Abstract
Background
The development and validation of Norfolk QOL-DN, a fiber-specific, quality-of-life tool for diabetic neuropathy, was published previously (Part 1). This study (Part 2) defines the psychometric properties of the German-translated Norfolk QOL-DN in a large multicenter (96 sites) population with neuropathy ranging from minimal to severe, comparing them with those in the original English/American version in a 30-center European/North American population with mild neuropathy; determines the power of the German-translated version in a five-staged diabetic peripheral neuropathy (DPN) German population to discriminate different levels of neuropathy severity; and establishes factors having the greatest impact on QOL.
Methods
One hundred eighty-six German patients were assessed: asymptomatic of DPN (n = 40), symptomatic (n = 46), DN with foot-ulcer history (n = 32), DN with amputations (n = 22), and DN amputation history (n = 46). German-translated Norfolk QOL-DN was administered to 177 patients with staged DN complications. German-translated Norfolk QOL-DN data were compared with QOL-DN data from the European/American study of 379 mild neuropathy patients. Exploratory factor analysis assessed factor structure consistency in the translated instrument. Ordinal regression analysis (polytomous universal model) was used to evaluate the association between factor scores and complication stages.
Results
The German translation identified the same five factors in more advanced neuropathy as in the English mild neuropathy population. Total QOL scores differed among each of the five neuropathy severity groups [analysis of variance p < 0.001, Tukey–Kramer post hoc, α = 0.05]. Two factors emerged as predictors of impaired QOL and disease severity: physical function/large fiber (Wald χ2 = 6.188, p = 0.013) and activities of daily living (ADL)(Wald χ2 = 9.098, p = 0.003).
Conclusions
Norfolk QOL-DN discriminates levels of neuropathy within and between populations. Physical functioning and ADL are the most important determinants of QOL. Early occurrence of orthostasis suggests a redefinition of autonomic neuropathy to be more symptom inclusive.
Keywords: ADLs, autonomic, large fiber, nerve fiber specific, QOL
Introduction
Pivotal to neuropathy research and its translation into clinical care is the notion that neuropathy is not a homogeneous disease; rather it is composed of a multitude of disorders affecting different types of nerve fibers and embracing many aspects of quality of life (QOL).1 The Quality of Life for Diabetic Neuropathy (QOL-DN) instrument in this study was composed of 46 items. Items 1–7 are nerve fiber-related symptoms (numbness, tingling/pins and needles, electric shocks, superficial peripheral pain, deep pain, weakness, and other symptoms). Items 8–11 inquire about duration of symptoms, symptoms at night, and current medications. Items 12–15 cover neuropathy diagnosis and related complications. Questions 8–15 are not included in the statistical analysis of the scales. With items 16–37, subjects respond to questions about the degree of physical problems that interfere with their activities of daily living (ADL). These items are scored on a five-point Likert scale (0, no problem; 4, severe problem). Items 38–46 are generic questions about health and not specific to neuropathy. They are also measured on a five-point Likert scale.
Our first objective was to define the factors in the German-translated version of the Norfolk QOL-DN and to determine whether it retained its psychometric properties after backward and forward translation by the MAPI Research Institute. (In the German-speaking focus group, the MAPI Research Institute paid strict attention to readability, possible ambiguity, and particularly functional equivalence, conferring with the authors on issues of interpretation of symptom descriptors, such as “deep pain,” “superficial pain,” and “electric shocks.”) After evaluating the translation equivalence of the two instruments,2 scale equivalence,3 and determining whether the measure assesses the same item cross culturally,4 we aimed to validate the German-translated QOL-DN, administering it in a German population with five stages of neuropathy, performing factor analysis on the results, and comparing its psychometric properties with those from the original English/America version in a 30-center European/North American population with stage 1, mild neuropathy (multicenter study).
The second objective of this study was to use the questionnaire in two different populations with differing stages of neuropathy to show that the Norfolk QOL-DN was able to discriminate different levels of neuropathy severity. It was our intention to use different patient populations to explore the strength of each factor and their impact on quality of life and to demonstrate that the same five factors emerged in the two populations utilizing this Norfolk QOL-DN scale. Although the tool has been translated into several different languages, it has not been tested previously in populations with more advanced stages of neuropathy complicated by foot ulcers nor have comparisons been made between the German version (forward and back translated by the MAPI Research Institute) and the original English version.
Our third objective was to establish which of the factors had the greatest impact on quality of life.
Results of the test–retest reliability of the English/American version of the Norfolk QOL-DN and its ability to discriminate between populations free of neuropathy and those with mild neuropathy were reported previously.5–7 The German version did not undergo this test–retest validation. However, it was thoroughly validated linguistically. First, it was validated linguistically in a forward translation by two independent translators. Thereafter, there was a “reconciliation” meeting between the forward translators and the local project manager. This was followed by a backward translation performed by an independent translator. After this, the source questionnaire was compared with the backward translation by the local translation team and the team at the MAPI Research Institute. Clinicians working in the field on this project then reviewed the translation. Administering the Norfolk QOL-DN to 379 patients with mild neuropathy revealed that large fiber/physical functioning and ADL were the most influential factors on QOL.8 Nerve fiber-specific functions for small fiber and autonomic nerve impairments had little effect in this population, presumably because of the relatively asymptomatic nature of mild neuropathy (Dyck stage 1).9 Further study in this population showed nerve impairments in specific QOL-DN domains, particularly physical functioning/large fiber and ADL (as mentioned earlier), correlated with objective measures of nerve function, making this instrument valuable as a means to determine the therapeutic efficacy of agents that target specific nerve fiber function in neuropathy.10,11
Since patients' own perceptions of their health status are now recognized and valued as an important measure, many tools for measuring QOL have become available. The most commonly used generic tools for measuring patients' perceptions of the effects of DN are the Medical Outcomes Study Short Forms (SF-20 and SF-36) and the Nottingham Health Profile.12,13 Most of these instruments are nonspecific, and those addressing diabetic neuropathy do not cover the full scope of neuropathy. Unlike diabetes QOL instruments, e.g., Diabetes Quality of Life14 and the diabetes symptom checklist,15 the Norfolk QOL-DN is neuropathy specific. It also differs from the NeuroQol16 by its capacity to separate different neuropathic disabilities attributed to the dysfunction of different nerve fibers—small, large, and autonomic.
Materials and Methods
Subject Characteristics of German Study Participants
In the German study, the German translation of the Norfolk QOL-DN was distributed to 186 neuropathy patients at 97 sites in Germany categorized into five groups, according to the German classification of five stages of neuropathy, G1 to G5. These are: stage G1, asymptomatic DN (n = 40), Dyck stage 1a; stage G2, symptomatic DN (n = 46), Dyck stage 1b-29; stage G3, DN with history of foot ulcers (n = 32); stage G4, DN with amputations (n = 22); and stage G5, DN with history of amputations (n = 26).7
Nine subjects with missing data were dropped from the analysis (N = 177). Of the 177 patients with diabetes (type 1, n = 31 and type 2, n = 146), there were 73 females (mean age, 69.9 ± 1.4) and 104 males (mean age, 64.5 ± 1.1), divided into five categories of neuropathy severity and complications of diabetes based on a questionnaire and a neurologic examination. Reflexes, pedal pulses, vibration, touch perception, sensitivity to heat and cold, and pain threshold were tested using reflex hammers, monofilaments, cotton swabs, and pin pricks, as described previously by Dyck9 and approved by the American Diabetes Association position statement.17 No electrophysiological data were collected. Thirty-eight individuals had asymptomatic neuropathy, 45 had symptomatic neuropathy, 30 had neuropathy with foot ulcers, 20 had amputations in 2002, and 44 had amputations before 2002. Table 1 displays demographic data. This population also had the following concomitant diseases: hypertension (81%), hypercholesterolemia (49%), coronary artery disease (38%), and dyslipidemia (37%). Other microvascular complications were retinopathy (28%) and nephropathy (25%).
Table 1.
Characteristics of Study Populationsa
| Characteristics of patients | Score | Normal |
|---|---|---|
| German Severe Neuropathy Group (N = 177) | ||
| Age (years, mean ± SE) | ||
| Females | 69.93 ± 1.42 | |
| Males | 64.49 ± 1.09 | |
| Gender (M:F, % male) | 104:73 (58.8%) | |
| Number of type 1 subjects | n = 31 (17.5%) | |
| Number of type 2 subjects | n = 146 (82.5%) | |
| Mean years since diagnosis with diabetes ± SE (years) | 15.82 ± 0.82 | |
| Females | 16.90 ± 1.31 | |
| Males | 15.04 ± 1.06 | |
| Subjects with asymptomatic neuropathy | n = 38 (21.5%) | |
| Subjects with symptomatic neuropathy | n = 45 (25.4%) | |
| Subjects with neuropathy in feet/foot ulcers | n = 30 (16.9%) | |
| Subjects with amputations in 2002 | n = 20 (11.3%) | |
| Subjects with amputations before 2002 | n = 44 (24.9%) | |
| Hemoglobin A1c (%) | 7.22 ± 0.08 | |
| Multicenter Mild Neuropathy Group (N = 379) | ||
| Age (years, mean ± SE) | 54.9 ± 0.49 | |
| Gender (M:F, % male) | 148:231 (39%) | |
| Height (centimeters, mean ± SE) | 175.66 ± 0.8 | |
| Weight (kilograms, mean ± SE) | 98.14 ± 1.73 | |
| Body mass index (mean ± SE) | 32.1 ± 0.96 | |
| Race distribution | ||
| White (n, % of group) | 315 (83.1%) | |
| Black (n, % of group) | 31 (8.2%) | |
| Hispanic (n, % of group) | 24 (6.3%) | |
| Other (n, % of group) | 9 (2.4%) | |
| Hemoglobin A1c (%) | 9.07 ± 1.73 | 5.0–6.05 |
| Total neuropathy score (mean ± SE), >20 = severe | 18.15 ± 0.96 | 0 |
| NISa,b sensory (mean ± SE)(total = 72) | 8.35 ± 0.34 | 0 |
| NIS reflexes (mean ± SE)(total = 17) | 3.30 ± 0.18 | 0 |
| NIS (LL)+7 new (mean ± SE)(total = 32) | 26.95 ± 0.75 | 0 |
| Nerve conduction velocity score (mean ± SE, normal deviates) | 4.74 ± 0.15 | 3.29 |
| Peroneal score (mean ± SE, normal deviates) | 5.19 ± 0.17 | 4.935 |
| Tibial score (mean ± SE, normal deviates) | 6.23 ± 0.17 | 4.935 |
| Sural score (mean ± SE, normal deviates) | 2.43 ± 0.06 | 3.29 |
| Vibration detection threshold score (mean ± SE, normal deviates) | 1.68 ± 0.08 | 1.645 |
| Heart rate variability score (high abnormality, mean ± SE, normal deviates) | 1.63 ± 0.11 | 1.645 |
Neuropathy measurements modified from Dyck.9 Normal deviates = <1.645 (95th percentile for each parameter measured)
NIS, nerve impairment score; LL, lower limbs; +7, added measures of nerve electrophysiology and autonomic nerve function.
Subject Characteristics of the Multicenter Study—Norfolk Data Set
Previously, data were obtained from 379 subjects with mild neuropathy (Dyck stage 1a,b). These subjects comprised the multicenter study comparison group. For the DN group, clinical parameters by physical examination and detailed neurologic evaluation to confirm the presence of neuropathy were used in Norfolk, Virginia (one of the study sites), as well as in the other international centers. Additionally, quantitative sensory,18 quantitative autonomic,19 and electrophysiologic testing were administered as described previously.20 Table 1 displays characteristics and measures of nerve dysfunction. In the Norfolk site there was a gender imbalance—39% males to 58% females. However, the impact of neuropathy on QOL was not gender dependent.
Procedure
In the German study, the Norfolk QOL-DN was self-administered to subjects one time at entry to the study. In the multicenter study comparison group, subjects completed the QOL-DN at the beginning and end of the study. The institutional review board at Eastern Virginia Medical School approved administration of the Norfolk QOL-DN. All subjects gave informed consent to participate in the study and agreed to the reporting of the information using deidentified data.
Factor Analysis and Statistical Methods in the German Sample
Exploratory factor analysis was used to characterize five factors in the German group. Thirty-six items from participants with complete data (n = 177) were entered into a principal components factor analysis. Five components were retained, in parallel to those extracted in the English-speaking group, and rotated orthogonally using the Varimax algorithm. Item-factor loadings were compared to those obtained from the corresponding analysis of the English-speaking sample. Factor scores from this rotation were saved and used in subsequent analyses.8
Ordinal regression using a polytomous universal model (PLUM) in SPSS assessed the association between factor scores on the Norfolk QOL-DN and clinically determined stage of neuropathy complication (rated 1–5). All five factors were initially entered as predictors of complication stage, but only two (physical function/large fiber and ADL) were significant predictors. The three nonsignificant factors were dropped and the regression model was refitted.
Descriptive statistical analyses were performed using total QOL-DN scores across the five factors derived from factor analysis for each of the five categories of neuropathy severity. Analysis of variance (ANOVA) examined differences between neuropathy severity groups on each of the factors. The Tukey–Kramer honestly significant difference method was used post hoc to assess significant pair-wise differences between QOL scores in each domain in the neuropathy groups of differing severity. Correlations between scores in the Norfolk QOL-DN with symptoms and objective measures of neuropathy were sought using Pearson's correlation coefficients when data were available. For all analyses, α was set at 0.05.
Results
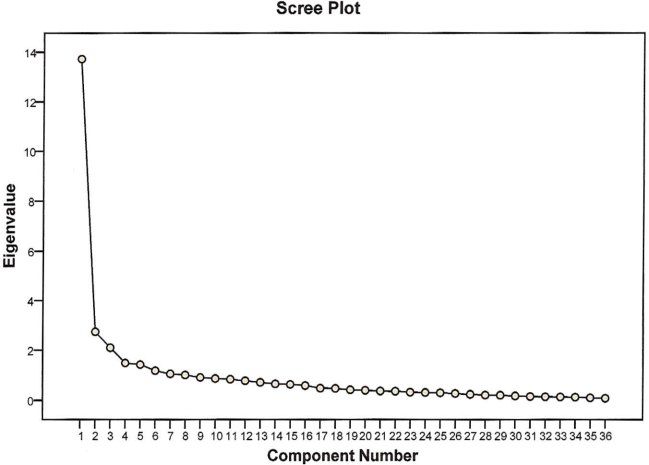
Results obtained from German study participants were compared to results obtained from 379 subjects (Norfolk data set) with mild neuropathy (stage 1) enrolled in the Multicenter Study Comparison Group. Five factors were extracted from the 36 Norfolk QOL-DN items. Although the number of factors extracted and rotated was driven by a priori criteria, visual inspection of the eigenvalue scree plot is suggestive of a five-factor solution, as the final deflection point of the scree curve occurs after the fifth component. Rotated factors accounted for about 60% of the response variance. Communalities, by item, ranged from 0.215 to 0.813 (note that communalities are equivalent to a lower bound of item reliability and may be low as a consequence of poor reliability, floor or ceiling effects, or other issues that diminish item reliability). Communalities are presented in Table 2. Communalities, by item, ranged from 0.341 to 0.816. Item-factor loadings for both the original study and the German sample are presented in Table 3. Although the number of factors extracted and rotated was driven by a priori criteria, visual inspection of the eigenvalue scree plot is suggestive of a five-factor solution, as the final deflection point of the scree curve occurs after the fifth component. Figure 1 shows the scree plot.
Table 2.
Communalitiesa
| List of abbreviated questions | Extraction |
|---|---|
| 1. Numbness | 0.621 |
| 2. Tingling | 0.676 |
| 3. Electric | 0.376 |
| 4. Unusual sensations | 0.341 |
| 5. Superficial pain | 0.515 |
| 6. Deep pain | 0.577 |
| 7. Weakness | 0.476 |
| 16. Pain kept awake? | 0.474 |
| 17. Touch of bed sheets bothered? | 0.524 |
| 18. Burned or injured and not felt? | 0.571 |
| 19. Symptom kept you from usual activities? | 0.644 |
| 20. Difficulty fine movements? | 0.550 |
| 21. Unsteady on feet? | 0.685 |
| 22. Problem getting out of chair? | 0.612 |
| 23. Problem walking downstairs? | 0.678 |
| 24. Unable to feel feet? | 0.587 |
| 25. Unable to tell hot/cold with hands? | 0.545 |
| 26. Unable to tell hot/cold with feet? | 0.416 |
| 27. Vomiting after meals? | 0.685 |
| 28. Diarrhea or loss of bowel control? | 0.456 |
| 29. Involuntary urination? | 0.414 |
| 32. Orthostasis? | 0.372 |
| 33. Difficulty bathing/showering? | 0.755 |
| 34. Difficulty dressing? | 0.748 |
| 35. Difficulty walking? | 0.789 |
| 36. Difficulty getting on/off toilet? | 0.753 |
| 37. Difficulty using eating utensils? | 0.530 |
| 38. Health—cut work or other time? | 0.720 |
| 39. Health—accomplished less? | 0.774 |
| 40. Health—limited in kind of activity? | 0.779 |
| 41. Health—difficulty performing activity/work? | 0.816 |
| 42. Your general health now? | 0.595 |
| 43. Compared to 3 months ago, health now is? | 0.564 |
| 44. Physical health interfered with normal social activity? | 0.561 |
| 45. Pain interfered with normal work? | 0.662 |
| 46. Weakness or shakiness interferes with normal work? | 0.642 |
Communalities ranged from 0.341 to 0.81. Questions 1–7 relate to symptoms, 16–37 relate to activities of daily living, and 38–46 relate to generic health over the past 4 weeks.
Table 3.
Similarity of the Composition and Item Loading within the Same Five Factors Identified in Patients from the Severe Neuropathy and Mild Neuropathy Groups
| German Severe Neuropathy Group | |
|---|---|
| Physical functioning/large fiber | Small fiber |
| 19. Symptoms kept from usual activities | 4. Unusual sensations |
| 21. Unsteady on feet | 18. Burned/injured self and can't feel it |
| 22. Getting out of chair | 25. Unable to feel hot/cold water with hands |
| 23. Problem walking downstairs | 26. Unable to feel hot/cold water with feet |
| 24. Unable to feel feet while walking | 43. General health compared to 3 months ago |
| 35. Difficulty walking | |
| 38. Cut down time spent on work/other activities | Symptoms |
| 39. Accomplished less than you would like | 1. Numbness |
| 40. Limited in work you could perform | 2. Tingling, pins, and needles |
| 41. Difficulty performing work/other activities | 3. Electric shocks |
| 42. Rating of current general health | 5. Superficial pain |
| 44. Physical health interferes with activities | 6. Deep pain |
| 45. Pain interferes with normal work | 7. Weakness |
| 46. Weakness/shakiness interferes with normal work | 16. Pain kept you awake at night |
| 17. Hyperalgesia | |
| Activities of daily living | |
| 20. Difficulty doing fine finger movements | Autonomic |
| 33. Difficulty bathing/showering | 27. Vomiting |
| 34. Difficulty dressing | 28. Diarrhea/bowel control |
| 36. Difficulty getting on/off toilet | 29. Involuntary urination |
| 37. Difficulty using eating utensils | 32. Orthostasis |
| Multicenter Mild Neuropathy Group | |
|---|---|
| Physical functioning/large fiber | Small fiber |
| 16. Pain kept you awake at night | 18. Burned/injured self and can't feel it |
| 19. Symptoms kept from usual activities | 24. Unable to feel feet while walking |
| 21. Unsteady on feet | 25. Unable to feel hot/cold water with hands |
| 22. Getting out of chair | 26. Unable to feel hot/cold water with feet |
| 23. Problem walking downstairs | |
| 35. Difficulty walking | Symptoms |
| 38. Cut down time spent on work/other activities | 1. Numbness |
| 39. Accomplished less than you would like | 2. Tingling, pins and needles |
| 40. Limited in work you could perform | 3. Electric shocks |
| 41. Difficulty performing work/other activities | 4. Unusual sensations |
| 42. Rating of current general health | 5. Superficial pain |
| 43. General health compared to 3 months ago | 6. Deep pain |
| 44. Physical health interferes with activities | 7. Weakness |
| 45. Pain interferes with normal work | 17. Hyperalgesia |
| 46. Weakness/shakiness interferes with normal work | |
| Activities of daily living | Autonomic |
| 20. Difficulty doing fine finger movements | 27. Vomiting |
| 33. Difficulty bathing/showering | 28. Diarrhea/bowel control |
| 34. Difficulty dressing | 32. Orthostasis |
| 36. Difficulty getting on/off toilet | |
| 37. Difficulty using eating utensils | |
Figure 1.
Scree plot.
Rotated factors accounted for 60% of the response variance. Table 4 shows the rotated component matrix. The first rotated factor extracted from both German and English data is composed of large fiber neuropathy symptoms and general physical health items. Remaining factors were extracted in a slightly different order in German and English samples, but the content reflected by the second and third factors is similar for both populations, with loadings of items related to ADL in one and specific symptoms of diabetic neuropathy in the other. The autonomic factor is defined more clearly by larger item loadings in the German sample. For the German group, the autonomic factor included four questions based on the following: vomiting, diarrhea/bowel control, involuntary urination, and orthostasis. It was apparent that patients free of objective neuropathic symptoms nonetheless could have orthostasis; hence, the early significance of positive autonomic scores in patients without somatic symptoms. The last extracted component evidenced some correspondence with small negative small fiber neuropathy symptoms (e.g., loss of feeling).
Table 4.
Rotated Component Matrix
| Component | |||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| 40.Health—limited activity | 0.845 | 0.140 | 0.170 | 0.113 | |
| 41. Health—difficulty activity | 0.841 | 0.191 | 0.260 | ||
| 39.Health—accomplish less | 0.822 | 0.264 | 0.112 | ||
| 38.Health—cut work or other time | 0.794 | 0.203 | 0.163 | 0.142 | |
| 19. Sxa kept from usual activities | 0.720 | 0.294 | 0.164 | 0.107 | |
| 45.Pain interferes with work | 0.701 | 0.350 | 0.201 | ||
| 23.Problem stairs—walking down | 0.693 | 0.421 | 0.102 | ||
| 35.ADL—walking | 0.687 | 0.227 | 0.515 | ||
| 21. Unsteady on feet | 0.675 | 0.216 | 0.400 | 0.129 | |
| 46.Weakness or shakiness interferes with work | 0.670 | 0.219 | 0.359 | 0.129 | |
| 44.Health interferes with social | 0.614 | 0.111 | 0.401 | ||
| 42. Your health now is… | 0.603 | 0.445 | 0.166 | ||
| 22.Problem chair—getting out | 0.569 | 0.520 | 0.131 | ||
| 2.(sx) Tingling | 0.134 | 0.786 | 0.190 | ||
| 1.(sx) Numbness | 0.207 | 0.744 | 0.147 | ||
| 5.(sx) Superficial pain | 0.239 | 0.639 | 0.226 | ||
| 6.(sx) Deep pain | 0.338 | 0.630 | 0.116 | 0.226 | |
| 3.(sx) Electric | 0.559 | 0.173 | 0.164 | ||
| 7.(sx) Weakness | 0.267 | 0.545 | 0.170 | 0.268 | |
| 17. Touch of bed sheets bothered | 0.154 | 0.541 | 0.173 | 0.412 | |
| 16. Pain kept awake | 0.398 | 0.466 | 0.147 | 0.183 | –0.210 |
| 26.Unable feet—hot/cold | 0.160 | 0.404 | 0.274 | 0.105 | 0.376 |
| 34.ADL—dressing | 0.440 | 0.735 | 0.101 | ||
| 36.ADL—toilet transfer | 0.446 | 0.115 | 0.731 | ||
| 33.ADL—bathing | 0.532 | 0.678 | |||
| 37. ADL—eating utensils | 0.142 | 0.260 | 0.634 | 0.193 | |
| 20.Difficulty fine movements | 0.272 | 0.290 | 0.521 | 0.346 | |
| 24. Unable to feel feet | 0.404 | 0.335 | 0.505 | 0.224 | |
| 27. Vomiting | 0.106 | 0.736 | 0.358 | ||
| 28.Diarrhea or bowel control | –0.105 | 0.657 | |||
| 29.Involuntary urination | 0.108 | 0.284 | 0.502 | ||
| 32.Fainting/dizziness | 0.296 | 0.277 | 0.155 | 0.426 | |
| 18. Injured and not felt | 0.118 | 0.182 | 0.462 | 0.549 | |
| 43.Compared to 3 months ago, health now is … | 0.321 | 0.235 | 0.372 | –0.513 | |
| 4.(sx) Unusual sensations | 0.199 | 0.289 | 0.211 | 0.413 | |
Symptoms
There was a striking similarity in composition and item loadings on the five factors identified from the English/American and German versions of the Norfolk QOL-DN. This was particularly clear in the physical function/large fiber and activities of daily living domains, which relate to fine motor skills. Of further note is the concordance between positive symptoms for both versions of the instrument. Patients with both mild and more severe neuropathy shared items in the small fiber scale identifying “cognitive loss” and those items in the symptom scale identifying “positive symptoms.” In the German sample, the question about “pain keeping you awake at night” featured as a symptom, whereas in the English/American version, this item cosegregated with physical functioning. This may simply reflect the more severe neuropathy in the German cohort. The fact that seven symptoms (numbness, tingling/pins and needles, electric shocks, superficial pain, deep pain, weakness, and hyperalgesia) appeared at all levels of severity of neuropathy suggests that they are early and important features of DN. The group labeled as “asymptomatic” of neuropathy was identified by objective measures of neuropathy (as mentioned earlier in the Materials and Methods section); however, the patients self-reported in the questionnaire the following symptoms: 55% had numbness, 47.5% had tingling/pins and needles, 10% had electric shocks, 32.5% had superficial pain, 32.5% had deep pain, 50% had weakness, and 27.5% had hyperalgesia. These symptoms are classified as “positive,” denoting that the nerves are still responsive. Even people with diabetes without neuropathy, as well as normal controls, have been shown to have some of these symptoms.21
The most notable difference between the two populations was in the autonomic domain. Scores in the German population were almost double those in the mild neuropathy comparison group, as shown in Figure 2. In general, autonomic features—orthostasis, vomiting, and diarrhea—are discerned as affecting QOL in more advanced stages of neuropathy. One question regarding involuntary urinating did not factor at all in the comparison group, but did in the German group using the German translation. Whether this reflects differences in comprehending the question or greater impact of neuropathy on bladder function needs to be resolved. A review of the questions showed that 37.5% of people in the “asymptomatic” group had a problem with orthostatic symptoms (e.g., dizziness upon standing, blurred vision). While this group was asymptomatic of
Figure 2.

Mean ± standard errors of absolute scores for the five factors in the German Severe Neuropathy and Multicenter Mild Neuropathy Groups. The total quality of life score was equivalent in the mild neuropathy multicenter group (total QOL = 27.3) and neuropathy group without symptoms (total QOL = 23.0); both groups were significantly different from all others. *Significantly different from all others except severity grade 1.**Significantly different from all others except severity grades 3 and 4. †Significantly different from all others except severity grade 4. ‡Significantly different from all others. ▪Significantly different from all others, showing that only patients with no symptoms have a significantly low small fiber score.
peripheral neuropathy, they had symptoms of autonomic neuropathy. Supine to standing blood pressure tests reveal postural difficulties, but do not sufficiently differentiate the nature of the autonomic involvement in patients with orthostatic symptoms. Previous literature has shown that autonomic symptoms occur later in the course of diabetes; however, other studies show that symptoms may be present with autonomic scores that appear to be normal.22 Orthostatic symptoms may not be caused by a drop in blood pressure, but may be related to tachycardia and/or bradycardia as a consequence of autonomic imbalance between sympathetic and parasympathetic function.
Ordinal regression using PLUM found that only the physical function/large fiber (Wald χ2 = 6.188, p = 0.013) and ADL (Wald χ2 = 9.098, p = 0.003, Nagelkerke pseudo R2 = 0.233) factors were significant predictors of stages of neuropathy complications. Figure 2 depicts significant differences in the mean score for the five subject groups of neuropathy severity, analyzed by ANOVA and Tukey–Kramer post hoc test (α = 0.05). The total QOL score of the neuropathy group without symptoms was significantly different from all other groups, whereas the total QOL score of groups with amputations was the highest. Since the quality of life for people with amputations is notably decreased,23 the tool unquestionably captured this decrease, showing its ability to discriminate between subject groups.
Breakdown of Total QOL Score and Each of the Five Factors as Shown in Figure 2
Total Quality of Life Score
This score was equivalent in the mild neuropathy multicenter group (total QOL = 27.3) and neuropathy group without symptoms (total QOL = 23.0); both groups were significantly different to all others.
Physical Functions/Large Fiber Score
This score was equivalent in the mild neuropathy multicenter group and neuropathy group without symptoms and significantly different to all groups. The neuropathy group with symptoms was significantly different from the group with amputations before 2002.
Symptom Score
The loss of toes or feet could account for loss of symptoms, making the amputations in the 2002 group similar to the neuropathy without symptoms group. Despite the amputations, the score was still higher in the amputations in the 2002 group than in the neuropathy without symptoms group due to some phantom pain and perhaps the loss of only one or two toes or fingers. It is interesting to note that even with mild neuropathy, the symptom score was maximized and statistically similar in all four severity groups, showing a ceiling effect.
Activities of Daily Living Scores
These scores were found to be equivalent in the group that had amputations before 2002 and amputations in 2002 and were statistically similar to the neuropathic feet/neuropathic ulcers group. Scores from these groups were significantly greater than the other three groups. The presence or history of an amputation or a foot ulcer had a significant impact on ADL.
Autonomic Score
Autonomic symptoms were present in all patients except those with mild neuropathy. Furthermore, four questions loaded into the autonomic factor for the German population (as opposed to three in the English version), which could also account for their slightly higher score.
Small Fiber Score
This score showed that the multicenter mild neuropathy group was significantly different from the neuropathy without symptoms group, but was statistically similar to all other groups. Thus only patients without symptoms have a significantly low small fiber score. Table 5 shows the variance explained by each factor and the cumulative variance.
Table 5.
Total Variance Explaineda
| Component | Total | % of variance | Cumulative % |
|---|---|---|---|
| 1 | 13.721 | 38.115 | 38.115 |
| 2 | 2.739 | 7.609 | 45.724 |
| 3 | 2.100 | 5.834 | 51.558 |
| 4 | 1.492 | 4.143 | 55.701 |
| 5 | 1.429 | 3.968 | 59.669 |
Extraction method: principal component analysis.
In summary, those subjects with the greatest neuropathy severity scored higher (worse) in the total QOL, physical functioning/large fiber domain, and ADL domain (accounting for 59.669% of the variance), whereas those with foot ulcers and neuropathy symptoms scored highest in the symptoms domain. Any amputation, recent or remote, causes deterioration in QOL. Discriminant validity of the Norfolk QOL-DN shown here in more severe neuropathy extends results of previous studies in three populations in Norfolk—normal controls, people with diabetes, and patients with mild diabetic neuropathy—clearly discriminating among these categories.7
Correlates of Norfolk QOL-DN and Other Measures of Neuropathy
We reported previously that the total Norfolk QOL-DN score correlated (Pearson) most strongly with the total neuropathy score (TNS)(r = 0.53, p = 0.0001) and then with the neuropathy impairment score in the lower limbs (NIS-LL+7) (r = 0.27, p = 0.0005) but not with individual indices of electrophysiology or quantitative sensory testing and quantitative autonomic function testing (p > 0.05). Of the QOL subscales, ADL were the greatest contributors to TNS (r = 0.58, p = 0.0001), mainly due to the effects of weakness (r = 0.38, p = 0.0001). Similarly, the large fiber domain correlated with weakness (r = 0.36) and NIS-LL+7 (r = 0.33, both p = 0.0001). Here we showed that in contrast to the large fiber domain, the small fiber domain correlated weakly with the TNS (r = 0.21, p = 0.007), prickling pain (r = 0.18, p = 0.03), and sensory amplitudes (r = 0.11, p = 0.03), as did autonomic with heat pain (r = 0.19, p = 0.03) and nerve conduction velocity (r = 0.23, p = 0.005). The strong correlation between the total neuropathy score and the total QOL-DN score, determined predominantly by the ADL score, is shown elsewhere.7 A strong relationship exists between weakness and total neuropathy scores on ADL.
Discussion
The German translation of Norfolk QOL-DN identified the same five factors as the English version: physical functioning/large fiber, ADL, symptoms, autonomic, and small fiber. The major driving force impacting QOL is physical functioning and ADL, accounting for 59.669% of the variance. ADL appear to be determined by fine motor functions as opposed to the more general crude requirements for physical functioning/large fiber. The symptoms category is driven by positive cognitive disturbances such as pain and numbness (for which there is no evidence of sensory loss, therefore, “positive”). The small fiber scores are less clearly defined between categories of neuropathy severity than in the other domains. Negative small fiber features reflect loss of cognitive perception, a feature of diabetes itself, as well as aging, or selective C-fiber loss. In the factor analysis of the German population, four items—orthostasis, vomiting, diarrhea/ constipation, and urination problems— were identified as constituting the autonomic factor. In the mild neuropathy population, three of these items featured weakly, and the item related to urination did not load at all. It appears that autonomic features occur in advanced stages of neuropathy, affecting QOL and accounting for more robust scores to autonomic questions in the German study.
Using the composite autonomic severity score, Low and colleagues22 reported that autonomic symptoms and deficits are common in diabetes; however, in mild neuropathy, the correlation between symptoms and deficits is overall weak, emphasizing the need to identify autonomic symptoms separately. There was also a significant correlation between autonomic scores in the Norfolk QOL-DN and heat pain thresholds, suggesting that the latter may be equivalent to a peripheral autonomic measure, although, in the Mayo clinic study, a correlation with sudorimetry was poor. Differences in populations and testing methods may account for this disparity.22
Exploratory factor analysis on results of 379 patients with mild neuropathy (stage 1) provided data for criterion validation.7 Here we showed that our tool also has the ability to discriminate between stage 1 neuropathy and populations with more severe neuropathy. The fact that physical functioning/large fiber and ADL domains comprise the majority of the questions and were the greatest contributors to the total score of the QOL-DN is relevant to observations that large fiber dysfunction causes weakness, instability, and a proclivity to falling and fractures.24,25
We conclude that the Norfolk QOL-DN is an important tool for measuring patients' own perception of the impact of diabetes and neuropathy on their physical and psychosocial functioning and may act as a guide in decision making toward altering the apparent health and functional status of individuals with neuropathy. This study does not address depression, which is a major feature of patients with diabetes and complications. The association between diabetic neuropathy and depressive symptoms has been confirmed and described in a study by Vileikyte and associates.26 We showed previously that impairments in these nerve fiber-specific domains correlate with objective measures of nerve functions,8,11 making this a useful instrument as a means to determine the therapeutic efficacy of nerve fiber-specific agents. The tool strongly reflects the total neuropathy score, embracing symptoms and neurologic examination. We showed, too, that electrophysiologic, quantitative sensory, and autonomic testing scores correlate weakly with QOL.7 Weakness is a prominent factor in impaired quality of life, particularly affecting large fiber involvement in physical functioning and ADL, whereas pain has a small impact on QOL. By relating neuropathic disabilities to different nerve fibers, it has the capacity for measuring the impact of future nerve fiber-specific neurotrophic therapies.17 With a large armamentarium of potential new therapies for nerve regeneration, enhanced large fiber function, and pain relief, a nerve fiber-specific quality tool for inventorying patient-reported quality of life outcomes captures aspects of DN not captured by standard measures. Autonomic symptoms appear early in the course of neuropathy and may not be due to organic structural damage to the autonomic nervous system, but to a functional imbalance of sympathetic and parasympathetic components. This emphasizes the need to resolve quality of life and activities of daily living for neuropathy into its fiber subtypes and even divisions within autonomic symptoms. Findings in this study suggest that the definition of autonomic neuropathy should be reevaluated to include symptoms previously ascribed only to a drop in blood pressure.
Acknowledgements
The authors sincerely thank Edward Bastyr III, M.D., Alan Oglesby, M.P.H., and Risa Hayes, Ph.D. for their valuable input; our thanks also to Rustem Samigullin for providing us with data from the multicenter study.
Abbreviations
- ADL
activities of daily living
- ANOVA
analysis of variance
- DN
diabetic neuropathy
- NIS-LL
neuropathy impairment score in the lower limbs
- QOL-DN
Quality of Life for Diabetic Neuropathy
- QOL
quality of life
- PLUM
polytomous universal model
- TNS
total neuropathy score
References
- 1.Vinik AI, Park TS, Stansberry KB, Pittenger GL. Diabetic neuropathies. Diabetologia. 2000;43(8):957–973. doi: 10.1007/s001250051477. [DOI] [PubMed] [Google Scholar]
- 2.Brislin R, Lonner W, Thorndike R. New York: Wiley; 1973. Cross-cultural research methods. [Google Scholar]
- 3.Hunt SM. Cross-cultural issues in the use of socio-medical indicators. Health Policy. 1986;6(2):149–158. doi: 10.1016/0168-8510(86)90004-7. [DOI] [PubMed] [Google Scholar]
- 4.Hayes R, Anderson R, Revicki D. The international assessment of health-related quality of life: theory, translation, measurement and analysis. New York: Rapid Communications; 1995. Psychometer evaluaton and interpretation of health-related quality of life data; pp. 103–114. [Google Scholar]
- 5.Vinik EJ, Stansberry KB, Zarrabi L, Witherspoon CAG, McNitt PM, Vinik AI. Development of a sensitive, specific quality of life inventory for peripheral neuropathy [abstract] Diabetes. 2000;49:A819. [Google Scholar]
- 6.Vinik E, Stansberry K, Doviak M, Ruck S, Vinik A. Norfolk Quality of Life (QOL) tool: scoring and reproducibility in healthy people, diabetic controls and patients with neuropathy. Diabetes. 2003;52(Suppl 1):A198. [Google Scholar]
- 7.Vinik E, Hayes R, Oglesby A, Bastyr E, Barlow P, Ford-Molvik S, Vinik A. The development and validation of the Norfolk QOL-DN, a new measure of patients' perception of the effects of diabetes and diabetic neuropathy. Diabetes Technol Ther. 2005;7(3):497–508. doi: 10.1089/dia.2005.7.497. [DOI] [PubMed] [Google Scholar]
- 8.Vinik EJ, Hayes C, Oglesby A, Vinik AI. Identification of factors in the nerve fiber specific quality of life (QOL-DN) inventory that reflect QOL and health status. Diabetes. 2004;53:A295. [Google Scholar]
- 9.Dyck PJ. Detection, characterization and staging of polyneuropathy: assessed in diabetes. Muscle Nerve. 1988;11(1):21–32. doi: 10.1002/mus.880110106. [DOI] [PubMed] [Google Scholar]
- 10.Vinik E, Stansberry K, Vinik A. Use of Neuropathy Quality of Life Tool (Norfolk QOL-DN) in a large clinical trial: comparison with symptom scores (NSS), nerve impairment (NISS), quantitative sensory (QST), autonomic (QAFT) tests and electrophysiology. American Diabetes Association 63rd Scientific Sessions. Diabetes. 2003;52(Suppl 1) [Google Scholar]
- 11.Vinik E, Stansberry KB, Oglesby A, Bastyr E, Vinik A. International Diabetes Federation (IDF) 18th International Diabetes Federation Congress. Paris, France: 2003. Norfolk Quality of Life (QOL) Tool: relationship between indices of QOL and specific nerve fiber symptoms and neurologic disabilities [abstract] [Google Scholar]
- 12.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473–483. [PubMed] [Google Scholar]
- 13.Benbow SJ, Wallymahmed ME, MacFarlane IA. Diabetic peripheral neuropathy and quality of life. QJM. 1998;91(11):733–737. doi: 10.1093/qjmed/91.11.733. [DOI] [PubMed] [Google Scholar]
- 14.Jacobson AM, de Groot M, Samson JA. The evaluation of two measures of quality of life in patients with type I and type II diabetes. Diabetes Care. 1994;17(4):267–274. doi: 10.2337/diacare.17.4.267. [DOI] [PubMed] [Google Scholar]
- 15.Grootenhuis PA, Snoek FJ, Heine RJ, Bouter LM. Development of a type 2 diabetes symptom checklist: a measure of symptom severity. Diabet Med. 1994;11(3):253–261. doi: 10.1111/j.1464-5491.1994.tb00268.x. [DOI] [PubMed] [Google Scholar]
- 16.Vileikyte L, Peyrot M, Bundy C, Rubin RR, Leventhal H, Mora P, Shaw JE, Baker P, Boulton AJ. The development and validation of a neuropathy- and foot ulcer-specific quality of life instrument. Diabetes Care. 2003;26(9):2549–2555. doi: 10.2337/diacare.26.9.2549. [DOI] [PubMed] [Google Scholar]
- 17.Boulton A, Vinik A, Arezzo J, Bril V, Feldman E, Freeman R, Malik R, Maser R, Sosenko J, Ziegler D American Diabetes Association. Diabetic neuropathies: a statement by the American Diabetes Association. Diabetes Care. 2005;28(4):956–962. doi: 10.2337/diacare.28.4.956. [DOI] [PubMed] [Google Scholar]
- 18.Vinik A, Mehrabyan A. Diabetic neuropathies. Med Clin North Am. 2004;88(4):947–999. doi: 10.1016/j.mcna.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 19.Vinik AI, Maser RE, Mitchell BD, Freeman R. Diabetic autonomic neuropathy. Diabetes Care. 2003;26(5):1553–1579. doi: 10.2337/diacare.26.5.1553. [DOI] [PubMed] [Google Scholar]
- 20.Witzke K, Vinik A. Diabetic neuropathy in older adults [abstract] Rev Endocr Metab Disord. 2005;6(2):117–127. doi: 10.1007/s11154-005-6724-7. [DOI] [PubMed] [Google Scholar]
- 21.Vinik A, Ullal J, Parson HK, Casellini CM. Diabetic neuropathies: clinical manifestations and current treatment options. Nat Clin Pract Endocrinol Metab. 2006;2(5):269–281. doi: 10.1038/ncpendmet0142. [DOI] [PubMed] [Google Scholar]
- 22.Low PA, Benrud-Larson LM, Sletten DM, Opfer-Gehrking TL, Weigand SD, O'Brien PC, Suarez GA, Dyck PJ. Autonomic symptoms and diabetic neuropathy: a population-based study. Diabetes Care. 2004;27(12):2942–2947. doi: 10.2337/diacare.27.12.2942. [DOI] [PubMed] [Google Scholar]
- 23.Willrich A, Pinzur M, McNeil M, Juknelis D, Lavery L. Health related quality of life, cognitive function, and depression in diabetic patients with foot ulcer or amputation. A preliminary study. Foot Ankle Int. 2005;26(2):128–134. doi: 10.1177/107110070502600203. [DOI] [PubMed] [Google Scholar]
- 24.Resnick HE, Stansberry KB, Harris TB, Tirivedi M, Smith K, Morgan P, Vinik AI. Diabetes, peripheral neuropathy, and old age disability. Muscle Nerve. 2002;25(1):43–50. doi: 10.1002/mus.1217. [DOI] [PubMed] [Google Scholar]
- 25.Vinik AI. Diabetic neuropathy, mobility and balance. Geriatric Times. 2003;4(1):13–15. [Google Scholar]
- 26.Vileikyte L, Leventhal H, Gonzalez JS, Peyrot M, Rubin RR, Ulbrecht JS, Garrow A, Waterman C, Cavanagh PR, Boulton AJ. Diabetic peripheral neuropathy and depressive symptoms: the association revisited. Diabetes Care. 2005;28(10):2378–2383. doi: 10.2337/diacare.28.10.2378. [DOI] [PubMed] [Google Scholar]