Abstract
There is growing recognition that obesity is common and represents a significant detriment to the health of companion animals in a manner similar to that by which it is affecting the human population. As is the case for other species, obesity appears to promote insulin resistance in horses and it is through this pathophysiological process that many of the adverse medical consequences of obesity are being characterized. Equine medical conditions that have been described in the context of obesity and insulin resistance differ from those in humans. Chronic human conditions that have been attributed to obesity and insulin resistance, such as atherosclerosis and diabetes mellitus, are rarely described in obese horses. Significant current interest is centered on the recognition that insulin resistance plays a role in the pathogenesis of laminitis, a potentially severe and debilitating cause of lameness in the equine species. Other equine medical conditions that are more likely in obese, insulin-resistant individuals include hyperlipemia (hepatic lipidosis) and developmental orthopedic disease (osteochondrosis). Pituitary pars intermedia dysfunction (equine Cushing's syndrome) represents another common endocrinopathic condition of older horses associated with insulin resistance. This review presents an introductory overview of the present understanding of obesity and insulin resistance and how these conditions may be associated with disease conditions in horses.
Keywords: Cushing's, horse, insulin resistance, laminitis, nutrition, obesity, pituitary gland
Obesity
In parallel with the human obesity epidemic, there is increasing recognition that obesity is common to many domestic animal species, including horses.1–3 Moreover, the reasons that domesticated animals develop obesity are broadly similar to those reasons that have been attributed to obesity in humans.4 Simply stated, contemporary husbandry practices are characterized by the provision of energy-rich rations to physically inactive horses. This short review is intended to highlight aspects of equine obesity and to draw contrasts between the medical risks associated with obesity in horses and humans.
Thriftiness
As a herbivorous species, horses evolved with a reliance on grass forage for their nutritional requirements. Accordingly, during the fall season, horses ingest increasing quantities of available forage and gain adiposity in preparation for the winter season when food tends to be relatively scarce. Stimulated appetite and adipogenesis at this time, along with the acquisition of a thick hair coat, are kindled in herbivores by an increase in the secretion of proopiomelanocortin (POMC) peptides from the hypothalamic–pituitary axis.5,6 These changes represent a critical survival mechanism that affords the provision of (stored) energy, in the form of body fat, throughout the winter months.
This seasonal outpouring of POMC peptides represents an important difference between herbivores and humans. In contrast to the human pituitary gland, there is a well-developed “intermediate lobe” (pars intermedia, PI) in the pituitary gland of herbivorous species.7 This PI plays a critical role in the endocrinological management of body condition in concert with seasonally dependent shifts in the availability of forage.5,6
In nature, the period of environmental harshness is finite and the acquired fat stores should be depleted prior to the onset of spring and the growth of new grass. In the healthy state, the acquisition of adipose tissue is therefore important for survival, but the acquisition of excessive adiposity and its chronic persistence exert diverse adverse effects on the health of the individual.8 Both insulin resistance (IR) and the development of a mild-to-moderate proinflammatory state have been regarded as key components of the survival mechanism during this limited period of environmental harshness and both appear to develop and resolve in parallel with the acquisition and depletion of additional adiposity at the onset and conclusion of winter.
Some animals, by virtue of the impositions of natural selection, have inherited genetic traits that have facilitated their survival through periods of environmental harshness. These animals are said to have inherited “thrifty genes.”9 Although it is likely that multiple diverse physiological processes contribute to the concept of thriftiness, IR appears to be an important component. A good example of the effect of natural selection on thriftiness is the Ossabaw Island swine.10
Following abandonment on a barrier island off the coast of Georgia (Ossabaw Island) by Spanish colonists approximately 500 years ago, isolated swine evolved a thrifty genotype in order to survive seasonal cycles of feasting and famine.11 When allowed to consume excess food in “modern” captivity, these Ossabaw Island swine quickly develop the highest levels of total body lipid of any mammal, IR and impaired glucose tolerance, hypertriglyceridemia, and hypercholesterolemia when compared to lean Ossabaw and domestic swine.12–14 Ossabaw Island swine develop coronary atherosclerotic lesions that are virtually indistinguishable from lesions in humans.15 To that end, these swine are currently being investigated as “large animal” models of metabolic syndrome and diabetes with relevance to human medicine.
The extent to which different breeds of horses have inherited thrifty genes is unknown, but it is reasonable to consider that some pony breeds, being more insulin resistant than horses, may represent equine-specific examples of this phenomenon.16–18 Interestingly, pony breeds are also predisposed to develop laminitis when compared with horses.19
Development and Recognition of Obesity in Horses
The prevalence of obesity in animals is underrecognized by both veterinary clinicians and owners.1 In fact, many animal owners deem a degree of obesity as normal, acceptable, and even desirable (Figure 1). In certain categories of the equine industry, horses are judged competitively by their physical characteristics. In these events, a degree of obesity is similarly often judged to be an advantage in the show ring.
Figure 1.
(A) Typical physical appearance of an obese pony. The body condition score for this pony is 9 out of 9 (maximal obesity score = 9). (B) Typical physical appearance of a thin horse. The body condition score for this pony is 2 out of 9 (maximal obesity score = 9).
There clearly exists a need for objective criteria by which horses might be “scored” in terms of whole-body adiposity. Whereas in the human context, the “body mass index” (BMI) has found utility in this respect, it has more recently been suggested that simply measuring the circumference of the (human) individual's waist is an excellent indicator of visceral adiposity.20 In the equine field, various methods have been recommended for the purpose of assessing the relative adiposity of equine patients, including the body condition score, the equine BMI, and the use of ultrasonography to assess subcutaneous fat thickness near the tail head.21–23 Determinations of the thickness of subcutaneous adipose repositories near the base of the tail and over the rump have been shown to be useful predictors of total body fat content when compared to determinations based on dissection of the carcass.24,25
The equine species evolved as a free-roaming herbivore that walked great distances on a daily basis, paying heed to potential predators, in order to find and ingest a sufficient and suitable quantity of forage. The quantity and type of available grassland species available to evolving horses differed significantly from that generally provided for horses today. The process of natural selection optimized the equine metabolism for feeding on “native” grassland species that were relatively low in sugar and starch content.
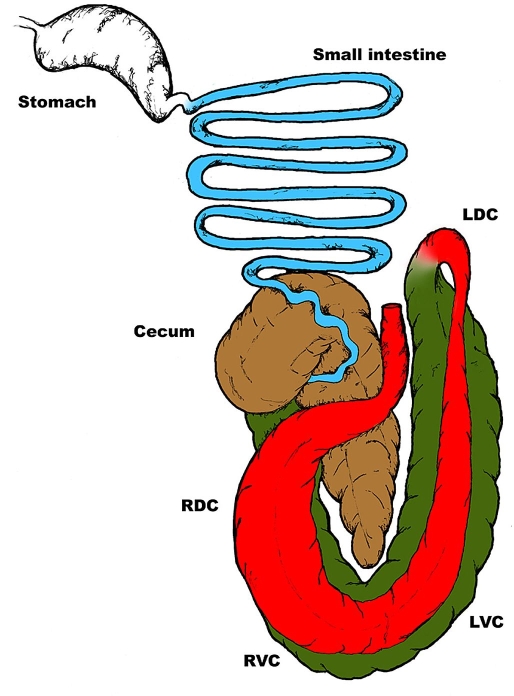
Like other mammals, horses lack enzymes that are capable of digesting the cell walls of plant species. The horse is a hindgut fermenting, herbivorous species; healthy horses exist in a symbiotic relationship with a substantial bacterial population (microbiota/flora) contained and maintained in their large intestine. The anatomy of the large intestine of horses is remarkably complex and differs considerably from that of humans (Figure 2). The equine large intestine may be divided into three parts likened to a series of fermentation chambers that provide for compartmentalized fermentation of forage. Upon entering the large intestine, digesta are first processed in the cecum, a very large chamber that is an anatomical cul-de-sac. Digesta are subsequently moved from the cecum into the chambers of the ascending colon (right ventral colon followed sequentially by the left ventral colon, the left dorsal colon, and the right dorsal colon). Digesta then move through the short transverse colon into the descending colon and are eventually passed as feces.
Figure 2.
Diagrammatic representation of the equine gastrointestinal tract. Note the extent and the capacity of the equine large intestine in contrast to its size and extent in humans. For purposes of diagrammatic clarity, the descending colon was excluded. RVC, right ventral colon; LVC, left ventral colon; LDC, left dorsal colon; RDC, right dorsal colon.
Using the process of fermentation, symbiotic bacteria degrade the structural carbohydrates of plant cell walls (cellulose, hemicellulose, and pectins) to short-chain (or volatile) fatty acids that are absorbed across the colonic epithelial lining and used for energy. In many respects, equine digestive processes evolved with emphasis on the efficient utilization of these “structural” carbohydrates (dietary fiber). Accordingly, plant species to which horses adapted through natural selection tend to be characterized by a relatively low nonstructural carbohydrate (NSC) content (sugars and starch). There is good evidence that provision of relatively high NSC-content rations to horses leads to significant diminishment in insulin sensitivity.3
The constraints imposed by the needs of modern society on the methods by which horses are accommodated and fed are such that a majority of domesticated horses are physically inactive and provided rations that are grossly excessive with regards to their nutritional energy requirements. Horse owners purchase energy-dense rations at the behest of attractive advertising and free advice afforded by the powerful and influential equine food manufacturing industry. Furthermore, forage sources (pastures, paddocks, and hay) used for feeding horses are commonly based on nutritionally improved grassland species that have been genetically enhanced for purposes of feeding food animal species (especially cattle) (http://www.safergrass.org). These forages are especially rich sources of sugars and starch (high NSC content) that have been optimized for rapid weight gain in food-bearing farm animal species.26
Evolution provided the horse with a metabolism that was best suited to its natural lifestyle before the influence of manmade domestication. It is therefore not surprising that domesticated horses develop obesity in light of the fact that they tend to be physically inactive and are provided with rations that are excessive in terms of energy (from the perspectives of both forage and grain).
Although there have been few published studies that report on the incidence of obesity, it is commonly believed, albeit anecdotally, that obesity is an underrecognized and common problem in the equine species. In one study, 45% of 319 randomly selected horses were scored subjectively as either “fat” or “very fat.” 1 Interestingly, in that study, the owners of the same horses generally underestimated the significance of obesity and failed to recognize its development in their animals. Based on the results of that study, the prevalence of obesity in horses exceeds that reported by others for pet cats (25.8%) and pet dogs (25.2%).27,28
Widespread recognition of the important relevance of obesity to health within the veterinary profession clearly lags that of its human medical counterpart. That said, many of the health risks associated with human obesity pertain to chronic diseases in a long-lived species. Domesticated animals rarely attain ages beyond 30 years.
Throughout the antecedent decade, there has been broad interest in the fact that IR represents a risk factor for laminitis, a common and debilitating cause for lameness in horses.29–32 However, the extent to which obesity contributes to worsening IR in horses, as it does in humans, has not yet been investigated as thoroughly.
Equine Health Consequences of Obesity
Some equine health consequences of obesity have been ascribed to the simple acquisition of excessive adipose tissue within the individual. Examples of adverse effects of obesity include exercise intolerance (reduced athleticism),33–35 thermoregulatory inefficiency,36 abnormal reproductive performance,37–39 and the development of benign lipomas in mesenteric adipose within the abdomen.40 Mesenteric lipomas are more likely to develop in obese horses and cause painful intestinal tract obstructions (known as “colic”). In some cases, mesenteric lipomas develop a long pedicle of attachment and are said to become “pedunculated.” Pedunculated lipomas tend to move within the abdomen in such a manner as to cause both obstruction and strangulation of obstructed intestine. Affected horses develop acute-onset severe pain, shock, and, without emergency surgical intervention, die.
New information points to the fact that, as clearly recognized in other species, obesity contributes to worsening IR in horses.41,42 Specific equine conditions that have been associated with IR may therefore be more likely in obese horses. Specifically, IR (and, by extension, obesity) has been implicated in the pathogenesis of laminitis,29–32 pituitary pars intermedia dysfunction (PPID),42–45 osteochondrosis,46 hyperlipemia,47 diabetes mellitus,48 and endotoxemia (systemic inflammation).49,50
The term “equine metabolic syndrome” (EMS) has been used to describe horses at risk for laminitis resulting from IR and (perhaps) obesity.32 In this regard, it is argued that laminitis represents a chronic equine medical condition analogous to human chronic conditions associated with IR and obesity (such as diabetes mellitus and cardiovascular diseases). Whereas the human metabolic syndrome definition51 includes the presence of IR, obesity, hypertension, a proinflammatory state, a prothrombotic state, and atherogenic dyslipidemia, application of the term EMS is generally applied to horses in which the following abnormalities are identified: IR, obesity, and signs of abnormality in the hoof (implicative for laminitis).32 A prominent and characteristic physical attribute of horses affected with EMS is the acquisition of adipose tissue within the crest of the neck (Figure 3). Interestingly, changes in the “thickness” of the affected neck have been linked to changes in the signs of clinical severity: as the neck thickens, signs of IR (such as laminitis) tend to increase (these signs decrease when treatments are associated with improvement in insulin sensitivity and a loss of neck crest thickness). It has been suggested that the circumference of the neck (compared with the horse's height or girth) might be a practical index for suspicion of IR (similar to the use of waist circumference as an indicator of high BMI and IR in obese human patients).52
Figure 3.
Physical appearance of the thickened neck that is commonly recognized as a sign of underlying insulin resistance.
Although evidence shows that EMS-affected horses develop hypertension, routine measurement of blood pressure is not generally undertaken in equine practice.53–55 Elevated circulating levels of some proinflammatory mediators have been identified in obese horses.56,57
As with the case for the human metabolic syndrome, substantial controversy has followed introduction of the EMS term into the equine medical field. There is yet still a lack of agreement regarding the criteria by which EMS should be defined. Clearly, substantial differences exist between EMS and its human counterpart. Use of the term EMS, implying that the principle and sole underlying endocrinopathic state is IR, helps differentiate affected horses from those affected with either hypothyroidism (extremely rare in adult horses) or PPID.32
Insulin Resistance in Horses
As with humans, medical problems in obese horses are often attributed to the consequences of IR. A complete discussion of the pathophysiology of IR is beyond the scope of this article. That IR likely plays a role in equine diseases has been recognized for many years.30 Substantial recent interest in the medical importance of IR has been significantly fueled by the recognition that it plays a role in the pathophysiology of laminitis.29,30 There is good evidence that genetic factors are important determinants as to whether specific breeds of horses are likely to develop greater or lesser degrees of IR.29 Other factors that contribute to worsening insulin sensitivity in other species, such as inflammation,56,57 obesity,41,58 dietary energy excess,3 age,43 physical inactivity,3,59 corticosteroids,60 and pregnancy,61 have been reported to some extent for horses.
Laminitis
Laminitis is an affliction of the hoof lamellar interface (HLI), the specialized dermoepidermal junction that serves to attach the hoof capsule to its underlying connective tissue and the distal phalanx. The importance of the structural integrity of the HLI is appreciated when one considers that it effectively bears the weight of the horse. Some conditions lead to rapid degradation of the HLI and cause physical separation of its dermoepidermal junction (by virtue of the weight-bearing function), a painful inflammatory state that often necessitates euthanasia and is referred to as acute laminitis (“founder”).62 Alternatively, chronic metabolic and endocrinological perturbations may cause subtle and progressive remodeling within the HLI that is referred to as chronic laminitis.63 Whether severe and extensive or mild, structural weakening of the HLI is a hallmark of the laminitic condition (Figure 4).
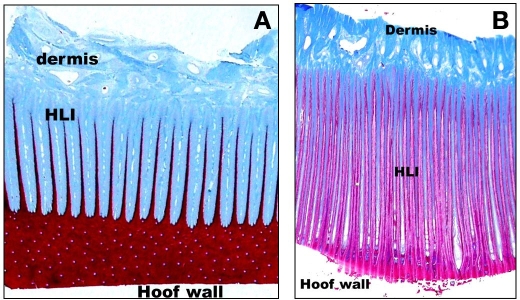
Figure 4.
(A) Low-powered, light microscopic appearance of the normal healthy hoof lamellar interface. The epidermal component has been stained brown and the dermal connective tissue component appears blue. Note the regular appearance of the interdigitating hoof lamellae that serves to facilitate attachment of the distal phalanx to the overlying hoof wall. HLI, hoof lamellar interface. (B) Low-powered, light microscopic appearance of the hoof lamellar interface obtained from a horse affected by chronic laminitis. The epidermal component has been stained magenta and the dermal connective tissue component appears blue. Note the irregular appearance of the interdigitating hoof lamellae. The lamellar structure has been distorted and attenuated. The net result of the structural remodeling process is a weakening of the attachment interface. HLI, hoof lamellar interface.
Laminitis is a potentially devastating complication of many disparate primary conditions. Acute laminitis commonly occurs during the treatment of severe gastrointestinal diseases such as obstructive intestinal strangulation and enterocolitis. Dietary indiscretions associated with the intake of large quantities of grain (starch) also commonly lead to colitis and acute laminitis (“grain overload”). Laminitis often arises in horses that are grazing forage pastures at times of the year when the NSC content of the grass is high.29 There is a well-documented risk for the development of laminitis during conditions in which the permeability of the intestinal lining is increased as a result of either disease or dietary indiscretion. Several plausible explanations for the risk of laminitis associated with disease of the alimentary tract have been proposed and include the absorption of bacterial toxins,64–66 cardiovascular derangements associated with systemic inflammation (ischemia of the HLI as a result of digital venoconstriction),67 that the HLI represents an equine-specific shock organ following translocation of colonic bacterial products into the blood (neutrophil granulocyte migration into the HLI appears to play a critical role in the pathogenesis of acute laminitis),68,69 and that IR develops as a component of a systemic inflammatory response to bacterial products (endotoxemia).70,71 Laminitis is also highly likely as a complication of other diseases associated with a systemic inflammatory response such as pleuropneumonia and endometritis.
However, a milder form of chronic laminitis is commonly identified in horses and ponies that may be attributed to underlying endocrinopathic influence. In this endocrinopathic manifestation of chronic laminitis, extensive, severe, and painful physical separation of the HLI is much less likely.63 Milder signs of subtle pain and remodeling of the HLI characterize endocrinopathic laminitis. Signs of abnormal hoof growth, including prominent growth lines and broadening of the white line zone, are typically evident in the affected hoof (Figure 5).
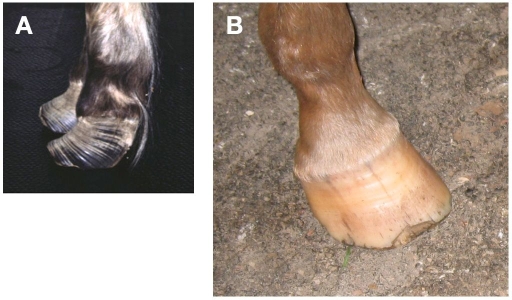
Figure 5.
(A) Physical appearance of the hoof of a horse affected by chronic laminitis. Note the prominent ridges at the surface of the hoof (“growth lines”) that tend to converge toward the front of the hoof. This physical appearance is characteristic of chronic laminitis. Broadening of the white line zone is a feature of chronic laminitis but it is evident when viewing the solar aspect of the hoof (not depicted here). (B) Physical appearance of a normal equine hoof.
The two most common endocrinopathic abnormalities associated with chronic laminitis are IR and the influence of excessive levels of corticosteroids.63 Injection of horses with synthetic glucocorticoids sometimes causes laminitis. Conditions associated with excessive circulating plasma cortisol concentrations (Cushing's syndrome), such as PPID, are commonly associated with laminitis.7
Despite substantial speculation, a satisfactory explanation for how and why laminitis develops as a result of either IR or glucocorticoids is presently lacking. The picture is made more complicated when one considers that glucocorticoids represent a potent cause of IR.72 Moreover, expression of the steroid converting enzyme 11-hydroxysteroid dehydrogenase-1 (11-HSD1) in abdominal adipose tissues is an important component of the human metabolic syndrome.73,74 This enzyme increases the localized tissue activity of cortisol, the active glucocorticoid, by ketoreduction of the inactive, but plentiful, circulating cortisone. As reported previously, the activity of 11-HSD1 in the equine HLI is increased in the presence of laminitis.75 Furthermore, it has also been reported that 11-HSD1 activity appears to be increased in subcutaneous adipose tissues obtained from the thickened neck of horses affected with EMS.76 The gene for the enzyme 11-HSD1 is likely one of many “thrifty” genes.
Aside from the possibility that endocrinopathic laminitis could be a result of the action of cortisol (increased by 11-HSD1), evidence also shows that insulin itself is directly toxic to the HLI. In a series of elegant experiments, administration of insulin to horses [chronic intravenous (IV) infusion working to maintain hyperinsulinemia over the course of several days] was shown to cause laminitis.77 Chronic hyperinsulinemia is a common finding in IR-affected horses at risk for laminitis. However, laminitis does not appear to be a complication of overt diabetes mellitus in the equine species.48
Pituitary Pars Intermedia Dysfunction
As noted earlier, the equine pituitary gland differs from that of humans in that there exists a comparatively well-developed intermediate lobe, the PI.7 The PI is important in herbivores because its secretory output contributes to readying the individual for winter.5 Melanotropes, the only secretory cell population in the PI, secrete POMC-derived peptide hormones (a different repertoire to the POMC peptides secreted by corticotropes in the pars distalis). Specifically, the POMC-derived secretory products of melanotropes include adrenocorticotrophic hormone (corticotropin), -endorphin, -melanocyte-stimulating hormone, and corticotropin-like intermediary peptide. In health, melanotrope secretory output is strictly inhibited by dopaminergic neurons in the periventricular aspects of the hypothalamus, and the day-to-day physiological contribution of PI-derived POMC peptides is regarded as minor.78,79
With age, these dopaminergic neurons tend to deteriorate as a result of oxidative stress damage.80 Loss of normal inhibitory dopaminergic influence on melanotropes leads to both POMC peptide hypersecretion and clonal expansion of melanotropes. The resulting clinical expression of persistent PI hyperfunction is referred to as PPID. Oxidative destruction of dopaminergic nerves in aged horses is somewhat similar to the oxidative loss of dopaminergic nerves in human patients affected by Parkinson's disease.81
A discussion of the clinical expression of PPID is beyond the scope of this article and the interested reader is directed to other sources of information.7 It has been suggested that PPID may arise as a result of chronic IR in obese horses and ponies that have been fed energy-dense rations over the course of many years.45 However, a satisfactory explanation for the development of oxidative damage to these critical dopaminergic nerves in the equine species has not yet been presented. Laminitis is a component of the clinical expression of both IR and PPID (Figure 6). It is also common that IR can be demonstrated readily in PPID-affected individuals.43
Figure 6.
Physical appearance of a pony affected by pituitary pars intermedia dysfunction (equine Cushing's syndrome) resulting from the unfettered secretion of POMC peptides from the intermediate lobe of the pituitary gland. Note that the hair coat has failed to shed out (inappropriate hirsutism) and that the front feet are affected by chronic laminitis.
Hyperlipemia Syndrome
Hyperlipemia syndrome results from excessive and pathological mobilization of lipid from excessive adipose tissue repositories in obese individuals. As befits their genetic tendency to IR, pony breeds, donkeys, and miniature horses are especially at risk for hyperlipemia.47 By definition, hyperlipemia implies that the plasma triglyceride concentration exceeds 500 mg/dl. Consequences of hyperlipemia include circulatory disturbances and organ failure (especially the liver and kidneys) as a result of fatty infiltration. The mortality associated with severe hyperlipemia is very high; mortality may be attributable to either hyperlipemia per se or various underlying causative disease processes. That liver disease results from the acquisition of lipid by hepatocytes as a result of an IR-associated triglyceride mobilization in obese horses is similar to nonalcoholic fatty liver disease in human patients, a common finding in metabolic syndrome.82
Diabetes Mellitus in Horses
Although horses and ponies commonly develop IR, it is rare that overt diabetes mellitus develops in this species.48 It appears that, in the face of significant IR, pancreatic secretion of insulin rarely fails during the lifetime of the horse. It has been suggested that, compared with their human counterpart, the life span of horses (<30 years) is less. Moreover, the ration of horses normally contains comparatively little fat (<4%). In contrast, IR is a common finding in obese cats and compared with horses it is associated more often with overt diabetes.83,84 It has also been suggested that comparatively low insulin sensitivity in carnivorous species may provide a survival advantage during periods of restricted food availability.84
Clinical Diagnosis of Insulin Resistance in Horses
Clinical suspicion of IR/EMS is based on results of the physical examination of the patient. Physical abnormalities commonly identified in EMS-affected patients include generalized or “regional” obesity, a thickened neck, and the presence of laminitis (based on either physical or radiographic abnormalities). It is important to eliminate PPID as the underlying cause of “regional” obesity and laminitis because the clinical appearance of PPID in teenage horses is similar to that of EMS. The extent to which EMS and PPID may be related is the subject of some controversy. It has been suggested that PPID may be an equine-specific complication of chronic IR.45
The best tests for IR include the frequently sampled IV glucose tolerance test and the euglycemic hyperinsulinemic clamp technique. Unfortunately, these tests are impractical for practicing veterinarians and less specific, alternative diagnostic approaches are generally recommended.
The easiest diagnostic test for IR is to simply determine the plasma insulin concentration. Compensatory hyperinsulinemia is a common finding in IR-affected equids (caution is needed when interpreting the results of single sample insulin determinations without due consideration of possible confounding factors). Serum insulin concentration can be influenced by many factors (in addition to the insulin sensitivity of the individual), including time since the animal was last fed, circulating cortisol concentration (diurnal variance, excitement, pain and stress, PPID), type of food on which the ration is based, reproductive status, and physiological status (fitness/illness). However, fasting concentrations of both insulin and glucose tend to be relatively constant and may be used to provide insight into the patient's insulin sensitivity. Horses and ponies affected with IR tend to be characterized by a high normal or slightly elevated plasma glucose concentration (reference range, 80–115 mg/dl) and hyperinsulinemia [reference range, <30 mU/ml (<220 pmol/liter)].
Practical clinical demonstration of IR may be achieved using either a simple (glucose:insulin) ratio or by calculating the reciprocal of the square root of insulin proxy. Both of these indices of patient insulin sensitivity yield similar correlations to measurements made using more complicated techniques.85
An alternative diagnostic test for IR in clinical patients is the combined intravenous glucose–insulin test (CGIT). Horses are tested by administering glucose and insulin (glucose, 150 mg/kg; insulin, 0.1 U/kg) and measuring the blood glucose concentration for 2 hours.86 Normal insulin sensitivity in horses is associated with a return of the plasma glucose concentration to baseline within 45 minutes. The CGIT represents a potentially practical clinical measurement of insulin sensitivity because it provides integrated information and more information than either the singular glucose tolerance test or an insulin sensitivity test (Figure 7).86
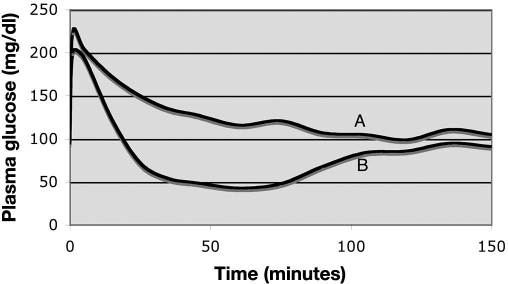
Figure 7.
Graph depicting the response of the plasma glucose concentration to administration of an IV bolus of glucose and an IV injection of insulin (combined insulin glucose test; details provided in the text). The graph depicts the response of two different animals. Insulin sensitivity is generally assessed as satisfactory for those individuals in which the plasma glucose concentration returns to its baseline value within 45 minutes of the outset of the protocol. In this case, horse A had poor insulin sensitivity and horse B had normal insulin sensitivity.
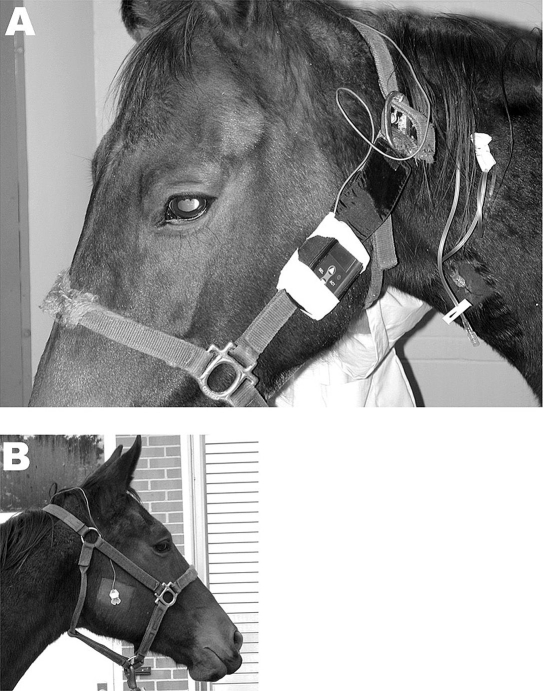
More recently we have adopted the use of interstitial tissue glucose monitoring to assess temporal changes in glucose concentration during the CGIT.87,88 The use of a small interstitial tissue probe eliminates the need for repetitive blood samples and patient handling stress (both of which might lead to iatrogenic elevations in the glucose concentration) (Figure 8).87,88
Figure 8.
(A) A horse instrumented with a continuous interstitial glucose monitor. Note that the instrument has been secured to the patient's head collar. (B) A horse instrumented with a continuous interstitial glucose monitor. Note that the probe has been inserted into the subcutaneous tissue on the right side of the horse's face.
Parallels between Obesity in Human and Equine Populations
Although dairy products, refined sugars, cereals, refined vegetable oils, and alcohol contribute 72% of the modern human diet, these nutritional components were not available to evolving humans and did not contribute to the natural selection pressures under which the human genome developed.89 Various “diseases of modern civilization” (such as those associated with obesity) have been attributed to discordance between our ancient, genetically determined biology and the nutritional, cultural, and activity patterns of contemporary Western populations.90,91 Specifically, consumption of recently introduced (refined) foods, coupled with a relatively low activity lifestyle, is at odds with our inherited genetic programming.90
Although the human genome is essentially unchanged since the emergence of modern human beings in East Africa thousands of years ago, humans remain genetically adapted for the foods consumed then.92 Modern humans have also inherited genes that evolved to support a physically active lifestyle, and physical inactivity in sedentary societies contributes directly to a risk of multiple chronic health disorders.91
Aspects of obesity in animals may afford some insight into human obesity. It could be argued that modern horses have also inherited genes that were evolved to support a physically active lifestyle and that physical inactivity (imposed by the needs of domestication and human convenience) also contributes to a risk of chronic disease, but the scientific evidence for the argument is yet still insubstantial. Similarly, modern horses are fed rations that are broadly different to that nutritional repertoire for which they are genetically adapted.
In this respect, significant parallels exist between the provision of refined, nutritionally dense rations to horses and the consumption of energy-dense refined foods by people in modern society. Beyond the provision of an excessive grain and pellet component in horse rations (with respect to nutritional requirements), modern horses are also fed forage items (pastures and hay) that have been enhanced genetically to suit the needs of the food animal industry. These forage sources are characterized by a relatively high nonstructural carbohydrate content (high starch and sugar content) and have been designed to promote weight gain, growth, and lactation in cattle (http://www.safergrass.org). Not surprisingly, when fed to inactive horses, these forage sources promote the development of obesity and their sugar content aggravates IR in affected individuals.
Conclusion
There is increasing awareness that obesity develops commonly in domesticated companion animals. Although there is substantial evidence regarding the fact that obesity represents a risk factor for complications and increased mortality for many human medical conditions, the effect of obesity on medical conditions in veterinary species has not been studied extensively. There is increasing interest in the role that IR plays in animal diseases. As is commonly reported in the human medical literature, the increasing incidence of obesity in horses might be linked to contemporary management practices that include prolonged periods of imposed physical inactivity and the provision of rations that are nutritionally excessive with regard to the animal's energy requirements. Moreover, further similarities to the human obesity situation might be drawn regarding the provision of diets for horses that contain substantial “refined” carbohydrates (nonstructural carbohydrates including sugars and starch), which are at odds with the nature of the food for which these species are genetically adapted.
For veterinarians, it is easy to engage in conversation with animal owners regarding the importance of obesity and its impact on health because there has been so much publicity and media interest. Talking to animal owners about the impositions of human-oriented animal confinement and exercise restrictions can readily be presented in the context of insufficient physical activity as a risk factor for human disease. Similarly, the increasing recognition that many common animal feeding practices are broadly inappropriate for nutritional requirements can be very effectively likened to aspects of unhealthy human nutritional practices in contemporary society.
To a greater and greater extent, veterinarians are discussing animal obesity in the broader context of what is presented to animal owners in the media regarding human obesity, physical inactivity, and unhealthy nutritional choices. Whereas humans make their own nutritional choices, obesity in animals is always a direct consequence of management decisions made on their behalf by animal owners. Curiously, in many cases, provision of a healthier ration for horses would represent a considerable cost saving for owners who often expend considerable financial resources on rations that, in terms of protein and energy, far exceed the animal's nutritional requirements. The extent to which obesity affects the general health of animals has been studied to a much lesser extent than it has for humans. Therefore, it is a very exciting time for veterinary scientists with interest in adipobiology, obesity, endocrinology, metabolism, and nutrition. It is important to emphasize that substantial differences exist among the pathophysiological consequences of obesity among different species. Facts that hold true for one species (such as humans) may not hold the same truth for animals.
Acknowledgement
The authors thanks Juliana Amorim and Don Connor for their assistance with Figure 2.
Abbreviations
- BMI
body mass index
- CGIT
combined intravenous glucose–insulin test
- EMS
equine metabolic syndrome
- HLI
hoof lamellar interface
- 11β-HSD1
11β-hydroxysteroid dehydrogenase-1
- IR
insulin resistance
- IV
intravenous
- NSC
nonstructural carbohydrate
- POMC
proopiomelanocortin
- PI
pars intermedia
- PPID
pituitary pars intermedia dysfunction
References
- 1.Wyse CA, McNie KA, Tannahil VJ, Murray JK, Love S. Prevalence of obesity in riding horses in Scotland. Vet Rec. 2008;162(18):590–591. doi: 10.1136/vr.162.18.590. [DOI] [PubMed] [Google Scholar]
- 2.Frank N, Elliott SB, Boston RC. Effects of long-term oral administration of levothyroxine sodium on glucose dynamics in healthy adult horses. Am J Vet Res. 2008;69(1):76–81. doi: 10.2460/ajvr.69.1.76. [DOI] [PubMed] [Google Scholar]
- 3.Pratt SE, Geor RJ, McCutcheon LJ. Effects of dietary energy source and physical conditioning on insulin sensitivity and glucose tolerance in standardbred horses. Equine Vet J Suppl. 2006;(36):579–584. doi: 10.1111/j.2042-3306.2006.tb05608.x. [DOI] [PubMed] [Google Scholar]
- 4.Johnson PJ, Ganjam VK, Messer NT, Turk JR, Keisler DH, Buff PR, Wiedmeyer CE. Proceedings of the 52nd Annual Convention of the American Association of Equine Practitioners. 2-6. TX: San Antonio; 2006. Dec, The obesity paradigm: an introduction to the emerging discipline of adipobiology; pp. 41–50. [Google Scholar]
- 5.Donaldson MT, McDonnell SM, Schanbacher BJ, Lamb SV, McFarlane D, Beech J. Variation in plasma adrenocorticotropic hormone concentration and dexamethasone suppression test results with season, age, and sex in healthy ponies and horses. J Vet Intern Med. 2005;19(2):217–222. doi: 10.1892/0891-6640(2005)19<217:vipahc>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 6.Ssewannyana E, Lincoln GA, Linton EA, Lowry PJ. Regulation of the seasonal cycle of beta-endorphin and ACTH secretion into the peripheral blood of rams. J Endocrinol. 1990;124(3):443–454. doi: 10.1677/joe.0.1240443. [DOI] [PubMed] [Google Scholar]
- 7.Messer NT, Johnson PJ. Evidence-based literature pertaining to thyroid dysfunction and Cushing's syndrome in the horse. Vet Clin North Am Equine Pract. 2007;23(2):329–364. doi: 10.1016/j.cveq.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 8.Fernandez-Real JM. Genetic predispositions to low-grade inflammation and type 2 diabetes. Diabetes Technol Ther. 2006;8(1):55–66. doi: 10.1089/dia.2006.8.55. [DOI] [PubMed] [Google Scholar]
- 9.Neel JV. Diabetes mellitus: a “thrifty” genotype rendered detrimental by “progress”? Am J Hum Genet. 1962;14:353–362. [PMC free article] [PubMed] [Google Scholar]
- 10.Dyson MC, Alloosh M, Boullion RD, Mokelke EA, Sturek M. Glucose intolerance and insulin resistance in Ossabaw compared to Yucatan swine. FASEB J. 2004;18:A1224–A1225. [Google Scholar]
- 11.Mayer JJ, Brisbin IL., Jr. Athens, GA: University of Georgia Press; 1991. Wild pigs of the United States: their history, morphology, and current status. [Google Scholar]
- 12.Buhlinger CA, Wangsness PJ, Martin RJ, Ziegler JH. Body composition, in vitro lipid metabolism and skeletal muscle characteristics in fast-growing, lean and in slow growing, obese pigs at equal age and weight. Growth. 1978;42(2):225–236. [PubMed] [Google Scholar]
- 13.Martin RJ, Gobble JL, Hartsock TH, Graves HB, Ziegler JH. Characterization of an obese syndrome in the pig. Proc Soc Exp Biol Med. 1973;143:198–203. doi: 10.3181/00379727-143-37285. [DOI] [PubMed] [Google Scholar]
- 14.Dyson M, Mokelke EA, Vuchetich J, Sturek M. Use of computed tomography to evaluate intra-abdominal fat stores in a swine model of the metabolic syndrome. FASEB J. 2005;19:A191–A192. [Google Scholar]
- 15.Gerrity RG, Natarajan R, Nadler JL, Kimsey T. Diabetes-induced accelerated atherosclerosis in swine. Diabetes. 2001;50(7):1654–1665. doi: 10.2337/diabetes.50.7.1654. [DOI] [PubMed] [Google Scholar]
- 16.Treiber KH, Kronfeld DS, Hess TM, Byrd BM, Splan RK, Staniar WB. Evaluation of genetic and metabolic predispositions and nutritional risk factors for pasture-associated laminitis in ponies. J Am Vet Med Assoc. 2006;228(10):1538–1545. doi: 10.2460/javma.228.10.1538. [DOI] [PubMed] [Google Scholar]
- 17.Field JR, Jeffcott LB. Equine laminitis–another hypothesis for pathogenesis. Med Hypotheses. 1989;30(3):203–210. doi: 10.1016/0306-9877(89)90062-5. [DOI] [PubMed] [Google Scholar]
- 18.Freestone JF, Beadle R, Shoemaker K, Bessin RT, Wolfsheimer KJ, Church C. Improved insulin sensitivity in hyperinsulinaemic ponies through physical conditioning and controlled feed intake. Equine Vet J. 1992;24(3):187–190. doi: 10.1111/j.2042-3306.1992.tb02812.x. [DOI] [PubMed] [Google Scholar]
- 19.Alford P, Geller S, Richardson B, Slater M, Honnas C, Foreman J, Robinson J, Messer M, Roberts M, Goble D, Hood D, Chaffin M. A multicenter, matched case-control study of risk factors for equine laminitis. Prev Vet Med. 2001;49(3-4):209–222. doi: 10.1016/s0167-5877(01)00188-x. [DOI] [PubMed] [Google Scholar]
- 20.Lee S, Bacha F, Gungor N, Arslanian SA. Waist circumference is an independent predictor of insulin resistance in black and white youths. J Pediatr. 2006;148(2):188–194. doi: 10.1016/j.jpeds.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 21.Henneke DR, Potter GD, Kreider JL, Yeates BF. Relationship between condition score, physical measurements and body fat percentage in mares. Equine Vet J. 1983;15(4):371–372. doi: 10.1111/j.2042-3306.1983.tb01826.x. [DOI] [PubMed] [Google Scholar]
- 22.Donaldson MT, McFarlane D, Jorgensen AJ, Beech J. Correlation between plasma alpha-melanocyte-stimulating hormone concentration and body mass index in healthy horses. Am J Vet Res. 2004;65(11):1469–1473. doi: 10.2460/ajvr.2004.65.1469. [DOI] [PubMed] [Google Scholar]
- 23.Kearns CF, McKeever KH, John-Alder H, Abe T, Brechue WF. Relationship between body composition, blood volume and maximal oxygen uptake. Equine Vet J Suppl. 2002;34:485–490. doi: 10.1111/j.2042-3306.2002.tb05470.x. [DOI] [PubMed] [Google Scholar]
- 24.Kane RA, Fisher M, Parrett D, Lawrence LM. Estimating fatness in horses. Proceedings of the 10th Equine Nutrition and Physiology Society Symposium; Fort Collins, CO: 1989. pp. 127–131. [Google Scholar]
- 25.Westervelt RG, Stouffer JR, Hintz HF, Schryver HF. Estimating fatness in horses and ponies. J Anim Sci. 1976;43:781–785. [Google Scholar]
- 26.Longland AC, Byrd BM. Pasture nonstructural carbohydrates and equine laminitis. J Nutr. 2006;136(7 Suppl):2099S–2102S. doi: 10.1093/jn/136.7.2099S. [DOI] [PubMed] [Google Scholar]
- 27.Allan FJ, Pfeiffer DU, Jones BR, Esslemont DH, Wiseman MS. A cross-sectional study of risk factors for obesity in cats in New Zealand. Prev Vet Med. 2000;46(3):183–196. doi: 10.1016/s0167-5877(00)00147-1. [DOI] [PubMed] [Google Scholar]
- 28.Robertson ID. The association of exercise, diet and other factors with owner-perceived obesity in privately owned dogs from metropolitan Perth, WA. Prev Vet Med. 2003;58(1-2):75–83. doi: 10.1016/s0167-5877(03)00009-6. [DOI] [PubMed] [Google Scholar]
- 29.Treiber KH, Kronfeld DS, Hess TM, Byrd BM, Splan RK, Staniar WB. Evaluation of genetic and metabolic predispositions and nutritional risk factors for pasture-associated laminitis in ponies. J Am Vet Med Assoc. 2006;228(10):1538–1545. doi: 10.2460/javma.228.10.1538. [DOI] [PubMed] [Google Scholar]
- 30.Coffman JR, Colles CM. Insulin tolerance in laminitic ponies. Can J Comp Med. 1983;47(3):347–351. [PMC free article] [PubMed] [Google Scholar]
- 31.Field JR, Jeffcott LB. Equine laminitis–another hypothesis for pathogenesis. Med Hypotheses. 1989;30(3):203–210. doi: 10.1016/0306-9877(89)90062-5. [DOI] [PubMed] [Google Scholar]
- 32.Johnson PJ. The equine metabolic syndrome peripheral Cushing's syndrome. Vet Clin North Am Equine Pract. 2002;18(2):271–293. doi: 10.1016/s0749-0739(02)00006-8. [DOI] [PubMed] [Google Scholar]
- 33.Kearns CF, McKeever KH, Kumagai K, Abe T. Fat-free mass is related to one-mile race performance in elite standardbred horses. Vet J. 2002;163(3):260–266. doi: 10.1053/tvjl.2001.0656. [DOI] [PubMed] [Google Scholar]
- 34.Lawrence LM, Jackson S, Kline K, Moser L, Powell D, Biel M. Observations on body weight and condition of horses in a 150-mile endurance ride. J Equine Vet Sci. 1992;12:320–324. [Google Scholar]
- 35.Garlinghouse SE, Burrill MJ. Relationship of body condition score to completion rate during 160 km endurance races. Equine Vet J Suppl. 1999;30:591–595. doi: 10.1111/j.2042-3306.1999.tb05290.x. [DOI] [PubMed] [Google Scholar]
- 36.Cymbaluk NF, Christison GI. Environmental effects on thermoregulation and nutrition of horses. Vet Clin North Am Equine Pract. 1990;6(2):355–372. doi: 10.1016/s0749-0739(17)30546-1. [DOI] [PubMed] [Google Scholar]
- 37.Henneke DR, Potter GD, Kreider JL. Body condition during pregnancy and lactation and reproductive efficiency of mares. Theriogenology. 1984;21:897–909. [Google Scholar]
- 38.Nagy P, Huszenicza G, Juhász J, Kulcsár M, Solti L, Reiczigel J, Abaváry K. Factors influencing ovarian activity and sexual behavior of postpartum mares under farm conditions. Theriogenology. 1998;50(7):1109–1119. doi: 10.1016/s0093-691x(98)00212-x. [DOI] [PubMed] [Google Scholar]
- 39.Fitzgerald BP, Reedy SE, Sessions DR, Vick MM, Murphy BA. Obesity disrupts the duration of the estrous cycle in the mare. J Anim Sci. 2003;81:102. doi: 10.2527/2004.8282321x. [DOI] [PubMed] [Google Scholar]
- 40.Garcia-Seco E, Wilson DA, Kramer J, Keegan KG, Branson KR, Johnson PJ, Tyler JW. Prevalence and risk factors associated with outcome of surgical removal of pedunculated lipomas in horses: 102 cases (1987-2002) J Am Vet Med Assoc. 2005;226(9):1529–1537. doi: 10.2460/javma.2005.226.1529. [DOI] [PubMed] [Google Scholar]
- 41.Hoffman RM, Boston RC, Stefanovski D, Kronfeld DS, Harris PA. Obesity and diet affect glucose dynamics and insulin sensitivity in Thoroughbred geldings. J Anim Sci. 2003;81(9):2333–2342. doi: 10.2527/2003.8192333x. [DOI] [PubMed] [Google Scholar]
- 42.Vick MM, Adams AA, Murphy BA, Sessions DR, Horohov DW, Cook RF, Shelton BJ, Fitzgerald BP. Relationships among inflammatory cytokines, obesity, and insulin sensitivity in the horse. J Anim Sci. 2007;85(5):1144–1155. doi: 10.2527/jas.2006-673. [DOI] [PubMed] [Google Scholar]
- 43.McGowan CM, Frost R, Pfeiffer DU, Neiger R. Serum insulin concentrations in horses with equine Cushing's syndrome: response to a cortisol inhibitor and prognostic value. Equine Vet J. 2004;36(3):295–298. doi: 10.2746/0425164044877288. [DOI] [PubMed] [Google Scholar]
- 44.Reeves HJ, Lees R, McGowan CM. Measurement of basal serum insulin concentration in the diagnosis of Cushing's disease in ponies. Vet Rec. 2001;149(15):449–452. doi: 10.1136/vr.149.15.449. [DOI] [PubMed] [Google Scholar]
- 45.Johnson PJ, Messer NT, Ganjam VK. Cushing's syndromes, insulin resistance and endocrinopathic laminitis. Equine Vet J. 2004;36(3):194–198. doi: 10.2746/0425164044877279. [DOI] [PubMed] [Google Scholar]
- 46.Ralston S. Hyperglycemia/hyperinsulinemia after feeding a meal of grain to young horses with osteochondrosis dissecans (OCD) lesions. Pferdeheilkunde. 1996;12:320–322. [Google Scholar]
- 47.Reid SW, Mohammed HO. Survival analysis approach to risk factors associated with hyperlipemia in donkeys. J Am Vet Med Assoc. 1996;209(8):1449–1452. [PubMed] [Google Scholar]
- 48.Johnson PJ, Scotty NC, Wiedmeyer C, Messer NT, Kreeger JM. Diabetes mellitus in a domesticated Spanish Mustang mare. J Am Vet Med Assoc. 2004;226(4):584–588. doi: 10.2460/javma.2005.226.584. [DOI] [PubMed] [Google Scholar]
- 49.Tóth F, Frank N, Elliott SB, Geor RJ, Boston RC. Effects of an intravenous endotoxin challenge on glucose and insulin dynamics in horses. Am J Vet Res. 2008;69(1):82–88. doi: 10.2460/ajvr.69.1.82. [DOI] [PubMed] [Google Scholar]
- 50.Jánosi S, Huszenicza G, Kulcsár M, Kóródi P. Endocrine and reproductive consequences of certain endotoxin-mediated diseases in farm mammals: a review. Acta Vet Hung. 1998;46(1):71–84. [PubMed] [Google Scholar]
- 51.Nilsson PM, Engström G, Hedblad B. The metabolic syndrome and incidence of cardiovascular disease in non-diabetic subjects–a population-based study comparing three different definitions. Diabet Med. 2007;24(5):464–472. doi: 10.1111/j.1464-5491.2007.02142.x. [DOI] [PubMed] [Google Scholar]
- 52.Carter RA, Geor RJ, Burton Staniar W, Cubitt TA, Harris PA. Vet J. 2008. Apr 25, Apparent adiposity assessed by standardised scoring systems and morphometric measurements in horses and ponies. [DOI] [PubMed] [Google Scholar]
- 53.Bailey SR, Habershon-Butcher JL, Ransom KJ, Elliott J, Menzies-Gow NJ. Hypertension and insulin resistance in a mixed-breed population of ponies predisposed to laminitis. Am J Vet Res. 2008;69(1):122–129. doi: 10.2460/ajvr.69.1.122. [DOI] [PubMed] [Google Scholar]
- 54.Garner HE, Coffman JR, Hahn AW, Ackerman N, Johnson JH. Equine laminitis and associated hypertension: a review. J Am Vet Med Assoc. 1975;166(1):56–57. [PubMed] [Google Scholar]
- 55.Rugh KS, Garner HE, Sprouse RF, Hatfield DG. Left ventricular hypertrophy in chronically hypertensive ponies. Lab Anim Sci. 1987;37(3):335–338. [PubMed] [Google Scholar]
- 56.Vick MM, Murphy BA, Sessions DR, Reedy SE, Kennedy EL, Horohov DW, Cook RF, Fitzgerald BP. Effects of systemic inflammation on insulin sensitivity in horses and inflammatory cytokine expression in adipose tissue. Am J Vet Res. 2008;69(1):130–139. doi: 10.2460/ajvr.69.1.130. [DOI] [PubMed] [Google Scholar]
- 57.Vick MM, Adams AA, Murphy BA, Sessions DR, Horohov DW, Cook RF, Shelton BJ, Fitzgerald BP. Relationships among inflammatory cytokines, obesity, and insulin sensitivity in the horse. J Anim Sci. 2007;85(5):1144–1155. doi: 10.2527/jas.2006-673. [DOI] [PubMed] [Google Scholar]
- 58.Firshman AM, Valberg SJ. Factors affecting clinical assessment of insulin sensitivity in horses. Equine Vet J. 2007;39(6):567–575. doi: 10.2746/042516407X238512. [DOI] [PubMed] [Google Scholar]
- 59.Powell DM, Reedy SE, Sessions DR, Fitzgerald BP. Effect of short-term exercise training on insulin sensitivity in obese and lean mares. Equine Vet J Suppl. 2002;(34):81–84. doi: 10.1111/j.2042-3306.2002.tb05396.x. [DOI] [PubMed] [Google Scholar]
- 60.Tiley HA, Geor RJ, McCutcheon LJ. Effects of dexamethasone on glucose dynamics and insulin sensitivity in healthy horses. Am J Vet Res. 2007;68(7):753–759. doi: 10.2460/ajvr.68.7.753. [DOI] [PubMed] [Google Scholar]
- 61.Fowder AL, Barnes RJ, Comline RS, Silver M. Pancreatic beta-cell function in the fetal foal and mare. J Endocrinol. 1980;87(2):293–301. doi: 10.1677/joe.0.0870293. [DOI] [PubMed] [Google Scholar]
- 62.Pass MA, Pollitt S, Pollitt CC. Decreased glucose metabolism causes separation of hoof lamellae in vitro: a trigger for laminitis? Equine Vet J Suppl. 1998;(26):133–138. doi: 10.1111/j.2042-3306.1998.tb05132.x. [DOI] [PubMed] [Google Scholar]
- 63.Johnson PJ, Messer NT, Slight SH, Wiedmeyer C, Buff P, Ganjam VK. Endocrinopathic laminitis in the horse. Clin Techniques Equine Pract. 2004;3(1):45–56. [Google Scholar]
- 64.Bailey SR, Menzies-Gow NJ, Marr CM, Elliott J. The effects of vasoactive amines found in the equine hindgut on digital blood flow in the normal horse. Equine Vet J. 2004;36(3):267–272. doi: 10.2746/0425164044877297. [DOI] [PubMed] [Google Scholar]
- 65.Bailey SR, Cunningham FM, Elliott J. Endotoxin and dietary amines may increase plasma 5-hydroxytryptamine in the horse. Equine Vet J. 2000;32(6):497–504. doi: 10.2746/042516400777584730. [DOI] [PubMed] [Google Scholar]
- 66.Milinovich GJ, Burrell PC, Pollitt CC, Bouvet A, Trott DJ. Streptococcus henryi sp. nov. and Streptococcus caballi sp. nov., isolated from the hindgut of horses with oligofructose-induced laminitis. Int J Syst Evol Microbiol. 2008;58(Pt 1):262–266. doi: 10.1099/ijs.0.65063-0. [DOI] [PubMed] [Google Scholar]
- 67.Allen D, Jr., Clark ES, Moore JN, Prasse KW. Evaluation of equine digital Starling forces and hemodynamics during early laminitis. Am J Vet Res. 1990;51(12):1930–1934. [PubMed] [Google Scholar]
- 68.Loftus JP, Belknap JK, Black SJ. Matrix metalloproteinase-9 in laminae of black walnut extract treated horses correlates with neutrophil abundance. Vet Immunol Immunopathol. 2006;113(3-4):267–276. doi: 10.1016/j.vetimm.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 69.Black SJ, Lunn DP, Yin C, Hwang M, Lenz SD, Belknap JK. Leukocyte emigration in the early stages of laminitis. Vet Immunol Immunopathol. 2006;109(1-2):161–166. doi: 10.1016/j.vetimm.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 70.Eades SC, Stokes AM, Johnson PJ, LeBlanc CJ, Ganjam VK, Buff PR, Moore RM. Serial alterations in digital hemodynamics and endothelin-1 immunoreactivity, platelet-neutrophil aggregation, and concentrations of nitric oxide, insulin, and glucose in blood obtained from horses following carbohydrate overload. Am J Vet Res. 2007;68(1):87–94. doi: 10.2460/ajvr.68.1.87. [DOI] [PubMed] [Google Scholar]
- 71.Bailey SR, Menzies-Gow NJ, Harris PA, Habershon-Butcher JL, Crawford C, Berhane Y, Boston RC, Elliott J. Effect of dietary fructans and dexamethasone administration on the insulin response of ponies predisposed to laminitis. J Am Vet Med Assoc. 2007;231(9):1365–1373. doi: 10.2460/javma.231.9.1365. [DOI] [PubMed] [Google Scholar]
- 72.Zarkovic M, Beleslin B, Ciric J, Penezic Z, Stojkovic M, Trbojevic B, Drezgic M, Savic S. Glucocorticoid effect on insulin sensitivity: a time frame. J Endocrinol Invest. 2008;31(3):238–242. doi: 10.1007/BF03345596. [DOI] [PubMed] [Google Scholar]
- 73.Kotelevtsev Y, Holmes MC, Burchell A, Houston PM, Schmoll D, Jamieson P, Best R, Brown R, Edwards CR, Seckl JR, Mullins JJ. 11beta-hydroxysteroid dehydrogenase type 1 knockout mice show attenuated glucocorticoid inducible responses and resist hyperglycemia on obesity or stress. Proc Natl Acad Sci USA. 1997;94(26):14924–14929. doi: 10.1073/pnas.94.26.14924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Masuzaki H, Paterson J, Shinyama H, Morton NM, Mullins JJ, Seckl JR, Flier JS. A transgenic model of visceral obesity and the metabolic syndrome. Science. 2001;294(5549):2166–2170. doi: 10.1126/science.1066285. [DOI] [PubMed] [Google Scholar]
- 75.Johnson PJ, Ganjam VK, Slight SH, Kreeger JM, Messer NT. Tissue-specific dysregulation of cortisol metabolism in equine laminitis. Equine Vet J. 2004;36(1):41–45. doi: 10.2746/0425164044864750. [DOI] [PubMed] [Google Scholar]
- 76.Schott II HC, Graves EA, Refsal KR, Nachreiner RJ, Eberhart SW, Johnson PJ, Slight SH, Messer NT, Ganjam VK. Treatment of pituitary pars intermedia dysfunction (classic Cushing's disease) and metabolic syndrome (peripheral Cushing's syndrome) in horses. In: Hillier A, Aiden P. Foster AP, Kwochka KW, editors. Advances in veterinary dermatology 5. Proceedings of the 5th World Congress of Veterinary Dermatology; Hofburg Conference Center, Vienna, Austria. Blackwell Publishing; 2004. pp. 159–169. [Google Scholar]
- 77.Asplin KE, Sillence MN, Pollitt CC, McGowan CM. Induction of laminitis by prolonged hyperinsulinaemia in clinically normal ponies. Vet J. 2007;174(3):530–535. doi: 10.1016/j.tvjl.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 78.Kemppainen R, Zerbe C, Sartin J. Regulation and secretion of proopiomelanocortin peptides from isolated perfused dog pituitary pars intermedia cells. Endocrinology. 1989;124(5):2208–2217. doi: 10.1210/endo-124-5-2208. [DOI] [PubMed] [Google Scholar]
- 79.Kemppainen RJ, Peterson ME. Regulation of alpha-melanocyte-stimulating hormone secretion from the pars intermedia of domestic cats. Am J Vet Res. 1999;60(2):245–249. [PubMed] [Google Scholar]
- 80.McFarlane D, Dybdal N, Donaldson MT, Miller L, Cribb AE. Nitration and increased alpha-synuclein expression associated with dopaminergic neurodegeneration in equine pituitary pars intermedia dysfunction. J Neuroendocrinol. 2005;17(2):73–80. doi: 10.1111/j.1365-2826.2005.01277.x. [DOI] [PubMed] [Google Scholar]
- 81.McFarlane D. Advantages and limitations of the equine disease, pituitary pars intermedia dysfunction as a model of spontaneous dopaminergic neurodegenerative disease. Ageing Res Rev. 2007;6(1):54–63. doi: 10.1016/j.arr.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 82.Daram SR, Boppidi H. Nonalcoholic fatty liver disease: hepatic manifestation of obesity and the metabolic syndrome. Postgrad Med. 2008;120(2):E01–E07. doi: 10.3810/pgm.2008.07.1800. [DOI] [PubMed] [Google Scholar]
- 83.Lawler DF, Larson BT, Ballam JM, Smith GK, Biery DN, Evans RH, Greeley EH, Segre M, Stowe HD, Kealy RD. Diet restriction and ageing in the dog: major observations over two decades. Br J Nutr. 2008;99(4):793–805. doi: 10.1017/S0007114507871686. [DOI] [PubMed] [Google Scholar]
- 84.Slingerland LI, Robben JH, van Haeften TW, Kooistra HS, Rijnberk A. Insulin sensitivity and beta-cell function in healthy cats: assessment with the use of the hyperglycemic glucose clamp. Horm Metab Res. 2007;39(5):341–346. doi: 10.1055/s-2007-976541. [DOI] [PubMed] [Google Scholar]
- 85.Treiber KH, Kronfeld DS, Hess TM, Boston RC, Harris PA. Use of proxies and reference quintiles obtained from minimal model analysis for determination of insulin sensitivity and pancreatic beta-cell responsiveness in horses. Am J Vet Res. 2005;66(12):2114–2121. doi: 10.2460/ajvr.2005.66.2114. [DOI] [PubMed] [Google Scholar]
- 86.Eiler H, Frank N, Andrews FM, Oliver JW, Fecteau KA. Physiologic assessment of blood glucose homeostasis via combined intravenous glucose and insulin testing in horses. Am J Vet Res. 2005;66(9):1598–1604. doi: 10.2460/ajvr.2005.66.1598. [DOI] [PubMed] [Google Scholar]
- 87.Wiedmeyer CE, Johnson PJ, Cohn LA, Meadows RL, Kerl ME, Tessman RK, Perlis J, DeClue AE. Evaluation of a continuous glucose monitoring system for use in veterinary medicine. Diabetes Technol Ther. 2005;7(6):885–895. doi: 10.1089/dia.2005.7.885. [DOI] [PubMed] [Google Scholar]
- 88.Wiedmeyer CE, Johnson PJ, Cohn LA, Meadows RL. Evaluation of a continuous glucose monitoring system for use in dogs, cats, and horses. J Am Vet Med Assoc. 2003;223(7):987–992. doi: 10.2460/javma.2003.223.987. [DOI] [PubMed] [Google Scholar]
- 89.Cordain L, Miller JB, Eaton SB, Mann N, Holt SH, Speth JD. Plant-animal subsistence ratios and macronutrient energy estimations in worldwide hunter-gatherer diets. Am J Clin Nutr. 2000;71(3):682–692. doi: 10.1093/ajcn/71.3.682. [DOI] [PubMed] [Google Scholar]
- 90.Cordain L, Eaton SB, Sebastian A, Mann N, Lindeberg S, Watkins BA, O'Keefe JH, Brand-Miller J. Origins and evolution of the Western diet: health implications for the 21st century. Am J Clin Nutr. 2005;81(2):341–354. doi: 10.1093/ajcn.81.2.341. [DOI] [PubMed] [Google Scholar]
- 91.Booth FW, Chakravarthy MV, Gordon SE, Spangenburg EE. Waging war on physical inactivity: using modern molecular ammunition against an ancient enemy. J Appl Physiol. 2002;93(1):3–30. doi: 10.1152/japplphysiol.00073.2002. [DOI] [PubMed] [Google Scholar]
- 92.Eaton SB. The ancestral human diet: what was it and should it be a paradigm for contemporary nutrition? Proc Nutr Soc. 2006;65(1):1–6. doi: 10.1079/pns2005471. [DOI] [PubMed] [Google Scholar]