Abstract
Background
This article provides a clinical update using a novel run-to-run algorithm to optimize prandial insulin dosing based on sparse glucose measurements from the previous day's meals. The objective was to use a refined run-to-run algorithm to calculate prandial insulin-to-carbohydrate ratios (I:CHO) for meals of variable carbohydrate content in subjects with type 1 diabetes (T1DM).
Method
The open-labeled, nonrandomized study took place over a 6-week period in a nonprofit research center. Nine subjects with T1DM using continuous subcutaneous insulin infusion participated. Basal insulin rates were optimized using continuous glucose monitoring, with a target fasting blood glucose of 90 mg/dl. Subjects monitored blood glucose concentration at the beginning of the meal and at 60 and 120 minutes after the start of the meal. They were instructed to start meals with blood glucose levels between 70 and 130 mg/dl. Subjects were contacted daily to collect data for the previous 24-hour period and to give them the physicianapproved, algorithm-derived I:CHO ratios for the next 24 hours. Subjects calculated the amount of the insulin bolus for each meal based on the corresponding I:CHO and their estimate of the meal's carbohydrate content. One- and 2-hour postprandial glucose concentrations served as the main outcome measures.
Results
The mean 1-hour postprandial blood glucose level was 104 ± 19 mg/dl. The 2-hour postprandial levels (96.5 ± 18 mg/dl) approached the preprandial levels (90.1 ± 13 mg/dl).
Conclusions
Run-to-run algorithms are able to improve postprandial blood glucose levels in subjects with T1DM.
Keywords: algorithm, insulin, prandial, run-to-run control, type 1 diabetes
Introduction
This article reports on the utility of using a novel run-to-run algorithm to optimize prandial insulin dosing based on sparse glucose measurements from the previous day's meals. Run-to-run control is used frequently in the chemical process industry to provide feedback control during manufacturing for processes that operate in a “batch” mode. We reported previously on the design, based on retrospective clinical data, of a novel run-to-run algorithm to optimize prandial insulin dosing.1 This study used a refined algorithm that allows for variable carbohydrate content in three meals per day. Results obtained show that the proposed run-to-run framework adjusting the insulin-to-carbohydrate ratios (I:CHO) works, being able to minimize the postprandial glycemic excursion while also minimizing hypoglycemic events.
Materials and Methods
Nine subjects with type 1 diabetes mellitus, who use continuous subcutaneous insulin infusion, were recruited (7 females/2 males, age 21–65 years, glycated hemoglobin A1c 7.1 ± 1.4%, body mass index 25.7 ± 5.7 kg/m2, duration of diabetes 14.8 ± 12.7 years). One female subject was withdrawn from the study due to urticaria requiring prednisone. Basal insulin infusion rates were optimized using continuous glucose monitoring,2 with a target of maintaining a fasting blood glucose (BG) of 90 mg/dl (5 mmol/liter). Inclusion criteria included having the diagnosis of type 1 diabetes for at least 1 year using a continuous subcutaneous insulin infusion pump with a rapid-acting insulin analog. Exclusion criteria included being pregnant or planning on becoming pregnant, being under 18 years of age, unwilling to perform repeated BG measurements, unwilling to take insulin as directed, or having abnormal thyroid, kidney, or liver function. The Cottage Health System Office of the Research Institutional Review Board approved the study, and informed, witnessed consent was obtained from all subjects.
Breakfast, lunch, and dinner meals of variable carbohydrate content were evaluated. The initial carbohydrate content of each meal was not restricted. The algorithm suggested an updated I:CHO for the same meal time for the following day. The total dose was then calculated based on the carbohydrate content of that meal. All prandial insulin was injected at initiation of the meal. The algorithm could also recommend that the subject decrease the carbohydrate content for the same mealtime on the following day.
Testing of the algorithm lasted 2 weeks. Using the OneTouch® UltraSmart® blood glucose monitoring system (LifeScan, Inc., Milpitas, CA), subjects monitored blood glucose concentrations at the beginning of the meal and at 60 and 120 minutes after the start of the meal. They were instructed to start meals with blood glucose levels between 70 and 130 mg/dl (3.9–7.2 mmol/liter).
Subjects were contacted daily to collect data for the previous 24-hour period and to give them the physician-approved, algorithm-derived I:CHO ratios for the next 24 hours. Subjects were asked to calculate the amount of the insulin bolus for each meal based on the I:CHO ratio and their estimate of the meal's carbohydrate content.
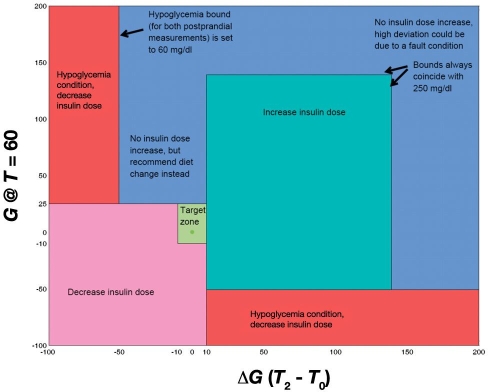
Each day was considered a “run.”1 A performance measure, ψk (k indicating the current day), was used to quantify the postprandial glucose excursion as a scalar quantity. A schematic of the decision regions and constraints of the algorithm can be seen in Figure 1. A corresponding performance measure, denoted by ψr, was used to quantify the ideal or target postprandial glucose response. In this case, the ideal is a response in which the BG remains unchanged from the value at the start of the meal (this corresponds to ψr = 0). The algorithm used the current I:CHO (υk) with the current and ideal performance measure and calculated the new I:CHO for the following day using
where the gain K is a tuning parameter (its selection is covered in Palerm and colleagues1).
Figure 1.
The algorithm takes different actions with respect to the adjustment of insulin depending on which region the performance measure falls into. The target zone (light green) requires no adjustment. The green region requires an increase in the insulin dose. The pink region requires a decrease in the insulin dose. The red region shows hypoglycemia, requiring a decrease in the insulin dose. The blue region, no change in insulin dose, recommends a lower carbohydrate content of the corresponding meal of the next run.
The performance measure is based on three BG determinations for each meal: preprandial blood glucose values (G0) and two postprandial values, reflecting peak excursion (G1, taken T1 minutes after the start of the meal) and 2 hours postprandial (G2, taken T2 minutes after the start of the meal). Postprandial determinations are targeted for 60 and 120 minutes after the start of the meal; however, exact timing is not necessary. For the 60-minute time point the algorithm uses the deviation from the start of the meal and is calculated considering the actual time of the measurement:
For the second postprandial measurement, deviation from the preprandial blood glucose is also used:
From these values, the performance measure is calculated as
A target zone was defined for the performance measure, bounded by
If the performance measure fell within this range, then the algorithm would not take any corrective action. A constraint was added to prevent the algorithm from changing the dose if either of the postprandial blood glucose measurements was greater than 250 mg/dl. The reason for this is that unless the initial I:CHO is grossly underestimated, the most likely reason for such high blood glucose levels would be a missed or late bolus or a gross underestimation of the carbohydrate content. Another constraint was for hypoglycemia (a blood glucose value lower than 60 mg/dl), in which the algorithm will cut back the insulin dose even if there is only one measurement available or if the second one cannot be used because there was additional action taken (e.g., consumption of carbohydrates or suspension of the basal insulin infusion).
Results
Nine subjects started and eight subjects completed the study. The mean carbohydrate content for the meal, at convergence, was breakfast: 34.7 ± 15.9 grams (0.517 ± 0.214 g/kg body weight), lunch: 47.7 ± 17.1 grams (0.636 ± 0.222 g/kg body weight), and dinner: 50.7 ± 20.6 grams CHO (0.680 ± 0.260 g/kg body weight). The defined target for convergence of the algorithm was defined as a deviation from the preprandial blood glucose of –10 to +35 mg/dl for the 60-minute postprandial BG value and of –10 to +27 mg/dl at the 120-minute postprandial blood glucose.
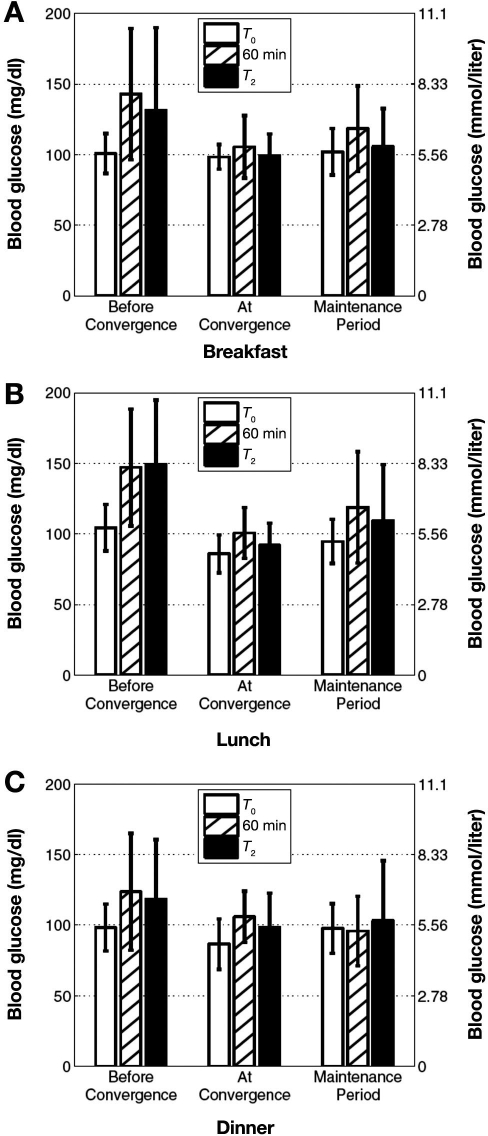
Table 1 shows average blood glucose levels in three distinct periods: before convergence, at convergence, and the maintenance period after convergence. The period “before convergence” extends from the first day, when the algorithm was initialized, to the day before the convergence target was achieved. “At convergence” was the day when this target was first achieved. The “maintenance period” covers all subsequent days following convergence. Figure 2 demonstrates graphically the average ± standard deviation (SD) blood glucose concentration for each meal during these three periods. For all three meals the difference in 1-hour postprandial blood glucose levels between the period before and after convergence was statistically significant (Mann–Whitney U test at a 0.05 significance level; p = 0.013, p = 0.023, and p = 0.0018 for breakfast, lunch, and dinner, respectively).
Table 1.
Average Blood Glucose Levels before Convergence, at Convergence, and after Convergencea
| Period before convergence | |||
|---|---|---|---|
| Breakfast | Lunch | Dinner | |
| Preprandial BG | 100.7 ± 14.2 (5.6 ±0.8) | 104.4± 16.4 (5.8 ± 0.9) | 98.3 ± 16.6 (5.5 ± 0.9) |
| BG at 60 min postprandial | 142.8 ± 46.6 (7.9 ±2.6) | 147.1 ± 41.4 (8.2 ± 2.3) | 123.7 ± 41.3 (6.9 ± 2.3) |
| BG at T2 postprandial | 131.4 ± 58.5 (7.3 ± 3.3) | 149.1 ± 45.7 (8.3 ± 2.5) | 118.4 ± 42.2 (6.6± 2.3) |
| Initial I:CHO ratio | 1 U per 9.9 ± 1.1 g CHO | 1 U per 10.3 ± 1.3 g CHO | 1 U per 9.7 ± 1.3 g CHO |
| At convergence | |||
| Breakfast | Lunch | Dinner | |
| Days to converge | 4.3 ± 2.7b | 2.8 ± 2.7 | 5.6 ± 3.2 |
| Preprandial BG | 98.3 ± 8.7 (5.5 ± 0.5) | 85.5 ± 13.5 (4.8 ± 0.8) | 86.5 ± 17.8 (4.8 ± 1.0) |
| BG at 60 min postprandial | 105.4 ± 22.1 (5.9 ± 1.2) | 100.7 ± 18.0 (5.6 ± 1.0) | 105.9 ± 18.1 (5.9 ± 1.0) |
| BG at T2 postprandial | 99.3 ± 15.2 (5.5 ± 0.8) | 91.9 ± 15.6 (5.1 ± 0.9) | 98.3 ± 24.3 (5.5 ± 1.4) |
| I:CHO ratio | 1 U per 8.4 ± 2.4 g CHO | 1 U per 9.2 ± 2.4 g CHO | 1 U per 9.2 ± 2.1 g CHO |
| Maintenance period after convergence | |||
| Breakfast | Lunch | Dinner | |
| Preprandial BG | 102.0 ± 16.4 (5.7 ± 0.9 | 94.6 ± 15.6 (5.3 ± 0.9 | 97.6 ± 17.7 (5.4 ± 1.0) |
| BG at 60 min postprandial | 118.4 ± 30.3 (6.6 ± 1.7) | 118.8 ± 39.5 (6.6 ± 2.2) | 95.8 ± 24.6 (5.3 ± 1.4) |
| BG at T2 postprandial | 105.6 ± 27.0 (5.9 ± 1.5) | 109.4 ± 39.6 (6.1 ± 2.2) | 103.2 ± 42.4 (5.7 ± 2.4) |
Average blood glucose levels in three distinct periods: before convergence, at convergence, and maintenance period after convergence. Blood glucose measurements are reported as mean ± SD in mg/dl (mmol/liter). Differences between the periods before and after convergence for the 1-hour postprandial glucose levels were statistically significant for all three meals (p = 0.013, p = 0.023, and p = 0.0018 for breakfast, lunch, and dinner, respectively).
One subject did not converge because of difficulty with the insulin infusion site on several mornings or not being able to measure blood glucose as required by the algorithm for this meal.
Figure 2.
For each of the meals (A, breakfast; B, lunch; C, dinner), the bar graph shows the blood glucose concentration average for the preprandial and two postprandial measurements for the periods before, at, and after convergence. For all three meals, the difference in 1-hour postprandial blood glucose levels between the period before and after convergence was statistically significant (Mann–Whitney U test at a 0.05 significance level; p = 0.013, p = 0.023, and p = 0.0018 for breakfast, lunch, and dinner, respectively).
The algorithm was able to safely improve postprandial blood glucose levels. The mean 1-hour postprandial blood glucose level was 104 ± 19 mg/dl. The 2-hour postprandial levels (96.5 ± 18 mg/dl) approached the preprandial levels (90.1 ± 13 mg/dl). There were a total of 12 hypoglycemic measurements (<55 mg/dl) reported over 230 meals. The mean glucose ± SD was 45.0 ± 5.0 mg/dl. There were no instances of severe hypoglycemia (defined as instances in which the subject required assistance). All subjects met convergence criteria except for one subject at breakfast, who had problems with her infusion site on several mornings or was not able to measure blood glucose at the appointed times for this meal as required by the algorithm.
It is of interest to note that the I:CHO for the lunch meal from previous testing of the run-to-run algorithm converged was similar to the I:CHO at convergence during this study (1 unit per 9.5 ± 2.3 and 1 unit per 9.2 ± 2.4 grams CHO, respectively). This also coincides with a completely independent approach to determining the correct I:CHO. Using the hyperinsulinemic–euglycemic clamp technique, Bevier and associates3 found the ratio for the lunch meal to be 1 unit per 9.3 ± 1.7 grams CHO.
Discussion
Determination of an adequate I:CHO remains a challenge for subjects with type 1 diabetes. To this end, the algorithm we have proposed provides a tool to help guide the continual adjustment to insulin dosing that is required.
This study provides a robust challenge of the algorithm; not only are all three meals considered, but the carbohydrate content of the meals is allowed to vary. Subjects were able to eat their usual type of meals. Once again, the algorithm was able to converge on the I:CHO and improve postprandial blood glucose levels throughout.
The incidence of hypoglycemia in this study did increase from that of initial testing, but is still lower than what is typically encountered in clinical practice. An advantage to the use of the algorithm is that by having the subject take blood glucose determinations at 1 and 2 hours into the postprandial period, any low level of blood glucose will most likely be identified before it becomes significant.
It must be noted that the cohort of subjects that participated in this study usually follow low carbohydrate diets. Therefore it is to be expected that the algorithm may not be able to maintain such tight bounds on postprandial blood glucose in the presence of meals with a large carbohydrate content. In theory, the algorithm should still work in such a population, although it may take longer to converge to optimal postprandial glucose levels. We are in the process of studying such a population in Spain.
A run-to-run framework can be used in a similar strategy to adjust the basal infusion rate profiles, which would provide for a comprehensive insulin dose adjustment framework.4 Such tools can help subjects with diabetes improve their glycemic control with only sparse measurements of blood glucose and fill the gap until a fully automated “artificial pancreatic β cell” that uses continuous glucose sensors becomes available.
Conclusion
In summary, we have shown the effectiveness and flexibility of this refined insulin-dosing algorithm. This algorithm has the potential to quantify the correct I:CHO ratios quickly and accurately and improve postprandial glycemia.
Acknowledgments
We thank Medtronic MiniMed and LifeScan for their generous support. We also thank all of our subjects for their participation, patience, and support.
Abbreviations
- BG
blood glucose
- I:CHO
insulin-to-carbohydrate ratio
- SD
standard deviation
- T1DM
type 1 diabetes mellitus
References
- 1.Palerm CC, Zisser H, Bevier WC, Jovanovic L, Doyle FJ. 3rd. Prandial insulin dosing using run-to-run control: application of clinical data and medical expertise to define a suitable performance metric. Diabetes Care. 2007;30(5):1131–1136. doi: 10.2337/dc06-2115. [DOI] [PubMed] [Google Scholar]
- 2.Zisser H, Bevier WC, Jovanovic L. Restoring euglycemia in the basal state using continuous glucose monitoring in subjects with type 1 diabetes mellitus. Diabetes Technol Ther. 2007;9(6):509–515. doi: 10.1089/dia.2007.0220. [DOI] [PubMed] [Google Scholar]
- 3.Bevier WC, Zisser H, Palerm CC, Finan DA, Seborg DE, Doyle FJ, Wollitzer AO, Jovanovic L. Calculating the insulin to carbohydrate ratio using the hyperinsulinaemic-euglycaemic clamp—a novel use for a proven technique. Diabetes Metab Res Rev. 2007;23(6):472–478. doi: 10.1002/dmrr.727. [DOI] [PubMed] [Google Scholar]
- 4.Palerm CC, Zisser H, Jovanovič L, Doyle FJ. A run-to-run control strategy to adjust basal insulin infusion rates in type 1 diabetes. J Process Control. 2008;18(3-4):258–265. doi: 10.1016/j.jprocont.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]