Abstract
Self-monitoring of blood glucose (SMBG) is an important adjunct to hemoglobin A1c (HbA1c) testing. This action can distinguish between fasting, preprandial, and postprandial hyperglycemia; detect glycemic excursions; identify and monitor resolution of hypoglycemia; and provide immediate feedback to patients about the effect of food choices, activity, and medication on glycemic control.
Pattern analysis is a systematic approach to identifying glycemic patterns within SMBG data and then taking appropriate action based upon those results. The use of pattern analysis involves: (1) establishing pre- and postprandial glucose targets; (2) obtaining data on glucose levels, carbohydrate intake, medication administration (type, dosages, timing), activity levels and physical/emotional stress; (3) analyzing data to identify patterns of glycemic excursions, assessing any influential factors, and implementing appropriate action(s); and (4) performing ongoing SMBG to assess the impact of any therapeutic changes made.
Computer-based and paper-based data collection and management tools can be developed to perform pattern analysis for identifying patterns in SMBG data. This approach to interpreting SMBG data facilitates rational therapeutic adjustments in response to this information. Pattern analysis of SMBG data can be of equal or greater value than measurement of HbA1c levels.
Keywords: diabetes, pattern, self-management, self-monitoring
Background
Diabetes is recognized by the World Health Organization and the International Diabetes Federation as a significant and growing health problem. In 2007, it was estimated that there were 246 million adults with diabetes throughout the world—an increase of more than 52 million since 2003.1 By 2025, the number of adults with diabetes is expected to rise to 380 million.1 This is considered a conservative figure.
People with diabetes are at increased risk for developing numerous complications, resulting in increased health care costs. In many countries, people with diabetes have a significantly decreased life expectancy,1 and there is a growing trend toward developing type 2 diabetes mellitus (T2DM) at much earlier ages than before. Thus, individuals and health care systems will be forced to deal with the devastating complications for many years.
With its severe complications and expensive treatments, diabetes has a profound impact on the physical, psychological, and financial well-being of individuals, their families, and our society.
Importance of Effective Diabetes Management and Overall Glucose Control
Large, randomized, controlled trials have clearly demonstrated a causal relationship between poor glycemic control and the development of microvascular disease.2,3 The link between effective diabetes management and reduced macrovascular disease has also been established.4,5 Studies by Gaede and colleagues showed that intensive management of all risk factors, including elevated lipids, blood pressure, and glycemia, had significant beneficial effects on cardiovascular-related deaths.6 This intensive therapy was also found to be cost-effective.7
Thus, all appropriate components of care (medical nutrition therapy, physical activity, weight loss regimens, pharmacologic interventions) should be implemented aggressively at the time of diagnosis.8 Clinicians should persistently monitor and titrate pharmacologic therapy until all glycemic goals are achieved.8
Importance of Addressing Postprandial Hyperglycemia
Although the current goal of diabetes therapy is to reduce time-averaged mean levels of hyperglycemia to prevent diabetic complications, hemoglobin A1c (HbA1c) only explains less than 25% of the variation in risk of developing complications.9,10 Postprandial hyperglycemia is now recognized as a significant risk factor for macrovascular disease, independent of HbA1c.11
Large epidemiological studies have shown a strong association between postprandial/postchallenge glycemia and cardiovascular risk and outcomes.12–14 A growing body of evidence also shows a causal relationship between postprandial hyperglycemia and oxidative stress,15 carotid IMT,16 and endothelial dysfunction,17,18 all of which are known markers of cardiovascular disease.
Although treating both fasting and postprandial hyperglycemia is important for achieving optimal glycemic control, only treatment with agents that specifically address postprandial glucose has been shown to reduce vascular events. A meta-analysis by Hanefeld and colleagues19 revealed significant positive trends in risk reduction for all selected cardiovascular event categories with patients treated with acarbose, an α-glucosidase inhibitor that specifically reduces postprandial glucose. Significant positive effects of postprandial glucose control have also been reported in patients treated with repaglinide, a rapid-acting insulin secretagogue that targets postprandial glucose.20
Several mechanisms are related to vascular damage. Numerous studies support the hypothesis of a causal relationship between hyperglycemia and oxidative stress.17,21–25 Current thinking proposes that hyperglycemia, free fatty acids, and insulin resistance feed into oxidative stress, protein kinase-C (PKC) activation, and advanced glycated end-product receptor (RAGE) activation, leading to vasoconstriction, inflammation, and thrombosis.26 Oxidative stress has been implicated as the underlying cause of both the macrovascular and microvascular complications associated with T2DM.27–29
Monnier and colleagues30 found that glucose fluctuations during postprandial periods and, more generally, during glucose swings exhibit a more specific triggering effect on oxidative stress than chronic sustained hyperglycemia. An earlier study by Schiekofer et al.31 demonstrated that glycemic variability promotes numerous steps in the inflammatory process. Most recently, El-Osta and colleagues9 demonstrated that transient hyperglycemic “spikes” cause persistent atherogenic effects during subsequent normoglycemia, thus providing additional evidence in support of postprandial hyperglycemia as an independent risk factor for diabetic complications. These studies suggest that interventional trials in T2DM should target not only HbA1c and mean glucose concentrations but also acute glucose swings. Addressing this metabolic abnormality is particularly important because postprandial hyperglycemia occurs frequently in individuals with type 1 diabetes mellitus (T1DM) and T2DM32–36 and can occur even when overall metabolic control appears to be adequate.33,35
Although there is still some controversy regarding postprandial glucose as an independent risk factor for vascular disease, the International Diabetes Federation guideline on postmeal glucose management provides a comprehensive analysis of the issue, along with the recommendation that postprandial glucose be treated aggressively.11 Moreover, the argument for managing postprandial glucose can be made simply by acknowledging that postprandial glucose is a component of HbA1c and, thus, must be treated.
Value and Utility of Self-Monitoring of Blood Glucose (SMBG)
Although HbA1c is perceived as the gold standard for monitoring glycemic control and serves as a surrogate for diabetes-related complications, it does not provide information about day-to-day and intra-day changes in glucose levels.37 Self-monitoring of blood glucose (SMBG) is an important adjunct to HbA1c because it can distinguish among fasting, preprandial, and postprandial hyperglycemia; detect glycemic excursions; identify and assist in monitoring resolution of hypoglycemia; and provide immediate feedback to patients about the effects of food choices, activity, and medication on glycemic control.37 HbA1c testing cannot make these distinctions or provide this information. Thus, SMBG is recognized as an important tool that guides glycemic management strategies and has the potential to improve problem-solving and decision-making skills for both the person with diabetes and his or her health care professional.8,38–40
Studies have clearly demonstrated the value of SMBG levels in the management of T1DM and insulin-treated T2DM.2,41 Using the American Association of Clinical Endocrinologists (AACE) road map42 with the help of SMBG, Lingvay and colleagues43 showed that in treatment-na metformin and insulin could achieve a normal HbA1c in a period of just 3 months. Other studies like the Treat-to-Target44 and 1-2-345 trials were able to achieve the targets by titration of insulin dose based on SMBG. Self-monitoring of blood glucose is also important in managing diabetes during pregnancy; it has been shown that intensive glycemic control significantly benefits fetal outcomes.46–50
Self-monitoring of blood glucose is recognized by leading medical organizations as an important tool in diabetes management, particularly in insulin-treated patients.8,39,51 However, some believe the utility of SMBG in non–insulin-treated T2DM remains controversial.52–57
Several studies involving non-insulin-treated T2DM have shown that therapeutic management programs that include structured SMBG use result in improved HbA1c control compared to programs without SMBG.54,57–62 Findings from three meta-analyses57,63,64 revealed that interventions that include SMBG levels result in an HbA1c reduction of 0.40% compared with interventions that do not include SMBG.57 When extrapolated to findings from the United Kingdom Prospective Diabetes Study,3 this decrease would be expected to reduce the risk of microvascular complications by approximately 14%.64
However, other studies have reported that SMBG has questionable value in non-insulin-treated patients.55,56,65–67 A study by Farmer and colleagues (DIGEM),55 which followed 453 non–insulin-treated patients for a median duration of 3 years, randomized patients into 3 groups: (1) control (usual care); (2) less intensive SMBG (meter training but no instruction for data interpretation); and (3) intensive SMBG (meter training with instruction for data interpretation and lifestyle modification). At the end of the study, researchers reported that there were no significant differences in improved HbA1c control among the 3 groups. However, it has been suggested that failure to show benefit from SMBG-use in non–insulin-treated diabetes in this study and others 56,68 may stem from a misapplication or misunderstanding of the true utility of SMBG as a tool to guide therapy, rather than as an independent therapeutic intervention.52,53,69
A key weakness of the DIGEM study was lack of information on the use of SMBG data to make pharmacologic treatment adjustments. Physicians would review SMBG data only when tests results of blood glucose were consistently >15 mmol/liter (270 mg/dl). Whether physicians ever saw any SMBG data from study subjects is unknown. In addition, the external validity of the study may have been constrained by the “floor effect” in that if levels of blood glucose are already well-controlled at baseline, additional improvements in HbA1c may be less easily achieved. According to the United Kingdom Guidelines, which were used in the study, “a target HbA1c should be set between 6.5% and 7.5% in patients with T2DM.”70 Because at least half of participants in all study groups were already at the goal (mean HbA1c 7.49–7.53%), achieving significant improvements in HbA1c would have been difficult. Further, there is also the possibility that there was little or no incentive to make changes in therapy to lower HbA1c.There was also an apparent lack of use of pharmacologic therapies that specifically target postprandial glucose, which may have, in fact, improved HbA1c levels. It has been demonstrated that the contribution of postprandial glucose to HbA1c becomes increasingly predominant as HbA1c falls below 8.4%.71 Another critical weakness of the study was the significant lack of adherence in the experimental groups; only 99 (67%) of the less intensive group and 79 (52%) of the more intensive group actually followed their prescribed SMBG regimens over the course of the study.
Also, the researchers failed to report important data relevant to SMBG values or use of those values by healthcare professionals. No data regarding SMBG values for the overall average, fasting/preprandial, or postprandial levels were provided. Thus, the frequency and/or severity of hypoglycemia or acute hyperglycemia (specifically, postprandial) is unknown. The researchers also provided no data regarding the number of patients whose SMBG results were reviewed by their physicians. In addition, it is unknown whether any changes in pharmacologic therapy were made by physicians based upon SMBG. Other studies have shown similar weaknesses.56
In summary, although many studies have reported negative findings regarding SMBG use in non–insulin-treated T2DM,55,56 they have, in fact, simply demonstrated that SMBG is valuable only when it is used effectively. Optimal SMBG use requires that both patients and health care professionals monitor, interpret and respond appropriately to acute glucose excursions and patterns of glycemia identified through SMBG.38,52,53 Table 1 presents proposed criteria for effective use of SMBG.
Table 1.
Proposed Criteria for Effective Use of SMBG53
| Use of SMBG may improve glycemic control in non–insulin-treated T2DM subjects when the following criteria are met: | |
| Subjects |
|
| Healthcare Professionals (HCPs) |
|
Episodic, Intensive Use of SMBG
Frequent monitoring of blood glucose is recommended in T1DM and insulin-treated T2DM. However, individuals who are treated with diet, exercise and/or oral or non-insulin injected (exenatide) antidiabetic medications may not require daily SMBG; pre- and postprandial testing on 2–3 days each week may be sufficient in some cases. However, it is valuable for all patients to perform intensive SMBG periodically to create data sets that facilitate identification of glucose patterns that are reflective of daily glycemic control.8 For example, patients may use a 7-point SMBG regimen, testing before and after each meal and at bedtime, over the course of 3 to 7 days. Or, patients may use a “staggered” regimen, testing before and after alternating meals (e.g., pre- and post-breakfast on Monday, pre- and post-lunch on Tuesday, etc.), over a 2- to 3-week period.37,72 By staggering SMBG measurements at different times on different days, patients can generate an accurate portrait of day-to-day glycemic excursions while avoiding the need to test many times in a single day.37
There are several situations in which intensive, episodic (short-term) SMBG can be beneficial to this patient group. These situations include times when patients: (1) have symptoms of hypoglycemia; (2) are undergoing adjustment in medication, nutrition, and/or physical activity; or (3) experience worsening HbA1c values. The AACE recommends episodic, intensive monitoring by patients with T2DM as a means to obtain comprehensive preprandial and 2-hour postprandial glucose measurements periodically and before clinician visits to guide adjustments in diabetes management.8
Pattern Analysis: Effective Use of SMBG Data
The key to effective use of SMBG in clinical practice is “pattern analysis,” regardless of the monitoring regimen.37 Pattern analysis is a systematic approach to identifying glycemic patterns within SMBG data and then taking appropriate action based upon those results.37 Using pattern analysis involves: (1) establishing pre- and postprandial glucose targets; (2) obtaining data on glucose levels, carbohydrate intake, medication administration (type, dosage, timing), activity levels, and physical/emotional stress; (3) analyzing data to identify patterns of glycemic excursions and assessing any influential factors; (4) implementing appropriate action(s); and (5) performing ongoing SMBG to assess the impact of any therapeutic changes made.73
Although clinicians have used pattern analysis in diabetes treatment since the early 1980s, only recently have studies looked at the impact of this approach on key clinical outcomes. A study by Cho and colleagues investigated the long-term effectiveness of an Internet-based glucose monitoring system (IBGMS) on glucose control in patients with T2DM.74 Patients in the intervention group logged onto a Website (http://www.biodang.com) at their convenience and uploaded their glucose levels (SMBG results). Patients were contacted by a clinician if there was a need to change medication or initiate lifestyle changes. Other information, such as current medication, weight, and blood pressure were also uploaded. At the end of 30 months, mean HbA1c in the intervention group was significantly lower than in patients who received conventional care (HbA1c [mean ± standard deviation], 6.9 ± 0.9% vs 7.5 ± 1.0%, p = .009).
Another study looked at the use of pattern analysis to predict severe hypoglycemia in patients with diabetes.75 The study followed 100 patients with T1DM (6 months) and 79 insulin-using patients with T2DM (4 months), during which time the patients' routine SMBG readings were downloaded from blood glucose meter memory. At 2-week intervals, patients reported occurrences of severe hypoglycemia, including the date and time of each occurrence. Analysis of the SMBG data and patient reports revealed that the clearest indicator of impending severe hypoglycemia was a significant increase in the relative risk for hypoglycemia as measured by the low blood glucose index, a proven mathematical calculation that provides an accurate assessment of severe hypoglycemia risk based upon SMBG data.76 The researchers concluded that severe hypoglycemia often follows a specific blood glucose fluctuation pattern that is identifiable from SMBG, which makes prediction of severe hypoglycemia possible. The ability to predict probable episodes of severe hypoglycemia based on blood glucose meter data has important clinical implications for helping patients avoid significant health consequences.
Although some may argue that fasting/preprandial blood glucose monitoring is sufficient for generating meaningful patterns of glycemic control, this is not the case; measuring only fasting/preprandial glucose cannot provide information about the timing, frequency, and/or degree of glucose excursions throughout the day, which is the whole point of pattern analysis. One cannot exclude postprandial testing from the regimen, particularly if one accepts that postprandial hyperglycemia is an independent risk factor for vascular disease. As demonstrated by Monnier,71 the influence of postprandial glucose becomes even more significant at lower HbA1c levels.
Moreover, the International Diabetes Federation is circulating a report that includes recommendations for the use of SMBG in non–insulin-treated T2DM. A key component of these recommendations is the value and utility of SMBG as a means of educating and motivating patients. Understanding how various foods, food portions, exercise, and other activities impact glucose levels is essential to an individual's willingness and ability to adopt healthy diabetes-related behaviors. Postprandial glucose testing is essential to obtaining this understanding.
SMBG Data Formats
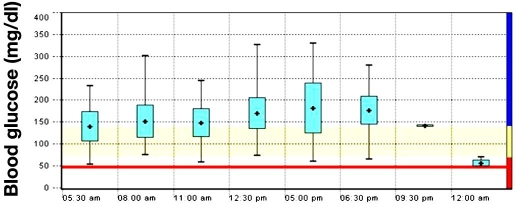
Most blood glucose meters can be used in tandem with software programs that allow downloading of blood glucose data into a wide variety of formats, which facilitate the clinician's ability to easily track and address glycemic trends. Formats offered by these software programs typically include: (1) an “electronic logbook” that lists blood glucose readings by date and time; (2) statistical summaries of average blood glucose values, number of tests performed, proportion of glucose levels above, below, and within the target range, frequency of testing by day of the week, and by time period within a given day, and the standard deviations, indicating glycemic variability, for specified time periods. Information is also presented in histograms, pie charts, and a modal day/week (a graphic that plots blood glucose values against a single 24-hour or 7-day period) (see Figure 1). One data management tool also provides information about patient risk for hypoglycemia and hyperglycemia using blood glucose risk indices.
Figure 1.
Day graph (modal day) from electronic data management tool. The graph provides detailed information on the blood glucose values and helps define the degree of glucose exposure and glucose variability over the reported time period. The blue boxes represent the standard deviation on mean blood glucose values, reflecting the glycemic variability.
The blood glucose risk indices capture the frequency and extent of the blood glucose fluctuations in a single number.77 For example, the low blood glucose index will be higher for subjects with a higher percentage of low blood glucose readings or who have more extreme hypoglycemic episodes. The high blood glucose index works in the same manner, indicating the frequency and/or extent of hyperglycemia.
With their ability to synthesize numerous test results into a wide variety of formats, blood glucose data management programs enable the clinician, patient, and other members of the management team to set goals, monitor progress from one clinic visit to another, identify problems, and evaluate interventions.78 However, because some blood glucose meters are limited in their ability to capture other key data (e.g., nutritional intake, physical activity, medication administration, stress levels, illness), clinicians are encouraged to use downloaded SMBG data in conjunction with written records in logbooks to evaluate glucose patterns and formulate more precise and efficacious therapeutic regimens.78 It is important to note that there are additional barriers to downloading SMBG data, such as incompatible software programs provided by the various manufacturers. Also, there is the issue of reimbursement for time spent downloading and interpreting data.
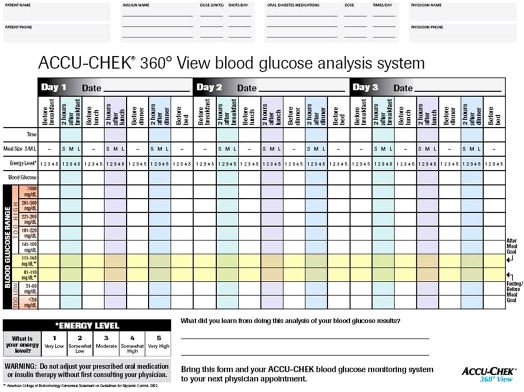
Although much of the focus has been on glucose data management software, new paper-based tools have also been developed to address clinical situations where computer-based pattern analysis is not desirable or practical. One such tool, the Accu-Chek®360° View blood glucose analysis system (Roche, Indianapolis, IN), collects three consecutive seven-point daily profiles, along with information about meal sizes and energy levels (Figure 2). An evaluation of the tool was conducted to determine if patients with T2DM could use and learn from the system.79 The evaluation showed that participants were willing and able to accurately complete 21 tests over 3 days of intensive blood glucose monitoring. Participants recorded blood glucose data with an average accuracy rate of 96%. Ninety percent of the participants indicated their willingness to repeat the monitoring on a quarterly basis, if asked to do so by their physician.
Figure 2.
Accu-Chek® 360° View blood glucose analysis system. The form is used to create a seven-point glucose profile over 3 days.Space is provided to plot and visualize blood glucose results. Guidelines on the form (rows highlighted in yellow) indicate pre- or postprandial blood glucose target ranges and ranges considered too high or low. Additional space is provided at the bottom to allow individuals to document what they learned from their testing.
This tool was also evaluated as part of an exploratory study that was conducted to determine whether primary care physicians could effectively use simple data collection tools to accurately identify glycemic abnormalities in episodic, intensive SMBG data from participants with non–insulin-treated T2DM, and whether use of these SMBG data would influence their therapeutic decisions.80 In the study, 23 case studies demonstrating several glycemic states (normoglycemia, elevated fasting glucose, elevated postprandial glucose, elevated glucose at all times, and hypoglycemia) were presented to 61 primary care physicians who evaluated the cases based upon HbA1c data alone, and then in combination with SMBG data. Participants were to identify the specific glucose pattern, determine the necessity for therapy change, and select specific therapeutic changes. Participant assessments were compared with assessments made by a panel of diabetes care specialists. The study showed that 78% of the participants identified the same primary blood glucose feature identified by the diabetes specialists; 93.8% agreed with the diabetes care specialists regarding the need for therapy modification. The majority (77 %) of participants changed the way they would manage the case after evaluating case studies with SMBG data made available to them. Overall, 86% of participants considered the SMBG data to be of equal or greater value than the HbA1c data; 71% of participants strongly agreed that they are now more likely to recommend episodic, high-frequency SMBG to their non–insulin-treated T2DM patients. A large, randomized clinical trial (NCT00674986) is currently underway to determine the impact of episodic, intensive SMBG monitoring on glycemic control in actual primary care practice.81
Conclusion
Diabetes is a significant and growing worldwide health concern.1 Uncontrolled diabetes is associated with debilitating microvascular and macrovascular complications.2,3 Intensive management of glycemia and other risk factors has been shown to reduce the development and/or progression of microvascular and macrovascular complications.2,3,6
The HbA1c test is valuable in assessing long-term glycemic control; however, it does not provide information about day-to-day changes in glucose levels.37 SMBG has been shown to be an important component of diabetes management through its ability to detect acute complications and assess intra-day glucose excursions.37 This is particularly important in light of studies that have shown postprandial hyperglycemia to be a risk factor for macrovascular disease, independent of HbA1c levels.12–14
Pattern analysis is a systematic approach to identifying glycemic patterns within SMBG data and then taking appropriate action based upon those results.31 The availability of computer-based and paper-based data collection and management tools facilitates more robust and efficient use of SMBG data, allowing clinicians and patients to quickly identify glycemic patterns and make more informed decisions about therapeutic adjustments that may be required.
Abbreviations
- AACE
American Association of Clinical Endocrinologists
- HbA1c
hemoglobin A1c
- T1DM
type 1 diabetes
- T2DM
type 2 diabetes
- SMBG
self-monitoring of blood glucose
References
- 1.International Diabetes Federation. Diabetes facts and figures [cited 2008 Nov 1] Available from: http://www.idf.org.
- 2.Diabetes Control and Complications Trial (DCCT) Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329(14):977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 3.UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352(9131):837–853. [PubMed] [Google Scholar]
- 4.Stratton IM, Adler AI, Neil HA, Matthews DR, Manley SE, Cull CA, Hadden D, Turner RC, Holman RR. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321(7258):405–412. doi: 10.1136/bmj.321.7258.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359(15):1577–1589. doi: 10.1056/NEJMoa0806470. [DOI] [PubMed] [Google Scholar]
- 6.Gaede P, Lund-Andersen H, Parving HH, Pedersen O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med. 2008;358(6):580–591. doi: 10.1056/NEJMoa0706245. [DOI] [PubMed] [Google Scholar]
- 7.Gaede P, Valentine WJ, Palmer AJ, Tucker DM, Lammert M, Parving HH, Pedersen O. Cost-effectiveness of Intensified versus conventional multifactorial intervention in type 2 diabetes: Results and projections from the Steno-2 study. Diabetes Care. 2008;31(8):1510–1515. doi: 10.2337/dc07-2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rodbard HW, Blonde L, Braithwaite SS, Brett EM, Cobin RH, Handelsman Y, Hellman R, Jellinger PS, Jovanovic LG, Levy P, Mechanick JI, Zangeneh F. AACE Diabetes Mellitus Clinical Practice Guidelines Task Force. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the management of diabetes mellitus. Endocr Pract. 2007;13(Suppl 1):1–68. doi: 10.4158/EP.13.S1.1. [DOI] [PubMed] [Google Scholar]
- 9.El-Osta A, Brasacchio D, Yao D, Pocai A, Jones PL, Roeder RG, Cooper ME, Brownlee M. Transient high glucose causes persistent epigenetic changes and altered gene expression during subsequent normoglycemia. J Exp Med. 2008;205(10):2409–2417. doi: 10.1084/jem.20081188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diabetes Control and Complications Trial (DCCT) Research Group. The relationship of glycemic exposure (HbA1c) to the risk of development and progression of retinopathy in the diabetes control and complications trial. Diabetes. 1995;44(8):968–983. [PubMed] [Google Scholar]
- 11.International Diabetes Federation Guideline Development Committee. Guideline for management of postmeal glucose. Brussels: International Diabetes Federation; 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sorkin JD, Muller DC, Fleg JL, Andres R. The relation of fasting and 2-h postchallenge plasma glucose concentrations to mortality: data from the Baltimore Longitudinal Study of Aging with a critical review of the literature. Diabetes Care. 2005;28(11):2626–2632. doi: 10.2337/diacare.28.11.2626. [DOI] [PubMed] [Google Scholar]
- 13.Levitan EB, Song Y, Ford ES, Liu S. Is nondiabetic hyperglycemia a risk factor for cardiovascular disease? A meta-analysis of prospective studies. Arch Intern Med. 2004;164(19):2147–2155. doi: 10.1001/archinte.164.19.2147. [DOI] [PubMed] [Google Scholar]
- 14.Cavalot F, Petrelli A, Traversa M, Bonomo K, Fiora E, Conti M, Anfossi G, Costa G, Trovati M. Postprandial blood glucose is a stronger predictor of cardiovascular events than fasting blood glucose in type 2 diabetes mellitus, particularly in women: lessons from the San Luigi Gonzaga Diabetes Study. J Clin Endocrinol Metab. 2006;91(3):813–819. doi: 10.1210/jc.2005-1005. [DOI] [PubMed] [Google Scholar]
- 15.Ceriello A, Quagliaro L, Piconi L, Assaloni R, Da RR, Maier A, Esposito K, Giugliano D. Effect of postprandial hypertriglyceridemia and hyperglycemia on circulating adhesion molecules and oxidative stress generation and the possible role of simvastatin treatment. Diabetes. 2004;53(3):701–710. doi: 10.2337/diabetes.53.3.701. [DOI] [PubMed] [Google Scholar]
- 16.Hanefeld M, Koehler C, Schaper F, Fuecker K, Henkel E, Temelkova-Kurktschiev T. Postprandial plasma glucose is an independent risk factor for increased carotid intima-media thickness in non-diabetic individuals. Atherosclerosis. 1999;144(1):229–235. doi: 10.1016/s0021-9150(99)00059-3. [DOI] [PubMed] [Google Scholar]
- 17.Williams SB, Goldfine AB, Timimi FK, Ting HH, Roddy MA, Simonson DC, Craeger MA. Acute hyperglycemia attenuates endothelium-dependent vasodilation in humans in vivo. Circulation. 1998;97(17):1695–1701. doi: 10.1161/01.cir.97.17.1695. [DOI] [PubMed] [Google Scholar]
- 18.Kawano H, Motoyama T, Hirashima O, Hirai N, Miyao Y, Sakamoto T, Kugiyama K, Ogawa H, Yasue H. Hyperglycemia rapidly suppresses flow-mediated endothelium-dependent vasodilation of brachial artery. J Am Coll Cardiol. 1999;34(1):146–154. doi: 10.1016/s0735-1097(99)00168-0. [DOI] [PubMed] [Google Scholar]
- 19.Hanefeld M, Cagatay M, Petrowitsch T, Neuser D, Petzinna D, Rupp M. Acarbose reduces the risk for myocardial infarction in type 2 diabetic patients: meta-analysis of seven long-term studies. Eur Heart J. 2004;25(1):10–16. doi: 10.1016/s0195-668x(03)00468-8. [DOI] [PubMed] [Google Scholar]
- 20.Esposito K, Giugliano D, Nappo F, Marfella R. Campanian Postprandial Hyperglycemia Study Group. Regression of carotid atherosclerosis by control of postprandial hyperglycemia in type 2 diabetes mellitus. Circulation. 2004;110(2):214–219. doi: 10.1161/01.CIR.0000134501.57864.66. [DOI] [PubMed] [Google Scholar]
- 21.Ceriello A, Falleti E, Motz E, Taboga C, Tonutti L, Ezsol Z, Gonano F, Bartoli E. Hyperglycemia-induced circulating ICAM-1 increase in diabetes mellitus: the possible role of oxidative stress. Horm Metab Res. 1998;30(3):146–149. doi: 10.1055/s-2007-978854. [DOI] [PubMed] [Google Scholar]
- 22.Cominacini L, Fratta PA, Garbin U, Campagnola M, Davoli A, Rigoni A, Zenti MG, Pastorino AM, Lo Cascio V. E-selectin plasma concentration is influenced by glycaemic control in NIDDM patients: possible role of oxidative stress. Diabetologia. 1997;40(5):584–589. doi: 10.1007/s001250050719. [DOI] [PubMed] [Google Scholar]
- 23.Nappo F, Esposito K, Cioffi M, Giugliano G, Molinari AM, Paolisso G, Marfella R, Giugliano D. Postprandial endothelial activation in healthy subjects and in type 2 diabetic patients: role of fat and carbohydrate meals. J Am Coll Cardiol. 2002;39(7):1145–1150. doi: 10.1016/s0735-1097(02)01741-2. [DOI] [PubMed] [Google Scholar]
- 24.Esposito K, Nappo F, Marfella R, Giugliano G, Giugliano F, Ciotola M, Quagliaro L, Ceriello A, Giugliano D. Inflammatory cytokine concentrations are acutely increased by hyperglycemia in humans: role of oxidative stress. Circulation. 2002;106(16):2067–2072. doi: 10.1161/01.cir.0000034509.14906.ae. [DOI] [PubMed] [Google Scholar]
- 25.Marfella R, Quagliaro L, Nappo F, Ceriello A, Giugliano D. Acute hyperglycemia induces an oxidative stress in healthy subjects. J Clin Invest. 2001;108(4):635–636. doi: 10.1172/JCI13727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gerich JE. Clinical significance, pathogenesis, and management of postprandial hyperglycemia. Arch Intern Med. 2003;163(11):1306–1316. doi: 10.1001/archinte.163.11.1306. [DOI] [PubMed] [Google Scholar]
- 27.Khatri JJ, Johnson C, Magid R, Lessner SM, Laude KM, Dikalov SI, Harrison DG, Sung HJ, Rong Y, Galis ZS. Vascular oxidant stress enhances progression and angiogenesis of experimental atheroma. Circulation. 2004;109(4):520–525. doi: 10.1161/01.CIR.0000109698.70638.2B. [DOI] [PubMed] [Google Scholar]
- 28.von Harsdorf R, Li PF, Dietz R. Signaling pathways in reactive oxygen species-induced cardiomyocyte apoptosis. Circulation. 1999;99(22):2934–2941. doi: 10.1161/01.cir.99.22.2934. [DOI] [PubMed] [Google Scholar]
- 29.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414(6865):813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 30.Monnier L, Mas E, Ginet C, Michel F, Villon L, Cristol JP, Colette C. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA. 2006;295(14):1681–1687. doi: 10.1001/jama.295.14.1681. [DOI] [PubMed] [Google Scholar]
- 31.Schiekofer S, Andrassy M, Chen J, Rudofsky G, Schneider J, Wendt T, Stefan N, Humpert P, Fritsche A, Stumvoll M, Schleicher E, Häring HU, Nawroth PP, Bierhaus A. Acute hyperglycemia causes intracellular formation of CML and activation of ras, p42/44 MAPK, and nuclear factor kappaB in PBMCs. Diabetes. 2003;52(3):621–633. doi: 10.2337/diabetes.52.3.621. [DOI] [PubMed] [Google Scholar]
- 32.Akbar DH. Sub-optimal postprandial blood glucose level in diabetics attending the outpatient clinic of a University Hospital. Saudi Med J. 2003;24(10):1109–1112. [PubMed] [Google Scholar]
- 33.Erlinger TP, Brancati FL. Postchallenge hyperglycemia in a national sample of U.S. adults with type 2 diabetes. Diabetes Care. 2001;24(10):1734–1738. doi: 10.2337/diacare.24.10.1734. [DOI] [PubMed] [Google Scholar]
- 34.Maia FF, Araujo LR. Efficacy of continuous glucose monitoring system (CGMS) to detect postprandial hyperglycemia and unrecognized hypoglycemia in type 1 diabetic patients. Diabetes Res Clin Pract. 2007;75(1):30–34. doi: 10.1016/j.diabres.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 35.Bonora E, Corrao G, Bagnardi V, Ceriello A, Comaschi M, Montanari P, Meigs JB. Prevalence and correlates of post-prandial hyperglycaemia in a large sample of patients with type 2 diabetes mellitus. Diabetologia. 2006;49(5):846–854. doi: 10.1007/s00125-006-0203-x. [DOI] [PubMed] [Google Scholar]
- 36.Esposito K, Ciotola M, Carleo D, Schisano B, Sardelli L, Di Tommaso D, Misso L, Saccomanno F, Ceriello A, Giugliano D. Post-meal glucose peaks at home associate with carotid intima-media thickness in type 2 diabetes. J Clin Endocrinol Metab. 2008;93(4):1345–1350. doi: 10.1210/jc.2007-2000. [DOI] [PubMed] [Google Scholar]
- 37.Dailey G. Assessing glycemic control with self-monitoring of blood glucose and hemoglobin A(1c) measurements. Mayo Clin Proc. 2007;82(2):229–235. doi: 10.4065/82.2.229. [DOI] [PubMed] [Google Scholar]
- 38.Austin MM, Haas L, Johnson T, Parkin CG, Parkin CL, Spollett G, Volpone MT. Self-monitoring of blood glucose: benefits and utilization. Diabetes Educ. 2006;32(6):835–836. 884–887. doi: 10.1177/0145721706295873. [DOI] [PubMed] [Google Scholar]
- 39.American Diabetes Association. Standards of medical care in diabetes–2008. Diabetes Care. 2008;31(Suppl 1):S12–S54. doi: 10.2337/dc08-S012. [DOI] [PubMed] [Google Scholar]
- 40.American Association of Clinical Endrocrinologists. The American Association of Clinical Endocrinologists Medical Guidelines for the Management of Diabetes Mellitus: the AACE system of intensive diabetes self-management–2000 update. Endocr Pract. 2000;6(1):43–84. [PubMed] [Google Scholar]
- 41.Abraira C, Colwell JA, Nuttall FQ, Sawin CT, Nagel NJ, Comstock JP, Emanuele NV, Levin SR, Henderson W, Lee HS. Veterans Affairs Cooperative Study on glycemic control and complications in type II diabetes (VA CSDM). Results of the feasibility trial. Veterans Affairs Cooperative Study in Type II Diabetes. Diabetes Care. 1995;18(8):1113–1123. doi: 10.2337/diacare.18.8.1113. [DOI] [PubMed] [Google Scholar]
- 42.Jellinger PS, Davidson JA, Blonde L, Grunberger G, Hellman R, Levy P. Road map to achieve glycemic goals. Jacksonville (FL): American Association of Clinical Endocrinologists; 2008. [DOI] [PubMed] [Google Scholar]
- 43.Lingvay I, Kaloyanova PF, Adams-Huet B, Salinas K, Raskin P. Insulin as initial therapy in type 2 diabetes: effective, safe, and well accepted. J Investig Med. 2007;55(2):62–68. doi: 10.2310/6650.2007.06036. [DOI] [PubMed] [Google Scholar]
- 44.Riddle MC, Rosenstock J, Gerich J. The treat-to-target trial: randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetic patients. Diabetes Care. 2003;26(11):3080–3086. doi: 10.2337/diacare.26.11.3080. [DOI] [PubMed] [Google Scholar]
- 45.Garber AJ, Wahlen J, Wahl T, Bressler P, Braceras R, Allen E, Jain R. Attainment of glycaemic goals in type 2 diabetes with once-, twice-, or thrice-daily dosing with biphasic insulin aspart 70/30 (The 1-2-3 study) Diabetes Obes Metab. 2006;8(1):58–66. doi: 10.1111/j.1463-1326.2005.00563.x. [DOI] [PubMed] [Google Scholar]
- 46.Goldberg JD, Franklin B, Lasser D, Jornsay DL, Hausknecht RU, Ginsberg-Fellner F, Berkowitz RL. Gestational diabetes: impact of home glucose monitoring on neonatal birth weight. Am J Obstet Gynecol. 1986;154(3):546–550. doi: 10.1016/0002-9378(86)90599-5. [DOI] [PubMed] [Google Scholar]
- 47.Jensen DM, Damm P, Moelsted-Pedersen L, Ovesen P, Westergaard JG, Moeller M, Beck-Nielsen H. Outcomes in type 1 diabetic pregnancies: a nationwide, population-based study. Diabetes Care. 2004;27(12):2819–2823. doi: 10.2337/diacare.27.12.2819. [DOI] [PubMed] [Google Scholar]
- 48.Jovanovic LG. Using meal-based self-monitoring of blood glucose as a tool to improve outcomes in pregnancy complicated by diabetes. Endocr Pract. 2008;14(2):239–247. doi: 10.4158/EP.14.2.239. [DOI] [PubMed] [Google Scholar]
- 49.Ylinen K, Aula P, Stenman UH, Kesaniemi-Kuokkanen T, Teramo K. Risk of minor and major fetal malformations in diabetics with high haemoglobin A1c values in early pregnancy. Br Med J. 1984;289(6441):345–356. doi: 10.1136/bmj.289.6441.345. (Clin Res Ed) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.De Veciana M, Major C, Morgan M, Asrat T, Toohey J, Lien J, Evans AT. Postprandial versus preprandial blood glucose monitoring in women with gestational diabetes mellitus requiring insulin therapy. N Engl J Med. 1995;333(19):1237–1241. doi: 10.1056/NEJM199511093331901. [DOI] [PubMed] [Google Scholar]
- 51.IDF Clinical Guidelines Task Force. Global guideline for Type 2 diabetes. Brussels: International Diabetes Federation; 2006. Available from: http://www.idf.org. [Google Scholar]
- 52.Klonoff DC. New evidence demonstrates that self-monitoring of blood glucose does not improve outcomes in type 2 diabetes–when this practice is not applied properly. J Diabetes Sci Technol. 2008;2(3):342–348. doi: 10.1177/193229680800200302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Parkin CG, Price D. Randomized studies are needed to assess the true role of self-monitoring of blood gluocse in noninsulin-treated type 2 diabetes. J Diabetes Sci Technol. 2007;1(4):595–602. doi: 10.1177/193229680700100419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Barnett AH, Krentz AJ, Strojek K, Sieradzki J, Azizi F, Embong M, Imamoglu S, Perusicová J, Uliciansky V, Winkler G. The efficacy of self-monitoring of blood glucose in the management of patients with type 2 diabetes treated with a gliclazide modified release-based regimen: A multicentre, randomized, parallel-group, 6-month evaluation (DINAMIC 1 study) Diabetes Obes Metab. 2008;10(12):1239–1247. doi: 10.1111/j.1463-1326.2008.00894.x. [DOI] [PubMed] [Google Scholar]
- 55.Farmer A, Wade A, Goyder E, Yudkin P, French D, Craven A, Holman R, Kinmonth AL, Neil A. Impact of self monitoring of blood glucose in the management of patients with non-insulin treated diabetes: open parallel group randomised trial. BMJ. 2007;335(7611):132. doi: 10.1136/bmj.39247.447431.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Davidson MB, Castellanos M, Kain D, Duran P. The effect of self monitoring of blood glucose concentrations on glycated hemoglobin levels in diabetic patients not taking insula blinded, randomized trial. Am J Med. 2005;118(4):422–425. doi: 10.1016/j.amjmed.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 57.Jansen JP. Self-monitoring of glucose in type 2 diabetes mellitus: a Bayesian meta-analysis of direct and indirect comparisons. Curr Med Res Opin. 2006;22(4):671–681. doi: 10.1185/030079906X96308. [DOI] [PubMed] [Google Scholar]
- 58.Guerci B, Drouin P, Grange V, Bougneres P, Fontaine P, Kerlan V, Passa P, Thivolet Ch, Vialettes B, Charbonnel B ASIA Group. Self-monitoring of blood glucose significantly improves metabolic control in patients with type 2 diabetes mellitus: the Auto-Surveillance Intervention Active (ASIA) study. Diabetes Metab. 2003;29(6):587–594. doi: 10.1016/s1262-3636(07)70073-3. [DOI] [PubMed] [Google Scholar]
- 59.Moreland EC, Volkening LK, Lawlor MT, Chalmers KA, Anderson BJ, Laffel LM. Use of a blood glucose monitoring manual to enhance monitoring adherence in adults with diabetes: a randomized controlled trial. Arch Intern Med. 2006;166(6):689–695. doi: 10.1001/archinte.166.6.689. [DOI] [PubMed] [Google Scholar]
- 60.Schwedes U, Siebolds M, Mertes G SMBG Study Group. Meal-related structured self-monitoring of blood glucose: effect on diabetes control in non-insulin-treated type 2 diabetic patients. Diabetes Care. 2002;25(11):1928–1932. doi: 10.2337/diacare.25.11.1928. [DOI] [PubMed] [Google Scholar]
- 61.Martin S, Schneider B, Heinemann L, Lodwig V, Kurth HJ, Kolb H, Scherbaum WA. Self-monitoring of blood glucose in type 2 diabetes and long-term outcome: an epidemiological cohort study. Diabetologia. 2006;49(2):271–278. doi: 10.1007/s00125-005-0083-5. [DOI] [PubMed] [Google Scholar]
- 62.Murata GH, Shah JH, Hoffman RM, Wendel CS, Adam KD, Solvas PA, Bokhari SU, Duckworth WC Diabetes Outcomes in Veterans Study (DOVES) Intensified blood glucose monitoring improves glycemic control in stable, insulin-treated veterans with type 2 diabetes: the Diabetes Outcomes in Veterans Study (DOVES) Diabetes Care. 2003;26(6):1759–1763. doi: 10.2337/diacare.26.6.1759. [DOI] [PubMed] [Google Scholar]
- 63.Sarol JN, Jr., Nicodemus NA, Jr., Tan KM, Grava MB. Self-monitoring of blood glucose as part of a multi-component therapy among non-insulin requiring type 2 diabetes patients: a meta-analysis (1966-2004) Curr Med Res Opin. 2005;21(2):173–184. doi: 10.1185/030079904X20286. [DOI] [PubMed] [Google Scholar]
- 64.Welschen LM, Bloemendal E, Nijpels G, Dekker JM, Heine RJ, Stalman WA, Bouter LM. Self-monitoring of blood glucose in patients with type 2 diabetes who are not using insula systematic review. Diabetes Care. 2005;28(6):1510–1517. doi: 10.2337/diacare.28.6.1510. [DOI] [PubMed] [Google Scholar]
- 65.Coster S, Gulliford MC, Seed PT, Powrie JK, Swaminathan R. Self-monitoring in Type 2 diabetes mellitus: a meta-analysis. Diabet Med. 2000;17(11):755–761. doi: 10.1046/j.1464-5491.2000.00390.x. [DOI] [PubMed] [Google Scholar]
- 66.Simon J, Gray A, Clarke P, Wade A, Neil A, Farmer A. Diabetes Glycaemic Education and Monitoring Trial Group. Cost effectiveness of self monitoring of blood glucose in patients with non-insulin treated type 2 diabetes: economic evaluation of data from the DiGEM trial. BMJ. 2008;336(7654):1177–1180. doi: 10.1136/bmj.39526.674873.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.O'Kane MJ, Bunting B, Copeland M, Coates VE ESMON study group. Efficacy of self monitoring of blood glucose in patients with newly diagnosed type 2 diabetes (ESMON study): randomised controlled trial. BMJ. 2008;336(7654):1174–1177. doi: 10.1136/bmj.39534.571644.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Miles P, Everett J, Murphy J, Kerr D. Comparison of blood or urine testing by patients with newly diagnosed non-insulin dependent diabetes: patient survey after randomised crossover trial. BMJ. 1997;315(7104):348–349. doi: 10.1136/bmj.315.7104.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Parkin CG, Hirsch IB. Self-monitoring of blood glucose cannot compensate for ineffective diabetes management. Am J Med. 2005;118(12):1448–1449. doi: 10.1016/j.amjmed.2005.05.023. [DOI] [PubMed] [Google Scholar]
- 70.McIntosh A, Hutchinson A, Home P, Brown F, Bruce A, Damerell A, Davis R, Field R, Frost G, Marshall S, Roddick J, Tesfaye S, Withers H, Suckling R, Smith S, Griffin S, Kaltenthaler E, Peters J, Feder G. Sheffield: ScHARR, University of Sheffield; 2001. Clinical guidelines and evidence review for Type 2 diabetes: management of blood glucose. [Google Scholar]
- 71.Monnier L, Lapinski H, Colette C. Contributions of fasting and postprandial plasma glucose increments to the overall diurnal hyperglycemia of type 2 diabetic patients: variations with increasing levels of HbA(1c) Diabetes Care. 2003;26(3):881–885. doi: 10.2337/diacare.26.3.881. [DOI] [PubMed] [Google Scholar]
- 72.Parkin C, Brooks N. Is postprandial glucose control important? Is it practical in primary care settings? Clin Diabetes. 2002;20:71–76. [Google Scholar]
- 73.Pearson J, Bergenstal R. Fine-tuning control: pattern management versus supplementation: View 1: Pattern management: an essential component of effective insulin management. Diabetes Spectrum. 2001;14:75–78. [Google Scholar]
- 74.Cho JH, Chang SA, Kwon HS, Choi YH, Ko SH, Moon SD, Yoo SJ, Song KH, Son HS, Kim HS, Lee WC, Cha BY, Son HY, Yoon KH. Long-term effect of the Internet-based glucose monitoring system on HbA1c reduction and glucose stability: a 30-month follow-up study for diabetes management with a ubiquitous medical care system. Diabetes Care. 2006;29(12):2625–2631.t. doi: 10.2337/dc05-2371. [DOI] [PubMed] [Google Scholar]
- 75.Cox DJ, Gonder-Frederick L, Ritterband L, Clarke W, Kovatchev BP. Prediction of severe hypoglycemia. Diabetes Care. 2007;30(6):1370–1373. doi: 10.2337/dc06-1386. [DOI] [PubMed] [Google Scholar]
- 76.Kovatchev BP, Cox DJ, Gonder-Frederick LA, Young-Hyman D, Schlundt D, Clarke W. Assessment of risk for severe hypoglycemia among adults with IDDM: validation of the low blood glucose index. Diabetes Care. 1998;21(11):1870–1875. doi: 10.2337/diacare.21.11.1870. [DOI] [PubMed] [Google Scholar]
- 77.Kovatchev BP, Cox DJ, Gonder-Frederick LA, Clarke W. Symmetrization of the blood glucose measurement scale and its applications. Diabetes Care. 1997;20(11):1655–1658. doi: 10.2337/diacare.20.11.1655. [DOI] [PubMed] [Google Scholar]
- 78.Hirsch IB. Blood glucose monitoring technology: translating data into practice. Endocr Pract. 2004;10(1):67–76. doi: 10.4158/EP.10.1.67. [DOI] [PubMed] [Google Scholar]
- 79.Childs B, Laan R. Development of a novel blood glucose analysis system for episodic bG monitoring in persons with type 2 diabetes. Diabetes. 2007;56(Suppl 1):427. [Google Scholar]
- 80.Polonsky W, Jelsovsky A, Panzera S, Parkin C, Wagner R. Diabetes Technol Ther. 2009. Primary care physicians identify and act upon glycemic abnormalities found in structured, episodic blood glucose monitoring data from non-insulin treated type 2 diabetes. (In press) [DOI] [PubMed] [Google Scholar]
- 81.ClinicalTrials.gov [homepage on the Internet] Bethesda (MD): National Institutes of Health; 2008. Episodic intensive blood glucose monitoring in non-insulin treated type 2 diabetes. Available from: http://clinicaltrials.gov/ct2/show/NCT00674986. [Google Scholar]