Abstract
Background
Therapeutic nonadherence is defined as the lack of equivalence between the behavior of the patients and their prescribed medical treatment. Consequences of nonadherence include not only health outcomes, but also cost saving. Thus, this issue gets paramount importance in contemporary medicine.
Method
The aim of this article is to discuss the relationships between technology and adherence by asking the following three questions. (1) How can technology be used to monitor patient adherence? (2) Considering the mechanisms of nonadherence in chronic diseases, is there room for technology in interventions aimed to improve patient adherence? (3) What about adherence to technology in diabetes care?
Results and Conclusion
Technology may help improve adherence to long-term therapies by (1) giving a concrete representation of adherence rewards, (2) overcoming immediate obstacles to adherence, such as the fear of hypoglycemia, and (3) providing an opportunity for patient–doctor conversations. This assumes, however, that both the patient and the doctor are convinced that technologies are useful.
Keywords: adherence, artificial pancreas, conversation, electronic reminders, hypoglycemia, memory, medication event monitoring systems, mental states, technology
Setting the Background: Nonadherence to Long-Term Therapies
Nonadherence to therapy is classically defined as the lack of concordance between the patients' behavior, e.g., taking medicines or following a diet, and the prescribed therapy.1 In diabetes care, this concerns not only medication, but also medical appointment attendance, changes in lifestyle (diet, exercise), the practice of self-monitoring of blood glucose (SMBG), the real use of continuous glucose monitoring (CGM) systems, measuring glycosylated hemoglobin A1c (HbA1c), having eye examinations as requested, and avoiding risk behaviors (smoking, alcohol, etc.). Nonadherence is a frequent phenomenon. For instance, a systematic review of the literature revealed that adherence to oral hypoglycemic agents for people with diabetes ranged from 36 to 93% only. Persistence, i.e., the proportion of patients who remained on treatment for 6–24 months, was also far from optimal: it ranged from 16 to 80%.2 Another review of the literature in diabetes care revealed that around two-thirds of patients were adherent to diet, but only 25% of them were adherent to advice concerning exercise, and only 7 % of the patients were found to be adherent to all the recommendations.3
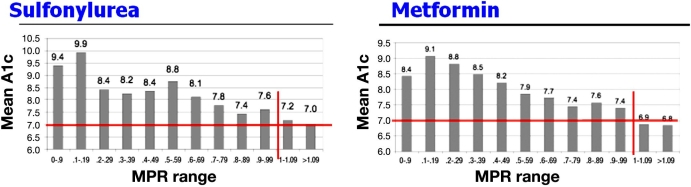
It is possible to demonstrate the deleterious effect of nonadherence on the efficiency of therapy. For instance, a study investigated the relationship between adherence to antihyperglycemic medication (sulfonylureas and metformin) and HbA1c in patients with diabetes. Medication adherence was assessed by determining the ratio of the total days supply of medication dispensed divided by the number of days of the evaluation period (medication possession ratio, MPR). HbA1c lower than 7.0% was observed most often in patients with MPR ≥1, with an impressive correlation between MPR and mean HbA1c level (Figure 1).4 It is also possible to show that nonadherence to medication represents a mortality risk factor. For instance, in the Beta-Blocker Heart Attack Trial, the mortality at 1 year after a first myocardial infarction was higher in patients in the placebo group (3.0 %) than in the beta-blocker group (1.4%). However, these figures were obtained in patients taking more than 75% of the tablets (either the beta blocker or the placebo). In nonadherent patients, the mortality rate in the beta-blocker group (4.2%) was actually higher than in patients adherent to the placebo and was highest in the nonadherent patients of the placebo group (7.0%).5 This puzzling observation was confirmed in a meta-analysis of 21 clinical trials.6 It can be concluded that nonadherent patients not only did not take their medication but also had other behaviors harmful to them; in general, adherers are healthy, with adherence being seen as a whole.7
Figure 1.
Adherence to oral antihyperglycemic medication and HbA1c.4 MPR is the ratio of the total days supply of medication dispensed divided by the number of days of the evaluation period.
It is therefore not surprising that improving adherence represents a major objective of health policies. Thus, in a 2003 report entitled “Adherence to Long Term Therapies, Evidence for Action” the World Health Organization (WHO) claimed that “increasing the effectiveness of adherence interventions may have a far greater impact on the health of the population than any improvement in specific medical treatment,”8 a quotation from a 2001 Cochrane review on interventions for helping patients to follow prescriptions for medications.9 However, attempts to improve adherence in chronic diseases have been disappointing so far: in a systematic review of 83 adherence interventions reported in 70 randomized, controlled clinical trials, only 36 were associated with improvements in adherence and only 25 interventions led to improvement in at least one treatment outcome.10
The aim of this article is to investigate the potential of technology to assist these efforts in improving adherence to long-term therapies. This question is at first glance puzzling, as it is aimed to investigate the interaction between technology and psychology. This article considers the following issues. (1) How can technology be used to monitor patient adherence? (2) Considering the mechanisms of nonadherence in chronic diseases, is there room for technology in interventions aimed at improving patient adherence? (3) What about adherence to technology in diabetes care?
Technology for Monitoring Adherence
The precise numbers quoted earlier must not cloud the difficulty of attempting to evaluate therapeutic adherence quantitatively. This evaluation generally uses physicians' assessments or the observations of patients' families concerning their behavior; questions addressed to patients themselves or patient's self-observation; pill count when patients are asked to bring back used packages of medication; determination of MPR; use of biological markers, such as the dose of medication in blood or urine; and, more recently, electronic surveillance systems that involve the placement of electronic circuits in the pill or eye drop bottles registering each use.11,12
Thus, “medication event monitoring systems” (MEMS) consist of standard pill bottles with microprocessors in the cap that record the timing and frequency of bottle openings (this recording does not prove, however, that the pill was actually taken). For instance, it is possible to provide an objective demonstration that the lunch pill is the pill missed most often.13 An interesting study14 investigated the effect of patients' perception of MEMS on adherence to treatment. Patients who, at the end of a study using MEMS to monitor adherence, agreed with the sentences “I used the MEMS every day,” “I felt comfortable using the MEMS in front of others,” or “I remembered to put my medication refills in the MEMS” were found to be more adherent than patients who disagreed. This observation may suggest that there may actually be a flaw in the use of these systems to monitor adherence, as patients adherent to MEMS may also be more adherent to medication, suggesting again that “adherence is a whole.”7 However, this may also suggest that the use of MEMS may represent per se an adherence intervention: indeed, in this study, approximately two-thirds of the participants stated that the MEMS helped them remember to take their medications.14
Using technology to assess patient adherence also represents a feature of tools commonly used in diabetes care. Thus, the addition of memory chips in glucometers capable of storing glucose determinations with corresponding times and dates made it possible to determine the level of reliability and accuracy of results recorded in patients' logbooks. A first study indicated that three-fourths of subjects had a significantly lower mean blood glucose level than was reported in the logbooks and revealed both underreporting and overreporting (addition of phantom values).15–17 Interestingly, another study found fewer discrepancies in individuals who were aware of the presence of the memory chip in the glucometer.18 In the same vein, the memory of insulin pumps can be used to detect missed boluses, a frequent phenomenon that correlates with glycemic control.19–21 Bolus alarms may represent a solution to this problem.22 To the best of our knowledge, the memory capacity of novel smart pens23 has not yet been used to detect missed insulin injections, i.e., to provide a quantitative evaluation of adherence to an insulin regimen.
Mechanisms of Nonadherence in Chronic Diseases: Room for Technology?
Information and Memory
The information that a patient must remember in order to take just one pill is actually complex and is made up of seven properties: the name of the drug, for which disease it is used, how to take it, the number of daily intakes, when to take it (before, during, or after a meal), dosage, and duration of the treatment. Actually, a study has shown that patients were unable, for a single drug, to remember more than 50% of the information given orally during a visit.24 This rate could obviously be even lower for a more complex regimen (polypharmacy), which is prescribed frequently in diabetes patients. This failure of memory may explain in part why the complexity of the therapeutic regimen has a major impact on therapeutic adherence, which represents a strong argument to favor combination medications in diseases such as diabetes care and hypertension.25,26
Taking medicine typically represents a condition where prospective memory is involved.27 According to Judi Ellis, “the core of a prospective memory task is an intention to act in a particular way at some specified moment in the future. When an intention is formed, one has to encode the content of that intention: the action (what to do); the retrieval criteria (when to do it) and the intent (that there is something you wish to do at some point). This content must be retained over a period of delay or retention interval (of minutes, hours, days etc.), then recalled and enacted when a situation that satisfies the retrieval criteria occurs. Clearly there is also some need for evaluating whether the intention has been fully or partially satisfied and to retain a record of this event.”28 A first approach used to compensate for patients' limited memory may consist of giving them either written information in the form of medicine reminder charts29 or pill dispensers.30 We already mentioned the interest of bolus alarms in smart pumps.22
The theory of prospective memory suggests involvement of a “research cue.” This “cue” may be event dependent, binding the task to a daily task (e.g., injecting insulin before going to bed), or time dependent, being as specific as possible (thus, it may be more specific to prescribe “take your tablet on Saturday evening” than “take your tablet once a week”). There may be room here for technology in the form of electronic reminders, such as electronic pill boxes.31 Interestingly, the theory of prospective memory also suggests that there is some need for evaluating whether the intention has been satisfied and to retain a record of this event. In this respect, novel insulin pens, which record the dose and timing of the last insulin injection, may be useful.32
Telecommunication through email or telephone may offer a way to improve adherence. For instance, a controlled study investigated the respective efficiency of improving visit attendance with either text messaging or mobile phone reminders 24–48 hours prior to a scheduled appointment. Attendance was better than in the control group of patients who did not receive any intervention.33 Similarly, mobile phone text messaging was found to be useful for smoking cessation in a study where participants allocated to the intervention group (active group) were sent regular personalized text messages providing information on symptoms to expect upon quitting, tips on how to avoid weight gain, cope with craving, avoid smoking triggers, motivational support such as success stories, or even distraction such as information on sports, fashion, and travels.34
However, a difficulty of this approach should not be overlooked as shown by a negative, but highly instructive, pilot study of an interactive voice response system aimed at improving medication refill compliance, in which not only patient complaints suggested that the system needed to be improved, such as “I received two reminder calls at 1:30 a.m. and did not appreciate that!,” but also that there is a risk that the technological nature of the approach is itself a source of rejection: “the machine made me sound stupid because it would ask me to answer when I already had” or “a machine is a machine. There is nothing human about that. I'd rather talk to a real person.”35 This brings us back to the importance of considering the mental mechanisms of nonadherence to long-term therapies.
Motivation and Intentionality
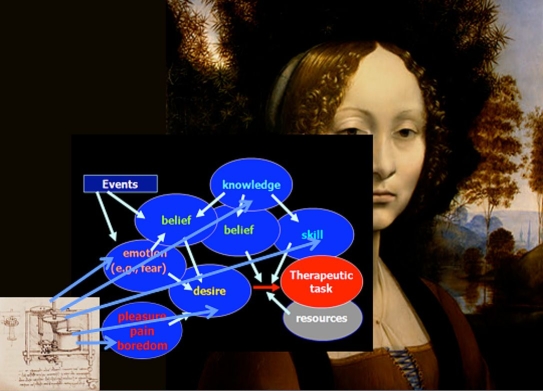
Figure 2 represents a tentative description of the mental processes leading to the acceptance of performing a therapeutic task (e.g., measuring blood glucose).7 Generally speaking, a patient may be nonadherent to part of her treatment because she (1) does not want to do it (deficit of desire), (2) does not know what to do and how to do it (deficit of knowledge and skills), (3) does not believe that she can do it, that it is necessary to do it, or that the advantages of doing it are more important than the benefits (lack of appropriate beliefs), (4) believes that it is dangerous to do it (effect of an emotion, fear), and (5) does not have the means to do it (lack of resources). Mental states such as pain, pleasure, or boredom may also be involved. As shown in Figure 2, technology may theoretically represent a way to improve adherence to therapy by acting on different targets—as an educational tool by providing the patient with knowledge and skills; specifically in the field of diabetes care by relieving the patient from the fear of hypoglycemia; or by promoting the desire to accomplish the therapeutic task.
Figure 2.
Technè and Psychè: mental states as targets of interventions aimed to improve adherence. The two artworks by Leonardo da Vinci symbolize the possibility of an interaction between technology and psychology.
Technology and Patient Education
Let us consider continuous glucose monitoring. “For the patient, the illustrative nature of the tracings may make them more meaningful than a logbook.”36 However, such a statement on the educational value of using a CGM system has not been evaluated in controlled studies.
A recent study evaluated the use of an algorithm (DATA, for DirecNet Applied Treatment Algorithm) provided to children using a continuous glucose monitoring system. Interestingly, the percentage of patients who used the algorithm at least 50% of the time in responding to alarms, or who used the algorithm most of the time or always to adjust premeal bolus, decreased between the 3rd and the 13th week of the study. Despite this, there was an increase in the percentage of patients who reported making changes to their carbohydrate-to-insulin ratios or to their basal insulin rates, suggesting that they were learning from their glucose patterns and were individualizing their treatment plans.37
This issue is important: if it was possible to really demonstrate that short-term (i.e., 3 months) use of the system does improve the ability of patients to implement functional insulin therapy on a long-term basis, this would pave the way to the definition of novel strategies for the use of these systems, with a possible impact on their refunding. Clearly there is a need for studies in this field that would refer to concepts developed in the field of patient education.
Technology and Fear of Hypoglycemia
Fear of hypoglycemia is the most feared complication of insulin therapy38,39 and is one of the reasons why patients with diabetes are reluctant to implement Diabetes Control and Complications Trial recommendations40 and to increase their insulin doses when their blood glucose level is high.41 However, only three studies have addressed the effect of using these systems regarding the fear of hypoglycemia. In two controlled studies, there was no difference between groups in the “fear of hypoglycemia” survey.42,43 Only in the first study did the CGM system group show a slightly but not significantly lower fear of hypoglycemia when comparing a 3-months score (56.3) to baseline score (61.8). In a recent nonrandomized study using the FreeStyle Navigator® CGM system, both children and parents agreed in the CGM satisfaction scale that the sensor “makes [them] feel safer knowing that [they] will be warned about low blood sugar before it happens.”44 Thus, further studies are needed to substantiate this point.
Technology and Motivation
In the minds of people having to follow long-term therapies, there may be a striking contrast between the objectives of treatment, which represents an abstract (“to avoid complications”) and long-term concept, and the representation of inaction, which is, in contrast, immediate and concrete, i.e., imagined readily in the form of a nap in front of the television set, an additional piece of cake, or the forbidden cigarette. The couple prevention/inaction, in other words, adherence/nonadherence, can thus be schematized in the form abstract–long term/concrete–short term. This association is not the effect of hazard: Yacov Trope and Nira Liberman worked out a theory, largely supported by several empirical studies, known as construal level theory.45 This theory suggests that the human mind tends to assign “high-level” criteria to remote events, in particular, abstraction; and “low-level” criteria to proximal events, in particular, a concrete description (thus, if I think in a remote event mode about reading, I will think of the fact that reading enriches my mind. If I think about reading tomorrow, I will think of the book that I am currently reading). This represents a major obstacle to adherence to long-term therapies,46 as people often prefer smaller rewards sooner to larger rewards later.47 Under this conceptual framework, technology may facilitate adherence by rendering its reward concrete and immediate: by using SMBG, CGM, or, more trivially, an accelerometer, adherence to insulin therapy or to exercise may be improved because people see what they did. However, again, this kind of hypothesis has not been tested and this remark points out how much empirical studies are needed to understand the “mechanism of action,” at the mental level, of implementing technology in medical care.
Technology against Boredom
“If somebody says that a task is mechanical, it does not mean that people are incapable of doing the task; it implies, though, that only a machine could do it over and over, without ever complaining, or feeling bored.” This sentence, from Douglas Hofstadter in “Gödel, Escher, Bach, an Eternal Golden Braid,” illustrates perfectly an aspect of adherence that cannot be overlooked: to be adherent is simply boring in the long term, which may explain why nonadherence is so frequent in chronic diseases. Technology may therefore represent a solution to the problem of nonadherence to long-term therapies by doing the task for the patient. This is why a true closed-loop insulin system is so eagerly waited for by patients: they would not have to measure blood glucose, to adjust insulin doses, and so on. Before these systems are available, patients may find assistance for the important and difficult task of adjusting insulin doses in smart pump technology48 or in telemedicine-based systems.49
Adherence to Technology
Adherence to technology may have an impact on its efficiency. This can be demonstrated by examining results of the recently published Juvenile Diabetes Research Foundation trial on continuous glucose monitoring. At least 6.0 days of sensor use per week was the average of 83% of patients older than 25 years, of 30% of 15- to 24-year-old patients, and of 50% of 8- to 14-year-old patients. An improvement in HbA1c was only observed in the first group of patients [delta over baseline: –0.53%, 95% confidence interval (CI) = –0.71 to 0.35, p < 0.001], but not in the two other groups of patients (0.08%, 95% CI –0.17 to 0.33, p = 0.52, and –0.13%, 95% CI –0.38 to 0.11, p = 0.29, respectively). Although this association was not discussed by the authors in the article,50 but in a subsequent letter to the journal, the lack of HbA1c improvement in children and teenagers was likely a consequence of poorer adherence observed in these ranges of age. Indeed, in another study, Hirsch and colleagues51 demonstrated a significant effect of adherence to the technology on metabolic control: each 10% increase in adherence was associated with a 41% increase in the probability of a 0.5% reduction in HbA1c, with this effect of adherence on HbA1c being significant (p = 0.0456).
Conclusion: The Doctors, Their Patients, and Technology
The aim of this article was to suggest that indeed, as claimed by the WHO, “increasing the effectiveness of adherence interventions may have a far greater impact on the health of the population than any improvement in specific medical treatment.” This statement can be applied to the development of drugs, as well as to other fields, such as cell therapy (poor adherence represents a critical issue in organ transplantation52) and, as shown earlier, in technology-based treatments, such as self-monitoring of blood glucose or continuous glucose monitoring. However, improving patient adherence is not simple, and it is necessary to understand why people are or are not adherent to medical prescriptions. We saw that the mechanisms imply the intervention of different mental states, which can be themselves the target of technology-based interventions (Figure 2).
However, as shown in Figure 2, desire plays a central role in the accomplishment of any action, and specifically of therapeutic actions. It is therefore important that both the patient and the health care provider are convinced of the utility of using the technology. In this respect, technology such as SMBG or CGM may represent a unique opportunity for a conversation between the doctor and his/her patient,53 which may in turn represent the true way toward an improvement in adherence.
A recent study demonstrated the importance of this conversation. It investigated the reasons of nonadherence to SMBG. Some verbatim are impressive: “Q: So did they ever ask to see your readings that you'd taken? A: Oh no, no. I don't even think they've asked me if I've got a meter.” “Well I'm filling out this book, nobody ever looks at it, and you go to the doctor, and they take your blood, and they can decide from what your levels are—so why am I inflicting this pain on myself for nothing?”54 In a recent critical review analyzing the key for success of SMBG, Klonoff and associates55 pointed out that “subjects and caregivers must use the SMBG monitoring equipment properly. Specific behavior by both parties is critical.” It is important to consider that nonadherence concerns not only patients, but doctors as well, who may not adhere to current guidelines, a phenomenon described as clinical inertia, which may have the same background as patient nonadherence.56
It is important to remember that almost all the interventions effective for improving patient adherence in long-term care were complex, including a combination of more convenient care, information, reminders, self-monitoring, manual telephone follow-up, reinforcement, counseling, family therapy, psychological therapy, crisis intervention, and supportive care.10 In conclusion, in this area, as in others, technè should not be seen as a panacea: it is only a part of the solution.
Abbreviations
- CGM
continuous glucose monitoring
- CI
confidence interval
- HbA1c
glycosylated hemoglobin A1c
- MEMS
medication event monitoring systems
- MPR
medication possession ratio
- SMBG
self-monitoring of blood glucose
- WHO
World Health Organization
References
- 1.Sackett DL. Introduction. In: Sackett DL, Haynes RB, editors. Compliance with therapeutic regimens. Baltimore, MD: John Hopkins University Press; 1979. [Google Scholar]
- 2.Cramer JA. A systematic review of adherence with medication for diabetes. Diabetes Care. 2004;27(5):1218–1224. doi: 10.2337/diacare.27.5.1218. [DOI] [PubMed] [Google Scholar]
- 3.McNabb WL. Adherence in diabetes: can we define it and can we measure it? Diabetes Care. 1997;20(2):216–218. doi: 10.2337/diacare.20.2.215. [DOI] [PubMed] [Google Scholar]
- 4.Lawrence DB, Ragucci KR, Long LB, Parris BS, Helfer LA. Relationship of oral antihyperglycemic (sulfonylurea or metformin) medication adherence and hemoglobin A1c goal attainment for HMO patients enrolled in a diabetes disease management program. J Manag Care Pharm. 2006;12(6):466–471. doi: 10.18553/jmcp.2006.12.6.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Horwitz RI, Viscoli CM, Berkman L, Donaldson RM, Horwitz SM, Murray CJ, Ransohoff DF, Sindelar J. Treatment adherence and risk of death after myocardial infarction. Lancet. 1990;336(8714):542–545. doi: 10.1016/0140-6736(90)92095-y. [DOI] [PubMed] [Google Scholar]
- 6.Simpson SH, Eurich DT, Majumdar SR, Padwal RS, Tsuyuki RT, Varney J, Johnson JA. A meta-analysis of the association between adherence to drug therapy and mortality. BMJ. 2006;333(7557):15. doi: 10.1136/bmj.38875.675486.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reach G. A novel conceptual framework for understanding adherence to long term therapies. Patient Preferences Adherence. 2008;2:7–20. [PMC free article] [PubMed] [Google Scholar]
- 8.WHO report. Adherence to long-term therapies, evidence for action. 2003:11. [Google Scholar]
- 9.Haynes RB. Interventions for helping patients to follow prescriptions for medications. Cochrane Database Syst Rev. 2001;(1) doi: 10.1002/14651858.CD000011. [DOI] [PubMed] [Google Scholar]
- 10.Haynes RB, Ackloo E, Sahota N, McDonald HP, Yao X. Interventions for enhancing medication adherence. Cochrane Database Syst Rev. 2008;(2):CD000011. doi: 10.1002/14651858.CD000011.pub3. [DOI] [PubMed] [Google Scholar]
- 11.Blackwell B, editor. Amsterdam: Harwood Academic Publishers; 1997. Treatment compliance and the therapeutic alliance; p. 6. [Google Scholar]
- 12.Costagliola D, Barberousse C. Comment mesurer l'observance. L'observance aux traitements contre le VIH/SIDA. Agence Nationale de Recherche sur le SIDA. 2001:33–42. [Google Scholar]
- 13.Winkler A, Teuscher AU, Mueller B, Diem P. Monitoring adherence to prescribed medication in type 2 diabetic patients treated with sulfonylureas. Swiss Med Wkly. 2002;132(27-28):379–385. doi: 10.4414/smw.2002.10036. [DOI] [PubMed] [Google Scholar]
- 14.Schoenthaler A, Ogedegbe G. Patients' perception of electronic monitoring devices affect medication adherence in hypertensive African Americans. Ann Pharmacother. 2008;42(5):647–652. doi: 10.1345/aph.1K640. [DOI] [PubMed] [Google Scholar]
- 15.Mazze RS, Pasmantier R, Murphy JA, Shamoon H. Self-monitoring of capillary blood glucose: changing the performance of individuals with diabetes. Diabetes Care. 1985;8(3):207–213. doi: 10.2337/diacare.8.3.207. [DOI] [PubMed] [Google Scholar]
- 16.Ziegler O, Kolopp M, Got I, Genton P, Debry G, Drouin P. Reliability of self-monitoring of blood glucose by CSII-treated patients with type I diabetes. Diabetes Care. 1989;12(3):184–188. doi: 10.2337/diacare.12.3.184. [DOI] [PubMed] [Google Scholar]
- 17.Williams CD, Scobie IN, Till S, Crane R, Lowy C, Sonksen PH. Use of memory meters to measure reliability of self blood glucose monitoring. Diabet Med. 1988;5(5):459–462. doi: 10.1111/j.1464-5491.1988.tb01028.x. [DOI] [PubMed] [Google Scholar]
- 18.Maeez RS, Shamoon H, Pasmantier R, Lucido D, Murphy J, Hartman K, Kuykendall Y, Lopatin W. Reliability of blood glucose monitoring by patients with diabetes mellitus. Am J Med. 1984;77(2):211–217. doi: 10.1016/0002-9343(84)90693-4. [DOI] [PubMed] [Google Scholar]
- 19.Burdick J, Chase HP, Slover RH, Knievel K, Scrimgeour L, Maniatis AK, Klingensmith GJ. Missed insulin meal boluses and elevated hemoglobin A1c levels in children receiving insulin pump therapy. Pediatrics. 2004;113(3 Pt 1):e221–e224. doi: 10.1542/peds.113.3.e221. [DOI] [PubMed] [Google Scholar]
- 20.Pańkowska E, Skórka A, Szypowska A, Lipka M. Memory of insulin pumps and their record as a source of information about insulin therapy in children and adolescents with type 1 diabetes. Diabetes Technol Ther. 2005;7(2):308–314. doi: 10.1089/dia.2005.7.308. [DOI] [PubMed] [Google Scholar]
- 21.Olinder AL, Kernell A, Smide B. Missed bolus doses: devastating for metabolic control in CSII-treated adolescents with type 1 diabetes. Pediatr Diabetes. 2008;10(2):142–148. doi: 10.1111/j.1399-5448.2008.00462.x. [DOI] [PubMed] [Google Scholar]
- 22.Chase HP, Horner B, McFann K, Yetzer H, Gaston J, Banion C, Fiallo-Scharer R, Slover R, Klingensmith G. The use of insulin pumps with meal bolus alarms in children with type 1 diabetes to improve glycemic control. Diabetes Care. 2006;29(5):1012–1015. doi: 10.2337/diacare.2951012. [DOI] [PubMed] [Google Scholar]
- 23.Ignaut DA, Venekamp WJ. HumaPen Memoir: a novel insulin-injecting pen with a dose-memory feature. Expert Rev Med Devices. 2007;4(6):793–802. doi: 10.1586/17434440.4.6.793. [DOI] [PubMed] [Google Scholar]
- 24.Higbee K. Patient recall of physicians prescription instructions. Hospital Formulary. 1982;17:553–556. [PubMed] [Google Scholar]
- 25.Leichtner SB. Combination medications in diabetes care: an opportunity that merits more attention. Clin Diabetes. 2003;21:175–217. [Google Scholar]
- 26.Schroeder K, Fahey T, Ebrahim S. Interventions for improving adherence to treatment in patients with high blood pressure in ambulatory settings. Cochrane Database Syst Rev. 2004;(2):CD004804. doi: 10.1002/14651858.CD004804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park DC, Kidder DP. Prospective memory and medication adherence. In: Brandimonte M, Einstein GO, McDaniel MA, editors. Prospective memory: theory and applications. Mahwah, NJ: Lawrence Erlbaum Associates; 1996. [Google Scholar]
- 28.Ellis J. Prospective memory and medicine taking. In: Myers LB, Midence K, editors. Adherence to treatment in medical conditions. Amsterdam: Harwood Academic Publishers; 1998. [Google Scholar]
- 29.Raynor DK, Booth TG, Blenkinsopp A. Effects of computer generated reminder charts on patients' compliance with drug regimens. BMJ. 1993;306(6886):1158–1161. doi: 10.1136/bmj.306.6886.1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haynes RB, McDonald HP, Garg AX. Helping patients follow prescribed treatment: clinical applications. JAMA. 2002;288(22):2880–2883. doi: 10.1001/jama.288.22.2880. [DOI] [PubMed] [Google Scholar]
- 31. Available from: http://www.medscape.com/viewarticle/559246.
- 32.Venekamp WJ, Kerr L, Dowsett SA, Johnson PA, Wimberley D, McKenzie C, Malone J, Milicevic Z. Functionality and acceptability of a new electronic insulin injection pen with a memory feature. Curr Med Res Opin. 2006;22(2):315–325. doi: 10.1185/030079906X80477. [DOI] [PubMed] [Google Scholar]
- 33.Leong KC, Chen WS, Leong KW, Mastura I, Mimi O, Sheikh MA, Zailinawati AH, Ng CJ, Phua KL, Teng CL. The use of text messaging to improve attendance in primary care: a randomized controlled trial. Fam Pract. 2006;23(63):699–705. doi: 10.1093/fampra/cml044. [DOI] [PubMed] [Google Scholar]
- 34.Rodgers A, Corbett T, Bramley D, Riddell T, Wills M, Lin RB, Jones M. Do u smoke after txt? Result of a randomised trial of smoking cessation using mobile phone text messaging. Tobacco Control. 2005;14(4):255–261. doi: 10.1136/tc.2005.011577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reidel K, Tamblyn R, Patel V, Huang A. Pilot study of an interactive voice response system to improve medication refill compliance. BMC Med Inform Decis Mak. 2008;8:46. doi: 10.1186/1472-6947-8-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Melki V, Ayon F, Fernandez M, Hanaire-Broutin H. Value and limitations of the Continuous Glucose Monitoring System in the management of type 1 diabetes. Diabetes Metab. 2006;32(2):123–129. doi: 10.1016/s1262-3636(07)70258-6. [DOI] [PubMed] [Google Scholar]
- 37.Buckingham B, Xing D, Weinzimer S, Fiallo-Scharer R, Kollman C, Mauras N, Tsalikian E, Tamborlane W, Wysocki T, Ruedy K, Beck R Diabetes Research In Children Network (DirecNet) Study Group. Use of the DirecNet Applied Treatment Algorithm (DATA) for diabetes management with a real-time continuous glucose monitor (the FreeStyle Navigator) Pediatr Diabetes. 2008;9(2):142–147. doi: 10.1111/j.1399-5448.2007.00301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Irvine AA, Cox D, Gonder-Frederick L. Fear of hypoglycemia: relationship to physical and psychological symptoms in patients with insulin-dependent diabetes mellitus. Health Psychol. 1992;11(2):135–138. doi: 10.1037//0278-6133.11.2.135. [DOI] [PubMed] [Google Scholar]
- 39.McCrimmon RJ, Frier BM. Hypoglycaemia, the most feared complication of insulin therapy. Diabete Metab. 1994;20(6):503–512. [PubMed] [Google Scholar]
- 40.Thompson CJ, Cummings JF, Chalmers J, Gould C, Newton RW. How have patients reacted to the implications of the DCCT? Diabetes Care. 1996;19(8):876–879. doi: 10.2337/diacare.19.8.876. [DOI] [PubMed] [Google Scholar]
- 41.Choleau C, Albisser AM, Bar-Hen A, Bihan H, Campinos C, Gherbi Z, Jomaa R, Aich M, Cohen R, Reach G. A novel method for assessing insulin dose adjustments by diabetic patients. J Diabetes Sci Technol. 2007;1(1):3–7. doi: 10.1177/193229680700100102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chase HP, Kim LM, Owen SL, MacKenzie TA, Klingensmith GJ, Murtfeldt R, Garg SK. Continuous subcutaneous glucose monitoring in children with type 1 diabetes. Pediatrics. 2001;107(2):222–226. doi: 10.1542/peds.107.2.222. [DOI] [PubMed] [Google Scholar]
- 43.Chase HP, Beck R, Tamborlane W, Buckingham B, Mauras N, Tsalikian E, Wysocki T, Weinzimer S, Kollman C, Ruedy K, Xing D. A randomized multicenter trial comparing the GlucoWatch Biographer with standard glucose monitoring in children with type 1 diabetes. Diabetes Care. 2005;28(5):1101–1106. doi: 10.2337/diacare.28.5.1101. [DOI] [PubMed] [Google Scholar]
- 44.Weinzimer S, Xing D, Tansey M, Fiallo-Scharer R, Mauras N, Wysocki T, Beck R, Tamborlane W, Ruedy K. Diabetes Research in Children Network (DirecNet) Study Group. FreeStyle navigator continuous glucose monitoring system use in children with type 1 diabetes using glargine-based multiple daily dose regimens: results of a pilot trial Diabetes Research in Children Network (DirecNet) Study Group. Diabetes Care. 2008;31(3):526–527. doi: 10.2337/dc07-1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Trope Y, Liberman N. Temporal construal. Psychol Rev. 2003;110(3):403–421. doi: 10.1037/0033-295x.110.3.403. [DOI] [PubMed] [Google Scholar]
- 46.Reach G. Obstacles to patient education in chronic diseases: a transtheoretical analysis. Patient Educ Counseling. doi: 10.1016/j.pec.2009.05.005. In press 2009. [DOI] [PubMed] [Google Scholar]
- 47.Ainslie G. Précis of Breakdown of Will. Behav Brain Sci. 2005;28(5):635–650. doi: 10.1017/S0140525X05000117. discussion 650-73. [DOI] [PubMed] [Google Scholar]
- 48.Zisser H, Robinson L, Bevier W, Dassau E, Ellingsen C, Doyle FJ, Jovanovic L. Bolus calculator: a review of four “smart” insulin pumps. Diabetes Technol Ther. 2008;10(6):441–444. doi: 10.1089/dia.2007.0284. [DOI] [PubMed] [Google Scholar]
- 49.Adaji A, Schattner P, Jones K. The use of information technology to enhance diabetes management in primary care: a literature review. Inform Prim Care. 2008;16(3):229–237. doi: 10.14236/jhi.v16i3.698. [DOI] [PubMed] [Google Scholar]
- 50.Tamborlane WV, Beck RW, Bode BW, Buckingham B, Chase HP, Clemons R, Fiallo-Scharer R, Fox LA, Gilliam LK, Hirsch IB, Huang ES, Kollman C, Kowalski AJ, Laffel L, Lawrence JM, Lee J, Mauras N, O'Grady M, Ruedy KJ, Tansey M, Tsalikian E, Weinzimer S, Wilson DM, Wolpert H, Wysocki T, Xing D Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group. Continuous glucose monitoring and intensive treatment of type 1 diabetes. N Engl J Med. 2008;359(14):1464–1476. doi: 10.1056/NEJMoa0805017. [DOI] [PubMed] [Google Scholar]
- 51.Hirsch IB, Abelseth J, Bode BW, Fischer JS, Kaufman FR, Mastrototaro J, Parkin CG, Wolpert HA, Buckingham BA. Sensor-augmented insulin pump therapy: results of the first randomized treat-to-target study. Diabetes Technol Ther. 2008;10(5):377–383. doi: 10.1089/dia.2008.0068. [DOI] [PubMed] [Google Scholar]
- 52.McAllister S, Buckner EB, Whiter-Williams C. Medication adherence after heart transplantation: adolescents and their issues. Prog Transplant. 2006;16(4):317–323. doi: 10.1177/152692480601600406. [DOI] [PubMed] [Google Scholar]
- 53.Clark NM, Cabana MD, Nan B, Gong ZM, Slish KK, Birk NA, Kaciroti N. The clinician-patient partnership paradigm: outcomes associated with physician communication behavior. Clin Pediatr (Phila) 2008;47(1):49–57. doi: 10.1177/0009922807305650. [DOI] [PubMed] [Google Scholar]
- 54.Peel E, Douglas M, Lawton J. Self monitoring of blood glucose in type 2 diabetes: longitudinal qualitative study of patients' perspectives. BMJ. 2007;335(7618):493–498. doi: 10.1136/bmj.39302.444572.DE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Klonoff DC, Bergenstal R, Blonde L, Boren SA, Church TS, Gaffaney J, Jovanovič L, Kendall DM, Kollman C, Kovatchev BP, Leippert C, Owens DR, Polonsky WH, Reach G, Renard E, Riddell MC, Rubin RR, Schnell O, Siminiero LM, Vigersky RA, Wilson DM, Wollitzer AO. Consensus report of the coalition for clinical research–self-monitoring of blood glucose. J Diabetes Sci Technol. 2008;2(6):1030–1053. doi: 10.1177/193229680800200612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Reach G. Patient non-adherence and healthcare-provider clinical inertia are clinical myopia. Diabetes Metab. 2008;34(4 Pt 1):382–385. doi: 10.1016/j.diabet.2008.02.008. [DOI] [PubMed] [Google Scholar]