Abstract
Introduction
AIDA is an interactive educational diabetes simulator that has been available without charge via the Internet for over 12 years. Recent articles have described the incorporation of a novel generic model of insulin absorption into AIDA as a way of enhancing its capabilities. The basic model components to be integrated have been overviewed, with the aim being to provide simulations of regimens utilizing insulin analogues, as well as insulin doses greater than 40 IU (the current upper limit within the latest release of AIDA [v4.3a]). Some preliminary calculated insulin absorption results have also recently been described.
Methods
This article presents the first simulated plasma insulin profiles from the integration of the generic subcutaneous insulin absorption model, and the currently implemented model in AIDA for insulin disposition. Insulin absorption has been described by the physiologically based model of Tarín and colleagues. A single compartment modeling approach has been used to specify how absorbed insulin is distributed in, and eliminated from, the human body. To enable a numerical solution of the absorption model, a spherical subcutaneous depot for the injected insulin dose has been assumed and spatially discretized into shell compartments with homogeneous concentrations, having as its center the injection site. The number of these compartments will depend on the dose and type of insulin. Insulin inflow arises as the sum of contributions to the different shells. For this report the first bench testing of plasma insulin determinations has been done.
Results
Simulated plasma insulin profiles are provided for currently available insulin preparations, including a rapidly acting insulin analogue (e.g., lispro/Humalog or aspart/Novolog), a short-acting (regular) insulin preparation (e.g., Actrapid), intermediate-acting insulins (both Semilente and neutral protamine Hagedorn types), and a very long-acting insulin analogue (e.g., glargine/Lantus), as well as for insulin doses up to 50 IU.
Discussion
The methodology to be adopted for implementing the generic absorption model within AIDA has been overviewed, and the first plasma insulin profiles based on this approach have been demonstrated. Ideas for future work and development are discussed. It is expected that an updated release of AIDA (v4.5), based on this collaborative approach, will become available for free—in due course—via the www.2aida.org Web site. Readers who wish to be informed when the new software is launched can join the very low volume AIDA announcement list by sending a blank email note to subscribe@2aida.org.
Keywords: absorption, computer, diabetes, insulin, model, simulation, software
Introduction
Diabetes clinical trials have clearly shown the need for intensive insulin therapy in order to maintain good glycemic control and reduce complications in patients with insulin-dependent (type 1) diabetes mellitus.1 However, such studies have also demonstrated the necessity for close attention to intensive insulin treatment, since this may lead to an increased risk of hypoglycemic episodes. Information technology may play an important role in optimizing subcutaneous insulin injection therapy. The ability to simulate the glucose–insulin regulatory system may be particularly useful to help patients understand management principles and acquire the skills needed to care for their disease efficiently and safely.
AIDA is an interactive educational diabetes simulator that has been in widespread general use for well over a decade.2–5 It has been positively assessed by a wide variety of different types of users.5,6 The software is available without charge from the www.2aida.org Web site as a noncommercial contribution to continuing diabetes education.
Although a range of other interactive simulation programs of glucose–insulin interaction in diabetes have been described in the literature,7–15 to date most of these do not seem to have been distributed so widely via the Internet, or been made particularly widely available. Indeed, in a number of cases it would seem that readers are entirely dependent on the authors' own descriptions of their prototypes in research articles, since no versions appear to be available for general use by others.9–12
By contrast, with AIDA, versions of the software have been freely available on the Web since 1996, and before that made available to researchers on diskette.16–18 This has led to a substantial experience with the software, worldwide, with well over 250,000 downloads of the program taking place since the program's original Internet launch.
AIDA's Glucoregulatory Model Structure
The glycemic response of an insulin-treated diabetic patient goes through transitory phases, leading to what may be considered a steady state blood glucose profile following a change in either the insulin regimen or the diet. The purpose of the AIDA approach has been to simulate these nontransient glycemic profiles along with the resulting plasma insulin responses over time. The simulation of these processes requires a mathematical model that describes insulin and glucose dynamics, and the manner in which these substances interact.
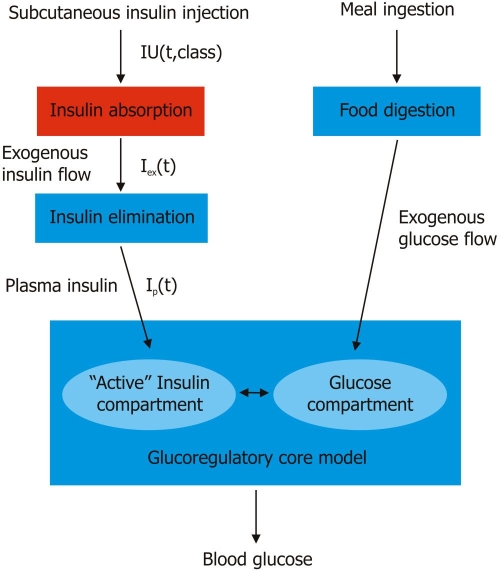
The AIDA model consists of interrelated glucose and insulin submodels (Figure 1) reflecting how insulin controls blood glucose levels. The submodel for insulin absorption following subcutaneous injections contains descriptions of insulin disposition (pharmacokinetics) and effect (pharmacodynamics). Modeling of glucose kinetics requires equations for glucose absorption and production/utilization in different organs/tissues. Eventually it is also envisaged in a future version of AIDA that equations which specify how blood glucose levels affect endogenous insulin secretion in noninsulin-dependent (type 2) diabetic patients will also be required.
Figure 1.
Block diagram of the overall AIDA v4 model structure3 incorporating the generic subcutaneous insulin absorption submodel described by Tarín and colleagues.19 IU = injection of international units of a class of insulin preparation. t = time. Iex = exogenous insulin flow profile. Ip = plasma insulin.
Since AIDA's original launch, new insulin analogues have appeared on the market and these are now in widespread general use. Inclusion of the whole spectrum of currently available insulin preparations within AIDA is of importance for maintaining its usability. For this purpose, a new absorption model able to cope with rapidly acting insulin analogues, as well as very-long acting insulin analogues, is being incorporated within AIDA. The adopted model should provide a suitable framework to characterize people with diabetes quantitatively, for educational purposes, and predict the blood glucose profile produced by an adjustment in diet and/or insulin dosage regimen with current therapies.
The model described draws on the generic subcutaneous Tarín model of insulin absorption,19,20 overviewed in earlier reports,21,22 and the Berger–Rodbard model8 of insulin kinetics that have been implemented in conjunction with a model of glucose dynamics, which describes the temporal evolution of the concentration of glucose in the blood.
In this latter glucose model, it is considered that glucose enters the bloodstream via intestinal absorption and hepatic glucose production, with glucose removal from the extracellular space occurring primarily by utilization in the various organs and tissues (liver and periphery). Additionally, glucose disappears by renal excretion if its concentration lies above a renal threshold. These glucose fluxes usually are complex functions of glucose and insulin levels.3 Plasma insulin is assumed to control glucose metabolism in the liver, while “active” insulin controls peripheral glucose uptake, mostly in muscle and adipose tissues.8
About Currently Available Insulin Preparations
It may be helpful to overview certain aspects of the insulin preparations that are intended for simulation in the updated AIDA software. Clearly insulin analogues, which have been developed over the last decade, need to be considered in order to modify the pharmacokinetics of injected insulin catered for within AIDA. These insulin analogues modify the structure of the human insulin molecule, to either delay or shorten the absorption time.
As reviewed by Bergenstal,23 when formulated for injection, regular insulin is present in solution as a hexamer. The insulin hexamer dissociates into dimers or monomers that are the forms which are efficiently absorbed into the bloodstream. It is the dissociation process, which takes 30–60 minutes, that determines the onset time and action curve of regular insulin.23
Various rapidly acting injectable insulins are available commercially (e.g., lispro and aspart), the amino acid changes for which result in alterations to the configuration of the insulin hexamer and changes in the molecular binding/association forces, generating a more weakly associated hexamer. Thus although each rapidly acting analogue still aggregates into an insulin hexamer, the analogue hexamer has a more rapid dissociation rate than regular insulin when injected into the subcutaneous tissue. This rapid dissociation of the hexamer into dimeric and monomeric forms leads to the more rapid onset, peak, and shorter duration of action of the rapidly acting insulin analogues.23
The time–action curves of regular insulins or rapidly acting insulin analogues can be prolonged in various ways that either (i) slow the absorption rate from the subcutaneous tissue or (ii) slow the clearance rate from the bloodstream.23 If either excess zinc (lente insulin) or the protein protamine [neutral protamine Hagedorn (NPH) insulin, or protamine suspensions of insulin lispro or aspart] is added to regular insulin or rapidly acting insulin analogue formulations, this reduces the rate of insulin absorption from the subcutaneous tissue into the bloodstream.23 Semilente insulin is an amorphous formulation of insulin with zinc added that exhibits a prompt intermediate-acting profile. Ultralente has been available in AIDA v4 since the outset.2,16 With ultralente, although there may be some insulin present 24 hours after injection, it is not sufficient to provide adequate basal coverage. Insulin glargine provides much better 24-hour coverage.23 Lente insulin is a preformed mixture of 30% Semilente insulin and 70% ultralente insulin.
As highlighted by Bergenstal,23 glargine is created by recombinant DNA techniques, resulting in changes in the amino acid structure of regular insulin. The very long-acting time–action curve of glargine is due to the fact that it has an isoelectric point such that in solution it has an acidic pH and when injected into the neutral pH of the subcutaneous tissue it forms microprecipitants, which are gradually absorbed over approximately 24 hours. Glargine, because of its acidic pH, cannot be mixed with other insulin preparations and must be injected separately and away from any other insulin being injected at the same time.23 Table 1 shows insulin action times for currently commonly available insulin preparations.
Table 1.
Standard Insulin Action Times of Currently Commonly Available Insulin Preparations (Although Considerable Intraindividual Variation Is Recognized)a
| Insulin | Onset of action | Peak action | Duration of clinically effective action |
|---|---|---|---|
| Rapidly acting | |||
| Lispro (Humalog®) | |||
| Aspart (Novolog®) | 5–15 min | 1–2 h | 4 h |
| Glulisine (Aprida®) | |||
| Short acting | |||
| Regular (Novolin®; Humulin®) | 30–45 min | 2–3 h | 5–8 h |
| Intermediate acting | |||
| NPH or lente | 2–4 h | 4–8 h | 10–16 h |
| Long acting | |||
| Ultralente (Humulin® U) | 3–5 h | 8–12 h | 16–20 h |
| Very long acting | |||
| Glargine (Lantus®) | 2 h | 8–16 h | 24 h |
| Insulin detemir | 3–4 h | 6–8 h | 20 h |
Derived from Bergenstal,23 with the kind approval of the publishers.Copyright © 2004, John Wiley & Sons Limited.Reproduced with permission.
Integrating a New, Generic Insulin Absorption Model into AIDA
An earlier report21 described the model components to be integrated to enhance the utility of the AIDA software, with the aim being to provide enhanced functionality and educational simulations of regimens utilizing insulin analogues, as well as insulin doses greater than 40 units.
The newly adopted insulin absorption model19,20 takes into consideration all the facts described in the previous section to derive a physiological generic model able to cope with most common insulin preparations and insulin analogues. A previous article22 provided some preliminary subcutaneous insulin absorption bench testing results for exogenous insulin flow data using the updated modeling approach. Subcutaneous injections of currently available insulin preparations, including rapidly acting insulin analogues (such as lispro/Humalog or aspart/Novolog), regular insulin preparations (e.g., Actrapid), intermediate-acting insulins (both Semilente and NPH types), and very long-acting insulin analogues (such as glargine/Lantus), were studied.22
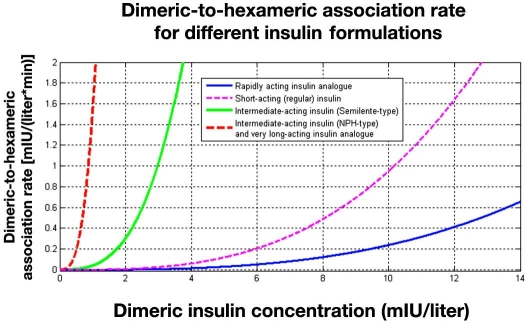
Hexameric and dimeric insulin dynamics are one of the main processes in the model that determine the onset time and action curve of the different insulin preparations. Figure 2 shows the dimeric-to-hexameric association rate for different insulin formulations as computed by the generic insulin absorption model adopted in AIDA.
Figure 2.
Graph summarizing the dimeric–hexameric association process utilized in the generic subcutaneous insulin absorption model to be implemented in AIDA v4.5. The resulting association rate is shown for the different insulin formulations. The association rate for an intermediate-acting insulin (NPH-type) preparation and a very long-acting insulin analogue is substantially greater than for a short-acting (regular) insulin preparation and a rapidly acting insulin analogue.
Furthermore, glargine microprecipitation is addressed in the novel absorption model by considering a new insulin state that degrades into hexameric form following a first order process with saturation. Diffusion in the subcutaneous tissue is also taken into account in the model.
By adding an adequate model of insulin kinetics/elimination, such as the one currently used in AIDA,3 simulated plasma insulin profiles may also be determined. In this article the first plasma insulin profiles are presented from integration of the generic subcutaneous insulin absorption model, and the currently implemented model in AIDA for insulin kinetics.
Methods
In people with insulin-dependent (type 1) diabetes, insulin inflow comes from subcutaneous injections; insulin being distributed in, and eliminated from, the blood. As shown in Figure 1, the output of the absorption model (Iex) represents the input (insulin inflow) into the systemic circulation, which can be calculated numerically for the different insulin classes according to the dose that has been injected.
Computing Plasma Insulin Levels Following a Single Injection
The kinetics of subcutaneous insulin absorption are described by the model of Tarín and colleagues.19 The differential equations constituting this physicochemically based model have been overviewed elsewhere.21 For AIDA a compartmental insulin model has been chosen to model insulin disposition. This contains separate compartments for plasma and “active” insulin. Insulin is removed from the plasma insulin compartment by hepatic degradation, while the “active” insulin compartment affects glycemic control and mimics the time delay of insulin action affecting various metabolic processes.8
The plasma insulin level, Ip, evolves according to the following differential equation:
| (1) |
where ke is the first order rate constant of insulin elimination, I exis the rate of insulin absorption (i.e., the output of the generic subcutaneous insulin absorption model [in international units (IU)/min]), BW is the patient's body weight, and Vi is the relative volume of insulin distribution (liters/kg), which comprises both plasma and interstitial spaces. Time is expressed in minutes.
The solution of Equation (1) specifies how plasma insulin evolves over time, provided that the dose and type of the insulin preparation have been specified and I exis known as the result of the numerical integration of the generic subcutaneous insulin absorption model. It is noted that Equation (1) only refers to insulin-dependent (type 1) diabetes mellitus; endogenous insulin secretion is omitted at present from this portion of the model.
Equation (2) is introduced to specify the buildup and deactivation of the “active” insulin pool, Ia, given as:
| (2) |
where k1 and k2 are first order rate constants used to account for the delay in insulin action. Representative parameters for Equations (1) and (2) have been taken from Berger and Rodbard8: ke = 0.090 min−1, k1 = 0.025 min−1, k2 = 0.021 min−1, and Vi = 0.142 l kg−1. Unless specified otherwise, a representative body weight of 70 kg has been used for the simulations shown in this article.
Computing Plasma Insulin Levels Corresponding to an Insulin Management Regimen
As will be evident from Equation (1), plasma insulin disposition is assumed to obey first order (linear) kinetics. This implies that the superposition principle applies for the resulting plasma insulin levels based on consecutive injections at physically separate injection sites. In other words, if the regimen consists of more than one injection component, then the overall rate of insulin absorption can be computed by adding together the different contributions of all daily injections and the preparations that they contain, based on injections being given into separate subcutaneous sites. This also means that the plasma insulin profile is the sum of individual profiles produced by the different injections constituting the regimen being applied.
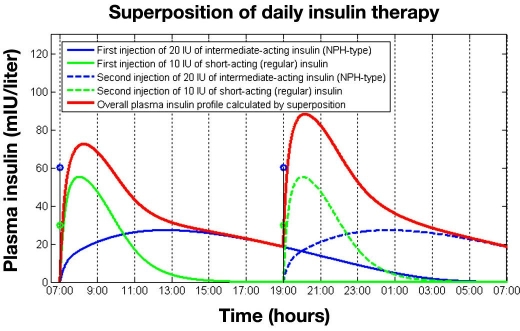
Figure 3 illustrates the superposition principle for plasma insulin levels, graphically. The graph shows simulated plasma insulin profiles for two injections of regular insulin and NPH-type insulin given at 7 am and 7 pm. The overall plasma insulin profile is calculated using the superposition principle as the summation of the individual curves and is shown by the thick line. As can be observed, at 7 pm some remaining insulin still exists from the previous basal injection, which accumulates with the basal-bolus injections done at that time, resulting in a greater peak insulin value compared to the previous period. Note that in this example the insulin level at 7 am (day 2) is very similar to the value computed at 7 pm on day 1 because neither the first regular insulin dose nor the first intermediate-acting insulin dose produces measurable insulin levels 24 hours after they were injected subcutaneously.
Figure 3.
A 24-hour plasma insulin profile showing the superposition principle in operation following the injection of regular insulin (10 IU) and intermediate-acting insulin (NPH type)(20 IU) given twice daily at 7 am and 7 pm.
The same approach can be used if different types of insulin are delivered and applies also for injections administered on separate days. For example, if the patient is given three regular insulin injections before the main meals with one intermediate-acting insulin injection given at bedtime on day 1, then the plasma insulin level will not be zero before breakfast on day 2.
This means that the contribution of the regular insulin injection before breakfast on day 2 will be superimposed on the insulin levels generated by injections administered on day 1. If a patient is managed with a particular insulin regimen, say “split and mix,” this accumulation of insulin levels needs to be considered as the so-called “steady state” daily plasma insulin profile achieved. In steady state the temporal pattern of plasma insulin theoretically repeats itself each day.
This steady state insulin profile, Iss corresponding to a given insulin regimen, is computed by using the superposition principle assuming 3 days to be enough to reach steady state conditions for most preparations and usual dosages, which produce negligible plasma insulin levels more than 48 hours after injection:
| (3) |
Equation (3) reflects that the steady state insulin response results from the composite effect of injections given for 3 subsequent days. It is evident that summation is not needed for rapidly acting insulin analogues and regular insulins, but is required for other, longer-acting insulin preparations whose absorption is prolonged, especially if large doses are administered.
Three days are typically enough to reach steady state conditions even when usual doses of glargine insulin are administered. It is noted, however, that Equation (3) should be extended when dealing with very high doses of glargine, which might produce measurable plasma insulin levels up to 96 hours after injection in some subjects. In these cases more than 3 days (say 4 days) are needed for the glucoregulatory system to reach steady state conditions.
Clearly the principle of superposition applies in case of linear kinetics, but an important additional prerequisite for the validity of its use is that contributions from the various injections are indeed comparable in terms of the expected metabolic effect per concentration unit. This holds for most of the insulin preparations considered, but corrections may be required when the model is to be extended to cover the insulin analogue detemir.
The simulation examples that follow have been carried out in MATLAB™ (v7.0.1, The MathWorks Inc., Natick, MA) using the numerical implementation of the absorption model described by Tarín and colleagues19,20 and the Runge–Kutta numerical integrator for the AIDA insulin kinetics/elimination model to simulate plasma insulin profiles. An upgrade to produce AIDA v4.5, which incorporates the new generic insulin absorption model, is in development. This revised model will be presented in a subsequent article, which will also help clinicians to see how differences in insulin absorption and plasma levels dynamics are reflected in different glycemic responses over time.
Results
This report demonstrates the first plasma insulin simulation results from the expanded, collaborative model.
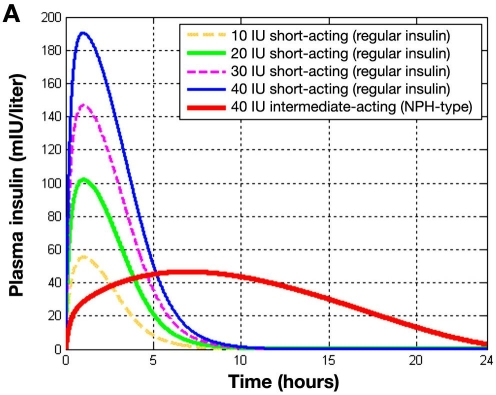
Figure 4A depicts plasma insulin levels over time after the subcutaneous injection of different doses of regular insulin compared to 40 IU of intermediate-acting (NPH-type) insulin as calculated by integration of the generic subcutaneous insulin absorption model19,20 and the original AIDA v4 insulin kinetics model. The curves generated clearly reflect both insulin absorption from the subcutaneous depot and insulin disposition/elimination, which follow once insulin has entered the systemic circulation.
Figure 4A.
Injection of different doses of regular insulin (10, 20, 30, and 40 IU) and a 40-IU intermediate-acting insulin (NPH type). Plasma insulin (in mIU/liter) versus time. Results reflect both insulin absorption and elimination kinetics.
As can be seen, the onset time of the two insulin formulations differs substantially; the plasma insulin shows a peak value at 1.5 hours after subcutaneous injection of regular insulin, independently of the administered dose, while the plasma insulin peak when injecting intermediate-acting insulin appears 7 hours after injection. In an earlier report,21 exogenous insulin flow data for a similar simulation were presented and it is noted that the current plasma insulin curves (Figure 4A) are very similar to the Iex insulin absorption profiles shown there.21 The dynamics of insulin disposition hardly show any influence on the time profile because insulin elimination is much faster than its absorption (i.e., absorption is the rate-limiting step for these classes of insulin preparations).
Comparable data for the existing AIDA v4.3a program were shown in Figure 2A of the earlier report,21 demonstrating the similarities for regular and intermediate-acting insulins. The advantage of this generic modeling development, however, manifests itself most with the modeling of insulin analogues (both rapidly acting and very long acting) and for insulin injection doses greater than 40 IU.
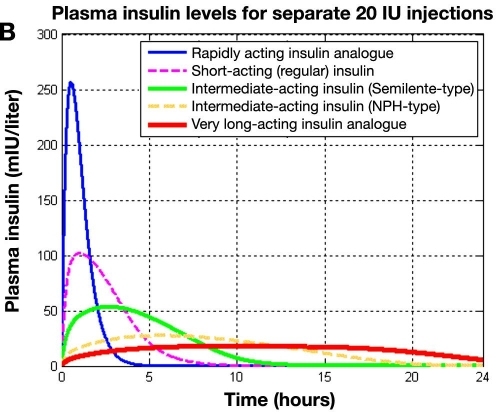
Figure 4B shows plasma insulin profiles as a function of time after subcutaneous injection for all the different classes of insulin preparations catered for in the new, subcutaneous insulin absorption model.19,20 A rapidly acting insulin analogue, a regular insulin preparation, intermediate-acting insulins (both Semilente and NPH types), and a very long-acting insulin analogue are compared. In all cases 20 IU was injected subcutaneously. Clearly, the rapidly acting insulin analogue shows a much higher and earlier peak than the regular insulin. The intermediate-acting insulins (both Semilente and NPH types) show a pronounced peak, while the very long-acting insulin analogue offers an almost flat profile. Also the onset time of the different insulin formulations differs substantially, with the rapidly acting insulin analogue showing the fastest onset time. As can be seen 5 hours after subcutaneous injection, the rapidly acting insulin analogue is fully absorbed, while the very long-acting insulin analogue has not even reached its maximum. This graph can be compared with Figure 6 in the earlier report 21 in which comparable exogenous insulin flow profile, Iex, data were shown.
Figure 4B.
. Simulated plasma insulin profiles for five classes of insulin preparations following five separate subcutaneous 20-IU injections. Plasma insulin (mIU/liter) versus time. Results reflect both insulin absorption and elimination kinetics.
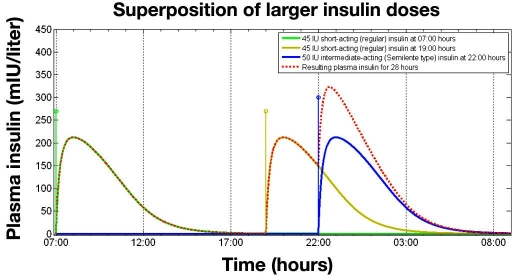
Figure 6.
Superposition of three insulin injections for an insulin-resistant patient: one injection of 45 IU of regular insulin at 7 am and a further regular insulin injection at 7 pm together with an injection of 50 IU of intermediate-acting insulin (Semilente type) at 10 pm (bedtime).
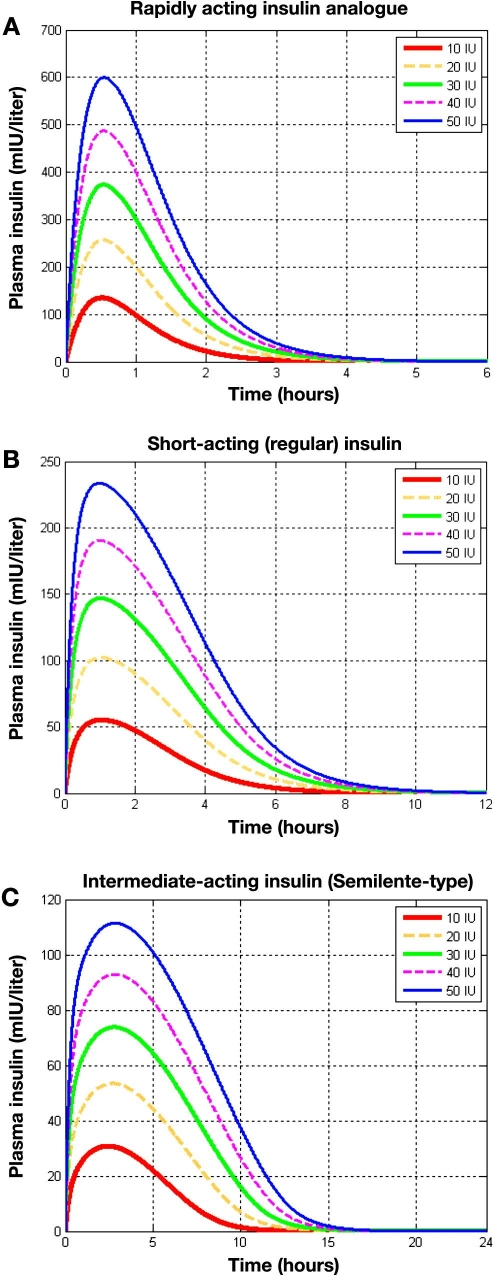
In Figure 5A the plasma insulin profiles for different doses of a rapidly acting insulin analogue are shown. Independent of the administered dose, the peak value of plasma insulin lies at 0.5 hour after injection as compared with a peak value of exogenous insulin flow approximately 0.3 hour after injection (Figure 8A in the earlier report21). The delay is due to the insulin kinetic model included for the plasma insulin determination.8 Of note, the new modeling approach (Figure 5A) allows insulin injections greater than 40 IU—the existing upper limit within AIDA v4.3a—to be simulated.
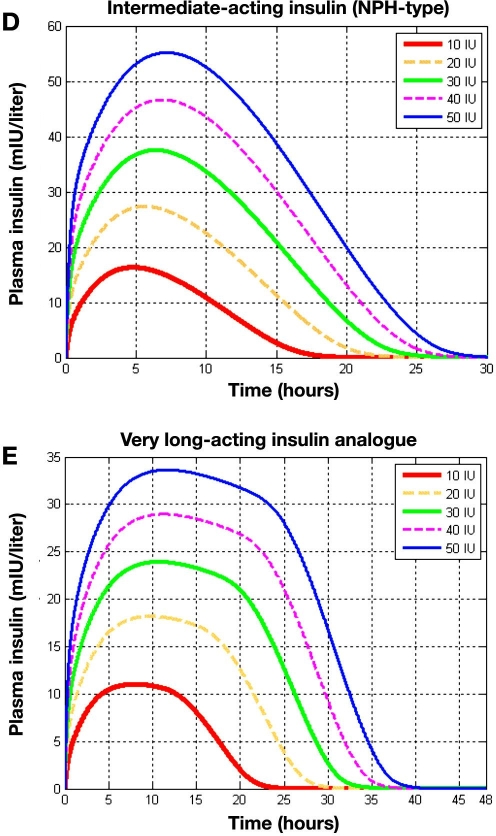
Figure 5.
Comparison of separate injections of 10, 20, 30, 40, and 50 IU for different classes of insulin preparations. Plasma insulin (mIU/liter) is shown for (A) a rapidly acting insulin analogue, (B) a regular insulin preparation, (C) an intermediate-acting insulin (Semilente type), (D) an intermediate-acting insulin (NPH type), and (E) a very long-acting insulin analogue.
Figure 5B shows plasma insulin levels for different doses of regular insulin. As with rapidly acting insulin it may be seen that the peak time is independent of the dose, around 1 hour. After approximately 9 hours the regular insulin has almost completely been eliminated from the blood.
Plasma insulin levels for different doses of Semilente insulin are shown in Figure 5C. It can be observed that 15 hours after the subcutaneous injection almost no insulin is remaining. In this graph it can also be seen that the peak value for plasma insulin occurs 2.5 hours after subcutaneous injection. Contrary to the examples for the previously shown insulin preparations, here a slight difference in peak times for the different doses, although not substantial, may be appreciated in the graph.
In Figure 5D plasma insulin levels for different doses of intermediate-acting insulin (NPH type) are plotted. In the case of this insulin preparation, the influence of the dose on the peak time becomes more apparent, with a 2.5-hour difference in the peak between 10 and 50 IU. The peak time varies between 5 and 7.5 hours. Insulin is essentially undetectable after around 17.5 hours for 10 IU and 27.5 hours for 50 IU.
A similar phenomenon is observed for plasma insulin levels for different doses of a very long-acting insulin analogue, as presented in Figure 5E and Figure 8C in the earlier report.21 From this figure it can be observed that during the 24 hours after injection plasma insulin levels show an almost flat profile. The very slow dynamics of the absorption process leads to a greater difference between dosages, such that for lower doses, 24 hours after injection, almost all the insulin is eliminated, while for higher doses it is not until 36 hours after injection that the insulin is eliminated.
The new modeling approach also permits the effects of multiple injections of larger doses of insulin to be simulated. Figure 6 shows the plasma insulin profile for an insulin-resistant patient receiving 45 IU of regular insulin at 7 am and 7 pm and 50 IU of Semilente at 10 pm.
Discussion
Using virtual people with diabetes provides a simulation opportunity to practice skills that might take months or years to acquire sufficiently by trial and error in real life. Providing a clinically useful virtual diabetes education platform is what the AIDA developers have been working toward for a number of years.2,22
One of the merits of the AIDA v4 diabetes simulation approach has been to present in detail each piece of knowledge that went into the AIDA model development and make available the resulting simulation tool without charge. This is one of the purposes of this article, and earlier reports,21,22 to disseminate information about the new diabetes modeling approach as widely and openly as possible, via the Internet. There are also plans to make the Turbo Pascal simulation engine source code of AIDA v4 open access and freely available via the Web24 to facilitate third-party freeware developments by other programmers.
The incorporation of the generic subcutaneous insulin absorption model of Tarín and colleagues19,20 alongside the existing model of insulin kinetics and disposal utilized within AIDA v4 from Berger and Rodbard8 is a step in the right direction and permits novel insulin analogues to be simulated by the new software, and the current insulin dose limit to be increased to at least 50 IU per injection.
The plasma insulin simulations presented in this article also show that the collaborative approach clearly gives encouraging data concerning the temporal patterns of insulin absorption.25 Nevertheless it is accepted that clinical studies and comparisons with actual measured insulin levels would be needed to establish the reliability of such an approach for simulations in practice. Thorough validation will clearly precede the implementation of the insulin disposition model presented in this trilogy of articles.
So far validation has been carried out against published data in the literature, but ongoing efforts are also directed to compare simulated profiles with yet unpublished data made available to the authors. Careful validation studies are particularly required with respect to glargine kinetics since most pharmacokinetic data in the public domain are from clinical studies in which lower-quality, nonspecific assays were used. In most recent clinical studies (not yet published) glargine was measured with a specific ELISA assay. These data could be used as a more reliable testbed for validating the revised absorption/disposition model of glargine.
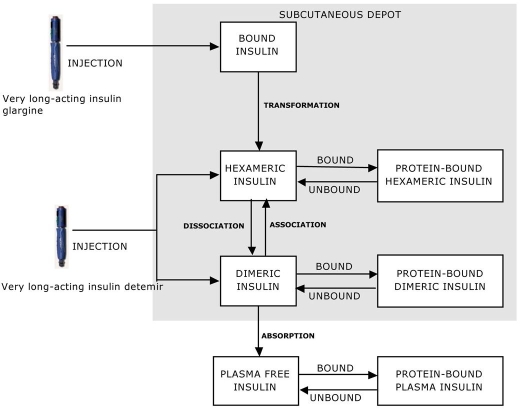
Very Long-Acting Insulin Analogues
Very long-acting insulin analogues have been found to be highly useful for providing a background basal insulin level. Insulin glargine, for example, has been shown to last 24 hours after subcutaneous injection and has been effective in once daily dosing regimens.26 Thus far long-acting analogue attention has focused on modeling insulin glargine rather than detemir. In the case of insulin glargine, additional amino acids are attached, causing precipitation of the molecule in neutral subcutaneous tissue, decreasing the rate of absorption. For insulin detemir, a fatty acid molecule is attached to the insulin chain by acetylation, which promotes binding of the insulin to albumin. In this way insulin absorption is prolonged and insulin clearance is decreased.
Detemir absorption is delayed by three mechanisms.27,28 The insulin remains liquid in the subcutaneous tissue, providing a greater surface area with reduced variability in absorption. There is strong binding to albumin at the injection site. In plasma, 98% remains bound to albumin, and in the target tissue 96% remains bound to albumin. This protects insulin detemir from liver clearance, which is only about 8%29 of the single pass extraction of human insulin.30 In the interstitial fluid, albumin reduces insulin receptor affinity and therefore results in the relatively low biological efficacy of the insulin analogue compared with human insulin.31 In addition, the fatty acid side chain interacts with neighboring insulin molecules and further prolongs the rate of absorption.
In the case of insulin glargine the delayed insulin absorption has been modeled by introducing an additional insulin state, the bound state,19 representing microprecipitations. At injection time, all injected insulin in the injection vial is considered to be in the bound form and then the diffusion of insulin through the subcutaneous depot, transformation between different insulin states (hexameric/dimeric), and absorption are described by the model.
For insulin detemir, the reduced rate of absorption might be tackled by introducing an additional state (collecting the insulin bound to albumin) into the model.19 The reduced insulin clearance could be dealt with by specifying a more comprehensive kinetic and dynamic insulin model as shown in Figure 7. All injected insulin in the injection vial would be considered to be in equilibrium between hexameric and dimeric forms. Once injected, the insulin would bind to albumin up to 98%, at which point a new equilibrium would be reached. Adequate model parameterization for insulin detemir would be crucial for reproducing a delayed absorption profile for this preparation.
Figure 7.
Detemir model structure, suggested for future work, that includes adaptation of the time constants defining the transformation rates of association/dissociation and the introduction of new states regarding insulin bound to albumin. These model modifications, together with adequate parameterization, should be able to take into account the delaying mechanisms of insulin detemir and allow reproduction of the delayed absorption profile of the preparation.
Relevant data investigating pharmacodynamics and pharmacokinetics of insulin detemir have been published,32 showing that insulin detemir provides a flat pharmaco-dynamic profile. In the Plank study, various relevant pharmacodynamic parameters such as the onset of action, end of action, duration of action, area under the curve, and time to maximum action were evaluated.32 It is possible on this basis that an updated version of the generic insulin absorption model could be developed and evaluated incorporating detemir insulin as well.
Future Work
A better understanding of the pharmacokinetics of different subcutaneously injected insulin preparations may be helpful for improving the design of intensified insulin treatment regimens and for increasing the predictive power of the algorithms used in a wearable artificial pancreas. However, insulin pharmacokinetics shows considerable variation not only between patients, but also within the same patient following repeated injections. Within-subject variation is far more substantial than hitherto appreciated and may not simply be explained as measurement error. The assessment of the source of variation is important for individualizing and optimizing insulin treatment. Hence there is interest to model such variability in insulin pharmacokinetics in future work.
Furthermore the incorporation of a more comprehensive generic model of insulin absorption within the AIDA software should be able to encourage the development of a range of further research projects.33
The action plan and the preliminary bench testing described previously,21,22 and the first simulated plasma insulin results overviewed earlier, are only the initial steps in the further development of AIDA. Future research may seek to extend the current absorption model of Tarín and colleagues19,20 not only to address the absorption of detemir, but also the subcutaneous infusion of insulin and the complex absorption profiles of mixtures of insulin preparations.
Modeling detemir pharmacokinetics is of special importance in order to clarify the difference between the clinical action of this long-acting analogue and that of glargine insulin. Clearly very similar action profiles may hide substantial differences in the underlying pharmacokinetics and pharmacodynamic actions of the two preparations. However, the basic structure of the absorption model overviewed here should be suitable to commence this research.
Insulin therapy in people with type 1 diabetes mellitus aims to mimic the pattern of endogenous insulin secretion present in healthy subjects. This pattern can be achieved, to some extent, by continuous subcutaneous insulin infusion (CSII) with an insulin pump administering an individually titrated basal insulin infusion and prandial insulin boluses. The generic absorption model could be extended to cater for subcutaneous insulin infusions where—in each time slice—insulin is entered into the innermost shell (i.e., sphere). When insulin is infused into the innermost shell, insulin already present in adjacent shells is pushed outward.
Motivated by the increasing use of CSII, there are plans to extend the insulin absorption model to also cater for insulin pump simulations.
Furthermore, modeling insulin absorption following the injection of mixtures of different insulin preparations remains a challenge. Available experimental data could also help evaluate such models, which should describe the complex interplay between the simultaneously injected components, as well as the various insulin forms interacting via association/dissociation processes.
With these envisaged extensions, the new insulin absorption model should allow the whole spectrum of currently available insulin preparations (including mixtures) and administration modes (both insulin injections and infusions) to be catered for within the AIDA v4 software.
As is evident from the foregoing, the current model revision has started with the incorporation of a generic, physiologically based model of insulin absorption. This absorption model will replace the Berger–Rodbard model8 adopted in the present release of AIDA (v4.3a), which includes a phenomenological (as opposed to a physiologically based) description of how insulin molecules leave the injection site and are absorbed into the bloodstream.
It is expected that an updated release of AIDA (v4.5), based on this collaborative approach, will become available for free—in due course—via the www.2aida.org Web site. Readers who wish to be informed when the new software is launched can join the very low volume AIDA announcement list by sending a blank email note to subscribe@2aida.org.
Acknowledgements
The Valencia group acknowledges the support of their work, in part, by the Spanish government under Grants DPI-2004-07167-C02-01 and DPI-2007-66728-C02-01 and by the European Union through FEDER funds. They also thank Professor Pfleiderer and Dr. Picó for their friendly support.
Abbreviations
- CSII
continuous subcutaneous insulin infusion
- Iex
exogenous insulin flow profile
- Ip
plasma insulin
- IU
international units [of insulin]
- NPH
neutral protamine Hagedorn
References
- 1.Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329(14):977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 2.Lehmann ED, Deutsch T. A physiological model of glucose-insulin interaction. IEEE EMBS Proceedings. 13th Annual International Conference of the IEEE Engineering in Medicine and Biology Society; 1991. pp. 2274–2275. [Google Scholar]
- 3.Lehmann ED, Deutsch T. A physiological model of glucose-insulin interaction in type I diabetes mellitus. J Biomed Eng. 1992;14(3):235–242. doi: 10.1016/0141-5425(92)90058-s. [DOI] [PubMed] [Google Scholar]
- 4.Lehmann ED. Diabetes moves onto the Internet. Lancet. 1996:347–1542. [Google Scholar]
- 5.Lehmann ED. University of London/Imperial College of Science, Technology and Medicine; 2004. Development, evaluation, and usage of ‘AIDA’– an interactive educational diabetes simulator. Ph. D. Thesis. [Google Scholar]
- 6.Lehmann ED, Chatu SS, Hashmy SSH. Retrospective pilot feedback survey of 200 users of the AIDA version 4 educational diabetes program. Diabetes Technol Ther. 2006;8(3):419–432. doi: 10.1089/dia.2006.8.419. and 8(5):602-8. [DOI] [PubMed] [Google Scholar]
- 7.Lehmann ED. Interactive educational simulators in diabetes care. Med Inform (Lond) 1997;22(1):47–76. doi: 10.3109/14639239709089834. [DOI] [PubMed] [Google Scholar]
- 8.Berger M, Rodbard D. Computer simulation of plasma insulin and glucose dynamics after subcutaneous insulin injection. Diabetes Care. 1989;12(10):725–736. doi: 10.2337/diacare.12.10.725. [DOI] [PubMed] [Google Scholar]
- 9.Sivitz WI, Davidson PC, Steed D, Bode B, Richardson P. Computer-assisted instruction in intense insulin therapy using a mathematical model for clinical simulation with a clinical algorithm and flow sheet. Diabetes Educ. 1989;15(1):77–79. doi: 10.1177/014572178901500120. [DOI] [PubMed] [Google Scholar]
- 10.Hedbrant J, Ludvigsson J, Nordenskjöld K. Särimner: a computer model of diabetes physiology for education of physicians and patients. Diabetes Res Clin Pract. 1991;14(2):113–122. doi: 10.1016/0168-8227(91)90117-v. [DOI] [PubMed] [Google Scholar]
- 11.Rutscher A, Salzsieder E, Fischer U. KADIS: model-aided education in type I diabetes. Karlsburg Diabetes Management System. Comput Methods Programs Biomed. 1994;41(3-4):205–215. doi: 10.1016/0169-2607(94)90055-8. [DOI] [PubMed] [Google Scholar]
- 12.Plougmann S, Hejlesen OK, Cavan DA. DiasNet–a diabetes advisory system for communication and education via the internet. Int J Med Inform. 2001 Dec;64(2-3):319–330. doi: 10.1016/s1386-5056(01)00214-3. [DOI] [PubMed] [Google Scholar]
- 13.Lehmann ED. Usage of a diabetes simulation system for education via the Internet. Int J Med Inform. 2003;69(1):63–69. doi: 10.1016/s1386-5056(02)00015-1. [DOI] [PubMed] [Google Scholar]
- 14.Biermann E, Mehnert H. DIABLOG: a simulation program of insulin-glucose dynamics for education of diabetics. Comput Methods Programs Biomed. 1990;32(3-4):311–318. doi: 10.1016/0169-2607(90)90114-o. [DOI] [PubMed] [Google Scholar]
- 15.Biermann E. DIACATOR: simulation of metabolic abnormalities of type II diabetes mellitus by use of a personal computer. Comput Methods Programs Biomed. 1994;41(3-4):217–229. doi: 10.1016/0169-2607(94)90056-6. [DOI] [PubMed] [Google Scholar]
- 16.Lehmann ED, Deutsch T, Carson ER, Sonksen PH. AIDA: An Interactive Diabetes Advisor. Computer Methods Prog Biomed. 1994;41(3):183–203. doi: 10.1016/0169-2607(94)90054-x. [DOI] [PubMed] [Google Scholar]
- 17.Lehmann ED, Hermanyi I, Deutsch T. Retrospective validation of a physiological model of glucose-insulin interaction in type 1 diabetes mellitus. Med Eng Phys. 1994;16(3):193–202. doi: 10.1016/1350-4533(94)90038-8. [Published erratum appears in Medical Eng Phys. 16(4):351-2] [DOI] [PubMed] [Google Scholar]
- 18.Lehmann ED, Deutsch T, Carson ER, Sonksen PH. Combining rule-based reasoning and mathematical modelling in diabetes care. Artif Intell Med. 1994;6(2):137–160. doi: 10.1016/0933-3657(94)90042-6. [DOI] [PubMed] [Google Scholar]
- 19.Tarín C, Teufel E, Picó J, Bondia J, Pfleiderer HJ. Comprehensive pharmacokinetic model of insulin Glargine and other insulin formulations. IEEE Trans Biomed Eng. 2005;52(12):1994–2005. doi: 10.1109/TBME.2005.857681. [DOI] [PubMed] [Google Scholar]
- 20.Tarín C, Bondia J, Teufel E, Vehí J. On spatial discretisation in the calculus of subcutaneous insulin absorption profiles. Proceedings of the 2nd European Modeling and Simulation Symposium (EMSS); 2006. pp. 379–384. [Google Scholar]
- 21.Lehmann ED, Tarín C, Bondia J, Teufel E, Deutsch T. Incorporating a generic model of subcutaneous insulin absorption into the AIDA v4 diabetes simulator. 1. A prospective collaborative development plan. J Diabetes Sci Technol. 2007;1(3):423–435. doi: 10.1177/193229680700100317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lehmann ED, Tarín C, Bondia J, Teufel E, Deutsch T. Incorporating a generic model of subcutaneous insulin absorption into the AIDA v4 diabetes simulator. 2. Preliminary bench testing. J Diabetes Sci Technol. 2007;1(5):780–793. doi: 10.1177/193229680700100525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bergenstal RM. Effective insulin therapy. In: DeFronzo RA, Ferrannini E, Keen H, Zimmet P, editors. International textbook of diabetes mellitus. 3rd. Chichester, UK: Wiley; 2004. pp. 995–1015. [Google Scholar]
- 24.Lehmann ED, Deutsch T. Open access availability of AIDA v4.3a diabetes simulator Turbo Pascal source code for non-commercial use [abstract] J Diabetes Sci Technol. 2007;1(2):A86. [Google Scholar]
- 25.Nucci G, Cobelli C. Models of subcutaneous insulin kinetics. A critical review. 2000;62(3):249–257. doi: 10.1016/s0169-2607(00)00071-7. [DOI] [PubMed] [Google Scholar]
- 26.Lepore M, Pampanelli S, Fanelli C, Porcellati F, Bartocci L, Di Vincenzo A, Cordoni C, Costa E, Brunetti P, Bolli GB. Pharmacokinetics and pharmacodynamics of subcutaneous injection of long-acting human insulin analog glargine, NPH insulin, and ultralente human insulin and continuous subcutaneous infusion of insulin lispro. Diabetes. 2000;49(12):2142–2148. doi: 10.2337/diabetes.49.12.2142. [DOI] [PubMed] [Google Scholar]
- 27.Whittingham JL, Havelund S, Jonassen I. Crystal structure of a prolonged-acting insulin with albumin-binding properties. Biochemistry. 1997;36(10):2826–2831. doi: 10.1021/bi9625105. [DOI] [PubMed] [Google Scholar]
- 28.Kurtzhals P, Havelund S, Jonassen I, Kiehr B, Larsen UD, Ribel U, Markussen J. Albumin binding of insulins acylated with fatty acids: characterization of the ligand-protein interaction and correlation between binding affinity and timing of the insulin effect in vivo. Biochem J. 1995;312(3):725–731. doi: 10.1042/bj3120725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zib I, Raskin P. Novel insulin analogues and its mitogenic potential. Diabetes Obes Metab. 2006;8(6):611–620. doi: 10.1111/j.1463-1326.2005.00567.x. [DOI] [PubMed] [Google Scholar]
- 30.Dea MK, Hamilton-Wessler M, Ader M, Moore D, Schäffer L, Loftager M, Vølund A, Bergman RN. Albumin binding of acylated insulin (NN304) does not deter action to stimulate glucose uptake. Diabetes. 2002;51(3):762–769. doi: 10.2337/diabetes.51.3.762. [DOI] [PubMed] [Google Scholar]
- 31.Hamilton-Wessler M, Ader M, Dea M, Moore D, Jorgensen PN, Markussen J, Bergman RN. Mechanism of protracted metabolic effects of fatty acid acylated insulin, NN304, in dogs: retention of NN304 by albumin. Diabetologia. 1999;42(10):1254–1263. doi: 10.1007/s001250051301. [DOI] [PubMed] [Google Scholar]
- 32.Plank J, Bodenlenz M, Sinner F, Magnes C, Goerzer E, Regitting W, Endahl LA, Draeger E, Zdravkovic M, Pieber TR. A double-blind, randomized, dose-response study investigating the pharmacodynamic and pharmacokinetic properties of long-acting insulin analog detemir. Diabetes Care. 2005;28(5):1107–1112. doi: 10.2337/diacare.28.5.1107. [DOI] [PubMed] [Google Scholar]
- 33.Lehmann ED. Research use of the AIDA www.2aida.org diabetes software simulation program: a review. Diabetes Technol Ther. 2003;5(3):425–438. doi: 10.1089/152091503765691938. and 5(4):641-51. [DOI] [PubMed] [Google Scholar]