Role of platelet-derived growth factor in wound healing. J Cell Biochem. 1991;45(4):319–326. doi: 10.1002/jcb.240450403. [DOI] [PubMed] [Google Scholar]
- 56.Thompson JA, Anderson KD, DiPietro JM, Zwiebel JA, Zametta M, Anderson WF, Maciag T. Site-directed neovessel formation in vivo. Science. 1988;241(4871):1349–1352. doi: 10.1126/science.2457952. [DOI] [PubMed] [Google Scholar]
- 57.Ziats NP, Miller KM, Anderson JM. In vitro and in vivo interactions of cells with biomaterials. Biomaterials. 1988;9(1):5–13. doi: 10.1016/0142-9612(88)90063-4. [DOI] [PubMed] [Google Scholar]
- 58.Arribas SM, Hinek A, González MC. Elastic fibres and vascular structure in hypertension. Pharmacol Ther. 2006;111(3):771–791. doi: 10.1016/j.pharmthera.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 59.Labat-Robert J, Bihari-Varga M, Robert L. Extracellular matrix. FEBS Lett. 1990;268(2):386–393. doi: 10.1016/0014-5793(90)81291-u. [DOI] [PubMed] [Google Scholar]
- 60.Anderson JM, Rodriguez A, Chang DT. Foreign body reaction to biomaterials. Semin Immunol. 2008;20(2):86–100. doi: 10.1016/j.smim.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Laurencin CT, Elgendy H. New York: John Wiley & Sons; 1994. The biocompatibility and toxicity of degradable polymeric materials: implication for drug delivery; pp. 27–46. [Google Scholar]
- 62.Sieminski AL, Gooch KJ. Biomaterial-microvasculature interactions. Biomaterials. 2000;21(22):2232–2241. doi: 10.1016/s0142-9612(00)00149-6. [DOI] [PubMed] [Google Scholar]
- 63.Rihova B. Immunocompatibility and biocompatibility of cell delivery systems. Adv Drug Deliv Rev. 2000;42(1-2):65–80. doi: 10.1016/s0169-409x(00)00054-5. [DOI] [PubMed] [Google Scholar]
- 64.Mikos AG, McIntire LV, Anderson JM, Babensee JE. Host response to tissue engineered devices. Adv Drug Deliv Rev. 1998;33(1-2):111–139. doi: 10.1016/s0169-409x(98)00023-4. [DOI] [PubMed] [Google Scholar]
- 65.Williams DF. Tissue-biomateial interactions. J Mater Sci. 1987;22:3421–3445. [Google Scholar]
- 66.Rickert D, Lendlein A, Peters I, Moses MA, Franke RP. Biocompatibility testing of novel multifunctional polymeric biomaterials for tissue engineering applications in head and neck surgery: an overview. Eur Arch Otorhinolaryngol. 2006;263(3):215–222. doi: 10.1007/s00405-005-0950-1. [DOI] [PubMed] [Google Scholar]
- 67.Santos MH, Valerio P, Goes AM, Leite MF, Heneine LG, Mansur HS. Biocompatibility evaluation of hydroxyapatite/collagen nanocomposites doped with Zn+2. Biomed Mater. 2007;2(2):135–141. doi: 10.1088/1748-6041/2/2/012. [DOI] [PubMed] [Google Scholar]
- 68.Kirkpatrick CJ, Bittinger F, Wagner M, Köhler H, van Kooten TG, Klein CL, Otto M. Current trends in biocompatibility testing. Proc Inst Mech Eng [H] 1998;212(2):75–84. doi: 10.1243/0954411981533845. [DOI] [PubMed] [Google Scholar]
- 69.Ito A, Kawamura H, Otsuka M, Ikeuchi M, Ohgushi H, Ishikawa K, Onuma K, Kanzaki N, Sogo Y, Ichinose N. Zinc-releasing calcium phosphate for stimulating bone formation. Mater Sci Eng C. 2002;22:21–25. [Google Scholar]
- 70.Webster TJ, Massa-Schlueter EA, Smith JL, Slamovich EB. Osteoblast response to hydroxyapatite doped with divalent and trivalent cations. Biomaterials. 2004;25(11):2111–2121. doi: 10.1016/j.biomaterials.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 71.Valerio P, Pereira MM, Goes AM, Leite MF. The effect of ionic products from bioactive glass dissolution on osteoblast proliferation and collagen production. Biomaterials. 2004;25(15):2941–2948. doi: 10.1016/j.biomaterials.2003.09.086. [DOI] [PubMed] [Google Scholar]
- 72.Sampaio BV, Goller G, Oktar FN, Valerio P, Goes AM, Leite MF. Biocompatibility evaluation of three different titanium-hydroxyapatite composites. Key Eng Mater. 2005;284-286:639–642. [Google Scholar]
- 73.ISO document 10993. Tests for cytotoxicity: in vitro methods. 1992. Dec, Biological compatibility of medical devices. Part 5. [Google Scholar]
- 74.Morrison C, Macnair R, MacDonald C, Wykman A, Goldie I, Grant MH. In vitro biocompatibility testing of polymers for orthopaedic implants using cultured fibroblasts and osteoblasts. Biomaterials. 1995;16(13):987–992. doi: 10.1016/0142-9612(95)94906-2. [DOI] [PubMed] [Google Scholar]
- 75.Ciapetti G, Granchi D, Verri E, Savarino L, Cavedagna D, Pizzoferrato A. Application of a combination of neutral red and amido black staining for rapid, reliable cytotoxicity testing of biomaterials. Biomaterials. 1996;17(13):1259–1264. [PubMed] [Google Scholar]
- 76.Dekker A, Panfil C, Valdor M, Richter H, Mittermayer Ch, Kirkpatrick CJ. Quantitative methods for in vitro cytotoxicity testing of biomaterials. Cells Mater. 1994;4:101–112. [Google Scholar]
- 77.Koschwanez HE, Reichert WM. In vitro, in vivo and post explantation testing of glucose-detecting biosensors: current methods and recommendations. Biomaterials. 2007;28(25):3687–3703. doi: 10.1016/j.biomaterials.2007.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lindner E, Cosofret VV, Ufer S, Buck RP, Kao WJ, Neuman MR, Anderson JM. Ion-selective membranes with low plasticizer content: electroanalytical characterization and biocompatibility studies. J Biomed Mater Res. 1994;28(5):591–601. doi: 10.1002/jbm.820280509. [DOI] [PubMed] [Google Scholar]
- 79.Marchant R, Hiltner A, Hamlin C, Rabinovitch A, Slobodkin R, Anderson JM. In vivo biocompatibility studies. I. The cage implant system and a biodegradable hydrogel. J Biomed Mater Res. 1983;17(2):301–325. doi: 10.1002/jbm.820170209. [DOI] [PubMed] [Google Scholar]
- 80.Kao WJ, McNally AK, Hiltner A, Anderson JM. Role for interleukin-4 in foreign-body giant cell formation on a poly(etherurethane urea) in vivo. J Biomed Mater Res. 1995;29(10):1267–1275. doi: 10.1002/jbm.820291014. [DOI] [PubMed] [Google Scholar]
- 81.Voskerician G, Liu CC, Anderson JM. Electrochemical characterization and in vivo biocompatibility of a thick-film printed sensor for continuous in vivo monitoring. IEEE Sensors J. 2005;5:1147–1158. [Google Scholar]
- 82.Ertefai S, Gough DA. Physiological preparation for studying the response of subcutaneously implanted glucose and oxygen sensors. J Biomed Eng. 1989;11(5):362–368. doi: 10.1016/0141-5425(89)90097-6. [DOI] [PubMed] [Google Scholar]
- 83.Papenfuss HD, Gross JF, Intaglietta M, Treese FA. A transparent access chamber for the rat dorsal skin fold. Microvasc Res. 1979;18(3):311–318. doi: 10.1016/0026-2862(79)90039-6. [DOI] [PubMed] [Google Scholar]
- 84.Rebrin K, Fischer U, Hahn von Dorsche H, von Woetke T, Abel P, Brunstein E. Subcutaneous glucose monitoring by means of electrochemical sensors: fiction or reality? J Biomed Eng. 1992;14(1):33–40. doi: 10.1016/0141-5425(92)90033-h. [DOI] [PubMed] [Google Scholar]
- 85.Gerritsen M, Jansen JA, Kros A, Vriezema DM, Sommerdijk NA, Nolte RJ, Lutterman JA, Van Hövell SW, Van der Gaag A. Influence of inflammatory cells and serum on the performance of implantable glucose sensors. J Biomed Mater Res. 2001;54(1):69–75. doi: 10.1002/1097-4636(200101)54:1<69::aid-jbm8>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 86.Gerritsen M, Jansen JA, Lutterman JA. Performance of subcutaneously implanted glucose sensors for continuous monitoring. Neth J Med. 1999;54(4):167–179. doi: 10.1016/s0300-2977(99)00006-6. [DOI] [PubMed] [Google Scholar]
- 87.Abel PU, von Woedtke T. Biosensors for in vivo glucose measurement: can we cross the experimental stage? Biosens Bioelectron. 2002;17(11-12):1059–1070. doi: 10.1016/s0956-5663(02)00099-4. [DOI] [PubMed] [Google Scholar]
- 88.Service RF. Can sensors make a home in the body? Science. 2002;297(5583):962–963. doi: 10.1126/science.297.5583.962. [DOI] [PubMed] [Google Scholar]
- 89.Ward WK, Jansen LB, Anderson E, Reach G, Klein JC, Wilson GS. A new amperometric glucose microsensor: in vitro and short-tern in vivo evaluation. Biosens Bioelectron. 2002;17(3):181–189. doi: 10.1016/s0956-5663(01)00268-8. [DOI] [PubMed] [Google Scholar]
- 90.Kyrolainen M, Rigsby P, Eddy S, Vadgama P. Bio-/haemocompatibility: implications and outcomes for sensors? Acta Anaesthesiol Scand Suppl. 1995;104:55–60. doi: 10.1111/j.1399-6576.1995.tb04255.x. [DOI] [PubMed] [Google Scholar]
- 91.Sharkawy AA, Klitzman B, Truskey GA, Reichert WM. Engineering the tissue which encapsulates subcutaneous implants. I. Diffusion properties. J Biomed Mater Res. 1997;37(3):401–412. doi: 10.1002/(sici)1097-4636(19971205)37:3<401::aid-jbm11>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 92.Wisniewski N, Klitzman B, Miller B, Reichert WM. Decreased analyte transport through implanted membranes: differentiation of biofouling from tissue effects. J Biomed Mater Res. 2001;57(4):513–521. doi: 10.1002/1097-4636(20011215)57:4<513::aid-jbm1197>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 93.Freeman CL, Mayhan KG, Picha GJ, Colton CK. Boston, MA: Materials Research Society Symposium; 1987. A study of mass transport resistance of glucose across rat capsular membranes; pp. 773–778. [Google Scholar]
- 94.Woodward SC. How fibroblasts and giant cells encapsulate implants: considerations in design of glucose sensors. Diabetes Care. 1982;5(3):278–281. doi: 10.2337/diacare.5.3.278. [DOI] [PubMed] [Google Scholar]
- 95.Reach G, Wilson GS. Can continuous glucose monitoring be used for the treatment of diabetes. Anal Chem. 1992;64(6):381A–386A. doi: 10.1021/ac00030a001. [DOI] [PubMed] [Google Scholar]
- 96.Bobbioni-Harsch E, Rohner-Jeanrenaud F, Koudelka M, de Rooij N, Jeanrenaud B. Lifespan of subcutaneous glucose sensors and their performances during dynamic glycaemia changes in rats. J Biomed Eng. 1993;15(6):457–463. doi: 10.1016/0141-5425(93)90058-7. [DOI] [PubMed] [Google Scholar]
- 97.Rebrin K, Fischer U, Hahn von Dorsche H, von Woetke T, Abel P, Brunstein E. Subcutaneous glucose monitoring by means of electrochemical sensors: fiction or reality. J Biomed Eng. 1992;14(1):33–40. doi: 10.1016/0141-5425(92)90033-h. [DOI] [PubMed] [Google Scholar]
- 98.Gilligan BJ, Shults MC, Rhodes RK, Updike SJ. Evaluation of a subcutaneous glucose sensor out to 3 months in a dog model. Diabetes Care. 1994;17(8):882–887. doi: 10.2337/diacare.17.8.882. [DOI] [PubMed] [Google Scholar]
- 99.Ju YM, Yu B, Koob TJ, Moussy Y, Moussy F. A novel porous collagen scaffold around an implantable biosensor for improving biocompatibility. I. In vitro/in vivo stability of the scaffold and in vitro sensitivity of the glucose sensor with scaffold. J Biomed Mater Res A. 2008;87(1):136–146. doi: 10.1002/jbm.a.31756. [DOI] [PubMed] [Google Scholar]
- 100.Koschwanez HE, Yap FY, Klitzman B, Reichert WM. In vitro and in vivo characterization of porous poly-L-lactic acid coatings for subcutaneously implanted glucose sensors. J Biomed Mater Res A. 2008 Jan 15; doi: 10.1002/jbm.a.31824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Moussy F, Harrison DJ, O'Brien DW, Rajotte RV. Performance of subcutaneously implanted needle-type glucose sensors employing a novel trilayer coating. Anal Chem. 1993;65(15):2072–2077. doi: 10.1021/ac00063a023. [DOI] [PubMed] [Google Scholar]
- 102.Valdes TI, Moussy F. A ferric chloride pre-treatment to prevent calcification of Nafion membrane used for implantable biosensors. Biosens Bioelectron. 1999;14(6):579–585. doi: 10.1016/s0956-5663(99)00023-8. [DOI] [PubMed] [Google Scholar]
- 103.Park JK, Tran PH, Chao JK, Ghodadra R, Rangarajan R, Thakor NV. In vivo nitric oxide sensor using non-conducting polymer-modified carbon fiber. Biosens Bioelectron. 1998;13(11):1187–1195. doi: 10.1016/s0956-5663(98)00078-5. [DOI] [PubMed] [Google Scholar]
- 104.Wisniewski N, Reichert M. Methods for reducing biosensor membrane biofouling. Colloids Surf B Biointerfaces. 2000;18(3-4):197–219. doi: 10.1016/s0927-7765(99)00148-4. [DOI] [PubMed] [Google Scholar]
- 105.Pappas N. An introduction to materials in medicine. New York: Academic Press; 1996. Biomaterials science; pp. 60–64. [Google Scholar]
- 106.Yu B, Wang C, Ju YM, West L, Harmon J, Moussy Y, Moussy F. Use of hydrogel coating to improve the performance of implanted glucose sensors. Biosens Bioelectron. 2008;23(8):1278–1284. doi: 10.1016/j.bios.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 107.Quinn CP, Pathak CP, Heller A, Hubbell JA. Photo-crosslinked copolymers of 2-hydroxyethyl methacrylate, poly(ethylene glycol) tetra-acrylate and ethylene dimethacrylate for improving biocompatibility of biosensors. Biomaterials. 1995;16(5):389–396. doi: 10.1016/0142-9612(95)98856-9. [DOI] [PubMed] [Google Scholar]
- 108.Espadas-Torre C, Meyerhoff ME. Thrombogenic properties of untreated and poly(ethylene oxide)-modified polymeric matrices useful for preparing intraarterial ion-selective electrodes. Anal Chem. 1995;67(18):3108–3114. doi: 10.1021/ac00114a003. [DOI] [PubMed] [Google Scholar]
- 109.Quinn CA, Connor RE, Heller A. Biocompatible, glucose-permeable hydrogel for in situ coating of implantable biosensors. Biomaterials. 1997;18(24):1665–1670. doi: 10.1016/s0142-9612(97)00125-7. [DOI] [PubMed] [Google Scholar]
- 110.Rigby G, Ahmed S, Horseman G, Vadgama P. In vivo glucose monitoring with open microflow–influences of fluid composition and preliminary evaluation in man. Anal Chim Acta. 1999;385:23–32. [Google Scholar]
- 111.Galeska I, Kim TK, Patil SD, Bhardwaj U, Chatttopadhyay D, Papadimitrakopoulos F, Burgess DJ. Controlled release of dexamethasone from PLGA microspheres embedded within polyacid-containing PVA hydrogels. AAPS J. 2005;7(1):E231–E240. doi: 10.1208/aapsj070122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hickey T, Kreutzer D, Burgess DJ, Moussy F. In vivo evaluation of a dexamethasone/PLGA microsphere system designed to suppress the inflammatory tissue response to implantable medical devices. J Biomed Mater Res. 2002;61(2):180–187. doi: 10.1002/jbm.10016. [DOI] [PubMed] [Google Scholar]
- 113.Hickey T, Kreutzer D, Burgess DJ, Moussy F. Dexamethasone/PLGA microspheres for continuous delivery of an anti-inflammatory drug for implantable medical devices. Biomaterials. 2002;23(7):1649–1656. doi: 10.1016/s0142-9612(01)00291-5. [DOI] [PubMed] [Google Scholar]
- 114.Patil SD, Papadimitrakopoulos F, Burgess DJ. Dexamethasone-loaded poly (lactic-co-glycolic) acid microspheres/poly (vinyl alcohol) hydrogel composite coatings for inflammation control. Diabetes Technol Ther. 2004;6(6):887–897. doi: 10.1089/dia.2004.6.887. [DOI] [PubMed] [Google Scholar]
- 115.Bhardwaj U, Sura R, Papadimitrakopoulos F, Burgess DJ. Controlling acute inflammation with fast releasing dexamethasone-PLGA microsphere/PVA hydrogel composites for implantable devices. J Diabetes Sci Technol. 2007;1(1):8–17. doi: 10.1177/193229680700100103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Macdonald RG, Panush RS, Pepine CJ. Rationale for use of glucocorticoids in modification of restenosis after percutaneous transluminal coronary angioplasty. Am J Cardiol. 1987;60(3):56B–60B. doi: 10.1016/0002-9149(87)90486-3. [DOI] [PubMed] [Google Scholar]
- 117.Hafezi-Moghadam A, Simoncini T, Yang Z, Limbourg FP, Plumier JC, Rebsamen MC, Hsieh CM, Chui DS, Thomas KL, Prorock AJ, Laubach VE, Moskowitz MA, French BA, Ley K, Liao JK. Acute cardiovascular protective effects of corticosteroids are mediated by non-transcriptional activation of endothelial nitric oxide synthase. Nat Med. 2002;8(5):473–479. doi: 10.1038/nm0502-473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Pepine CJ, Hirshfeld JW, Macdonald RG, Henderson MA, Bass TA, Goldberg S, Savage MP, Vetrovec G, Cowley M, Taussig AS. A controlled trial of corticosteroids to prevent restenosis after coronary angioplasty. M-HEART Group. Circulation. 1990;81(6):1753–1761. doi: 10.1161/01.cir.81.6.1753. [DOI] [PubMed] [Google Scholar]
- 119.Fournier E, Passirani C, Colin N, Breton P, Sagodira S, Benoit JP. Development of novel 5-FU-loaded poly(methylidene malonate 2.1.2)-based microspheres for the treatment of brain cancers. Eur J Pharm Biopharm. 2004;57(2):189–197. doi: 10.1016/S0939-6411(03)00146-2. [DOI] [PubMed] [Google Scholar]
- 120.Johns G, Corbo G, Thanoo BC, Maskiewicz R. Microsphere formulation: broad applicability of a continuous formation process for manufacture of sustained-release injectable microspheres. Drug Deliv Technol. 2004;4:60–63. [Google Scholar]
- 121.Hassan CM, Peppas NA. Structure and applications of poly(vinyl alcohol) hydrogels produced by conventional crosslinking or by freezing/thawing methods. Adv Polymer Sci. 2000;153:37–65. [Google Scholar]
- 122.Bourke SL, Al-Khalili M, Briggs T, Michniak BB, Kohn J, Poole-Warren LA. A photo-crosslinked poly(vinyl alcohol) hydrogel growth factor release vehicle for wound healing applications. AAPS PharmSci. 2003;5(4):E33. doi: 10.1208/ps050433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Klueh U, Dorsky DI, Kreutzer DL. Use of vascular endothelial cell growth factor gene transfer to enhance implantable sensor function in vivo. J Biomed Mater Res A. 2003;67(4):1072–1086. doi: 10.1002/jbm.a.20041. [DOI] [PubMed] [Google Scholar]
- 124.Norton LW, Koschwanez HE, Wisniewski NA, Klitzman B, Reichert WM. Vascular endothelial growth factor and dexamethasone release from nonfouling sensor coatings affect the foreign body response. J Biomed Mater Res A. 2007;81(4):858–869. doi: 10.1002/jbm.a.31088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ward WK, Wood MD, Casey HM, Quinn MJ, Federiuk IF. The effect of local subcutaneous delivery of vascular endothelial growth factor on the function of a chronically implanted amperometric glucose sensor. Diabetes Technol Ther. 2004;6(2):137–145. doi: 10.1089/152091504773731320. [DOI] [PubMed] [Google Scholar]
- 126.Norton LW, Tegnell E, Toporek SS, Reichert WM. In vitro characterization of vascular endothelial growth factor and dexamethasone releasing hydrogels for implantable probe coatings. Biomaterials. 2005;26(16):3285–3297. doi: 10.1016/j.biomaterials.2004.07.069. [DOI] [PubMed] [Google Scholar]
- 127.Patil SD, Papadmitrakopoulos F, Burgess DJ. Concurrent delivery of dexamethasone and VEGF for localized inflammation control and angiogenesis. J Control Release. 2007;117(1):68–79. doi: 10.1016/j.jconrel.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 128.Gaspardone A, Versaci F. Coronary stenting and inflammation. Am J Cardiol. 2005;96(12A):65L–70L. doi: 10.1016/j.amjcard.2005.09.064. [DOI] [PubMed] [Google Scholar]
- 129.Kornowski R, Hong MK, Tio FO, Bramwell O, Wu H, Leon MB. In-stent restenosis: contributions of inflammatory responses and arterial injury to neointimal hyperplasia. J Am Coll Cardiol. 1998;31(1):224–230. doi: 10.1016/s0735-1097(97)00450-6. [DOI] [PubMed] [Google Scholar]
- 130.Wu P, Grainger DW. Drug/device combinations for local drug therapies and infection prophylaxis. Biomaterials. 2006;27(11):2450–2467. doi: 10.1016/j.biomaterials.2005.11.031. [DOI] [PubMed] [Google Scholar]
- 131.Tanabe K, Regar E, Lee CH, Hoye A, van der Giessen WJ, Serruys PW. Local drug delivery using coated stents: new developments and future perspectives. Curr Pharm Des. 2004;10(4):357–367. doi: 10.2174/1381612043453289. [DOI] [PubMed] [Google Scholar]
- 132.Yamaguchi T. Drug-eluting stent trial. Cardiac Practice. 2004;15:417–419. [Google Scholar]
- 133.Schwartz RS, Chronos NA, Virmani R. Preclinical restenosis models and drug-eluting stents: still important, still much to learn. J Am Coll Cardiol. 2004;44(7):1373–1385. doi: 10.1016/j.jacc.2004.04.060. [DOI] [PubMed] [Google Scholar]
- 134.Robinson KA, Chronos NA, Schieffer E, Palmer SJ, Cipolla GD, Milner PG, King SB., 3rd Endoluminal local delivery of PCNA/cdc2 antisense oligonucleotides by porous balloon catheter does not affect neointima formation or vessel size in the pig coronary artery model of postangioplasty restenosis. Cathet Cardiovasc Diagn. 1997;41(3):348–353. doi: 10.1002/(sici)1097-0304(199707)41:3<348::aid-ccd17>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 135.Stone GW, Moses JW, Ellis SG, Schofer J, Dawkins KD, Morice MC, Colombo A, Schampaert E, Grube E, Kirtane AJ, Cutlip DE, Fahy M, Pocock SJ, Mehran R, Leon MB. Safety and efficacy of sirolimus- and paclitaxel-eluting coronary stents. N Engl J Med. 2007;356(10):998–1008. doi: 10.1056/NEJMoa067193. [DOI] [PubMed] [Google Scholar]
- 136.Schoen FJ, Levy RJ. Calcification of tissue heart valve substitutes: progress toward understanding and prevention. Ann Thorac Surg. 2005;79(3):1072–1080. doi: 10.1016/j.athoracsur.2004.06.033. [DOI] [PubMed] [Google Scholar]
- 137.Golomb G, Wagner D. Characterization and anticalcification effects of implantable polyurethane matrices containing amorphous dispersion of bisphosphonic acid. Clin Mater. 1991;8:33–42. [Google Scholar]
- 138.Golomb G, Dixon M, Smith MS, Schoen FJ, Levy RJ. Controlled-release drug delivery of diphosphonates to inhibit bioprosthetic heart valve calcification: release rate modulation with silicone matrices via drug solubility and membrane coating. J Pharm Sci. 1987;76(4):271–276. doi: 10.1002/jps.2600760402. [DOI] [PubMed] [Google Scholar]
- 139.Johnston TP, Bove EL, Bolling SF, Schoen FJ, Boyd JA, Golomb G, Levy RJ. Local controlled release of 1-hydroxyethylidene diphosphonate using silicone-rubber matrices. Effects of sterilization on in vitro release and in vivo efficacy. ASAIO Trans. 1988;34(3):835–838. [PubMed] [Google Scholar]
- 140.Johnston TP, Schoen FJ, Levy RJ. Prevention of calcification of bioprosthetic heart valve leaflets by Ca2+ diphosphonate pretreatment. J Pharm Sci. 1988;77(9):740–744. doi: 10.1002/jps.2600770903. [DOI] [PubMed] [Google Scholar]
- 141.Levy RJ, Wolfrum J, Schoen FJ, Hawley MA, Lund SA, Langer R. Inhibition of calcification of bioprosthetic heart valves by local controlled-release diphosphonate. Science. 1985;228(4696):190–192. doi: 10.1126/science.3919445. [DOI] [PubMed] [Google Scholar]
- 142.Hirsch D, Drader J, Pathak YV, Yee R, Schoen FJ, Levy RJ. Synergistic inhibition of the calcification of glutaraldehyde pretreated bovine pericardium in a rat subdermal model by FeCl3 and ethanehydroxydiphosphonate: preincubation and polymeric controlled release studies. Biomaterials. 1993;14(9):705–711. doi: 10.1016/0142-9612(93)90069-e. [DOI] [PubMed] [Google Scholar]
- 143.Johnston TP, Webb CL, Schoen FJ, Levy RJ. Assessment of the in vitro transport parameters for ethanehydroxy diphosphonate through a polyurethane membrane. A potential refillable reservoir drug delivery device. ASAIO J. 1992;38(3):M611–M616. doi: 10.1097/00002480-199207000-00109. [DOI] [PubMed] [Google Scholar]
- 144.Pathak YV, Boyd J, Levy RJ, Schoen FJ. Prevention of calcification of glutaraldehyde pretreated bovine pericardium through controlled release polymeric implants: studies of Fe3+, Al3+, protamine sulphate and levamisole. Biomaterials. 1990;11(9):718–723. doi: 10.1016/0142-9612(90)90034-n. [DOI] [PubMed] [Google Scholar]
- 145.Webb CL, Schoen FJ, Flowers WE, Alfrey AC, Horton C, Levy RJ. Inhibition of mineralization of glutaraldehyde-pretreated bovine pericardium by AlCl3. Mechanisms and comparisons with FeCl3, LaCl3, and Ga(NO3)3 in rat subdermal model studies. Am J Pathol. 1991;138(4):971–981. [PMC free article] [PubMed] [Google Scholar]
- 146.Vyavahare NR, Jones PL, Hirsch D, Schoen FJ, Levy RJ. Prevention of glutaraldehyde-fixed bioprosthetic heart valve calcification by alcohol pretreatment: further mechanistic studies. J Heart Valve Dis. 2000;9(4):561–566. [PubMed] [Google Scholar]
- 147.Vyavahare NR, Hirsch D, Lerner E, Baskin JZ, Zand R, Schoen FJ, Levy RJ. Prevention of calcification of glutaraldehyde-crosslinked porcine aortic cusps by ethanol preincubation: mechanistic studies of protein structure and water-biomaterial relationships. J Biomed Mater Res. 1998;40(4):577–585. doi: 10.1002/(sici)1097-4636(19980615)40:4<577::aid-jbm9>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 148.Bottio T, Thiene G, Pettenazzo E, Ius P, Bortolotti U, Rizzoli G, Valfré C, Casarotto D, Valente M. Hancock II bioprosthesis: a glance at the microscope in mid-long-term explants. J Thorac Cardiovasc Surg. 2003;126(1):99–105. doi: 10.1016/s0022-5223(03)00131-4. [DOI] [PubMed] [Google Scholar]
- 149.David TE, Armstrong S, Sun Z. The Hancock II bioprosthesis at 12 years. Ann Thorac Surg. 1998;66(6 Suppl):S95–S98. doi: 10.1016/s0003-4975(98)01099-6. [DOI] [PubMed] [Google Scholar]
- 150.Ohri R, Hahn SK, Hoffman AS, Stayton PS, Giachelli CM. Hyaluronic acid grafting mitigates calcification of glutaraldehyde-fixed bovine pericardium. J Biomed Mater Res A. 2004;70(2):328–334. doi: 10.1002/jbm.a.30088. [DOI] [PubMed] [Google Scholar]
- 151.Lee WK, Park KD, Kim YH, Suh H, Park JC, Lee JE, Sun K, Baek MJ, Kim HM, Kim SH. Improved calcification resistance and biocompatibility of tissue patch grafted with sulfonated PEO or heparin after glutaraldehyde fixation. J Biomed Mater Res. 2001;58(1):27–35. doi: 10.1002/1097-4636(2001)58:1<27::aid-jbm40>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 152.Park KD, Yun JY, Han DK, Jeong SY, Kim YH, Choi KS, Kim HM, Kim HJ, Kim KT. Chemical modification of implantable biologic tissue for anti-calcification. ASAIO J. 1994;40(3):M377–M382. doi: 10.1097/00002480-199407000-00026. [DOI] [PubMed] [Google Scholar]
- 153.Golomb G, Ezra V. Covalent binding of protamine by glutaraldehyde to bioprosthetic tissue: characterization and anticalcification effect. Biomater Artif Cells Immobilization Biotechnol. 1992;20(1):31–41. doi: 10.3109/10731199209117856. [DOI] [PubMed] [Google Scholar]
- 154.Golomb G, Ezra V. Prevention of bioprosthetic heart valve tissue calcification by charge modification: effects of protamine binding by formaldehyde. J Biomed Mater Res. 1991;25(1):85–98. doi: 10.1002/jbm.820250107. [DOI] [PubMed] [Google Scholar]
- 155.Bernacca GM, Dimitri WR, Fisher AC, Mackay TG, Wheatley DJ. Chemical modification of bovine pericardium and its effect on calcification in the rat subdermal model. Biomaterials. 1992;13(6):345–352. doi: 10.1016/0142-9612(92)90038-p. [DOI] [PubMed] [Google Scholar]
- 156.Jee KS, Kim YS, Park KD, Kim YH. A novel chemical modification of bioprosthetic tissues using L-arginine. Biomaterials. 2003;24(20):3409–3416. doi: 10.1016/s0142-9612(03)00204-7. [DOI] [PubMed] [Google Scholar]
- 157.Kim HJ, Bae JW, Kim CH, Lee JW, Shin JW, Park KD. Acellular matrix of bovine pericardium bound with L-arginine. Biomed Mater. 2007;2(3):S111–S116. doi: 10.1088/1748-6041/2/3/S05. [DOI] [PubMed] [Google Scholar]
- 158.Andre-Frei V, Chevallay B, Orly I, Boudeulle M, Huc A, Herbage D. Acellular mineral deposition in collagen-based biomaterials incubated in cell culture media. Calcif Tissue Int. 2000;66(3):204–211. doi: 10.1007/s002230010041. [DOI] [PubMed] [Google Scholar]
- 159.Golomb G, Wagner D. Development of a new in vitro model for studying implantable polyurethane calcification. Biomaterials. 1991;12(4):397–405. doi: 10.1016/0142-9612(91)90008-x. [DOI] [PubMed] [Google Scholar]
- 160.Shumakov VI, Rosanova IB, Vasin SL, Salomatina LA, Sevastianov VI. Biomaterial calcification without direct material-cell interaction. ASAIO Trans. 1990;36(3):M181–M184. [PubMed] [Google Scholar]
- 161.Schwenter F, Bouche N, Pralong WF, Aebischer P. In vivo calcium deposition on polyvinyl alcohol matrix used in hollow fiber cell macroencapsulation devices. Biomaterials. 2004;25(17):3861–3868. doi: 10.1016/j.biomaterials.2003.10.030. [DOI] [PubMed] [Google Scholar]
- 162.Tomashefski JF, Jr, Cohen AM, Doershuk CF. Longterm histopathologic follow-up of bronchial arteries after therapeutic embolization with polyvinyl alcohol (Ivalon) in patients with cystic fibrosis. Hum Pathol. 1988;19(5):555–561. doi: 10.1016/s0046-8177(88)80204-1. [DOI] [PubMed] [Google Scholar]
- 163.Du C, Cui FZ, Zhang W, Feng QL, Zhu XD, de Groot K. Formation of calcium phosphate/collagen composites through mineralization of collagen matrix. J Biomed Mater Res. 2000;50(4):518–527. doi: 10.1002/(sici)1097-4636(20000615)50:4<518::aid-jbm7>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 164.Levy RJ, Schoen FJ, Sherman FS, Nichols J, Hawley MA, Lund SA. Calcification of subcutaneously implanted type I collagen sponges. Effects of formaldehyde and glutaraldehyde pretreatments. Am J Pathol. 1986;122(1):71–82. [PMC free article] [PubMed] [Google Scholar]


