Abstract
Background
Glucagon counterregulation (GCR) is a key protection against hypoglycemia that is compromised in diabetes. In β-cell-deficient rats, GCR pulsatility can be amplified if insulin (INS) or somatostatin (SS) are infused in the pancreatic artery and then switched off during hypoglycemia. The data indicate that these signals act by different mechanisms, and here we analyze the differences between the two switch offs (SOs) and predict the GCR-amplifying effect of their individual or combined application.
Methods
A minimal control network (MCN) of α/δ-cell interactions is approximated by differential equations to explain the GCR response to a SO and test in silico the hypotheses: (i) INS SO suppresses basal and pulsatile, while SS SO blocks only pulsatile glucagon release and (ii) simultaneous application of the two switch offs will augment the individual GCR response.
Results
The mechanism postulated in (i) explains the differences in the GCR responses between the SOs. The MCN predicts that simultaneous application of INS and SS decreases basal glucagon but increases post-SO amplitude, thus doubling the response of GCR achieved by each of the individual signals.
Conclusion
The current analyses predict that INS and SS SOs improve defective GCR in β-cell deficiency through different but complementary mechanisms and suggest SO strategies to maximally enhance GCR in type 1 diabetes by simultaneous manipulation of the network control. These results are clinically relevant, as they could have application to design of an artificial pancreas by providing ways to augment GCR that would not require glucagon infusion.
Keywords: counterregulation, feedback, glucagon, hypoglycemia, intrapancreatic network, mathematical model
Introduction
Glucagon counterregulation (GCR) prevents dangerous glucose declines and is a key element in maintaining glucose homeostasis.1,2 It is impaired in insulin (INS)-dependent diabetes by an unknown mechanism, and this defect prevents the effective treatment of diabetes,3,4 especially when it is accompanied by a loss of epinephrine counterregulation. Therefore, a major challenge in the struggle to find a better treatment for type 1 diabetes is to understand the mechanisms of GCR compromise associated with β-cell deficiency. The so-called “switch-off” (SO) hypothesis posits that α-cell activation during hypoglycemia requires both the availability and rapid decline of intraislet INS.1 Thus the defects in GCR response to hypoglycemia in type 1 diabetes are attributed to loss of an INS SO inhibitory signal from the β cells that is a rapid termination of α-cell suppression by INS. Based on numerous prior reports5–10 suggesting network pancreatic regulation and recent in vitro and in vivo results supporting the SO hypothesis,11–14 we have recently suggested that an intrapancreatic minimal control network (MCN) of interactions relating to major islet cell types regulates the pancreatic output and can explain key elements of the GCR control.15 Our in vivo experiments and theoretical-modeling results supported and extended the SO hypothesis by demonstrating that, in streptozotocin-treated rats, the impaired GCR can be enhanced if INS or somatostatin (SS) are locally infused and switched off at hypoglycemia.15 Based on these results, we proposed that GCR develops, at least partially, as a pulsatile rebound response to the disinhibition of the α cells, which are under the control of at least one β-cell-independent feedback mechanism (operating within the postulated MCN). A direct consequence of these assumptions is the inference that any signal that can locally suppress the α-cell activity can augment the pulsatile GCR if it is rapidly removed during hypoglycemia.16
In our experiments,15 we observed some unexpected differences in the response to the two SO signals, INS, and SS. In particular, we detected approximately 30% higher absolute response to an INS SO as compared to SS SO, which was accompanied also with higher levels prior to SO (see Figure 3 in Reference 15). In this prior proof-of-concept study, the group sizes were quite small (N = 6 in both intervention groups) and both comparisons were not significant. However, the pre-SO level comparison was close to being significant at p = .07. Given these findings, the primary goal of the current study is to test in silico whether the previously mentioned differences (assuming that they exist) between the responses to the two signals can be explained in the framework of the postulated MCN and, in particular, whether the model proposed in Reference 15 can be modified in a way that it can still reproduce the key predictions for GCR control reported in Reference 15 and simultaneously propose a mechanism for the expected differences in the actions of the two SOs. Such an outcome would further support the concept that the postulated connectivity unifies major intrapancreatic system-level endocrine GCR control mechanisms. Establishing a theoretical basis for the differences in the two SO mechanisms would be clinically relevant since it will justify an experimental effort to explore possibilities for their simultaneous application. Accordingly, the secondary goal of this work is to use model-based analysis to predict whether the GCR-amplifying effects of the combined local infusion of INS and SS followed by their SO during hypoglycemia will be higher than the effect achieved by their individual application.
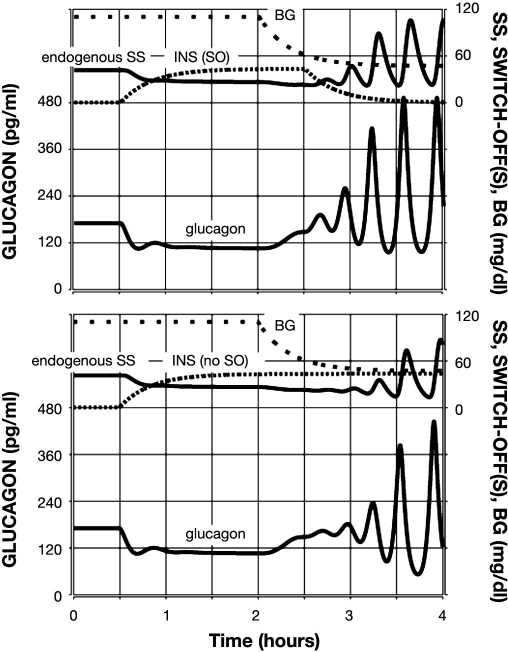
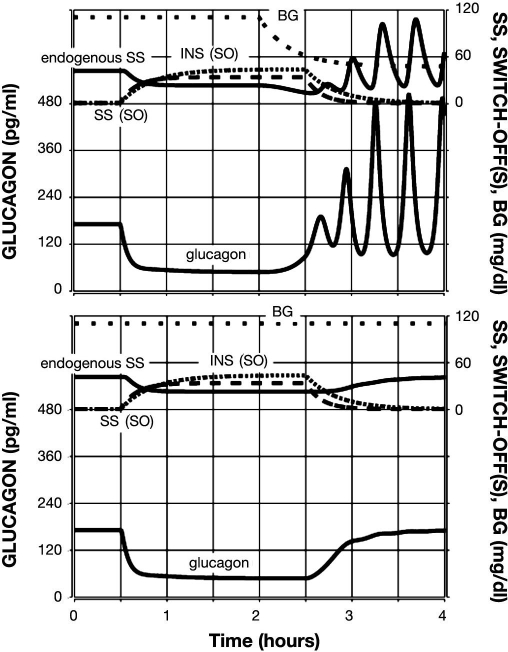
Figure 3.
Model-predicted GCR response to glucose decline and an intrapancreatic infusion of INS that has been switched off (top) or not switched off (bottom) during hypoglycemia. Glucagon is shown by the lower black line; endogenous SS is shown by the upper black line; INS infusion is represented by a densely dotted line; and BG is shown by a sparsely dotted line.
Methods
Review of the Glucagon Counterregulation Network Control
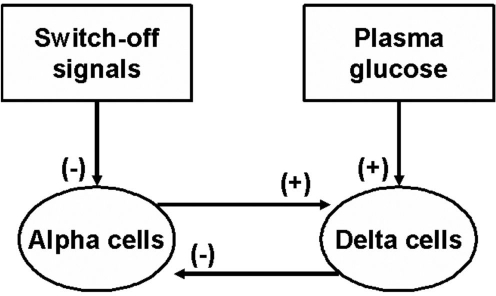
Figure 1 summarizes the postulation in Reference 15 MCN of glucagon secretion in β-cell deficiency via selected interactions between plasma glucose, SO signals (INS or SS), α cells, and δ cells.
Figure 1.
Minimal intrapancreatic regulation of GCR by SO signals in β-cell deficiency.
In brief, these relationships are based on experimental evidence reported in the following (for details see Reference 15):
δ-cell inhibition of α-cells: Multiple studies both in vivo and in vitro have shown this inhibitory relationship by SS of glucagon α-cell release;5,6,17–26
α-cell stimulation of δ-cells: A variety of studies indicate that glucagon stimulates release of SS;9,10,18,27–32
Glucose stimulation of δ-cells: Hyperglycemia has repeatedly been shown to increase SS secretion by δ-cells;29,33–35 and
Insulin inhibition of α-cells: Endogenous and exogenous INS inhibits α-cell glucagon release.7,36–39
Dynamic Approximation of the Minimal Control Network
Similar to Reference 15, we formalize the network shown in Figure 1 with a set of coupled delayed nonlinear differential equations that describe the rate of change of the system components and their interaction (see References 16, 40–43 for more details on modeling endocrine feedback networks). The model equations are:
| (1), |
| (2), |
Here, GL(t), SS(t), BG(t),I1(t), and I2(t) denote the concentrations of glucagon, SS, blood glucose (BG), and exogenous SO signal(s) (acting on the pulsatile or/and the basal glucagon secretion), respectively, and the derivative is with respect to time t. We note that Equation (1) differs from the analogous model equation in Reference 15 to reflect the assumption that different SO signals may have a different impact on glucagon secretion and may suppress the basal and/or δ-cell-regulated α-cell release differently (discussed later).
Model Specifics
In the construction of the model equations, we pursued the following strategy (see Reference 16 for more details). We assume that the rate of change of glucagon and SS depends on two processes: secretion and ongoing elimination. The elimination is assumed to be proportional to the concentration, and the effect is approximated by the terms -kGLGL and -kSSSS. Here, the coefficients kGL and kSS are called coefficients of elimination and are related to the half-lives of the corresponding hormones by the formula, (half-life) = Ln 2/(coefficients of elimination). The remaining terms in the right-hand side of the equations represent the secretion component. In the case of glucagon [Equation (1)], the secretion term has two parts: a SS-regulated component and a basal component (SS independent). In the case of SS [Equation (2)], there is no basal secretion, and the model assumes two independent components, the first of which positively regulated by glucagon and the second by glucose. The regulation of glucagon by SS is
which is a positive, nonlinear, sigmoid, decreasing function that many authors use to simulate negative regulation. In this formulation, it is assumed that SS exerts its effect with some lag (DSS). The coefficient tSS is called a threshold and corresponds to the half-maximal inhibitory dose (ID50) of SS. The parameter nSS controls the slope of the response and is called a Hill coefficient. The dose-response Hill function takes values from 0 to 1 and is multiplied by a parameter rGL, which is called the secretion rate and is related to the maximal attainable glucagon concentration by the formula, (maximal attainable glucagon concentration) = rGL / kGL. Analogously, the secretion rate of SS is presented by the sum of two stimulatory Hill functions multiplied by corresponding secretion rates, which mediates the (independent) stimulation of SS by glucagon and glucose. The thresholds tGL and tBG correspond to the half-maximal stimulatory doses (SD50) of glucagon and glucose, respectively. The Hill coefficients nGL and nBG model the slope of the corresponding dose-response effect. We also note that the action of glucagon on SS is assumed to be exerted after certain delay (DGL).
As noted earlier, both the basal and the regulated secretion components in Equation (1) are multiplied by
the model the suppressive effects of exogenous INS or SS exerted on glucagon (see the section titled Model-Based Simulations for more details).
Strategy for Model Parameter Determination
There is no sufficient experimental information to determine most of the model parameters. Therefore, here and also in Reference 15, they have been functionally determined to guarantee that the models can approximate several key experimental observations. In the case of our earlier model,15 these were restricted to
Glucagon pulsatility during hypoglycemia (after a SO) with pulses recurring approximately every 15–20 min as suggested by the results of the pulsatility deconvolution analysis performed in Reference 15, which showed that the SO groups have, on average, 2.4 (INS SO) and 2.6 (SS SO) pulses in the 45 min post-SO interval. Results from other laboratories also document pulsatility of pancreatic hormones during hypoglycemia.44
Pronounced (more than three-fold increase over baseline) pulsatile glucagon response following a SO of glucagon suppressing signals during hypoglycemia.
Restriction of the GCR enhancement by a SO signal by high glucose conditions.
Lack of GCR response to glucose decline in the face of an absent SO signal.
Suppression of pulsatile GCR if a glucagon-suppressing signal is intrapancreatically infused but not switched off during hypoglycemia.
Here, we chose the model parameters in a way that the model can still predict (i)–(v) and can also account for two new features of the GCR response suggested by the experimental observations reported in Reference 15:
vi. A 30% higher GCR response to INS SO versus SS SO.
vii. Better glucagon suppression by SS before the SO as compared to suppression by INS.
The half-life of glucagon was assumed to be approximately 2 min to match the results of the pulsatility analysis (kGL = 20h−1). The half-life of SS in the pancreas has been functionally determined to be longer than the half-life of glucagon (kSS = 10h−1). The delays in the system were functionally determined (together with the potencies and sensitivities discussed later) to guarantee that glucagon pulses occur at intervals of ∼20 min to correspond to the number of pulses after the SO point detected in the pulsatility analysis:15 DSS = DGL = 1.8 min. The remaining parameters used in the simulations were also determined functionally, and the concentrations presented are in arbitrary units. These units can, however, be easily rescaled to match real concentrations. In particular, we used release rates rSS = 400, bSS = 40, rGL = 80, and rbasal = 0.3 for the basal secretion of glucagon (concentration/h); potencies tBG = 65, tSS = 1.3, and tGL = 0.07 (concentration); sensitivities (Hill coefficients) nBG = 5, nSS = 3.2, and nGL = 3.4. To integrate the equations, we used a Runge–Kutta 4 algorithm and performed numerical simulations to test the system response to external SO signals that suppress and release α-cell activity under different conditions.
We note that some of the model parameters (including the functional half-life of SS) have been changed from our earlier model.15 These changes were necessary to guarantee that the model can approximate the new requirements (vi) and (vii). They also caused an alteration in the overall behavior of the system, which now oscillates only after the SO signal is removed and cannot oscillate on its own. To our knowledge, there is no experimental evidence to support either behavior, and here we decided to avoid the oscillations in the basal state to emphasize the fact that the feedback between glucagon and SS is important for the model to drive pulsatile secretion only during counterregulation (see Reference 44). Other factors may also contribute to the pulsatility of the pancreatic hormones, especially during the basal state.45–48
Model-Based Simulations
Simulation of Blood Glucose Decrease
To simulate euglycemia followed by hypoglycemia the rate of change of BG was approximated by
Rate of elimination = 3 h−1 and rate of secretion = 3 × 110 mg/dl/h or 3 × 47 mg/dl/h were chosen as parameters to provide gradual decrease in BG concentration to <50 mg/dl in a way that at the time of SO (t = 2.5), BG = 60 mg/dl. Thereby, the chosen model simulates a BG drop from 110 to 60 mg/dl in 30 min.
Simulation of Combined Infusion Experiments
Infusion of a SO signal was modeled by an additional equation (one for each SO signal): ISS′ = -kSS,IISS + rSS,I and/or IINS′ = -kINS,IIINS + rINS,I. The infusion rates rSS,I and rINS,I (concentration/hour) were assumed ≠ 0 only from t = 0.5 to 2.5. For the SS SO signal, we chose kSS,I = 6 and rSS,I = 600 (for 0.5 < t < 2.5), and for INS, kINS,I = 3 and rINS,I = 13 (for 0.5 < t < 2.5). The elimination and infusion rates (kSS,I, kINS,I and rSS,I, rINS,I, respectively) for each of the exogenous SO signals were functionally determined as follows. The apparent elimination rates were determined to guarantee gradual increase of the glucagon secretion following the SOs as observed in vivo.15 The infusion rates were determined to guarantee a difference in the pre-SO glucagon levels between INS and SS similar to the observed in the experiments.15 The functional half-life of the infused SO signals were assumed to be longer than the half-life of the endogenously released hormones, thereby accounting for possible slower delivery of the “functional” signal.
When infusion of a SS SO signal was modeled, we assumed I1 = 0 and I2 = ISS, which approximates the hypothesis that exogenous SS suppresses the pulsatile but not the basal glucagon release. In the case of INS SO, I1 = IINS and I2 = IINS/3. The formulation assumes that exogenous INS suppresses both the pulsatile and basal glucagon release (the latter inhibition is assumed three-fold less potent).
Finally, modeling the combined infusion of INS and SS SOs was straightforward: I1 = IINS and I2 = ISS + IINS/3.
We note that the approximation of the effect of exogenous SS on the system is based on the assumption that endogenous and exogenous SS has different mechanisms of regulating the α cells, and they do not necessarily occur in one and the same pool/compartment or by one and the same receptors. In fact, in the postulated network, endogenous SS represents the combined negative effect of the δ cells on the α cells and is required only to guarantee that the α cells are feedback regulated by a BG-stimulated pancreatic factor (see the discussion in Reference 15). It is possible that the δ cells control the α cells by common gap junctions49 and not by the secretion in the pancreatic circulation SS, which has been postulated to have limited effect on the α cells.7,8
To justify the difference in the postulated action between SS and INS SOs, we note the following. The concept of interpreting GCR as a pulsatile rebound based on reciprocal action between pancreatic glucagon and SS implies that a decrease in basal glucagon will effectively increase the glucagon pulsatile activity by allowing the glucagon-stimulated SS to reach lower nadirs. Therefore, in this work we assumed that the amplification of pulsatile GCR by INS SO is mediated by two system-level processes simultaneously: a rebound response of the system due to disinhibition and a release of the system by providing permissive SS levels due to decrease in basal glucagon. On the other hand, the enhancement of GCR by SS SO is assumed on a system level to be primarily a rebound response, which requires better suppression of glucagon secretion by the SO signal.
Results and Discussion
The results of the simulations are presented as follows. First, we have shown that the proposed MCN model, which has changed significantly since initially introduced in Reference 15, is still consistent with the important observation that in β-cell deficiency, the GCR response is defective. Next, we tested the model response to an intrapancreatic infusion of INS or SS signals that were either switched off or not. Then the GCR response to a combined INS plus SS SO signal was tested under two different circumstances: either with or without ongoing hypoglycemia. Finally, we tested the importance of the SO during hypoglycemia of any one of the two signals to the overall amplification of the GCR response by the combined SO.
Minimal Glucagon Counterregulation Response to Glucose Decline in the Face of Absent Switch-Off Signal in β-Cell Deficiency
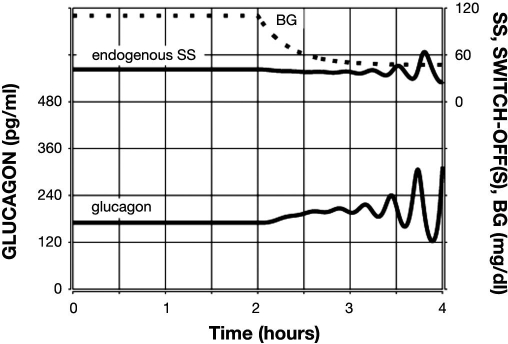
The plot in Figure 2 shows the predicted lack of glucagon response to hypoglycemia if a SO signal is missing—a key observation reported in our experimental results15 and elsewhere.11,13 The system responds with a less than 50% increase in the pulse amplitude of glucagon in the 1 h interval after BG reaches 60 mg/dl, which agrees with our experimental observations15 and shows that the model satisfies Condition (iv).
Figure 2.
Model-predicted lack of glucagon response to glucose decline alone in β-cell deficiency. Glucagon is shown by the lower black line; endogenous SS is shown by the upper black line; and BG is shown by a sparsely dotted line.
Glucagon Counterregulation Response to Insulin Switch-Off Signal
First, we tested the model response to a 2 h intra-pancreatic infusion of INS switched off at hypoglycemia (BG = 60 md/dl). The infusion was initiated at time t = 0.5 h (arbitrary time units) and switched-off at t = 2.5 h. A simulated gradual BG decline started at t = 2 h, and BG = 60 mg/dl at the SO point. The model response is depicted in Figure 3 (top panel) and illustrates a rebound glucagon secretion after the SO reaching an almost three-fold increase in glucagon in the 1 h period after the SO as compared to the pre-SO levels, which is similar to that experimentally observed in Reference 15. For comparison, Figure 3 (bottom panel) depicts the GCR response if the intrapancreatic INS was not switched off during hypoglycemia. In this case, the GCR response is delayed (∼30 min) and reduced an ∼1.5-fold increase in glucagon pulse amplitude achieved at the very end of the 1 h interval after BG reaches 60 mg/dl. This result agrees with the observations reported in Reference 11, which demonstrate a lack of significant increase in glucagon in this 1 h interval if INS is not switched off. Thus the model satisfies Conditions (i), (ii), and (v) in regards to INS. We also note that in Reference 11, the authors did not monitor the glucagon response beyond this 1 h interval. Therefore, the (delayed) GCR response during hypoglycemia in the simulation when intrapancreatic INS was not switched off should be considered as a model-based prediction.
Glucagon Counterregulation Response to a Somatostatin Switch-Off Signal
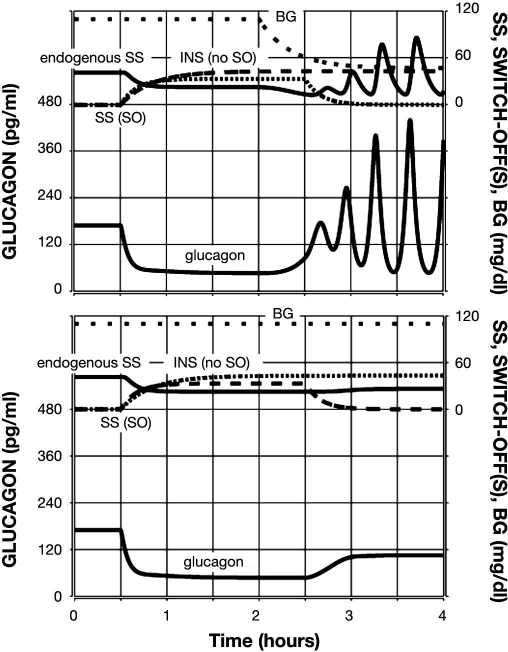
In a similar simulation, we tested the model response to an intrapancreatic infusion of SS, which was either switched off or not during hypoglycemia (BG = 60 md/dl). Figure 4 (top) summarizes the results and shows that this SO signal causes a similar three-fold increase in glucagon in the 1 h period after the SO as compared to the pre-SO levels. However, this was accompanied by almost 30% lower pre-SO levels as compared to the action of the INS intrapancreatic signal. The model also predicts an outcome that has not been tested experimentally, that if the signal were not switched off, the GCR response would be suppressed to a less than 50% increase in glucagon concentration [see Figure 4 (bottom)]. A comparison between the top panels in Figures 3 and 4 reveals that, even though the fold increase in the 1 h following the SO was the same with INS and SS, the absolute levels achieved in the same 1 h interval were approximately 25% higher with INS as compared to SS SO. These outcomes agree well with the reported15 differences in the responses to the two signals (Figure 3 in Reference 15). They also show that the model satisfies Conditions (i), (ii), and (v) in regards to SS and Conditions (vi) and (vii) in regards to the differences between INS and SS SO.
Figure 4.
Model-predicted GCR response to glucose decline and an intrapancreatic infusion of SS that has been switched off (top) or not switched off (bottom) during hypoglycemia. Glucagon is shown by the lower black line; endogenous SS is shown by the upper black line; exogenous SS infusion is shown by a dashed line; and BG is shown by a sparsely dotted line.
The following simulations should be considered as entirely model-predicted outcomes since experiments involving the simultaneous infusion of SO signals has not been performed yet.
Glucagon Counterregulation Response to a Combined Insulin and Somatostatin Switch-Off Signal
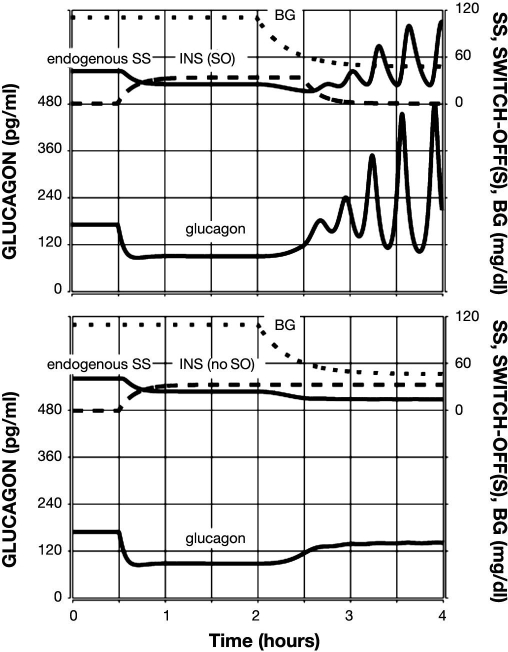
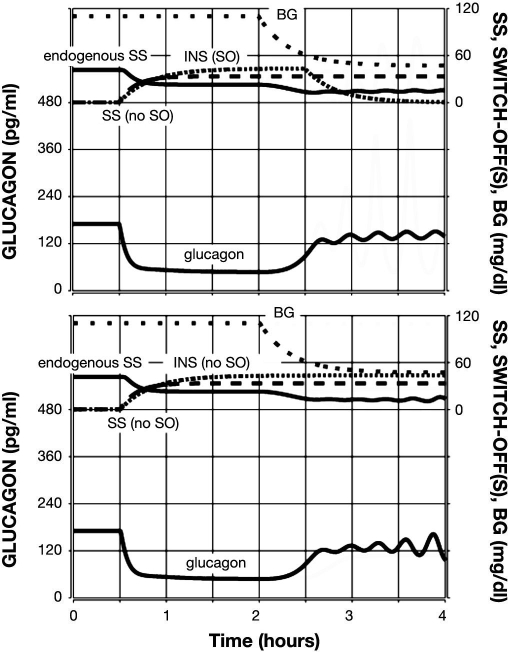
The model response to a combined intrapancreatic infusion of INS and SS switched off during hypoglycemia (BG = 60 md/dl) is illustrated in Figure 5 (top panel). The plot illustrates an obvious improvement in the fold GCR response as compared to the responses to individual signals: an almost six-fold increase in glucagon in the 1 h period after the SO as compared to the pre-SO concentration. This is due to a significant decrease in the pre-SO glucagon accompanied by an absolute response to the SO, which is higher than the responses to both of the individual SOs. The bottom panel of Figure 5 summarizes the approximately three-fold increase in response to a combined SO, assuming that no hypoglycemia was present. In this simulation, the SO causes the glucagon levels to return to the typical levels when no intrapancreatic signal is infused (from t = 0 to 0.5 h in all simulations). Therefore, this increase may not have biological meaning, given the relatively low absolute increase.
Figure 5.
Model-predicted GCR response to glucose decline (top) or lack of decline (bottom) and a combined INS and SS SO. Glucagon is shown by the lower black line; endogenous SS is shown by the upper black line; INS infusion is represented by a densely dotted line; exogenous SS infusion is shown by a dashed line; and BG is shown by a sparsely dotted line.
Glucagon Counterregulation Response to Somatostatin Switch-Off Signal in the Face of Constant Intrapancreatic Insulin Infusion
The simulations shown in Figure 3 (bottom panel) predict that if INS is intrapancreatically infused but not switched off at hypoglycemia, the GCR response during the first hour after hypoglycemia is suppressed. However, if BG remains low, after some lag glucagon, pulsatility develops almost to the same extent as in the SO case [Figure 3 (top)]. Our next two simulations are designed to test whether the additional application of SS SO can overcome the suppression of GCR due to the delay in glucagon increase caused by the persisting intra-pancreatic INS infusion. The model response is illustrated in Figure 6. The top panel shows that the infusion and SO of SS causes a left shift in time of the GCR response as compared to the GCR response depicted in Figure 3 (bottom). There is also an improvement in the fold GCR response relative to the pre-SO concentration due to the additional decrease in the pre-SO glucagon levels. The bottom panel of Figure 6 summarizes the GCR response to the same infusion/SO strategy but with no hypoglycemia. In this case, the model predicts less than a two-fold increase in glucagon following the SO, which forces glucagon to return to lower-than-typical concentrations due to the continuing suppression of α-cell secretion by the intrapancreatic INS infusion.
Figure 6.
Model-predicted GCR response to glucose decline (top) or lack of decline (bottom) and a combined INS and SS intrapancreatic infusion in which SS but not INS has been switched off during hypoglycemia. Glucagon is shown by the lower black line; endogenous SS is shown by the upper black line; INS infusion is represented by a densely dotted line; exogenous SS infusion is shown by a dashed line; and BG is shown by a sparsely dotted line.
Effective Glucagon Counterregulation Enhancement by a Combined Intrapancreatic Signal Requires Switch-Off of Somatostatin
In our final simulations, we tested whether the predicted suppression of GCR caused by a failure to SO intrapancreatic SS [Figure 4 (bottom)] can be overcome by additional infusion of intrapancreatic INS. Figure 7 summarizes the outcome in this experiment in which neither a SO (top panel) nor a lack of SO (bottom panel) of INS was able to effectively restore the GCR response.
Figure 7.
Model-predicted suppression of GCR response to glucose decline and a combined INS and SS intrapancreatic infusion in which INS but not SS has been switched off (top) or both have not been switched off (bottom) during hypoglycemia. Glucagon is shown by the lower black line; endogenous SS is shown by the upper black line; INS infusion is represented by a densely dotted line; exogenous SS infusion is shown by a dashed line; and BG is shown by a sparsely dotted line.
Discussion
At least three mechanisms have been proposed to explain the GCR:49 (i) glucose decline directly stimulates the α cells; (ii) the response is mediated by removal of an inhibitory INS β-cell signal (SO hypothesis); and (iii) central and/or local autonomic inputs direct α-cell activation. The decline of GCR parallels the progression of type 145,50,51 and (possibly) type 2 diabetes,52 but the underlying mechanism is not clearly delineated. The SO hypothesis1,53 attributes the defect in GCR in INS-deficient diabetes to loss of an INS SO signal from the β cells and is supported by recent in vivo and in vitro results.11–14 Our recent study15 supports the hypothesis that multiple α-cell inhibiting intrapancreatic signals switched off during hypoglycemia enhance the pulsatile GCR via a local feedback mechanism. The model-based analysis predicts that the mechanism behind the SO involves a rebound glucagon release triggered by the disinhibition of a putative α–δ cell feedback network as postulated in the MCN (Figure 1). In the experiments, we detected a nonsignificant ∼30% higher GCR response to an INS SO as compared to SS SO, and the pre-SO levels trended higher in the INS group (p = .07). In this work, we seek to explain these possible differences by postulating dissimilar MCN mechanisms of suppression of the α cells by INS and SS. In particular, exogenous INS was assumed to suppress both the pulsatile and basal glucagon release [(the last two terms in the right-hand side of Equation (1)], while exogenous SS was supposed to affect only the basal glucagon release [the second term in the right-hand side of Equation (1)]. To explore the consistency of this assumption with the experimental results, we utilized dynamic modeling to approximate the hypothesized connectivity. The specific construct differs in some of the system parameters from the construct initially presented,15 with changes required to describe the new physiological assumptions and guarantee that the model can approximate the new Requirements (vi) and (vii). They were also required since the impact of the exogenous SO signals on the MCN is modeled differently to approximate the major model assumption in this work that exogenous INS and SS exert their SO effect by different mechanisms. As a result of these parameter changes, the overall behavior of the system is now different form the behavior described by our earlier model. For example, now the system cannot oscillate on its own (timeline t = 0 to 0.5 in Figures 2–7) and requires the SO signal to be removed. To our knowledge, there is no sufficient experimental evidence to support either pulsatile behavior, and here we decided to avoid the model oscillations in the basal state to emphasize the fact that the feedback between glucagon and SS is important for the model to drive pulsatile secretion only during counterregulation (see Reference 44). Other factors may also contribute to the pulsatility of the pancreatic hormones, especially during the basal state,45–48 and they are not described by the current model.
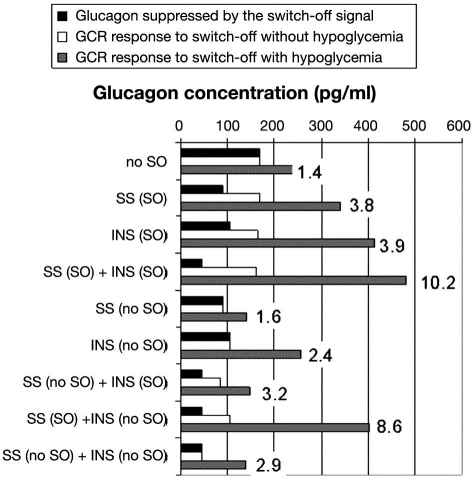
To illustrate the relationship between the responses to different SO strategies, we summarize in Figure 8 some of the outcomes in the model-assisted simulations presented earlier. At the top of the graph, we show baseline results without any SO signals. The black bar illustrates the glucagon level before t = 2 h, which is the time when glucagon would be maximally suppressed if a SO signal were present. The white and gray bars illustrate the maximal glucagon response in the 1 h interval from t = 2.5 to 3.5 h without (white) and with (gray) hypoglycemia stimulus. This interval corresponds to the 1 h interval after a SO in all other simulations. The black and white bars are the same since glucagon levels remain unchanged if there is no hypoglycemia. Each subsequent set of three bars indicates these effects with single SO, combined SO, and the mixture of SO and no SO for the two signals, INS and SS. Thus the bar graph gives the following glucagon concentrations: (i) glucagon suppressed by the intrapancreatic SO signal (black bars: the glucagon concentration immediately before the onset of BG decline at t = 2 h; at that time, glucagon is maximally suppressed by the intrapancreatic infusion and not affected by the decline in glucose); (ii) GCR response to a SO if hypoglycemia was not induced (white bars: the maximal glucagon concentrations achieved within a 1 h interval after the SO); and (iii) GCR response if hypoglycemia was induced (gray bars: the maximal glucagon concentrations achieved within a 1 h interval after the SO). The graph also includes the maximal fold increase in glucagon in response to a SO during hypoglycemia relative to the glucagon levels before the onset of BG decline. Note that we present the fold increase differently than in our earlier paper,15 where the comparison was relative to the glucagon levels at the time of SO, which are higher than the levels before the onset of BG decline.
Figure 8.
Summary of the model-predicted GCR responses to different SO signals with or without simulated hypoglycemia. The term no SO means the signal was not switched off.
Since the current model is different from the initial construct,15 our first simulation establishes that the new model still predicts a minimal GCR response to hypoglycemia if a SO signal is absent (Figure 2). As shown in Figure 8 (the top group of bars: no SO), the model predicts that if there is no SO, the GCR response is only an approximately 1.4-fold increase in glucagon levels in the 1 h period following the point at which BG reaches 60 mg/dl, relative to the levels before the onset of BG decline. This outcome is consistent with that previously observed11,15 and shows a minimal GCR response to a saline SO, which confirms that the current modified model is consistent with the experimental observations. We also point out that comparisons between the black and white bars in the second and third bar groups in Figure 8 show that the model simulations predict restriction of the GCR enhancement by a SO signal by high-glucose conditions [thus satisfying Condition (iii)], which, in the case of INS, agrees with the available experimental data.11
In the mathematical (in silico) simulation of the GCR response to each of the two individual SOs, INS and SS, the hypothesized differences in the action between these signals resulted in different post-SO increases in GCR and pre-SO decreases in glucagon as previously observed (Figure 3 in Reference 15): (i) glucagon concentration at the time of SO of INS (t = 2.5 h) is similar to the glucagon levels before the infusion of the intrapancreatic signal (beginning at t = 0.5 h) (Figure 3); (ii) glucagon concentration at the time of SO of INS is higher than the glucagon concentration at the time of SO of SS (Figure 4); and (iii) an approximately 20% higher maximal GCR response to INS versus SS SO occurs in the 1 h interval after the SO (Figure 8) [compare the gray bars INS (SO) versus SS(SO)].
The current model appears to represent an adequate extension of the MCN construct previously proposed,15 because it meets two criteria: (i) it continues to explain key features of the GCR response established experimentally by our laboratory15 and others,11 which were explained by the earlier model [Conditions (i)–(vi)]; and (ii) additionally, it accounts for the specific GCR responses to individual SO signals [Conditions (vi) and (vii)]. The assumed mechanism of action of exogenous INS and SS predicts one possible explanation for the differences observed in the experiments, but other scenarios may be possible. For example, one may try to approximate the differences in the responses to different SOs by assuming unequal functional half-life of the exogenous signal. In this regard, a longer half-life might result in a delayed GCR after the SO. At this point, experimental data does not exist to precisely delineate the exact intrapancreatic actions of different SO signals, and future studies involving simultaneous frequent portal sampling for the major islet peptides would be required to address this issue. Thus our MCN model not only replicates experimental findings, but it predicts that additional experiments are required to precisely show the involved mechanisms.
The model-predicted GCR enhancement by a simultaneous infusion and SO of INS and SS [Figure 5 (top)] reveals that it is expected that the double SO will combine two clinically relevant outcomes: marked suppression of glucagon during euglycemia and robust GCR response to hypoglycemia if the SO coincides with low BG levels. In view of possible clinical applications, it is critical that the amplitude of the SO-triggered GCR response is glucose dependent. This is evident in the lower panel of Figure 5, which shows that if a double SO occurs during euglycemia, the GCR response will be markedly suppressed. The prediction is significant, given the possibility that a glucose detection device of an artificial pancreas may incorrectly signal a low BG event while the actual glucose concentrations are normal (or even high). As shown in Figure 8 [SS(SO) + INS(SO)], the fold increase with respect to the glucagon concentrations suppressed by both high glucose and intrapancreatic infusion of the combined signal is more than ten-fold, which is approximately 2.5-fold higher than the GCR enhancement achieved by each of the individual SOs [SS(SO) or INS(SO)]. Accordingly, the combined SO strategy is an attractive GCR enhancement strategy. An alternative scenario, in which INS and SS are infused intrapancreatically but only SS is switched off during hypoglycemia, will guarantee the same suppression of glucagon during normoglycemia and similar enhancement of GCR by the SO as compared to the double SO strategy (Figure 8). In addition, the GCR response to a SO during euglycemia will be approximately 50% lower than the response to the double SO, which could further improve the dose-dependent control of GCR by plasma glucose. A strategy for GCR enhancement based on infusion of INS and SS and SO of only INS (or lack of SO) is clearly inappropriate due to its failure to evoke significant increase in glucagon (Figure 8). Clearly a SO of INS cannot overcome the repression exerted on the system by SS.
The possibility that INS and SS SOs act via different mechanisms could present clinically relevant combined SO strategies for enhancement of defective GCR in INS-deficient diabetes. In particular, not only is glucagon important for hypoglycemia defense, it is also implicated in hyperglycemia, because it is inappropriately elevated in patients with diabetes.54–56 In recent years, treatment strategies that involve computer control of INS pumps in “artificial pancreas” and “closed-loop systems” started to emerge. In some studies, glucagon was suggested as a protection against hypoglycemia,57 and it is thought that reduction of inappropriate hyperglucagonemia may be part of the therapeutic action of some drugs such as incretins. However, adding glucagon in pharmacological doses may increase system instability by contributing to hyperglycemia, which could potentially worsen INS resistance, require increased amounts of exogenous INS, and render the system difficult to control. Thus it would be important for control algorithms in an artificial pancreas to restore glucagon secretion to a more normal state during both hyper- and hypoglycemia. Therefore, developing strategies that combine suppression of endogenous glucagon during hyper- and euglycemia with strong endogenous glucose-dependent GCR response to hypoglycemia merit serious consideration. Achieving this goal by a combination of signals may have the advantage that lower doses of each signal can be used. This may permit their pharmacological application and avoid systemic complications and, ultimately, the need of intrapancreatic delivery. In this regard, however, there are certain important challenges related to close-loop systems that rely on subcutaneous delivery. In the context of the concepts presented here, they are mainly related to the inability to promptly terminate the impact of the infused signals that continues to be delivered from the subcutaneous tissue even after the pump has stopped the infusion. Mechanistically, this is equivalent to an increase of the effective half-life of the signal and will attenuate the rebound after a SO. Therefore, in future studies, attention should be also placed on the impact of the dynamics of the withdrawal of the SO signal on the GCR enhancement.
Conclusions
This work continues our “hybrid” approach to the investigation of the GCR mechanisms in β-cell deficiency initiated in Reference 15, which combined experimental and modeling studies. Here the focus is placed on a model-based analysis of the experimentally detected differences in the actions of two intrapancreatic signals, INS and SS, as they both individually enhance the glucagon response to hypoglycemia after a SO. The analysis shows that the difference between the two SO signals can be explained on a system level within the framework of a postulated MCN of glucagon release. This further supports the concept that the postulated connectivity unifies major GCR control mechanisms. The construct was used further to compare different strategies of manipulating the intrapancreatic network to enhance the defective GCR response in β-cell deficiency by a SO. A good potential of a combined SO to amplify the benefits provided by each of the individual signals was demonstrated. Therefore, the results support the hypothesis that α-cell inhibiting intrapancreatic signals switched off during hypoglycemia may act in concert to enhance the pulsatile GCR via a local feedback mechanism. The predictions are clinically relevant as they could have application to artificial pancreas design by providing combined strategies to augment GCR that would not require glucagon infusion.
Abbreviations
- BG
blood glucose
- GCR
glucagon counterregulation
- INS
insulin
- MCN
minimal control network
- SO
switch off
- SS
somatostatin
References
- 1.Cryer PE, Gerich JE. Relevance of glucose counterregulatory systems to patients with diabetes: critical roles of glucagon and epinephrine. Diabetes Care. 1993;6(1):95–99. doi: 10.2337/diacare.6.1.95. [DOI] [PubMed] [Google Scholar]
- 2.Gerich JE. Lilly lecture 1988. Glucose counterregulation and its impact on diabetes mellitus. Diabetes. 1988;37(12):1608–1617. doi: 10.2337/diab.37.12.1608. [DOI] [PubMed] [Google Scholar]
- 3.Cryer PE. Hypoglycemia is the limiting factor in the management of diabetes. Diabetes Metab Res Rev. 1999;15(1):42–46. doi: 10.1002/(sici)1520-7560(199901/02)15:1<42::aid-dmrr1>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 4.Cryer PE. Hypoglycemia: the limiting factor in the glycaemic management of type I and type II diabetes. Diabetologia. 2002;45(7):937–948. doi: 10.1007/s00125-002-0822-9. [DOI] [PubMed] [Google Scholar]
- 5.Brelje TC, Scharp DW, Sorenson RL. Three-dimensional imaging of intact isolated islets of Langerhans with confocal microscopy. Diabetes. 1989;38(6):808–814. doi: 10.2337/diab.38.6.808. [DOI] [PubMed] [Google Scholar]
- 6.Kleinman R, Gingerich R, Wong H, Walsh J, Lloyd K, Ohning G, De Giorgio R, Sternini C, Brunicardi FC. Use of the Fab fragment for immunoneutralization of somatostatin in the isolated perfused human pancreas. Am J Surg. 1994;167(1):114–119. doi: 10.1016/0002-9610(94)90061-2. [DOI] [PubMed] [Google Scholar]
- 7.Samols E, Stagner JI. Intra-islet regulation. Am J Med. 1988;85(5A):31–35. doi: 10.1016/0002-9343(88)90395-6. [DOI] [PubMed] [Google Scholar]
- 8.Samols E, Stagner JI. Islet somatostatin—microvascular, paracrine, and pulsatile regulation. Metabolism. 1990;39(9 Suppl 2):55–60. doi: 10.1016/0026-0495(90)90212-u. [DOI] [PubMed] [Google Scholar]
- 9.Stagner JI, Samols E, Marks V. The anterograde and retrograde infusion of glucagon antibodies suggests that A cells are vascularly perfused before D cells within the rat islet. Diabetologia. 1989;32(3):203–206. doi: 10.1007/BF00265095. [DOI] [PubMed] [Google Scholar]
- 10.Stagner JI, Samols E, Bonner-Weir S. beta—-alpha—-delta pancreatic islet cellular perfusion in dogs. Diabetes. 1988;37(12):1715–1721. doi: 10.2337/diab.37.12.1715. [DOI] [PubMed] [Google Scholar]
- 11.Zhou H, Tran PO, Yang S, Zhang T, LeRoy E, Oseid E, Robertson RP. Regulation of alpha-cell function by the beta-cell during hypoglycemia in Wistar rats: the “switch-off” hypothesis. Diabetes. 2004;53(6):1482–1487. doi: 10.2337/diabetes.53.6.1482. [DOI] [PubMed] [Google Scholar]
- 12.Hope KM, Tran PO, Zhou H, Oseid E, Leroy E, Robertson RP. Regulation of alpha-cell function by the beta-cell in isolated human and rat islets deprived of glucose: the “switch-off” hypothesis. Diabetes. 2004;53(6):1488–1495. doi: 10.2337/diabetes.53.6.1488. [DOI] [PubMed] [Google Scholar]
- 13.Zhou H, Zhang T, Oseid E, Harmon J, Tonooka N, Robertson RP. Reversal of defective glucagon responses to hypoglycemia in insulin-dependent autoimmune diabetic BB rats. Endocrinology. 2007;148(6):2863–2869. doi: 10.1210/en.2006-1375. [DOI] [PubMed] [Google Scholar]
- 14.Zhou H, Zhang T, Harmon JS, Bryan J, Robertson RP. Zinc, not insulin, regulates the rat α-cell response to hypoglycemia in vivo. Diabetes. 2007;56(4):1107–1112. doi: 10.2337/db06-1454. [DOI] [PubMed] [Google Scholar]
- 15.Farhy LS, Du Z, Zeng Q, Veldhuis PP, Johnson ML, Brayman KL, McCall AL. Amplification of pulsatile glucagon counterregulation by switch-off of alpha-cell-suppressing signals in streptozotocin-treated rats. Am J Physiol Endocrinol Metab. 2008;295(3):E575–E585. doi: 10.1152/ajpendo.90372.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Farhy LS. Modeling of oscillations in endocrine networks with feedback. Methods Enzymol. 2004;384:54–81. doi: 10.1016/S0076-6879(04)84005-9. [DOI] [PubMed] [Google Scholar]
- 17.Brunicardi FC, Atiya A, Moldovan S, Lee TC, Fagan SP, Kleinman RM, Adrian TE, Coy DH, Walsh JH, Fisher WE. Activation of somatostatin receptor subtype 2 inhibits insulin secretion in the isolated perfused human pancreas. Pancreas. 2003;27(4):e84–e89. doi: 10.1097/00006676-200311000-00019. [DOI] [PubMed] [Google Scholar]
- 18.Brunicardi FC, Kleinman R, Moldovan S, Nguyen TH, Watt PC, Walsh J, Gingerich R. Immunoneutralization of somatostatin, insulin, and glucagon causes alterations in islet cell secretion in the isolated perfused human pancreas. Pancreas. 2001;23(3):302–308. doi: 10.1097/00006676-200110000-00012. [DOI] [PubMed] [Google Scholar]
- 19.Cejvan K, Coy DH, Efendic S. Intra-islet somatostatin regulates glucagon release via type 2 somatostatin receptors in rats. Diabetes. 2003;52(5):1176–1181. doi: 10.2337/diabetes.52.5.1176. [DOI] [PubMed] [Google Scholar]
- 20.Klaff LJ, Taborsky GJ., Jr. Pancreatic somatostatin is a mediator of glucagon inhibition by hyperglycemia. Diabetes. 1987;36(5):592–596. doi: 10.2337/diab.36.5.592. [DOI] [PubMed] [Google Scholar]
- 21.Kleinman R, Gingerich R, Ohning G, Wong H, Olthoff K, Walsh J, Brunicardi FC. The influence of somatostatin on glucagon and pancreatic polypeptide secretion in the isolated perfused human pancreas. Int J Pancreatol. 1995;18(1):51–57. doi: 10.1007/BF02825421. [DOI] [PubMed] [Google Scholar]
- 22.Ludvigsen E, Olsson R, Stridsberg M, Janson ET, Sandler S. Expression and distribution of somatostatin receptor subtypes in the pancreatic islets of mice and rats. J Histochem Cytochem. 2004;52(3):391–400. doi: 10.1177/002215540405200310. [DOI] [PubMed] [Google Scholar]
- 23.Portela-Gomes GM, Stridsberg M, Grimelius L, Oberg K, Janson ET. Expression of the five different somatostatin receptor subtypes in endocrine cells of the pancreas. Appl Immunohistochem Mol Morphol. 2000;8(2):126–132. doi: 10.1097/00129039-200006000-00007. [DOI] [PubMed] [Google Scholar]
- 24.Schuit FC, Derde MP, Pipeleers DG. Sensitivity of rat pancreatic A and B cells to somatostatin. Diabetologia. 1989;32(3):207–212. doi: 10.1007/BF00265096. [DOI] [PubMed] [Google Scholar]
- 25.Strowski MZ, Parmar RM, Blake AD, Schaeffer JM. Somatostatin inhibits insulin and glucagon secretion via two receptors subtypes: an in vitro study of pancreatic islets from somatostatin receptor 2 knockout mice. Endocrinology. 2000;141(1):111–117. doi: 10.1210/endo.141.1.7263. [DOI] [PubMed] [Google Scholar]
- 26.Tirone TA, Norman MA, Moldovan S, DeMayo FJ, Wang XP, Brunicardi FC. Pancreatic somatostatin inhibits insulin secretion via SSTR-5 in the isolated perfused mouse pancreas model. Pancreas. 2003;26(3):e67–e73. doi: 10.1097/00006676-200304000-00025. [DOI] [PubMed] [Google Scholar]
- 27.Brunicardi FC, Elahi D, Andersen DK. Splanchnic neural regulation of somatostatin secretion in the isolated perfused human pancreas. Ann Surg. 1994;219(3):258–266. doi: 10.1097/00000658-199403000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dumonteil E, Magnan C, Ritz-Laser B, Ktorza A, Meda P, Philippe J. Glucose regulates proinsulin and prosomatostatin but not proglucagon messenger ribonucleic acid levels in rat pancreatic islets. Endocrinology. 2000;141(1):174–180. doi: 10.1210/endo.141.1.7230. [DOI] [PubMed] [Google Scholar]
- 29.Epstein S, Berelowitz M, Bell NH. Pentagastrin and glucagon stimulate serum somatostatin-like immunoreactivity in man. J Clin Endocrinol Metab. 1980;51(6):1227–1231. doi: 10.1210/jcem-51-6-1227. [DOI] [PubMed] [Google Scholar]
- 30.Hayashi M, Yamada H, Uehara S, Morimoto R, Muroyama A, Yatsushiro S, Takeda J, Yamamoto A, Moriyama Y. Secretory granule-mediated co-secretion of L-glutamate and glucagon triggers glutamatergic signal transmission in islets of Langerhans. J Biol Chem. 2003;278(3):1966–1974. doi: 10.1074/jbc.M206758200. [DOI] [PubMed] [Google Scholar]
- 31.Tapia-Arancibia L, Astier H. Glutamate stimulates somatostatin release from diencephalic neurons in primary culture. Endocrinology. 1988;123(5):2360–2366. doi: 10.1210/endo-123-5-2360. [DOI] [PubMed] [Google Scholar]
- 32.Utsumi M, Makimura H, Ishihara K, Morita S, Baba S. Determination of immunoreactive somatostatin in rat plasma and responses to arginine, glucose and glucagon infusion. Diabetologia. 1979;17(5):319–323. doi: 10.1007/BF01235888. [DOI] [PubMed] [Google Scholar]
- 33.Fujitani S, Ikenoue T, Akiyoshi M, Maki T, Yada T. Somatostatin and insulin secretion due to common mechanisms by a new hypoglycemic agent, A-4166, in perfused rat pancreas. Metabolism. 1996;45(2):184–189. doi: 10.1016/s0026-0495(96)90051-7. [DOI] [PubMed] [Google Scholar]
- 34.Göpel SO, Kanno T, Barg S, Rorsman P. Patch-clamp characterisation of somatostatin-secreting -cells in intact mouse pancreatic islets. J Physiol. 2000;528(Pt 3):497–507. doi: 10.1111/j.1469-7793.2000.00497.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hermansen K, Christensen SE, Orskov H. Characterization of somatostatin release from the pancreas: the role of potassium. Scand J Clin Lab Invest. 1979;39(8):717–722. doi: 10.3109/00365517909108162. [DOI] [PubMed] [Google Scholar]
- 36.Ishihara H, Maechler P, Gjinovci A, Herrera PL, Wollheim CB. Islet β-cell secretion determines glucagon release from neighboring α-cells. Nat Cell Biol. 2003;5:330–335. doi: 10.1038/ncb951. [DOI] [PubMed] [Google Scholar]
- 37.Ito K, Maruyama H, Hirose H, Kido K, Koyama K, Kataoka K, Saruta T. Exogenous insulin dose-dependently suppresses glucopenia-induced glucagon secretion from perfused rat pancreas. Metabolism. 1995;44(3):358–362. doi: 10.1016/0026-0495(95)90166-3. [DOI] [PubMed] [Google Scholar]
- 38.Ravier MA, Rutter GA. Glucose or insulin, but not zinc ions, inhibit glucagon secretion from mouse pancreatic alpha cells. Diabetes. 2005;54(6):1789–1797. doi: 10.2337/diabetes.54.6.1789. [DOI] [PubMed] [Google Scholar]
- 39.Van Schravendijk CF, Foriers A, Van den Brande JL, Pipeleers DG. Evidence for the presence of type I insulin-like growth factor receptors on rat pancreatic A and B cells. Endocrinology. 1987;121(5):1784–1788. doi: 10.1210/endo-121-5-1784. [DOI] [PubMed] [Google Scholar]
- 40.Farhy LS, Straume M, Johnson ML, Kovatchev B, Veldhuis JD. A construct of interactive feedback control of the GH axis in the male. Am J Physiol Regul Integr Comp Physiol. 2001;281(1):R38–R51. doi: 10.1152/ajpregu.2001.281.1.R38. [DOI] [PubMed] [Google Scholar]
- 41.Farhy LS, Straume M, Johnson ML, Kovatchev B, Veldhuis JD. Unequal autonegative feedback by GH models the sexual dimorphism in GH secretory dynamics. Am J Physiol Regul Integr Comp Physiol. 2002;282(3):R753–R764. doi: 10.1152/ajpregu.00407.2001. [DOI] [PubMed] [Google Scholar]
- 42.Farhy LS, Veldhuis JD. Putative GH pulse renewal: periventricular somatostatinergic control of an arcuate-nuclear somatostatin and GH-releasing hormone oscillator. Am J Physiol Regul Integr Comp Physiol. 2004;286(6):R1030–R1042. doi: 10.1152/ajpregu.00473.2003. [DOI] [PubMed] [Google Scholar]
- 43.Farhy LS, Veldhuis JD. Joint pituitary-hypothalamic and intrahypothalamic autofeedback construct of pulsatile growth hormone secretion. Am J Physiol Regul Integr Comp Physiol. 2003;285(5):R1240–R1249. doi: 10.1152/ajpregu.00086.2003. [DOI] [PubMed] [Google Scholar]
- 44.Genter P, Berman N, Jacob M, Ipp E. Counterregulatory hormones oscillate during steady-state hypoglycemia. Am J Physiol. 1998;275(5 Pt 1):E821–E829. doi: 10.1152/ajpendo.1998.275.5.E821. [DOI] [PubMed] [Google Scholar]
- 45.Bergsten P. Pathophysiology of impaired pulsatile insulin release. Diabetes Metab Res Rev. 2000;16(3):179–191. doi: 10.1002/1520-7560(200005/06)16:3<179::aid-dmrr115>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 46.Bergsten P. Role of oscillations in membrane potential, cytoplasmic Ca2+, and metabolism for plasma insulin oscillations. Diabetes. 2002;51(Suppl 1):S171–S176. doi: 10.2337/diabetes.51.2007.s171. [DOI] [PubMed] [Google Scholar]
- 47.Berts A, Liu YJ, Gylfe E, Hellman B. Oscillatory Ca2+ signaling in somatostatin-producing cells from the human pancreas. Metabolism. 1997;46(4):366–369. doi: 10.1016/s0026-0495(97)90048-2. [DOI] [PubMed] [Google Scholar]
- 48.Jaspan JB, Lever E, Polonsky KS, Van Cauter E. In vivo pulsatility of pancreatic islet peptides. Am J Physiol. 1986;251(2 Pt 1):E215–E226. doi: 10.1152/ajpendo.1986.251.2.E215. [DOI] [PubMed] [Google Scholar]
- 49.Taborsky GJ, Jr., Ahrén B, Havel PJ. Autonomic mediation of glucagon secretion during hypoglycemia: implications for impaired alpha-cell responses in type 1 diabetes. Diabetes. 1998;47(7):995–1005. doi: 10.2337/diabetes.47.7.995. [DOI] [PubMed] [Google Scholar]
- 50.Gerich JE, Langlois M, Noacco C, Karam JH, Forsham PH. Lack of glucagon response to hypoglycemia in diabetes: evidence for an intrinsic pancreatic alpha cell defect. Science. 1973;182(108):171–173. doi: 10.1126/science.182.4108.171. [DOI] [PubMed] [Google Scholar]
- 51.Hoffman RP, Arslanian S, Drash AL, Becker DJ. Impaired counterregulatory hormone responses to hypoglycemia in children and adolescents with new onset IDDM. J Pediatr Endocrinol. 1994;7(3):235–244. doi: 10.1515/jpem.1994.7.3.235. [DOI] [PubMed] [Google Scholar]
- 52.Segel SA, Paramore DS, Cryer PE. Hypoglycemia-associated autonomic failure in advanced type 2 diabetes. Diabetes. 2002;51(3):724–733. doi: 10.2337/diabetes.51.3.724. [DOI] [PubMed] [Google Scholar]
- 53.Banarer S, McGregor VP, Cryer PE. Intraislet hyperinsulinemia prevents the glucagon response to hypoglycemia despite an intact autonomic response. Diabetes. 2002;51(4):958–965. doi: 10.2337/diabetes.51.4.958. [DOI] [PubMed] [Google Scholar]
- 54.Müller WA, Faloona GR, Unger RH. Hyperglucagonemia in diabetic ketoacidosis. Its prevalence and significance. Am J Med. 1973;54(1):52–57. doi: 10.1016/0002-9343(73)90083-1. [DOI] [PubMed] [Google Scholar]
- 55.Reaven GM, Chen YD, Golay A, Swislocki AL, Jaspan JB. Documentation of hyperglucagonemia throughout the day in nonobese and obese patients with noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab. 1987;64(1):106–110. doi: 10.1210/jcem-64-1-106. [DOI] [PubMed] [Google Scholar]
- 56.Unger RH, Orci L. Glucagon and the A cell: physiology and pathophysiology. N Engl J Med. 1981;304(25):1518–1524. doi: 10.1056/NEJM198106183042504. [DOI] [PubMed] [Google Scholar]
- 57.El-Khatib FH, Jiang J, Gerrity RG, Damiano ER. Pharmacodynamics and stability of subcutaneously infused glucagon in a type 1 diabetic swine model in vivo. Diabetes Technol Ther. 2007;9(2):135–144. doi: 10.1089/dia.2006.0006. [DOI] [PubMed] [Google Scholar]