Abstract
Background
Now emerging as an important risk factor for type 1 diabetes, vitamin D deficiency is also associated with obesity, metabolic syndrome, and type 2 diabetes and has been identified as a potential cardiometabolic risk factor. A simple, accurate screening test for 25-hydroxy vitamin D [25(OH)D] deficiency is needed. We developed a liquid chromatography/tandem mass spectrometry assay for 25-hydroxy vitamin D2 [25(OH)D2] and 25-hydroxy vitamin D3 [25(OH)D3] in dried blood spots.
Method
Blood spots were collected by finger stick simultaneously with serum samples obtained by venipuncture from healthy volunteers. Disks punched from the dried blood spots were sonicated with an internal standard solution of deuterated 25(OH)D3 (26,26,26,27,27,27-d6). Methanol was added to precipitate proteins prior to extraction with hexane. The extracted samples were dried and reconstituted in 50:50 methanol:H2O before injection into a Varian 320-MS TQ mass spectrometer.
Results
Blood spot assay precision was good over the reportable range: interassay coefficients of variation were 13, 13, and 11% at concentrations of 14, 26, and 81 ng/ml, respectively, for 25-hydroxy vitamin D3 and 12% at 23 ng/ml for 25(OH)D2. The 25(OH)D3 assay was linear from 3.5 to 75 ng/ml (R > 0.99). Blood spot and serum values showed excellent correlation for 25(OH)D2 (R = 0.90, n = 54) and 25(OH)D3 (R = 0.91, n = 83).
Conclusions
This blood spot assay for 25(OH)D2 and 25(OH)D3 provides a convenient and cost-effective alternative to serum assays and can be automated. This may be valuable in large-scale screening for risk of type 1 diabetes, for cardiometabolic risk screening, and for monitoring vitamin D supplementation
Keywords: blood spot, cardiometabolic risk, diabetes, liquid chromatography, tandem mass spectrometry, screening, vitamin D
Introduction
Vitamin D deficiency is becoming a significant health concern as low blood levels of its main metabolite, 25-hydroxyvitamin D [25(OH)D], have been linked with a growing number of preventable diseases. While extreme deficiency has long been known as the cause of rickets in children and osteomalacia in adults, even nonrachitic levels of deficiency are now known to increase the risk of some autoimmune diseases, particularly type 1 diabetes1 and multiple sclerosis, as well as type 2 diabetes, cardiovascular disease, stroke, some cancers, and infectious diseases.2
Studies have shown that low 25(OH)D levels may contribute to an overall cardiometabolic risk associated with diabetes or hypertension. Two nested case-control studies in Finland with a follow-up of 22 years found higher 25(OH)D levels in men than in women and a significantly reduced incidence of type 2 diabetes in the highest quartile of 25(OH)D levels in the men.3 In New Zealand, a cross-sectional survey of Caucasian and Polynesian workers found significantly lower serum 25-hydroxy vitamin D3 [25(OH)D3] levels in those with type 2 diabetes and impaired glucose tolerance compared with controls, with the lowest levels being found in the Polynesians.4 An Italian study of 459 type 2 diabetic outpatients compared with age- and sex-matched controls found a higher prevalence of hypovitaminosis D in the diabetic outpatients. Among the diabetic outpatients, those classified as having hypovitaminosis D (levels <20 ng/ml) were more likely to be women and to have higher hemoglobin A1c, triglycerides, C-reactive protein, and fibrinogen than those with higher 25(OH)D levels,5 suggesting a greater cardiometabolic risk. Randomized trials are needed to show whether vitamin D supplementation can indeed reduce the risk of diabetes and/or cardiovascular disease.6 Indications are positive: after 20 years of follow-up in 83,779 women in the Nurses' Health Study,7 a comparison of the highest and lowest vitamin D and calcium intake categories showed a 33% lower risk of type 2 diabetes in women taking >1200 mg/day calcium and >800 IU/day vitamin D compared with <600 mg/day and 400 IU/day, respectively.
Type 1 diabetes has been linked consistently with vitamin D deficiency. In Sweden's nationwide Diabetes Incidence Study, plasma 25(OH)D levels were significantly lower in type 1 diabetic individuals at diagnosis than in controls. When levels in men and women were compared, the men had lower levels than women at diagnosis, which may possibly be relevant to the overall higher incidence of type 1 diabetes in men than in women.8 In a study in the sunnier climate of Brisbane, Australia, adolescents with type 1 diabetes were found to be more than three times as likely to have vitamin D deficiency than a control group.9 Both of these studies found that the low 25(OH)D levels in the diabetic patients persisted several years after diagnosis.
A birth-cohort study using data from 10,366 children born in 1966 in northern Finland, 81 of whom had developed diabetes, found a relative risk of 0.12 for the development of type 1 diabetes in children who regularly took 2000 IU/day vitamin D compared to no supplementation and a relative risk of 0.22 compared to those taking less than 2000 IU/day.10 Studies such as this led to recommendations to increase daily vitamin D intake to 2000 IU/day (50 μg/day) in infants and children susceptible to vitamin D deficiency.11–13 A 2008 meta-analysis of five observational studies found a significantly reduced risk of type 1 diabetes in infants receiving vitamin D supplements compared to no supplementation, with the risk being lower with increasing vitamin D dosage.14
Simple methods for screening for vitamin D deficiency are required, both to identify children and adults at risk of diabetes and to establish the potential benefit of supplementation in order to reduce future diabetes and/or cardiometabolic risk.
The naturally occurring form of vitamin D, found in cod liver oil and manufactured endogenously by the action of sunlight on 7-dehydrocholesterol in the skin, is vitamin D3 (cholecalciferol), whereas the predominant prescription form used to treat deficiency in the United States is the plant-derived vitamin D2 (ergocalciferol). These are metabolized in the liver to 25(OH)D3 and 25-hydroxyvitamin D2 [25(OH)D2], respectively. These very similar molecules are widely accepted as the circulating form of the vitamin that best represents functional vitamin D status.
Assays for 25(OH)D have been hampered by its extremely hydrophobic nature, its association with vitamin D binding protein in blood, and the very close similarity between 25(OH)D 2and 25(OH)D3, all of which have contributed to significant variations between laboratories and between methods.15 We have developed a method for assaying dried blood spots using liquid chromatography/tandem mass spectrometry (LC-MS/MS), a method that has been found to distinguish accurately between 25(OH)D 2and 25(OH)D 3in serum.16,17 Because serum is the standard medium available to health care providers for the assessment of 25(OH)D levels, serum was, therefore, used as a reference for comparison with blood spot samples in our LC-MS/MS assay.
Methods
Blood spots were collected by finger stick from healthy volunteers, dropped onto filter paper cards (Whatman), and dried. A serum sample was obtained simultaneously from each volunteer by venipuncture. Four 6-mm disks were punched from the dried blood spots (Wallac Multipuncher) into glass tubes. According to Whatman 510(k) product literature, each 6-mm disk from a dried blood spot contains the equivalent of approximately 4.9 μl of serum. For the serum assay, 10 μl of serum was pipetted directly into the tubes. Extraction prior to the assay was carried out as follows: with the exception of the 30-minute sonication, serum and blood spots were treated in the same manner. The tubes were vortexed and sonicated for 30 minutes after adding 500 μl of an aqueous solution of deuterated d6-25(OH)D3 (26,26,26,27,27,27-d6, Medical Isotopes, Inc., Pelham, NH) as an internal standard (IS). To precipitate proteins in the sample, 500 μl of methanol was added to each tube. Hexane (1 ml) was added to extract the lipophilic compounds, and samples were vortexed briefly. After centrifuging the tubes at 1500 g for 10 minutes, the hexane layer was removed and evaporated. Samples were reconstituted by adding 200 μl of 50% methanol and were transferred to 1-ml vials ready for injection. The LC-MS/MS was performed using a Varian 320-MS TQ mass spectrometer, connected to a Varian 212-LC binary pump system and a Varian 430 autosampler with cooling capability (Varian Inc., Palo Alto, CA). A Varian Pursuit 3u PFP 50 × 2.0-mm column with an attached appropriate Metaguard pre-column was used at a flow rate of 0.2 ml/min at 50°C. The mobile phase contained 0.1% formic acid and 2 mM ammonium acetate and ramped from 70 to 95% methanol over the 10-minute run. 25(OH)D3, 25(OH)D2, and d6-25(OH)D3 (IS) were analyzed by electrospray ionization tandem mass spectrometry in the positive-ion mode and the conditions were as follow: housing temperature, 55°C; manifold temperature, 42°C; needle voltage, 5400 V; spray shield voltage, 600 V; drying gas temperature, 200°C; drying gas pressure, 25.0 psi; nebulizer pressure, 60.0 psi; and collision-induced dissociation gas pressure, 2.00 mTorr. Capillary voltages were 40.7 V for 25(OH)D3, 39.1 V for d6-25(OH)D 3(IS), and 37.6 V for 25(OH)D2, and the monitored transitions included m/z 401.3 → 365.3 and 383.3 with collision energies (CE) of 8 and 7 V, respectively, for 25(OH)D3, 407.3 → 371.3 and 389.3 with CE of 8.5 and 7.5 V, respectively, for IS, and 413.3 → 377.3 and 395.3 with CE of 7.5 and 7.5 V, respectively, for 25(OH)D2.
To assess interassay precision, one replicate of each sample was prepared per day over a span of 8 days. Samples were stored at –20°C and extracted each day for assay as described earlier.
Results
The levels of 25(OH)D2 and 25(OH)D3in the blood spots were calibrated to equivalent serum levels using six serum samples and blood spots collected at the same time as the sera from those same individuals. These samples were chosen from the pool of healthy volunteer samples to represent a range between 10 and 100 ng/ml for 25(OH)D 3and between 2 and 36 ng/ml for 25(OH)D2. The serum assay was validated internally and found to be linear to over 1000 ng/ml. Results from our LC-MS/MS serum assay correlated well (R2 = 0.99) with values obtained for the same samples sent to other accredited laboratories. In addition, we participate in the Vitamin D External Quality Assessment Scheme, which provides pooled serum control samples for the purpose of optimizing assay performance and monitoring accuracy.
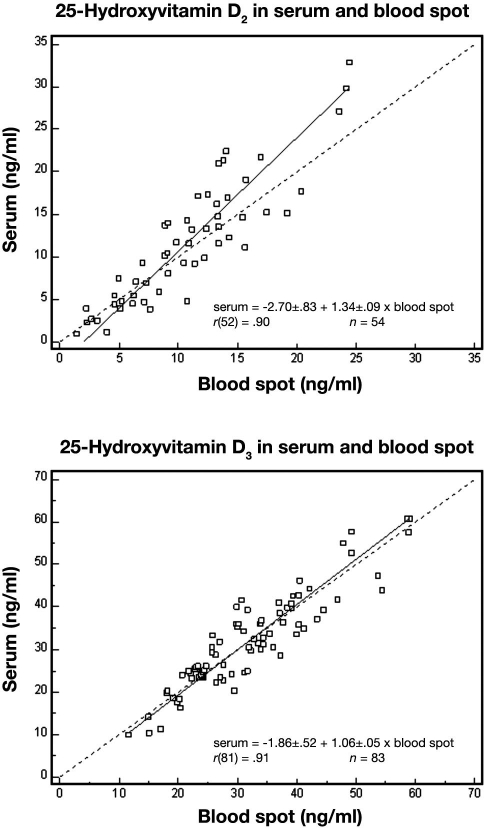
Precision of the blood spot assay was good over the reportable range: interassay coefficients of variation were 13, 13, and 11% at concentrations of 14, 26, and 81 ng/ml, respectively, for 25(OH)D3 and 12% at 23 ng/ml for 25(OH)D2. The 25(OH)D3 assay was linear in blood spot samples from 3.5 to 75 ng/ml (R > 0.99). Blood spot and serum values showed excellent correlation (see Figure 1) for 25(OH)D2 (R = 0.90, n = 54) and 25(OH)D3 (R = 0.91, n = 83).
Figure 1.
Correlation between simultaneously collected blood spot and serum levels of 25-hydroxyvitamin D2 and 25-hydroxyvitamin D3 in healthy volunteers. In each case, the dotted line represents identity and the solid line is the Deming fit.
A Deming regression (using MedCalc 9.2.1) for 25(OH)D3 indicated that the intercept [–1.86 ± 1.52, 95% confidence interval (CI) –4.89 to 1.16] did not depart significantly from 0 and that the slope (1.06 ± 0.05, 95% CI 0.96 to 1.16) did not depart significantly from 1. However, for 25(OH)D2, both the intercept (–2.70 ± 0.83, 95% CI –4.36 to –1.04) and the slope (1.34 ± 0.09, 95% CI 1.17 to 1.51) departed significantly from identity. This latter finding suggests the need for a 25(OH)D2 conversion (blood spot to serum) for application purposes.
Dried blood spot samples from one volunteer, which were stored at room temperature, showed no significant loss of analyte detection on repeated analysis over more than 4 months (see Table 1).
Table 1.
To Assess Sample Stability, 25(OH)D2 and 25(OH)D3 Levels in Blood Spots Stored at Room Temperature Were Assayed on 12 Occasions over a 4-Month Perioda
| Days after sample collection | 25(OH)D2 concentration (ng/ml) | 25(OH)D3 concentration (ng/ml) |
|---|---|---|
| 0 | 10.8 | 5.1 |
| 6 | 11.3 | 6.2 |
| 35 | 10.4 | 5.8 |
| 47 | 11.3 | 4.7 |
| 64 | 10.8 | 5.4 |
| 68 | 11.3 | 4.6 |
| 78 | 12.3 | 6.1 |
| 83 | 12.5 | 5.6 |
| 89 | 11.6 | 6.3 |
| 98 | 13.2 | 5.7 |
| 102 | 13.0 | 4.9 |
| 125 | 10.7 | 6.5 |
| Mean (coefficient of variation) | 11.6 (8.1%) | 5.6 (11.7%) |
Blood spots were all obtained from one volunteer, who provided enough blood samples for this long-term study.
Discussion
Vitamin D deficiency has been linked with hypertension, diabetes mellitus, obesity, metabolic syndrome, and cardiometabolic risk18–20; significant inverse correlations were seen between 25(OH)D levels and abdominal adiposity, hypertriglyceridemia, and hyperglycemia in one large population study, which showed increasing odds of having metabolic syndrome as vitamin D levels decreased.20 The association of vitamin D with cardiovascular disease including stroke and congestive heart failure through diabetes and hypertension has also been suggested.6 Precise mechanisms by which vitamin D can protect against the development of both type 1 and type 2 diabetes are still being elucidated. Vitamin D deficiency impairs insulin synthesis and secretion, possibly as a result of the role of vitamin D in the regulation of plasma calcium levels, as well as its direct effects on pancreatic β cells, and high doses of vitamin D have been protective against autoimmune diseases in animal models, suggestive of a role in immune modulation.21–24
The present study aimed at developing a simple, convenient, and accurate method for measuring vitamin D in dried blood spot. The ability to use blood spot samples has significant advantages over conventional serum assays. Blood spots can be collected conveniently at home and, once dry, are stable at room temperature for several weeks prior to analysis. The low sample volume required makes this method ideal also for pediatric screening, as enough sample can be obtained during routine heel stick procedures in infants. Vitamin D deficiency is of particular concern in infants because of inadequate levels in breast milk as a result of widespread deficiency in nursing mothers, especially those who are dark-skinned,25 which has implications for risk of type 1 diabetes and other autoimmune diseases in childhood. The potential risk of subsequent development of type 1 diabetes in infants and children who have a deficiency in 25(OH)D has resulted in recommendations for vitamin D supplementation in nursing mothers as well as infants and children.13,14
Of particular advantage for the laboratory staff involved in blood collection and analysis, dried blood spots present only a minimal biohazard, as most infectious agents are inactivated by drying. Moreover, this allows for ease of shipment throughout the world without the requirements for biohazard labeling and packaging necessary for a liquid blood sample. The dried blood spot methodology is applicable to large population studies for epidemiological research, as well as for regular monitoring of vitamin D status in people at risk of deficiency, particularly in remote areas where access to a phlebotomy service is difficult.
Our LC-MS/MS method using dried blood spot samples showed excellent sensitivity and precision and a good correlation between serum and blood spot levels in the same individuals. The effect of an abnormal hematocrit on this correlation was not explored in this study, but because of the possible implication of red blood cell concentration in an assay involving dried whole blood, we did an experiment on samples containing a range of red blood cell concentrations and a known amount of 25(OH)D3. We found very little change in levels within a range of 40–60% hematocrit, covering the range that would be seen in a normal population. Other studies have shown good serum/blood spot correlations and <±10% effect of hematocrit on assay results within a hematocrit range of 0.31 to 0.61 for prostate-specific antigen26 and within a hematocrit range of 0.2 to 0.62 for insulin-like growth factor (IGF) I and IGF-binding protein III.27 Variations in hematocrit should have little relevance for vitamin D screening, which is used most often in a relatively healthy population: vitamin D deficiency generally has no immediate symptoms, whereas correcting a deficiency can help prevent more serious future health issues.
Like most LC-MS/MS assays, our method does not differentiate between 25(OH)D and its C-3 epimer. Using an adapted LC-MS/MS assay, this epimer has been found to represent between 8.7 and 61.1% of the total 25(OH)D in 22.7% of infants less than 1 year old, but not in older infants or adults, undergoing routine testing at the Mayo Clinic.17 However, its prevalence in a population of healthy infants and its clinical relevance have not been fully explored. We have also been able to adapt our LC-MS/MS assay using an attenuated run time, which separates out the C-3 epimer. This adaptation may be useful for determining the significance of the presence of this epimer when studying children under 1 year old.
Differentiation between 25(OH)D2and 25(OH)D3 is important for monitoring vitamin D therapy, as vitamin D2 is the predominant prescription form. The half-life of 25(OH)D2 is shorter than that of 25(OH)D3 and it binds less well to the vitamin D binding protein, making it less potent and, therefore, required to be administered at much higher doses than vitamin D3.28 Some currently used assays have a diminished capacity to detect 25(OH)D2, which can lead to dangerous overdosing when attempting to monitor therapy with vitamin D2.15,28 The ability to distinguish the two forms is, therefore, necessary to monitor therapy and avoid toxicity, particularly when very large doses of vitamin D2 are given by injection biweekly or monthly, resulting in temporarily very high serum levels.
It may also be significant, when assessing the value of retrospective population studies, to pay attention to the form of vitamin D used for supplementation and whether the assay used was able to detect both forms in stored serum samples. For example, a study of a small sample from two similar populations in Finland and in the neighboring Karelian Republic of Russia found no difference in serum vitamin D levels, despite an overall sixfold higher incidence of type 1 diabetes in the Finnish population in general.29 Vitamin D supplementation is strongly recommended in both areas because of their northern latitude, but the study did not indicate whether this was mostly vitamin D3 (found in cod liver oil, used widely in Scandinavia) or vitamin D2, and the number of children sampled who actually went on to develop diabetes is not yet known. The assay used in the study is advertised as detecting 75% of 25(OH)D2 and 100% of 25(OH)D3. If the Russian children were supplemented with vitamin D2, the measured 25(OH)D levels could, therefore, be an underestimate of their blood levels. While the specifics of supplements used in these areas have not been reported, it is nevertheless a factor to be considered when examining vitamin D status in supplementing populations. An assay that detects 100% of both 25(OH)D2 and 25(OH)D3 accurately helps researchers assess the actual vitamin D status of such populations more objectively.
Whether conducting research on the epidemiology of diabetes and cardiometabolic risk and its connection with vitamin D status or establishing an adequate vitamin D supplementation regimen for patients either at risk of or already having diabetes, a simple test for vitamin D status is a potential asset to both the patient and the health care professional. The blood spot assay described here combines convenient, minimally invasive testing with an accurate determination of both vitamin D2 and vitamin D3 levels, giving the clinician a full picture of a patient's vitamin D status and ensuring that blood levels are optimal.
Conclusion
This LC-MS/MS method, with its excellent reliability in blood spot samples, combined with the convenience of blood spot sample collection, has enormous potential as a screening tool for diabetes and cardiometabolic risk and can be used for large-scale epidemiological studies. Moreover, the ability to distinguish between 25(OH)D 2and 25(OH)D3 allows accurate monitoring of vitamin D supplementation in adults, nursing mothers, and young children who are deficient, as well as providing reliable assessments of actual vitamin D status in supplementing populations.
Acknowledgment
The authors thank Danielle Moore of ZRT Laboratory for her help with collection and organization of samples for this study.
Abbreviations
- CE
collision energy
- CI
confidence interval
- IGF
insulin-like growth factor
- IS
internal standard
- LC-MS/MS
liquid chromatography/tandem mass spectrometry, [25(OH)D] 25-hydroxy vitamin D, [25(OH)D2] 25-hydroxy vitamin D2, [25(OH)D3] 25-hydroxy vitamin D3
References
- 1.Mathieu C, Badenhoop K. Vitamin D and type 1 diabetes mellitus: state of the art. Trends Endocrinol Metab. 2005;16(6):261–266. doi: 10.1016/j.tem.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 2.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 3.Knekt P, Laaksonen M, Mattila C, Härkänen T, Marniemi J, Heliövaara M, Rissanen H, Montonen J, Reunanen A. Serum vitamin D and subsequent occurrence of type 2 diabetes. Epidemiology. 2008;19(5):666–671. doi: 10.1097/EDE.0b013e318176b8ad. [DOI] [PubMed] [Google Scholar]
- 4.Scragg R, Holdaway I, Singh V, Metcalf P, Baker J, Dryson E. Serum 25-hydroxyvitamin D3 levels decreased in impaired glucose tolerance and diabetes mellitus. Diabetes Res Clin Pract. 1995;27(3):181–188. doi: 10.1016/0168-8227(95)01040-k. [DOI] [PubMed] [Google Scholar]
- 5.Cigolini M, Iagulli MP, Miconi V, Galiotto M, Lombardi S, Targher G. Serum 25-hydroxyvitamin D3 concentrations and prevalence of cardiovascular disease among type 2 diabetic patients. Diabetes Care. 2006;29(3):722–724. doi: 10.2337/diacare.29.03.06.dc05-2148. [DOI] [PubMed] [Google Scholar]
- 6.Michos ED, Melamed ML. Vitamin D and cardiovascular disease risk. Curr Opin Clin Nutr Metab Care. 2008;11(1):7–12. doi: 10.1097/MCO.0b013e3282f2f4dd. [DOI] [PubMed] [Google Scholar]
- 7.Pittas AG, Dawson-Hughes B, Li T, Van Dam RM, Willett WC, Manson JE, Hu FB. Vitamin D and calcium intake in relation to type 2 diabetes in women. Diabetes Care. 2006;29(3):650–656. doi: 10.2337/diacare.29.03.06.dc05-1961. [DOI] [PubMed] [Google Scholar]
- 8.Littorin B, Blom P, Schölin A, Arnqvist HJ, Blohmé G, Bolinder J, Ekbom-Schnell A, Eriksson JW, Gudbjörnsdottir S, Nyström L, Ostman J, Sundkvist G. Lower levels of plasma 25-hydroxyvitamin D among young adults at diagnosis of autoimmune type 1 diabetes compared with control subjects: results from the nationwide Diabetes Incidence Study in Sweden (DISS) Diabetologia. 2006;49(12):2847–2852. doi: 10.1007/s00125-006-0426-x. [DOI] [PubMed] [Google Scholar]
- 9.Greer RM, Rogers MA, Bowling FG, Buntain HM, Harris M, Leong GM, Cotterill AM. Australian children and adolescents with type 1 diabetes have low vitamin D levels. Med J Aust. 2007;187(1):59–60. doi: 10.5694/j.1326-5377.2007.tb01130.x. [DOI] [PubMed] [Google Scholar]
- 10.Hyppönen E, Läärä E, Reunanen A, Järvelin MR, Virtanen SM. Intake of vitamin D and risk of type 1 diabetes: a birth-cohort study. Lancet. 2001;358(9292):1500–1503. doi: 10.1016/S0140-6736(01)06580-1. [DOI] [PubMed] [Google Scholar]
- 11.Harris S. Can vitamin D supplementation in infancy prevent type 1 diabetes? Nutr Rev. 2002;60(4):118–121. doi: 10.1301/00296640260085868. [DOI] [PubMed] [Google Scholar]
- 12.Harris SS. Vitamin D and type 1 diabetes. Am J Clin Nutr. 2004;79(5):889–890. doi: 10.1093/ajcn/79.5.889. author reply 890. [DOI] [PubMed] [Google Scholar]
- 13.Harris SS. Vitamin D in type 1 diabetes prevention. J Nutr. 2005;135(2):323–325. doi: 10.1093/jn/135.2.323. [DOI] [PubMed] [Google Scholar]
- 14.Zipitis CS, Akobeng AK. Vitamin D supplementation in early childhood and risk of type 1 diabetes: a systematic review and meta-analysis. Arch Dis Child. 2008;93(6):512–517. doi: 10.1136/adc.2007.128579. [DOI] [PubMed] [Google Scholar]
- 15.Hollis BW. Editorial: The determination of circulating 25-hydroxyvitamin D: no easy task. J Clin Endocrinol Metab. 2004;89(7):3149–3151. doi: 10.1210/jc.2004-0682. [DOI] [PubMed] [Google Scholar]
- 16.Saenger AK, Laha TJ, Bremner DE, Sadrzadeh SM. Quantification of serum 25-hydroxyvitamin D(2) and D(3) using HPLC-tandem mass spectrometry and examination of reference intervals for diagnosis of vitamin D deficiency. Am J Clin Pathol. 2006;125(6):914–920. doi: 10.1309/J32U-F7GT-QPWN-25AP. [DOI] [PubMed] [Google Scholar]
- 17.Singh RJ, Taylor RL, Reddy GS, Grebe SK. C-3 epimers can account for a significant proportion of total circulating 25-hydroxyvitamin D in infants, complicating accurate measurement and interpretation of vitamin D status. J Clin Endocrinol Metab. 2006;91(8):3055–3061. doi: 10.1210/jc.2006-0710. [DOI] [PubMed] [Google Scholar]
- 18.Botella-Carretero JI, Alvarez-Blasco F, Villafruela JJ, Balsa JA, Vázquez C, Escobar-Morreale HF. Vitamin D deficiency is associated with the metabolic syndrome in morbid obesity. Clin Nutr. 2007;26(5):573–580. doi: 10.1016/j.clnu.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 19.Schwalfenberg G. Vitamin D and diabetes: improvement of glycemic control with vitamin D3 repletion. Can Fam Physician. 2008;54(6):864–866. [PMC free article] [PubMed] [Google Scholar]
- 20.Ford ES, Ajani UA, McGuire LC, Liu S. Concentrations of serum vitamin D and the metabolic syndrome among U.S. adults. Diabetes Care. 2005;28(5):1228–1230. doi: 10.2337/diacare.28.5.1228. [DOI] [PubMed] [Google Scholar]
- 21.Mathieu C, Gysemans C, Giulietti A, Bouillon R. Vitamin D and diabetes. Diabetologia. 2005;48(7):1247–1257. doi: 10.1007/s00125-005-1802-7. [DOI] [PubMed] [Google Scholar]
- 22.Mathieu C, Badenhoop K. Vitamin D and type 1 diabetes mellitus: state of the art. Trends Endocrinol Metab. 2005;16(6):261–266. doi: 10.1016/j.tem.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 23.Luong K, Nguyen LT, Nguyen DN. The role of vitamin D in protecting type 1 diabetes mellitus. Diabetes Metab Res Rev. 2005;21(4):338–346. doi: 10.1002/dmrr.557. [DOI] [PubMed] [Google Scholar]
- 24.Palomer X, González-Clemente JM, Blanco-Vaca F, Mauricio D. Role of vitamin D in the pathogenesis of type 2 diabetes mellitus. Diabetes Obes Metab. 2008;10(3):185–197. doi: 10.1111/j.1463-1326.2007.00710.x. [DOI] [PubMed] [Google Scholar]
- 25.Hollis BW, Wagner CL. Assessment of dietary vitamin D requirements during pregnancy and lactation. Am J Clin Nutr. 2004;79(5):717–726. doi: 10.1093/ajcn/79.5.717. [DOI] [PubMed] [Google Scholar]
- 26.Hoffman BR, Yu H, Diamandis EP. Assay of prostate-specific antigen from whole blood spotted on filter paper and application to prostate cancer screening. Clin Chem. 1996;42(4):536–544. [PubMed] [Google Scholar]
- 27.Diamandi A, Khosravi MJ, Mistry J, Martinez V, Guevara-Aguirre J. Filter paper blood spot assay of human insulin-like growth factor I (IGF-I) and IGF-binding protein-3 and preliminary application in the evaluation of growth hormone status. J Clin Endocrinol Metab. 1998;83(7):2296–2301. doi: 10.1210/jcem.83.7.4923. [DOI] [PubMed] [Google Scholar]
- 28.Houghton LA, Vieth R. The case against ergocalciferol (vitamin D2) as a vitamin supplement. Am J Clin Nutr. 2006;84(4):694–697. doi: 10.1093/ajcn/84.4.694. [DOI] [PubMed] [Google Scholar]
- 29.Viskari H, Kondrashova A, Koskela P, Knip M, Hyöty H. Circulating vitamin D concentrations in two neighboring populations with markedly different incidence of type 1 diabetes. Diabetes Care. 2006;29(6):1458–1459. doi: 10.2337/dc06-2559. [DOI] [PubMed] [Google Scholar]