Abstract
Introduction
This article reviews glycated albumin (GA) as a potential intermediate-term glycation index to fill the gap between self-monitoring of blood glucose (SMBG) and hemoglobin A1c testing in diabetes management. The introduction gives an assessment of available short-, medium-, and long-term glycemic indicators.
Methodologies and Utility
Methods of GA measurement are summarized, and the variance of normal and diabetic GA values are discussed. Greatest uniformity in GA measurement is generally associated with immunoassay and the newer affinity chromatography methodologies utilized by reference laboratories. Utility of GA measurement includes its value as a marker for glycation, its substantial relationship to diabetes complications such as nephropathy and coronary artery disease, and as an unambiguous indicator of glycemic control in diabetes patients undergoing hemodialysis. Studies support the utility of GA in detecting short-term changes in glycemic control, and GA testing has been strongly recommended for gestational diabetes.
Results and Discussion
The results of a survey with mailings to over 3500 diabetes care professionals primarily in the United States are outlined and analyzed (margin of error: +/-6.5%, 95% confidence). Respondents strongly supported the need for a test for intermediate glycemic control as well as the utility of a rapid GA test as a monthly glycemic indicator.
Conclusions
Such a test, as yet unavailable, could increase compliance and enhance empowerment among diabetes patients. It also has the potential to reduce the number of recommended SMBG tests, which may result in significant health care cost savings.
Keywords: gestational diabetes, glycated albumin, glycation, glycation index, rapid assay, type 2 diabetes
Introduction
Diabetes monitoring for protein glycation, an essential element for the long-term control of the complications of diabetes mellitus, is currently managed by a combination of daily self-monitoring of blood glucose (SMBG) measurements and physician-assessed hemoglobin A1c (A1C) levels every 3–6 months. The literature has called into question the effective utility of both those methodologies for large numbers of type 2 diabetes patients. After a brief assessment of available short-, medium-, and long-term glycemic indicators, this article reviews glycated albumin (GA) as a potential intermediate glycation index for diabetes control. Methods for the measurement of GA are summarized, accompanied by discussion of the discrepancies between the values generated by different methods. The results of a survey of endocrinologists as well as published literature provide an assessment of the degree of utility and acceptance that GA would have as a new monthly management tool for diabetes.
National survey data in 19891 showed that only 40% of type 1 diabetes patients and 26% of type 2 diabetes patients (33% of all diabetes patients) performed SMBG testing at least once a day. Later studies have disputed the conventional wisdom of a positive link between daily blood glucose testing and improved glycemic control for type 2 diabetes patients. The 2007 Fremantle study of 1286 type 2 diabetes patients over 5 years concluded that neither SMBG testing nor its frequency was associated with glycemic benefit in type 2 diabetes patients regardless of treatment.2 A 2006 study of nearly 3000 type 2 diabetes patients on either oral antidiabetic drugs or a restricted diet found no benefit from SMBG for metabolic control in either group (part of a study of 24,500 type 1 and type 2 patients in Germany and Austria).3 In a 2007 study, no improvement in glycemic control was found in noninsulin treated diabetes patients, even when given training and encouragement.4
The literature may be broken down into glycemic indicators that provide information over the long, medium, and short term. The utility of A1C as a long-term glycemic indicator that correlates levels of glycated hemoglobin in blood with blood sugar levels has been thoroughly reviewed.5 Since most hemoglobin resides in the red blood cell, which has a half-life of approximately 120 days, the relative amount of glycated hemoglobin in a patient's blood becomes a living record of glycemia over a period of a few months. The A1C test has become a gold standard, because it has been shown to reliably predict the risk of developing diabetes-related complications. Although a recent report recommends devising a protocol to employ A1C as a screening or diagnostic tool for diabetes, it has not yet been accepted by the clinical community and official associations and societies.6 Recent efforts addressing reference method standardization have left two areas of uncertainty about A1C as previously discussed by Jeffcoate:7 biological variability and clinical variability. This review emphasized concern for myriad influences on erythrocyte lifespan variability and cautioned that the relationship of A1C monitoring to microvascular disease in type 2 diabetes patients is not strongly established.
Short-term indicators are no longer limited to measurements of glucose in blood, plasma, serum, or other body fluids. Serum 1,5-anhydroglucitol (1,5-AG) is a nonprotein marker for monitoring short-term glycemic control and has been used for more than a decade in Japan under the name GlycoMark™. It represents diabetic status over a 24 hr period, because it reflects glucose's competitive inhibition of 1,5-AG reabsorption in the kidney tubule.8 In a recent study involving type 1 and type 2 diabetes patients showing good to moderate control, 1,5-AG was found to reflect postprandial glycemic excursions more robustly than either A1C or the intermediate indicator, fructosamine (FA).9 It is not recommended for monitoring gestational diabetes, however, since renal hemodynamics are not stable during pregnancy. Another short-term marker, apolipoprotein B, is a component of low-density lipoproteins (LDLs) that becomes glycated. This protein is of special interest because of its involvement in atherogenesis. Due to the 3–5 day circulating half-life of LDLs, the glycated LDL level reflects mean glycemia over the preceding week.10
Over the past 15–20 years, many published reports have described the measurement of serum protein indicators, specifically FA and GA, as a method to assess glycemic status over intermediate periods (2–4 weeks) that reflect the half-lives of these respective molecules in serum. Fructosamine (named for the chemical similarity to fructose) refers to the sum of all ketoamine linkages resulting from the glycation of circulating serum proteins. An assay for FA was found to be easily automated and thus relatively inexpensive to perform, and FA measurement remains popular in some countries outside the United States. A rapid test for FA was scrutinized by the Food and Drug Administration in 1997, and a few clinical studies with mixed results11–13 were reported, but the subsequent tortuous commercial path for this test has curtailed its availability in the United States, and it is no longer available as a commercial rapid diagnostic test. However, experience with FA testing has shown the potential value of an intermediate index to retrospectively evaluate changes in diet and exercise habits, to allow faster evaluation of changes in medication dosages and other control measures, and to serve as an inexpensive screening test for impaired glycemic control.14
Methodologies of Glycated Albumin Measurement
An increasing amount of attention has been focused on the use of GA as an intermediate indicator of glycemic status. Albumin is the largest component of the plasma proteins, representing more than 80% of the total molecules and 60% of the total plasma protein concentration. Several methods are presently employed in the isolation and quantification of GA. These include (a) enzymatic assay, (b) high-performance liquid chromatography (HPLC) and affinity chromatography, (c) immunoassay, including quantification by radio-immunoassay, (d) enzyme-linked immunosorbent assay (ELISA), (e) enzyme-linked boronate immunoassay (ELBIA), (f) colorimetry, and (g) electrochemical. In the United States, only 6 out of over 50 large commercial clinical laboratories contacted perform GA testing (Table 1). Five of these six laboratories employ affinity chromatography and state reference values for GA in the range of 0.6–3.0%. The sixth laboratory performs an enzymatic assay with a GA reference range of 11–16%.
Table 1.
United States Clinical Reference Laboratories Offering Glycated Albumin Testing
| Name | Web Site | Method Used | Glycated Albumin Reference Range |
|---|---|---|---|
| Laboratory Alliance of Central New York, LLC | www.laboratoryalliance.com | Affinity column chromatography, turbidimetry, boronate affinity | 0.6–3.0% |
| ARUP Reference Laboratory | www.aruplab.com | Boronate affinity chromatography, turbidimetry | 0.6–3.0% |
| Reference Laboratory at the Cleveland Clinic | http://referencelab.clevelandclinic.org | Boronate affinity chromatography, turbidimetric immunoassay | 0.6–3.0% |
| Pacific Biometrics, Inc. | www.pacbio.com | Enzymatic | 11–16% |
| Ohio University Medical Center Laboratory | www.oumedical.com | Boronate affinity chromatography, turbidimetric immunoassay | 0.6–3% |
| Quest Diagnostics, Inc. | http://cas2.questdiagnostics.com | Affinity column chromatography | 0.8–1.4% |
Nearly half the scientific and clinical reports relevant to GA measurement reviewed over the past 30 years describe normal GA values of 2.6% or lower, in line with the majority of reference labs. About a quarter of the reports indicate normal values in the range of 5–9% and another quarter report normal values in the range of 10–20%, the latter in line with the single enzymatic reference determination. Overall, typical diabetic GA values are 2–5 times above normal values for a given reporting method.
Throughout the literature, separation by affinity chromatography is associated with disparate normal and diabetic GA% values. Values for normal individuals range from means of 0.6 to 8.6% and values for diabetic individuals from means of 1.4 to 16.59%. In general, older reports (1980–1985) involving affinity chromatography are associated with higher GA values than more recent reports, which suggests that lower values represent a refinement in technique. The results of boronate-affinity chromatography may be skewed because of imprecise reckoning of glycated molecules vis-à-vis binding sites on the resin.15 This may explain the tighter reference range (0.8–1.4%) in the one commercial laboratory using affinity column chromatography without boronate (Table 1). This is in contrast to immunoassays using monoclonal antibodies with specificity for each molecule. Monoclonal isolation associated with ELISA as well as immunonephelemetry (turbidimetric) and gel electrophoresis with bromcresol green (BCG) produced GA% values in the lower range of reported values.16
Criticisms of quantification of GA by thiobarbituric acid (TBA) and BCG assays have appeared in older literature.15,17 Less complicated colorimetric assays have replaced the TBA method,18 including nitroblue tetrazolium19 and 2-keto-glucose with hydrazine.20 Refinement of BCG has progressed to the Hitachi automated BCG-dye binding analyzer, yielding results in agreement with a reported method for separating and quantifying nonglycated human serum albumin using lateral chromatography and immunofluorescence.21
The ELBIA method22 generated moderately high GA values. HPLC generated the highest values, both for older methodologies, where incomplete separation may relate to sample size,23 and for the newer enzymatic methodologies.24–26 The latter is the basis for the Lucica GA-L Glycoalbumin assay kit. Kouzuma et al.24 have reported good correlation between GA measurement by this assay and values obtained from HPLC. In updated versions of this assay,25,26 quantification by bromcresol purple dye is associated with considerably lower values than those originally obtained with BCG. A further refinement of this enzymatic amino acid elimination system26 has been targeted as a point-of-care application.
In summary, greatest uniformity in GA measurement is generally associated with immunoassay and the newer affinity chromatography methodologies typically found in United States reference laboratories.
Utility of Glycated Albumin
Cohen and Clements16 declared that the concentration of GA in serum, with a half-life of 12–19 days, would be an excellent index of recent ambient glycemia. Albumin can be measured in the blood with fewer issues than FA and would fill the time gap between SMBG and A1C at approximately 1 month. In addition to directly measuring the effects of hyperglycemia on the most prevalent plasma protein, GA has been directly implicated as a causal factor in several major complications of diabetes, especially in nephropathy due to its interaction with receptors on mesangial cells.27 In blockade experiments with mice, the albuminuria and mesangial expansion that are known hallmarks of diabetic nephropathy have been shown to be controlled by the interaction of GA with mesangial cell receptors, independent of the actions of hyperglycemia. In the cardiovascular system, macrophages in the artery walls can also recognize GA via specific receptors and stimulate an inflammatory response, ultimately leading to the evolution of atheroma plaques. Pu et al.28 showed that GA levels are significantly higher in coronary artery disease (CAD) patients, and unlike A1C, GA is a significant predictor of CAD in type 2 diabetes patients. Increased GA levels in the blood have been implicated in diabetic retinopathy29 and via the cerebrospinal fluid in Alzheimer's disease.30 Of particular interest are recent reports involving hypothyroidism31 and glucose oscillations,32 where A1C failed to track the diabetic episode, but a shorter term index did.
Although GA testing may initially be viewed as adjunctive to A1C for diabetes management, immediate applications are apparent for gestational diabetes and type 2 diabetes patients. A recent clinical study comparing GA and A1C in type 2 diabetes patients over 16 weeks found that GA decreased more rapidly than A1C as glycemic control improved, and the ratio of GA to A1C was higher in the hyperglycemic state than when diabetes was well controlled.33 These results support the utility of GA in detecting short-term changes in glycemic control, a finding supported by the recommendation that a GA test be adopted immediately for gestational diabetes.34 Recent studies of diabetes patients undergoing hemodialysis in Japan35 and the United States36 concluded that the A1C test underestimated glycemic control when erythropoietin was used, potentially affecting almost 90% of dialysis patients, while GA testing provided more accurate estimates for those patients.
Endocrinologist Survey: Results and Discussion
Epinex Diagnostics (Irvine, CA) conducted a survey in 2005–2006 to determine the feasibility of a rapid diagnostic test based on a GA index. The survey was intended to provide a baseline for diagnostic procedures, especially as they are currently applied to type 2 diabetes patients. It was intended to test physician support for the use of a monthly index for glycation as a component of diabetes management in a number of key areas, including gestational diabetes, geriatric monitoring, and undiagnosed or asymptomatic screening.
A total of more than 3500 surveys were sent to a select group of endocrinologists, diabetes specialists, researchers, and general practitioners. Two hundred fifteen responses were received and evaluated, representing a margin of error from random survey sample calculations analysis of 6.5% at a 95% confidence level. Respondents were evenly distributed across the United States, including Puerto Rico. Approximately 4% of respondents were from Canada, Europe, Asia, and Latin America. Eighty-eight percent of respondents have an M.D. degree, and 59% designated endocrinologist as their specialization, followed in frequency by diabetologist (18%), internal medicine (15%), and a few family practitioners and geriatricians. Virtually all the respondents treat diabetes patients on a regular basis, with the majority (54%) averaging ten or more patients per day.
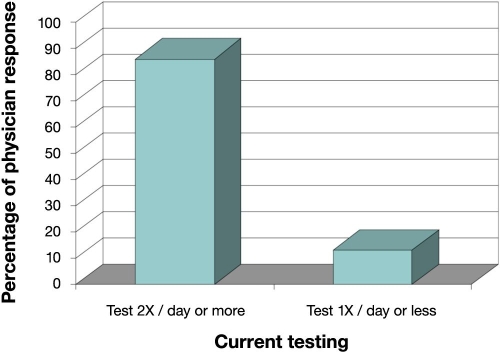
Respondents indicated that current monitoring standards for diabetes involve a combination of SMBG procedure and A1C measurement. The survey indicated that type 2 diabetes patients are most often asked to measure blood glucose twice (49%) or thrice (37%) daily for a total of (86%) (Figure 1). Compliant type 2 patients on diet and medical maintenance, however, are asked to measure blood glucose twice daily (42%) and once (35%) daily, with only 11% indicating three or more times. Over 90% of respondents perform the A1C test 3–4 times per year in all diabetes patients. Sixty percent of respondents regard the A1C test as the gold standard of diabetes management, but 31% note that it is occasionally misleading and only approximately 12% encourage A1C over-the-counter (OTC) testing. Despite some obvious advantages associated with OTC and self-testing, including engendering patient empowerment, many physicians are concerned about the accuracy of OTC A1C tests.
Figure 1.
Endocrinologist survey—Recommendation for Self-Monitoring of Blood Glucose Testing for Type 2 Patients.
The history of FA testing has served as a springboard to something better in intermediate glycemic control, judging from clinical test results and the overall response in this survey, in which 64% agreed there was a need for intermediate glycemic control and only 13% disagreed. Nearly 90% of the respondents were aware of the FA test, and nearly 60% indicated that they had used the test and/or knew colleagues who had used the test. Of those knowledgeable of the test, 63% considered it reliable and accurate. The ability of FA to diagnose diabetes has been historically criticized37–42 for yielding false positives and having a lack of sensitivity. In 1991, Narayanan43 summarized several potential interference agents for early FA assays, and a decade later, colorimetric determination of FA was criticized as being susceptible to interference factors in blood, such as lipid.44 At present, unreliable results appear due to the fact that a collection of proteins, some of which vary rapidly and considerably during intercurrent disease, are being simultaneously assessed without the benefit of normal baseline data and a standardized relationship to total protein.
The concept of a rapid test in which the measurement of GA is compared to total albumin (glycation index: percent of GA to total albumin) from a single drop of blood was explained to the survey respondents. Over half gave a favorable response to a test for short-to-intermediate glycemic status and control. Overall, 69% thought such a test would be “very good” for tracking gestational diabetes, and only 5% indicated “not at all.” When asked specifically about a GA index, 88% responded positively: 26% rated it excellent, 40% very good, and 22% good for tracking gestational diabetes. In addition, just over half of all respondents answered in the affirmative for GA monitoring geriatric patients. These patterns did not change when only the researchers—22% of survey respondents—were considered. Seventy-three percent of respondents envisioned a positive contribution for an OTC version of a rapid GA test. Included in this subtotal were 34% of the total respondents who viewed GA as complementary to A1C and fingerstick glucose and another 18% who felt it would contribute to personal empowerment. The balance of the subtotal (21% of the total) believed that an OTC version of GA would enhance diabetes management, the same percentage who recommended OTC A1C for diabetes management. Seventy-four percent recommended that diabetes patients be tested for GA, and 61% indicated an interest in participating in a focus group on the subject.
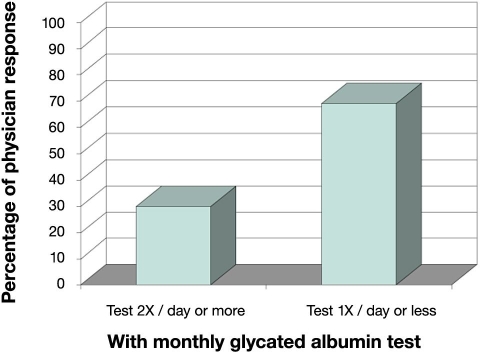
According to the responses given by endocrinologists to the Epinex survey, 13% of diabetes patients are asked to measure glucose once daily or less (Figure 1). With the advent of a successful monthly GA test, 69% would be asked to measure glucose once or less daily (Figure 2). This reduction of 56% from 86% to 30% in daily SMBG testing (Figure 2) indicates the potential for a very large factor in healthcare savings. While the short-term information provided by SMBG is vital to all diabetes patients and cannot be replaced by GA, it is important to consider that diabetes management over the long term should be improved by a more representative indicator of the disease processes involved. The highest percentage (38%) of respondents indicated that monthly GA status would relegate SMBG testing in compliant diabetes patients to once daily, and 50% would recommend a GA test ahead of a fasting blood glucose or an oral glucose tolerance test for low-to-mid level diabetes risk patients. Regarding an OTC GA test, more than half (52%) felt it would positively affect diabetes management largely as a complement to finger stick glucose and A1C. The remaining respondents were approximately equally divided between “fostering a sense of personal empowerment” (19%), “no contribution” (13%), and “harmful if used incorrectly” (12%).
Figure 2.
Endocrinologist survey—Recommendation for Self-Monitoring of Blood Glucose Testing for Type 2 Patients.
It is clear from this survey that the establishment of a GA index for diabetes care has the potential to significantly impact diabetes monitoring as an adjunct to A1C and SMBG. It may also have an impact on screening and diagnosing prediabetes or people suffering from metabolic syndrome. Reducing the number of recommended blood glucose tests in lieu of GA measurement, i.e., reducing the number of times people have to stick themselves, should increase compliance with testing and improve patient care and outcome. It also represents an enormous potential saving in healthcare cost in that the survey results support a solid economic argument regarding a shift away from more expensive glucose testing in favor of a reliable and relatively inexpensive GA test.
Conclusions
Given the expanding diabetes population, endocrinologists are increasingly recognizing the need for an intermediate glycemic indicator. A GA test has the potential to significantly impact diabetes monitoring and fulfill this need. With a turnover time in plasma of 2–3 weeks, GA provides a glycation update on a monthly basis. GA has been shown to be more accurate than the existing A1C “gold standard” for diabetes patients undergoing hemodialysis, a major segment of the diabetes population. Levels of GA have also been shown to have a substantial relationship to certain types of diabetes complications such as nephropathy and CAD. With more and more studies questioning SMBG as an effective method for monitoring type 2 diabetes, GA has the potential to provide a reliable and effective alternative. It has already been recognized as an ideal marker for gestational diabetes, with potential clinical applications for diabetes patients undergoing hemodialysis and diabetes patients with CAD. An available GA rapid test as a monthly indicator of glycation could bring about a significant reduction in daily SMBG, resulting in substantial healthcare cost savings as well as an increase in patient compliance. Availability of such a test to the patient depends on reconciling the disparate results seen with various measurement methodologies. There is currently no rapid GA test for intermediate glycation available to physicians or patients.
Acknowledgements
David Trasoff of Corporate Communications, Epinex Diagnostics, Inc. and Jaycee Delizo, Bo Du, and Christy Purnajo of the Department of Research and Development, Epinex Diagnostics, Inc.
Abbreviations
- 1,5-AG
1,5-anhydroglucitol
- A1C
hemoglobin A1c
- BCG
bromcresol green
- CAD
coronary artery disease
- ELBIA
enzyme-linked boronate immunoassay
- ELISA
enzyme-linked immunosorbent assay
- FA
fructosamine
- GA
glycated albumin
- HPLC
high-performance liquid chromatography
- LDL
low-density lipoprotein
- OTC
over-the-counter
- SMBG
self-monitoring of blood glucose
- TBA
thiobarbituric acid
References
- 1.Goldstein DE, Little RR, Lorenz RA, Malone JI, Nathan D, Peterson CM, Sacks DB. Tests of glycemia in diabetes. Diabetes Care. 2004;27(7):1761–1773. doi: 10.2337/diacare.27.7.1761. [DOI] [PubMed] [Google Scholar]
- 2.Davis WA, Bruce DG, Davis TM. Does self-monitoring of blood glucose improve outcome in type-2 diabetes? The Fremantle Diabetes Study. Diabetologia. 2007;50(3):510–515. doi: 10.1007/s00125-006-0581-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schütt M, Kern W, Krause U, Busch P, Dapp A, Grziwotz R, Mayer I, Rosenbauer J, Wagner C, Zimmermann A, Kerner W, Holl RW DPV Initiative. Is the frequency of self-monitoring of blood glucose related to long-term metabolic control? Multicenter analysis including 24,500 patients from 191 centers in Germany and Austria. Exp Clin Endocrinol Diabetes. 2006;114(7):384–388. doi: 10.1055/s-2006-924152. [DOI] [PubMed] [Google Scholar]
- 4.Farmer A, Wade A, Goyder E, Yudkin P, French D, Craven A, Holman R, Kinmonth AL, Neil A. Impact of self monitoring of blood glucose in the management of patients with non-insulin treated diabetes: open parallel group randomized trial. BMJ. 2007;335(7611):132. doi: 10.1136/bmj.39247.447431.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldstein DE, Little RR, Lorenz RA, Malone JI, Nathan DM, Peterson CM American Diabetes Association. Tests of glycemia in diabetes. Diabetes Care. 2003;26(Suppl1):S106–S108. doi: 10.2337/diacare.26.2007.s106. [DOI] [PubMed] [Google Scholar]
- 6.Saudek CD, Herman WH, Sacks DB, Bergenstal RM, Edelman D, Davidson MB. A new look at screening and diagnosing diabetes mellitus. J. Clin. Endocrinol Metab. 2008;93(7):2447–2453. doi: 10.1210/jc.2007-2174. [DOI] [PubMed] [Google Scholar]
- 7.Jeffcoate SL. Diabetes control and complications: the role of glycated hemoglobin, 25 years on. Diabet Med. 2004;21(7):657–665. doi: 10.1046/j.1464-5491.2003.01065.x. [DOI] [PubMed] [Google Scholar]
- 8.Buse JB, Freeman JL, Edelman SV, Jovanovic L, McGill JB. Serum 1,5-anhydoglucitol (GlycoMark): a short-term glycemic marker. Diabetes Technol Ther. 2003;5(3):355–363. doi: 10.1089/152091503765691839. [DOI] [PubMed] [Google Scholar]
- 9.Dungan KM, Buse JB, Largay J, Kelly MM, Button EA, Kato S, Wittlin S. 1,5-anhydroglucitol and postprandial hyperglycemia as measured by continuous glucose monitoring system in moderately controlled patients with diabetes. Diabetes Care. 2006;29(6):1214–1219. doi: 10.2337/dc06-1910. [DOI] [PubMed] [Google Scholar]
- 10.Lyons TJ, Baynes JW, Patrick JS, Colwell JA, Lopes-Vierlla MF. Glycosylation of low density lipoprotein in patients with type 1 (insulin dependent) diabetes: correlations with other parameters of glycemic control. Diabetologia. 1986;29(10):685–689. doi: 10.1007/BF00870276. [DOI] [PubMed] [Google Scholar]
- 11.Edelman SV, Callahan P, Deeb LC. Multisite evaluation of a new diabetes self-test for glucose and glycated protein (fructosamine) Diabetes Technol Ther. 2000;2(2):233–238. doi: 10.1089/15209150050025195. [DOI] [PubMed] [Google Scholar]
- 12.Carter AW, Borchardt N, Cooney M, Greene D. Dual-test monitoring of hyperglycemia using daily glucose and weekly fructosamine values. Diabetes Technol Ther. 2001;3(3):399–403. doi: 10.1089/15209150152607178. [DOI] [PubMed] [Google Scholar]
- 13.Lindsey CC, Carter AW, Mangum S, Greene D, Richardson A, Brown S, McCandless B. A prospective, randomized, multicentered controlled trial to compare the annual glycemic and quality outcomes of patients with diabetes mellitus monitored with weekly fructosamine testing versus usual care: a 3-month interim analysis. Diabetes Technol Ther. 2002;4(5):637–642. doi: 10.1089/152091502320798268. [DOI] [PubMed] [Google Scholar]
- 14.Carter AW, Borchardt N, Cooney M, Greene D. Dual test diabetes screening project: screening for poor glycemic control in a large workplace population. Diabetes Technol Ther. 2000;2(4):529–536. doi: 10.1089/15209150050501934. [DOI] [PubMed] [Google Scholar]
- 15.Johnson RN, Baker JR. Inaccuracy in measuring glycated albumin concentration by thiobarbituric acid colorimetry and by boronate chromatography. Clin Chem. 1988;34(7):1456–1459. [PubMed] [Google Scholar]
- 16.Cohen MP, Clements RS. Measuring glycated proteins: clinical and methodological aspects. Diabetes Technol Ther. 1999;1(1):57–70. doi: 10.1089/152091599317585. [DOI] [PubMed] [Google Scholar]
- 17.Silver AC, Lamb E, Cattell WR, Dawnay AB. Investigation and validation of the affinity chromatography method for measuring glycated albumin in serum and urine. Clin Chim Acta. 1991;202(1-2):11–22. doi: 10.1016/0009-8981(91)90251-7. [DOI] [PubMed] [Google Scholar]
- 18.Kumar A, Rao P, Pattabiriman TN. A colorimetric method for the estimation of serum glycated proteins based on differential reduction of free and bound glucose by sodium borohydride. Biochem Med Metab Biol. 1988;39(3):296–304. doi: 10.1016/0885-4505(88)90089-8. [DOI] [PubMed] [Google Scholar]
- 19.Mashiba S, Uchida K, Okuda S, Tomita S. Measurement of glycated albumin by the nitroblue tetrazolium colorimetric method. Clin Chim Acta. 1992;212(1-2):3–15. doi: 10.1016/0009-8981(92)90133-b. [DOI] [PubMed] [Google Scholar]
- 20.Kobayashi K, Yoshimoto K, Hirauchi K, Uchida K. A novel colorimetric method for determination of glycated protein based on 2-keto-glucose release with hydrazine. Biol Pharm Bull. 1993;16(2):195–198. doi: 10.1248/bpb.16.195. [DOI] [PubMed] [Google Scholar]
- 21.Choi S, Choi EY, Kim DJ, Kim JH, Kim TS, Oh SW. A rapid, simple measurement of human albumin in whole blood using a fluorescence immunoassay (I) Clin Chim Acta. 2004;339(1-2):147–156. doi: 10.1016/j.cccn.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 22.Ikeda K, Sakamoto Y, Kawaski Y, Miyake T, Tanaka K, Urata T, Katayama Y, Ueda S, Horiuchi S. Determination of glycated albumin by enzyme-linked boronate immunoassay (ELBIA) Clin Chem. 1998;44(2):256–263. [PubMed] [Google Scholar]
- 23.Cohen MP. Caution: interpretation of results of HPLC assay for serum glycated albumin. Diabetologia. 1991;34(10):766. doi: 10.1007/BF00401527. [DOI] [PubMed] [Google Scholar]
- 24.Kouzuma T, Usami T, Yamakoshi M, Takahashi M, Imamura S. An enzymatic method for the measurement of glycated albumin in biological samples. Clin Chim Acta. 2002;324(1-2):61–71. doi: 10.1016/s0009-8981(02)00207-3. [DOI] [PubMed] [Google Scholar]
- 25.Kouzuma T, Uemastu Y, Usami T, Imamura S. Study of glycated amino acid elimination reaction for an improved enzymatic glycated albumin measurement method. Clin Chim Acta. 2004;346(2):135–143. doi: 10.1016/j.cccn.2004.02.019. [DOI] [PubMed] [Google Scholar]
- 26.Yamaguchi M, Kambe S, Eto T, Yamakoshi M, Kouzuma T, Suzuki N. Point of care testing system via enzymatic method for the rapid efficient assay of glycated albumin. Biosens Bioelectron. 2005;21(3):426–432. doi: 10.1016/j.bios.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 27.Gugliucci A. Glycation as the glucose link to diabetic complications. J Am Osteopath Assoc. 2000;100(10):621–634. [PubMed] [Google Scholar]
- 28.Pu LJ, Lu L, Shen WF, Zhang Q, Zhang RY, Zhang JS, Hu J, Yang Zk, Ding FH, Chen QJ, Shen J, Fang DH, Lou S. Increased serum glycated albumin level is associated with the presence and severity of coronary artery disease in type 2 diabetic patients. Circ J. 2007;71(7):1067–1073. doi: 10.1253/circj.71.1067. [DOI] [PubMed] [Google Scholar]
- 29.Okumura A, Mitamura Y, Namekata K, Nakamura K, Harada C, Harada T. Glycated albumin induces activation of activator-protein-1 in retinal glial cells. Jpn J Ophthalmol. 2007;51(3):236–237. doi: 10.1007/s10384-007-0431-8. [DOI] [PubMed] [Google Scholar]
- 30.Shuvaev VV, Laffont I, Serot JM, Fujii J, Taniguchi N, Siest G. Increased protein glycation in cerebrospinal fluid of Alzheimer's disease. Neurobiol Aging. 2001;22(3):397–402. doi: 10.1016/s0197-4580(00)00253-0. [DOI] [PubMed] [Google Scholar]
- 31.Moriyama K, Kanamoto N, Hataya Y, Nanbu T, Hosoda K, Arai H, Nakao K. A case of type 2 diabetes mellitus developing hypothyroidism discovered as a result of discrepancy between glycated hemoglobin and glycated albumin values. Diabetes Res Clin Pract. 2006;71(3):227–232. doi: 10.1016/j.diabres.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 32.Monier L, Mas E, Ginet C, Michel F, Villon L, Cristol JP, Colette C. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA. 2006;295(14):1681–1687. doi: 10.1001/jama.295.14.1681. [DOI] [PubMed] [Google Scholar]
- 33.Takahashi S, Uchino H, Shimizu T, Kanazawa A, Tamura Y, Sakai K, Watada H, Hirose T, Kawamori R, Tanaka Y. Comparison of glycated albumin (GA) and glycated hemoglobin (HbA1c) in type 2 diabetic patients: usefulness of GA for evaluation for short-term changes in glycemic control. Endocr J. 2007;54(1):139–144. doi: 10.1507/endocrj.k06-103. [DOI] [PubMed] [Google Scholar]
- 34.Hicks JM, Haeckel R, Price CP, Lewandrowski K, Wu AH. Recommendations and opinions for the use of point-of-care testing for hospitals and primary care: summary of a 1999 symposium. Clin Chim Acta. 2001;303(1-2):1–17. doi: 10.1016/s0009-8981(00)00400-9. [DOI] [PubMed] [Google Scholar]
- 35.Inaba M, Okuno S, Kumeda Y, Yamada S, Imanishi Y, Tabata T, Okamura M, Okada S, Yamakawa T, Ishimura E, Nishizawa Y Osaka CKD Expert Research Group. Glycated albumin is a better glycemic indicator than glycated hemoglobin values in hemodialysis patients with diabetes: effect of anemia and erythropoietin injection. J Am Soc Nephrol. 2007;18(3):896–903. doi: 10.1681/ASN.2006070772. [DOI] [PubMed] [Google Scholar]
- 36.Peacock TP, Shihabi ZK, Bleyer AJ, Dolbare EL, Byers JR, Knovich MA, Calles-Escandon J, Russell GB, Freedman BI. Comparison of glycated albumin and hemoglobin A(1c) in diabetic subjects on hemodialysis. Kidney Int. 2008;73(9):1062–1068. doi: 10.1038/ki.2008.25. [DOI] [PubMed] [Google Scholar]
- 37.Baker JR, O'Connor JP, Metcalf PA, Lawson MR, Johnson RN. Clinical usefulness of estimation of serum fructosamine concentration as a screening test for diabetes mellitus. Br Med J (Clin Res Ed) 1983;287(6396):863–867. doi: 10.1136/bmj.287.6396.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shima K, Abe F, Chikakiyo H, Ito N. The relative value of glycated albumin, hemoglobin A1c and fructosamine when screening for diabetes mellitus. Diabetes Res Clin Pract. 1989;7(4):243–250. doi: 10.1016/0168-8227(89)90011-9. [DOI] [PubMed] [Google Scholar]
- 39.Guillausseau PJ, Charles MA, Paolaggi F, Timsit J, Chanson P, Peynet J, Godard V, Eschwege E, Rousselet F, Lubetzki Comparison of HbA1 and fructosamine in diagnosis of glucose-tolerance abnormalities. Diabetes Care. 1990;13(8):898–900. doi: 10.2337/diacare.13.8.898. [DOI] [PubMed] [Google Scholar]
- 40.Tsuji I, Nakamoto K, Hasegawa T, Hisashige A, Inawashiro H, Fukao A, Hisamichi S. Receiver operating characteristic analysis on fasting plasma glucose, HbA1c, and fructosamine on diabetes screening. Diabetes Care. 1991;14(11):1075–1077. doi: 10.2337/diacare.14.11.1075. [DOI] [PubMed] [Google Scholar]
- 41.Kasezawa N, Kiyose H, Ito K, Iwatsuka T, Kawai H, Goto Y, Kondo K, Sasamori N, Suzuki K, Suzuki T. Criteria for screening diabetes mellitus using serum fructosamine level and fasting plasma glucose level. The Japanese Society of Multiphasic Health Testing and Services (JMHT), Fructosamine Working Committee. Methods Inf Med. 1993;32(3):237–240. [PubMed] [Google Scholar]
- 42.Cefalu WT, Ettinger WH, Bell-Farrow AD, Rushing JT. Serum fructosamine as a screening test for diabetes in the elderly: a pilot study. J Am Geriatr Soc. 1993;41(10):1090–1094. doi: 10.1111/j.1532-5415.1993.tb06457.x. [DOI] [PubMed] [Google Scholar]
- 43.Narayanan S. Laboratory monitoring of gestational diabetes. Ann Clin Lab Sci. 1991;21(6):392–401. [PubMed] [Google Scholar]
- 44.Carter AW. Home fructosamine testing: is its demise premature? Diabetes Technol Ther. 2002;4(5):643–644. doi: 10.1089/152091502320798277. [DOI] [PubMed] [Google Scholar]