Abstract
Despite the availability of modern insulin injection devices with needles that are so sharp and thin that practically no injection pain takes place, it is still the dream of patients with diabetes to, for example, swallow a tablet with insulin. This is not associated with any pain and would allow more discretion. Therefore, availability of oral insulin would not only ease insulin therapy, it would certainly increase compliance. However, despite numerous attempts to develop such a “tablet” in the past 85 years, still no oral insulin is commercially available. Buccal insulin is currently in the last stages of clinical development by one company and might become available in the United States and Europe in the coming years (it is already on the market in some other countries). The aim of this review is to critically describe the different approaches that are currently under development. Optimal coverage of prandial insulin requirements is the aim with both routes of insulin administration (at least with most approaches). The speed of onset of metabolic effect seen with some oral insulin approaches is rapid, but absorption appears to be lower when the tablet is taken immediately prior to a meal. With all approaches, considerable amounts of insulin have to be applied in order to induce therapeutically relevant increases in the metabolic effect because of the low relative biopotency of buccal insulin. Unfortunately, the number of publications about clinical–experimental and clinical studies is surprisingly low. In addition, there is no study published in which the variability of the metabolic effect induced (with and without a meal) was studied adequately. In summary, after the failure of inhaled insulin, oral insulin and buccal insulin are hot candidates to come to the market as the next alternative routes of insulin administration.
Keywords: buccal insulin, insulin formulations, insulin therapy, oral insulin
Introduction
To apply insulin without breaking the skin barrier by a needle and/or to allow better coverage of prandial or basal insulin requirements are of the main reasons for the continuous search for alternative routes of insulin administration (ARIA). Inhalation of insulin is one such alternative to subcutaneous (SC) injection/infusion of insulin. This route of insulin administration allows the application of a sufficient amount of insulin prior to a meal to achieve sufficient prandial metabolic control; however, Exubera, as the first and until now only inhaled insulin with a market approval, was not a market success due to insufficient uptake in the market. The withdrawal of Exubera from the market for this reason and the discontinuation of most other inhaled insulin development programs have drowned most of the hopes in this direction.1 Another alternative to SC injections is the use of microneedles for insulin delivery. With intradermal injection or infusion of regular human insulin or a rapid-acting insulin analog, a rapid onset of action along with a meal can be achieved.2 The aim of this review is to critically review our current knowledge about oral insulin and buccal insulin, two other quite promising developments for ARIA, with a clear focus on presenting data from clinical–experimental and clinical studies. Most other reviews about this topic discuss oral insulin or buccal insulin only briefly and/or are more focused on preclinical aspects.3–7 In these reviews, the details of the obstacles for oral insulin are also described.
Publications about Oral Insulin and Buccal Insulin
Due to the lack of good original publications about the different developments that are pursued, this review is relatively brief. A literature search resulted in 226 original publications and 38 reviews (hits with the term “oral insulin” in PubMed on December 11, 2008). These numbers look impressive at first glance; however, a review of these publications reveals that only seven papers present results from clinical–experimental or clinical studies with oral insulin. One article is in Russian, one is from 1991 with three healthy subjects, and the five others are from companies no longer active in this field of research (i.e., Cortecs, Nobex, Emisphere). From the 38 reviews, only 3 were published in recent years and are focused on oral insulin. The vast majority of the publications deal with early pharmacological developments—with in vitro or animal studies only—and were reported in respective journals. It appears as if, each and every year, 5–10 such early pharmacological studies were published, but only a very small number (none?) of the presented approaches enter clinical development. It might very well be that, in many cases, factors like an unpleasant taste or other nonacceptable side effects are the roadblocks for such developments. A number of other publications are focused on the use of oral insulin for the prevention of diabetes, i.e., on the immunological effects of insulin, and not on its therapeutic use.
More or less, the same holds true for the publications on “buccal insulin” (94 original publications and 29 reviews). Most of the studies report the results from animal studies (most often in dogs and rats). Besides the six studies in humans reported by Generex (discussed later), no other clinical studies were reported since 1994. It might very well be that there are more publications in other journals (e.g., pharmacological journals); however, one would assume that results of studies with humans are reported in journals covered by PubMed.
It appears as if there are more reviews (like this one) about oral/buccal insulin than original publications reporting clinical data. One reason for this “reluctance” to publish data might be that this is a highly competitive area of research; unfortunately, this is combined with a number of limitations in the freedom of communication.
Selection of Companies That Will Be Presented in This Review
A number of companies have claimed to be developing an oral insulin formulation. However, it is not easy to evaluate the level of activity of these companies. It appears as if some companies have vanished or are not interested in this topic currently (e.g., AutoImmune, Biosante, Coremed, Cortecs, Eligen, Nobex, and Protein Delivery). Most of the developments of these companies have failed in phase II clinical studies, showing insufficient metabolic control in patients with diabetes. The definition of “activity” used for the selection of companies presented subsequently in this review is that they have presented new data from clinical–experimental or clinical studies between 2006–2009 at scientific meetings. Five companies working on oral insulin fulfill these criteria: Emisphere, United States; Biocon, India; Diabetology, United Kingdom; Diasome, United States; and Oramed, Israel (Table 1). According to the selection criteria used, the approaches followed by other companies that have presented no human data thus far (e.g., Access Pharma, United States; Apollo, Australia; and Merrion, Ireland) will not be presented. Whereas a number of companies try to develop an oral insulin formulation, there is only one company developing a buccal insulin formulation (i.e., Oral-Lyn, Generex Biotechnology Corporation, Toronto, Canada).
Table 1.
Comparison of Oral Insulin Companies That Will Be Presented in This Review (in Alphabetical Order)
| Oral insulin companies | Dose (U) | Total daily dose | Functional bio-availability | Mechanism | Hepatic targeting |
|---|---|---|---|---|---|
| Biocon | 300–500 | 1200–2000 | Low | Active transport | No |
| Diabetology | 150/300 | 600–1200 | Low | Active transport | No |
| Diasome | 5 | 20 | High | Tight junction | Yes |
| Emisphere | 300 | 1200 | Low | Active transport | No |
| Oramed | 236 | 944 | Low | Active transport | Possible |
| (modified after Diasome) | |||||
A more rigid definition—focus on published studies only—would have reduced even further the number of companies that can be presented in this review. Most of these companies are relatively small, with the exception of Biocon. The limited economical resources of these companies are most probably the best explanation for the small number of (good) clinical–experimental and clinical studies that have been performed with a given development (with wide differences between the companies). In our review of published studies, including those presented as abstracts/posters only, it appears as if most of the studies performed were not performed according to the standards of Good Clinical Practice (GCP). Additionally, many have used an unusual study design (e.g., no appropriate control group or highly selected groups of patients). In summary, the validity of many of these studies is at least dubious.
History
Almost immediately after the invention of insulin in 1922, practically all possible routes of insulin administration were studied by scientists/clinicians full of imagination. Therefore, the first reported studies about attempts to develop an oral insulin formulation or a buccal insulin formulation go back into these early years of insulin therapy. In another review about oral insulin published in 1993, Berger8 reported the results of most probably the first study with an oral insulin formulation, stating, “On August 7, 1922, Dr. Joslin started SC insulin therapy on a 42-year-old nurse. Between October 25 and October 31, 1922, he conducted a formal study on the efficacy of an oral insulin preparation which had been prepared for him by the Eli Lilly Company. Despite a stepwise increase in the dosage of orally administered insulin, the metabolism of this nurse re-deteriorated, and after 1 week Dr. Joslin discontinued the experiment. Similar results were obtained with another patient in early 1923.”
The conclusion drawn from these observations and an analysis of more than 125 publications on the subject of oral insulin between 1924 and 1980 by Berger is quite clear already from the title of the review: “Oral insulin 1922–1992: the history of continuous ambition and failure.” This author also highlighted that “even [if] it might become possible to get some orally ingested insulin intact through the intestinal mucosa, the dosage and timing problems would be of such magnitude that the attempts to follow-up on the dream of an oral insulin substitution must still appear highly unrealistic to any clinical diabetologist.”
Advantages of Oral Insulin
Despite this very negative view, the attractiveness of this route of insulin administration is so high that research continues. As stated earlier, for patients with diabetes, it is quite attractive to swallow an insulin pill. The hope is that this convenience would lead to a better compliance of the patients toward the start and maintenance of an insulin therapy. The hope is that this increased compliance in turn leads to better metabolic control, reducing the risk of development of diabetes-related complications with all their consequences. However, it is not only an improvement in the quality of life that makes oral insulin attractive. If the insulin would be absorbed in the gut, this peptide would be (like all other amino acids and nutritional components) transferred directly toward the liver with the bloodstream draining the gut. At the liver level, the exogenously applied insulin would control hepatic glucose production to the same extent, as this is induced by endogenously secreted insulin in healthy subjects. This more “physiological insulin delivery” would be associated with reduced peripheral hyperinsulinemia (as is the case with SC insulin administration).
Limitations of Pharmacokinetic Parameters with Oral Insulin
Due to the fact that at least 50% of the insulin that reaches the liver level is degraded inside the liver (first-pass hepatic insulin extraction), measurements of insulin levels in the peripheral blood after administration of an oral insulin formulation do not reflect the pharmacokinetic (PK) properties in the same manner that we are used to with SC insulin administration. This had to be taken into account when PK summary measures were discussed with oral insulin, at least when it came to parameters describing maximal concentration levels. This fact also had to be kept in mind when numbers about the relative bioavailability of an oral insulin formulation (comparison of serum insulin levels achieved after SC injection of a given dose of a prandial insulin formulation versus oral administration of a given dose of an oral insulin formulation) were provided; instead, the relative biopotency should be reported. This requires performance of adequately designed glucose clamp studies. Unfortunately, most companies do not employ this standard approach to evaluate the pharmacodynamic (PD) properties and thereby the biopotency of their novel oral insulin formulations. In view of its mechanism of action, measurements of the suppression of hepatic glucose production by means of stable tracers would also be of high relevance. However, this was not employed in any of the studies reported thus far (only in a study performed by Nobex9), most probably due to cost reasons. The PD properties of oral insulin can also be assessed by meal challenge studies, i.e., measurement of the differences in postprandial glycemic excursions with standardized meals and different insulin administrations.
Obstacles for Oral Insulin
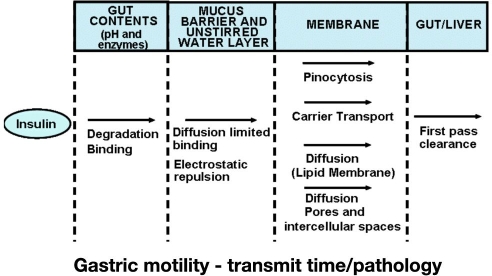
In view of the attractiveness of this route of insulin administration and all the failures in the past, it is obvious that the barriers built up by Mother Nature for oral insulin must be extremely high. The peptide insulin must survive transit through the gastrointestinal tract (GI) in order to allow absorption into the bloodstream (Figure 1).3 It is the job of the gut to destroy proteins into amino acids that are absorbed via the epithelium in the GI but to avoid uptake of potentially dangerous proteins. The low pH in the stomach and the activities of peptidases in the GI usually degrade/destroy all peptides.
Figure 1.
Factors having an impact on insulin absorption after oral application of insulin.
If insulin molecules make it intact into the gut (experimentally, a solution containing insulin can be applied by means of a catheter directly into the gut), they have to pass the wall of the jejunum, which is a single—thick—mucus layer with a tight barrier of epithelial cells. Mucus is secreted by the underlying mucosal goblet cells and is continuously excreted at the apical side of enterocyte. It is a dynamic structure exhibiting a viscosity gradient such that the viscosity of mucus increases from the bottom (as a liquid to be excreted) to the top (as a gel to act as a permeability barrier). From a chemical point of view, diffusion of insulin into the mucus lining the gut should be rather easy since both are of hydrophilic nature. However, intermolecular interactions between the functional groups of both insulin and mucins (such as COOH, OH, and NH2) cannot be underestimated, especially through hydrogen bonds between both proteins. From a physical point of view, diffusion of a protein such as insulin (which has a molecular weight of approximately 6000 Da) in the mucus layer is hampered because of the high viscosity of the latter. Therefore, the diffusion coefficient of native insulin in the intestinal mucus is most probably quite low.
The question is then how the insulin molecules are actually transferred across this wall into the bloodstream; there is no insulin-specific transfer mechanism. Potentially different routes (Figure 1) can account for this transfer. The basic idea of the majority of the oral insulin development approaches presented in the following sections is to use one or more of these transfer mechanisms for other substances to bring insulin along with them into the bloodstream. The approaches presented in the following sections differ significantly in this respect; this appears to have a profound impact on the rapidity of absorption/onset of metabolic effect and the amount of insulin that is intact absorbed into the bloodstream.
It appears as if, with most developments, the high barriers for oral insulin are associated with a considerable loss of insulin; only a relatively small number of insulin molecules make it successfully into the portal blood. In order to induce a clinically meaningful metabolic effect, i.e., a decrease in blood glucose, a high amount of insulin must be applied. It is clear that, for a practically usable therapy with oral insulin (this holds true for buccal insulin as well), insulin must be absorbed in as good as possible reproducible quantities at defined time points after application. If no comparable timing of absorption from dosing to dosing (especially when combined with meals) can be achieved, not only may postprandial metabolic control vary, but it also carries the inherent risk of late postprandial hypoglycemic events in case the applied insulin absorption is delayed after a meal. Again, the number of clinical–experimental studies with an appropriate methodology to study postprandial glycemic excursions devoted to these questions is low.
One can assume that a key factor for the timing of insulin absorption and the amount of insulin absorbed is gastric motility, as this massively influences the transit time inside the GI with oral-enteric (ingested) insulin application but clearly not with oral-buccal application. A number of different factors have an impact on the motility, e.g., different drugs and other antidiabetes drugs, and diabetes itself (i.e., diabetes gastroparesis). Even with relatively small differences in the transfer rates/absorption rates, a considerable variability in the metabolic effect induced may take place. Therefore, it is annoying that no data have been published thus far about the intraindividual variability (which is much more important than the interindividual variability) of insulin action after oral or buccal application with repeated dosing of an identical insulin dose in the same subjects under controlled conditions.
Usually, we assume that ingested insulin is absorbed in the gut only. However, a certain amount is probably absorbed directly from the stomach mucosa. Even if there apparently are no data supporting this, the very rapid absorption kinetics of some of the oral insulin formulations presented suggest at least some absorption from the stomach.
Oral Insulin: Emisphere
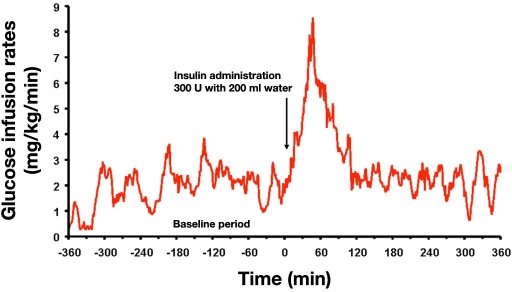
Since the late 1990s, this U.S.-based company has tried to develop an oral insulin formulation. However, it appears as if these activities were stopped per a statement on the company's homepage. From 2001–2004, this company has performed a number of phase I / IIa clinical–experimental studies evaluating the time-action profile of this oral insulin formulation, the dose-response relationship, and the impact of a meal on insulin absorption. In a proof of concept study, different doses of insulin in combination with an agent that interacts with insulin in a manner that promotes the uptake in the gut and meanwhile protects the insulin were administered orally as a single dose to 12 non-insulin dependent patients with type 2 diabetes and 4 control subjects. In all cases, a glucose-lowering effect was demonstrated, preceded by an increase in plasma insulin levels.10 Studying the metabolic effect of this oral insulin formulation with a glucose clamp approach, allowing a precise measurement of the blood-glucose-lowering effect, showed that swallowing a capsule with 300 U insulin together with 200 ml of water induced a clear increase in glucose infusion rates (GIR) to keep blood glucose at the target level within 30–60 min after intake by a patient with type 2 diabetes (Figure 2).11
Figure 2.
Glucose infusion rates necessary to keep blood glucose constant after swallowing a capsule with 300 U of an oral insulin formulation (Emisphere) in a glucose clamp setting in a patient with type 2 diabetes.11
This study documented for the first time the PD properties of an oral insulin formulation in comparison to SC-injected regular insulin. It also proved that therapeutically relevant amounts of insulin are absorbed rapidly after administration. The rapid increase and decrease in the metabolic effect induced should allow good coverage of prandial insulin requirements. At the same time, it should reduce the risk of developing late postprandial hypoglycemic events.
It should be mentioned that the relative biopotency of this rapidly absorbed oral insulin formulation was 20% when taking into account the areas under the GIR in the first 60 min after administration; however, the relative biopotency was only 3% when using longer time intervals (0–6 h). Even if, for optimal control of postprandial excursions, the immediate effect is of great relevance, calculation of the relative biopotency over restricted time periods can be misleading and should be used with caution.
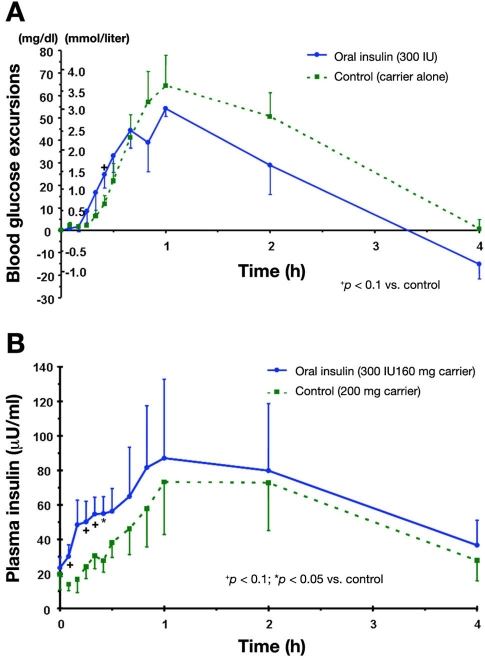
In a randomized, controlled, double-blind, parallel group pilot study in 13 patients (7 treated, 6 controls) with type 2 diabetes well controlled with dietary treatment, the safety and efficacy of treatment with this oral insulin formulation was studied for two weeks.12 Each subject received either 300 IU insulin + 160 mg carrier or 200 mg carrier alone (administered as 2 tablets 10 min before main meals and before bedtime). In comparison to the control group, blood glucose levels of the patients treated with oral insulin were significantly lower after an oral glucose tolerance test in comparison to baseline but not in comparison to the control group. The oral insulin was well tolerated, i.e., no side effects or hypoglycemic events were observed.
In another study, an improved formulation of this oral insulin was studied in eight patients with type 2 diabetes.13 Administration of two tablets (each 150 IU insulin + 80 mg carrier) or placebo (200 mg carrier alone) in a subgroup of four patients, which consumed a mixed meal (441 kcal, 66% carbohydrates), induced a more rapid increase in insulinemia (Figure 3B), which was accompanied by lower postprandial excursions (Figure 3A). However, in view of the small sample size, it is not surprising that most differences did not reach statistical significance.
Figure 3.
(A) Blood glucose excursions and (B) serum insulin levels after a test meal in four patients with type 2 diabetes with 300 IU oral insulin or placebo.
In 2006, Emisphere performed a 90-day double-blind phase II clinical study in India with 145 patients with type 2 diabetes on oral antidiabetes drugs (OADs; Metformin).14 The patients were randomized in four different groups and treated with three different insulin doses or placebo. No significant differences in metabolic control [hemoglobin A1c (HbA1c)] between the groups were observed despite treatment with up to 1000 U of oral insulin per day. The negative outcome of this study was explained with problems in conducting the study adequately. Unfortunately, the results of this study were never presented in a full publication. Most probably it was the negative outcome of this study that hampered cooperation with a big pharmaceutical company and stopped this development despite a lot of effort for a number of years.
Oral Insulin: Biocon
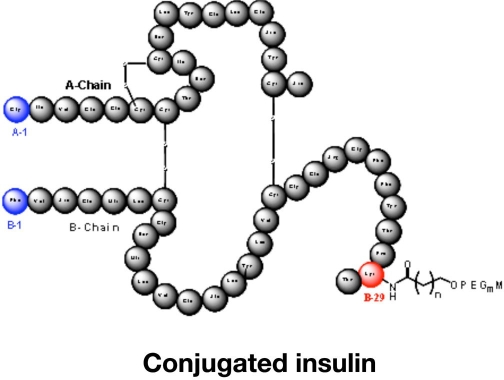
This large Indian-based pharmaceutical company has taken over the oral insulin technology developed by Nobex.15–17 Thus the formulation of their current oral insulin candidate (IN-105) is based on several years of development with oral insulin analogs, including HIM2. IN-105 is a human insulin molecule conjugated on position B29 with polyethylene glycol via an acyl chain (Figure 4). The current formulation for IN-105 is a second-generation tablet, which is declared to be simple to manufacture, uses readily available excipients, and has an attractive stability profile at ambient conditions. It appears as if Biocon is intensively working on this development.
Figure 4.
Primary structure of the conjugated oral insulin IN-105.
IN-105 is declared to have the following characteristics:
improved half-life in the digestive tract and improved absorption,
lower immunogenicity as compared to insulin,
lower mitogenicity as compared to insulin,
retains a similar pharmacological activity as insulin, and
conserves safety profile and good clearance profile as compared to insulin.
Due to the fact that IN-105 is an insulin analog, safety aspects are of relevance. Extensive preclinical studies in different species have shown no issues in acute dose toxicity studies or in 14-, 90-, and 180-day chronic toxicity studies. Also, genotoxicity/mutagenicity/reproductive toxicity and teratogenicity studies have shown nothing.
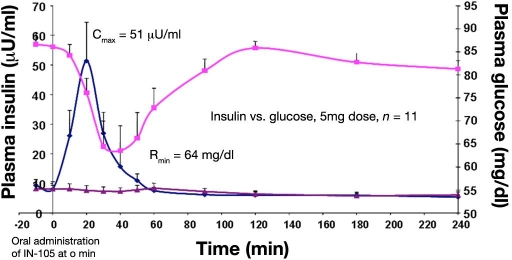
Pharmacokinetic and PD studies (measured as a decline in blood glucose) in healthy subjects have shown that IN-105 is absorbed rapidly and produces a corresponding drop in blood glucose (Figure 5). Maximal circulating insulin levels after oral administration of 5 mg IN-105 at t = 0 min were observed after 20 min. The maximum drop in glucose occurred at 40 min after oral administration. However, the rapid decline in blood glucose will have induced a counter regulatory response that induces an increase in glycemia per se. The limitations of PK measurements with oral insulin have already been mentioned.
Figure 5.
Increase in mean plasma insulin levels and subsequent decrease of plasma glucose in 11 healthy subjects after oral administration of 5 mg IN-105 at t = 0 min.
Data from an ascending-dose study with IN-105 in patients with type 2 diabetes showed a significant decrease in 2 h postprandial glucose excursions in a dose-related manner. Application of single doses of placebo or IN-105 10, 15, 20, and 30 mg tablets on five separate study days prior to a mixed 600 kcal breakfast showed a proportional absorption of the drug. A serum average Cmax of 350 mU/liter was reached at 30 min postdosing at the highest dose. The resulting decrease in blood glucose also showed linearity with respect to the dose. The 2 h postprandial increase in glycemia rises over baseline was 15, 24, 31, and 50 mg/dl lower than the corresponding rise for placebo.
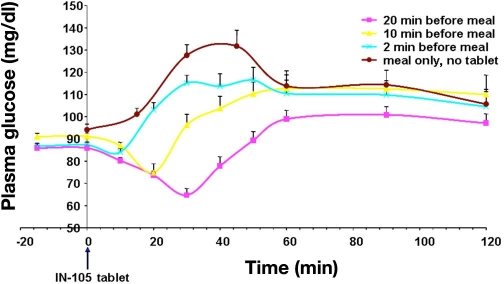
Another study in 14 healthy subjects evaluated the effect of timing of oral insulin administration on meal-related glucose excursions (Figure 6). IN-105 administration at various times before a meal (single meal, high carbohydrate diet [carbohydrate ∼62%]) in two different doses (5 and 10 mg) showed maximum PD effect in reducing glucose level when the tablet is taken 20 min before the meal. Drug intake 10 or 5 min before the meal resulted in higher postprandial glycemic excursions. As the experience with the Emisphere approach showed, the performance of such studies is crucial. If the absorption of the oral insulin from the gut is hampered by a meal, this reduces the biopotency even more.
Figure 6.
Increase in mean plasma glucose levels of 14 healthy subjects after a meal. On the different study days, IN-105 was applied with a different time interval prior to the start of the meal.
Longer-term, 6-month studies are planned in patients with type 2 diabetes to understand the impact of chronic IN-105 dosing on metabolic control.
Oral Insulin: Diabetology
This small U.K.-based company with an ambitious name has had an oral insulin formulation (CapsulinTM) in development for a number of years that is not a new chemical entity (in contrast to, for example, the Biocon development). This should enable a simpler approval procedure by the regulatory authorities. The dry powder mixture, which contains insulin, stabilizer, and solubilizer (“generally regarded as safe”/Pharmacopeia excipients), is packaged in an enteric coated capsule (with 150 U) that protects the insulin from gastric degradation. The capsule is declared to pass intact through the stomach to the small intestine. The coating shall dissolve in the jejunum in an area with neutral pH, and the capsule content is subsequently released. The excipients (an aromatic alcohol and a solubilization aid) are supposed to enhance insulin absorption through the intestinal mucosal layer.
Diabetology has performed some early clinical–experimental proof-of-concept studies in healthy subjects and patients with type 1 diabetes and more recently a phase IIa randomized, open, crossover study in 16 patients with type 2 diabetes.18,19 One group of eight patients participated on two glucose clamp study days with 150 U Capsulin on one day and SC injection of 12 IU regular insulin on the other day. The other group of patients received 300 U Capsulin on one study day and also 12 IU regular insulin the other day. In the 10 days between the two clamps, patients were instructed to swallow one capsule in morning and one in the evening (300 U per day) 60 min prior to breakfast and evening meal (no placebo control). For the 6 h period of the glucose clamp study days, the glucose requirements to keep blood glucose constant for both doses of Capsulin (150 and 300 U) was ∼50% that of the 12 IU SC regular insulin injected into the abdomen. Therefore, no dose-related effect was observed with Capsulin. From the time-action profiles, it appears as if 150 and 300 U Capsulin had comparable intersubject variability to SC regular insulin. The onset of the metabolic effect with Capsulin and regular insulin was slow, i.e., maximal GIR were observed after several hours. This might hamper prandial insulin coverage; however, this was not studied until now. In addition, after 6 h, a significant amount of metabolic effect (even if the absolute level was low with 1 mg/kg/min) was still present, which can induce late postprandial hypoglycemic events.
Substitution of the oral agents by Capsulin for a 10-day period did not compromise fasting blood glucose levels; however, the level of metabolic control established was mediocre with average glucose levels around 9 mmol/liter.20 That no adverse effects (e.g., hypoglycemia) were observed can be interpreted as a good sign; however, it can also mean that the metabolic effect induced was suboptimal.
Oral Insulin: Diasome
The approach followed by this small U.S.-based company is a novel insulin delivery system that can be used for oral and SC insulin delivery. The key components of this are hepatic-directed vesicles loaded with insulin (HDV-I) that were developed some years ago.21 These vesicles are composed of liposomes (<150 nm diameter) that contain insulin attached to a specific proprietary hepatocyte-targeting molecule (HTM). The HTM is proposed to selectively target the delivery of the encapsulated insulin to the hepatocytes similar to normal insulin physiology. This oral HDV-I should be stable at low pH and in blood. It should be small enough (20–50 nm) to cross membrane barriers and to avoid enzymatic degradation. In contrast to all other oral insulin formulations, HDV-I is declared to have a high biopotency, i.e., it is formulated as an oral gel capsule with 5 U insulin only.
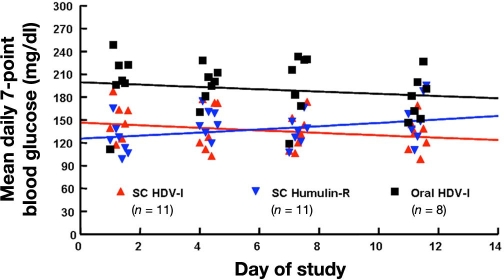
More recent studies were performed in a large diabetes research center in patients with type 2 diabetes22 and type 1 diabetes.23 Figure 7 shows the results of a small single-blind placebo-controlled trial in six patients with type 2 diabetes with current diagnosis of the disease (residual endogenous insulin secretion). Patients swallowed capsules with different insulin content while also taking their usual OADs 30–45 min prior to breakfast (60 g carbohydrates). While adding oral insulin to the treatment improves postprandial glycemic excursions in comparison to placebo, escalating doses of this oral insulin does not induce a further improved metabolic control. It might be that, with the lowest dose already, a full suppression of hepatic glucose production was established; however, it might also be that only a certain amount of insulin was absorbed despite an increase in dose. In the randomized, double-blind (for injectable insulin arms only, SC regular human insulin and SC HDV-I), open-label (oral HDV-I) study with 30 patients with type 1 diabetes, the metabolic control (average glycemia) achieved was worse with oral insulin in comparison to the two patient groups with SC insulin treatment (Figure 8).
Figure 7.
Area under the postprandial blood glucose profiles of six patients with type 2 diabetes while escalating doses of oral HDV-I were applied.22 SEM, standard error of the mean.
Figure 8.
Scatter plot of the mean daily seven-point blood glucose values for subjects in the three different treatment groups on days 1, 4, 7, and 11.23 Each point on the graph is the mean of 11 subjects for the injection groups and 8 subjects for the oral treatment group.
Beginning in 2009, a large long-term study is being conducted in 40 U.S. centers with 230 patients (placebo controlled). Diasome has also initiated a food-effect study to determine the optimal time for dosing. Unfortunately, the results of these studies are not presently available. The data presented thus far give no clear understanding of the PK/PD properties of this oral insulin formulation. One would also like to see data of the variability of absorption of insulin from the gut and the subsequent metabolic effect induced.
Oral Insulin: Oramed
This relatively small Israel-based company started their development recently; however, some of the people working for this company have a long-standing interest in this topic and have worked for other companies that have tried to develop an oral insulin formulation before. Oramed presented data from a single-blind, open-label study with eight healthy subjects in which all subjects received a single dose of an oral capsule on four separate visits.24 The capsules contained 8 mg (216 U) insulin and differed in the concentration of the excipients added to the insulin powder. The capsules were administered in the morning after an 8 h overnight fast. Only one formulation showed positive results, i.e., a reduction in blood glucose and elevation of plasma insulin (accompanied by a reduction in C-peptide) occurred in five of the eight participants some hours after swallowing the capsule. The curves of one subject are shown in Figure 9.
Figure 9.
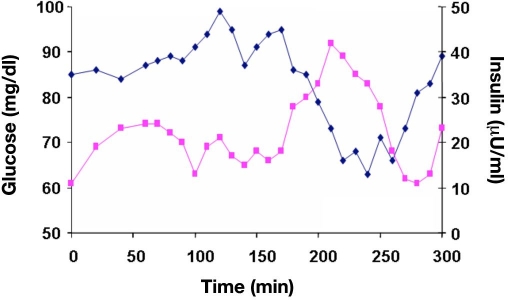
Changes in blood glucose (measured by a blood glucose meter) and plasma insulin in one healthy subject after swallowing a capsule with 216 U insulin and excipients at t = 0 min that promote insulin uptake.24
Oral Insulin: Low Biopotency = High Amounts of Insulin Required
What are the consequences of the low biopotency seen with nearly all approaches presented here? A typical patient with type 2 diabetes on insulin therapy in Germany25 applies a total daily SC insulin dose of 80 IU. As 1 mg of pure insulin powder contains 27 IU, such a patient applies 3 mg insulin per day. This corresponds to approximately 1 g of insulin per year. Assuming that 10 million patients with diabetes are on insulin therapy worldwide, this translates into an amount of 10 tons of insulin per year. With a relative biopotency of 10% with oral insulin (a quite positive assumption in most cases), this means 100 tons per year. This in turn would require a massive increase in production capacity of the insulin manufacturing companies. However, with modern bio-technological production methods (and other attempts for novel insulin production, e.g., by means of plants) this is not a fundamental obstacle but means relatively high costs. At the same time, one has to acknowledge that the sheer costs of goods with insulin are not that high than with other, more complex peptides.
Oral Insulin: (Potential) Side Effects
With oral insulin, considerable amounts of insulin (and other excipients) have to be applied. The question is, can this insulin induce side effects? If there is an even distribution and degradation of most of the insulin taking place in the stomach, the concentration of insulin in the lower GI should be low. Nevertheless, it might be that locally high insulin concentrations in the GI show up. The question is, can such high levels of a growth-promoting substance like insulin induce cancer development or enhance the development of existing tumors? It is known that there is a modestly increased risk of colorectal cancer in SC-insulin-treated patients with type 2 diabetes but not in patients with type 1 diabetes. Also, in animal studies with application of high doses of oral insulin over prolonged periods of time during insulin-tolerization studies, no carcinogenic effects have been observed.
Even if insulin has no side effects, one has to acknowledge that the other substances (very different chemical compounds) added to improve uptake of insulin in the gut might have safety issues, especially if taken repeatedly over prolonged periods of time. The absorption enhancers added to oral insulin formulations such as chemical solubilizers (i.e., sodium lauryl sulfate) or even biological ones (i.e., bile salts) have the ability to extract and solubilize lipids (such as those of the cell membrane) and threaten the integrity of cell structures even on a short/medium term.
In view of the history with inhaled insulin, all potential risks (even if there is only a theoretical risk) of oral insulin require careful consideration. One has to acknowledge the high costs for such investigations, as many patients must be involved and followed up on for long periods of time. Nevertheless, it might not only save the companies a lot of money in the long run, but also—most importantly—avoid risks for patients. Clearly, this also holds true for potential safety aspects of buccal insulin.
Buccal Insulin
Drug delivery via the buccal mucosa has a number of advantages, such as
presystemic metabolism in the GI and liver is avoided;
good accessibility;
the drug is in direct contact with the mucosa, avoiding loss in any other liquid, allowing establishment of a high drug concentration gradient across the mucosa favoring drug diffusion into the underlying tissue;
possibility to localize the drug according to the permeability features of the target area;
relatively large surface for absorption (100–200 cm2);
level of vascularization is very high in some areas;
weak variations of pH (≠GI);
buccal enzymatic activity is mainly intracellular and less developed than in other mucosae; and
the buccal mucosa can be considered as quite robust since it can undergo chemical, physical, and mechanical stresses;
but also a number of drawbacks, such as
the buccal mucosa is not an absorptive organ (≠intestinal mucosa). Its structural histological and biochemical features are those of a lining but not absorptive mucosa (pluristratified epithelium and intercellular barrier of permeability), thus promoting absorption from the buccal mucosa is a challenge by definition;
-
there exists great variations of permeability among the different areas of the oral mucosa:
sublingual area is thin and nonkeratinized, highly permeable (high drug input);
cheek mucosa is thicker and nonkeratinized, fairly permeable (low but sustained drug input); and
the palate is thin epithelium but highly keratinized, negligible permeability (up to now, because studies on the topic are quite scarce).
In summary, the continuous, but variable, saliva flow and the robust multilayered structure of the oral epithelium constitute an effective barrier to penetration of drugs.26,27,38 Despite this, the first attempts to utilize the buccal mucosa for insulin absorption were made as early as 1925. A number of attempts have been made over time (with one exception tested in animals only) to improve buccal insulin absorption by adding absorption enhancers or to modify the lipophilicity of insulin. Not only was the effectiveness of these measures poor, but the variability of the induced effect was considerable. One also has to acknowledge that studies about buccal drug delivery using rats as an animal model are of no value since the whole buccal mucosa of rodents is known to be highly keratinized. Therefore, the permeability of their buccal tissue is weak to insignificant while that of humans is quite high in the sublingual nonkeratinized area (high levels of polar glycosylated lipids impeding the densification of the intercellular space) to negligible in the keratinized hard palate. The only animal model that can be of use when studying the human buccal permeability are pigs. The massive differences in the permeability among the different areas of the oral mucosa (mentioned earlier) also explain why absorption depends on the exact place of localization of a given drug. Thus, if an insulin formulation is sprayed in the open mouth toward the throat, it cannot reach the sublingual area, which is closed by the ventral side of the tongue during application. Therefore, it is of no surprise that, in a small number of patients with type 1 diabetes using an aqueous human insulin spray, a reduction in blood glucose was observed, but only after multiple applications.26 Insulin administered into the mouth is not oral insulin (if swallowed, it is rapidly degraded in the stomach) and not pulmonary insulin (the particle size allows no transfer into the alveoli): it is a buccal insulin.
Oral-lyn
Generex is a Canadian- and U.S.-based company that has a number of products in development for a range of indications, all based on RapidMist, the company's “advanced buccal drug delivery technology, is composed of a proprietary formulation and a proprietary device design that is able to deliver drugs through the buccal mucosa safely” (www.Generex.com/technology.php). Clearly, this company is also aware of the difficulty to get larger molecules across the inner lining of the mouth, but they believe they have the right combination of ingredients—a surfactant, a solubilizer, a micelle-creating agent, and emulsifying agents—all “generally regarded as safe” excipients prepared in the right way to allow it to penetrate predicatively.27 In a sense, all these agents have the same definition: a surfactant is a solubilizer in which one is a micelle-creating agent that is itself an emulsifying agent. These substances are necessary to transport micellized insulin either across lipoidal cell membrane or across the lipid components of the intercellular permeability barrier. Surfactants must be added to the Oral-lyn formulation to enhance insulin absorption. However, the surfactants remain unknown (nature? quantity?), and it seems that no information is available about the long-term mucosal tolerance toward these-known ingredients (especially above their critical micellar concentration). Such information is of utmost importance since buccal insulin is to be given daily for life.
Oral-lyn is a liquid formulation of human regular insulin with a spray propellant for prandial insulin therapy. The insulin formulation is stable at room temperature for more than six months. The formulation results in an aerosol with relatively large micelles (85% of that having a mean size >10 µm) and therefore cannot go into the lungs. Once the tasteless, odorless product is sprayed into the mouth by use of a propeller, the RapidMist device is claimed to get through the superficial layers of the mucosa toward the blood vessels and get right into the bloodstream. The device used to spray the Oral-lyn insulin droplets of uniform size with high speed (100 mph) into the mouth looks like an asthma device. Each 28 ml canister contains 400 U of regular human insulin. The advantage of this is that there is no intimidation for people using it (http://industry.bnet.com/pharma/1000242/). Each puff is claimed to deliver 10 U insulin to the human body (with an absorption rate of 10%, discussed later, this corresponds to 1 U). Thus application of >10 U insulin for a meal becoming active requires 10 puffs; this undertaking can be considered time-consuming and not user friendly. The insulin is claimed to be released from the device as a metered dose, identical from first puff to last. However, appropriate dosing of this buccal insulin requires some sort of training.
Unfortunately, no description of the exact mechanism of insulin uptake by this route is available.27 This is a bit surprising in view of the number of well-respected diabetologists working for Generex who are involved in the clinical–experimental and clinical trials. A clear understanding of the “science” behind this novel route of insulin administration (i.e., more detailed in vitro/in vivoabsorption studies and more research about the mechanism of mucosal transport) would be quite helpful to achieve a widespread acceptance of this novel approach in the scientific community. The product is on the market in a number of countries (e.g., Ecuador and India) and has pending registrations in a number of others.
Clinical–Experimental and Clinical Studies with Oral-lyn
While focusing here on full publications, ignoring a number of abstracts (mainly from one group of researchers from Ecuador) and a letter,28 the first publication about the PK and PD properties of Oral-lyn was a review article.29 In the latter, the results of a number of studies with patients with type 1 and type 2 diabetes were presented in brief. Ten puffs (100 U) of this buccal insulin in a double-blind, crossover meal study induced a more rapid increase in insulinemia than SC injection of 10 IU of regular human insulin. It was not outlined if this was a double-dummy study. Peak insulin levels were observed after 40–60 min in “most” patients receiving Oral-lyn. Due to the fact that the areas under the curves (AUC) under the plasma insulin profiles were comparable between these two routes of insulin administration, it was assumed that the relative bioavailability was in the range of 10%. The increase in glycemia was highest on the study day without insulin application (placebo). For patients with type 1 diabetes, this increase without a prandial insulin application was surprisingly small. With Oral-lyn, the glycemia was comparable to that with SC insulin in the first 120 min (despite the more rapid increase in insulinemia) and remained higher thereafter. However, it is difficult to interpret the study results without more detailed information about the study design, i.e., basal insulin therapy, comparability of preprandial glycemia and insulinemia, and meal composition. This buccal insulin was also studied in patients with type 2 diabetes treated with insulin, treated with oral agents, and failing diet and exercise. The outcome of these studies was positive for Oral-lyn. However, as described earlier, it is not easy to accept these data without more details.
In a research article published in the same journal, the results of a proof-of-concept study in patients with type 2 diabetes were presented.30 In this open-label, crossover, randomized study performed in Canada and South America, 23 obese patients on insulin therapy (mean body mass index = 35.3 kg/m2, mean HbA1c = 7.9%) received 100 U Oral-lyn on one study day and an injection of 0.1 IU/kg rapid-acting insulin analog (insulin Lispro) SC on the other study day 10 min prior to a breakfast (360 calories, Sustacal liquid meal). The 30 and 60 min postprandial blood glucose levels were lower with the buccal insulin, which is in accordance with the more rapid increase in insulinemia with Oral-lyn. However, the fasting blood glucose (i.e., preprandial glycemia) was allowed to be in the range of 72–144 mg/dl. Even if the mean values were comparable, in view of the impact of the preprandial values on the height of the postprandial glycemic excursions, it is crucial to have identical starting values in each patient on all study days. It appears as if the more rapid increase in insulinemia—accompanied by a rapid decrease thereafter—seen with buccal insulin induces a relatively short-lived metabolic effect (lower initial postprandial increase in glycemia) but after a 60–90 min glycemia increase to a similar high level on both study days. The postprandial glycemic control was not optimal on both study days, i.e., with SC injection of a rapid-acting insulin analog in a sufficient dose, one would assume to see lower excursions. However, it might be that the patients were so obese that the absorption from the SC insulin depot was delayed (the increase in insulinemia with Lispro was from a high starting value of 50 to 80 µU/ml after 60 min). It is also not clear if some metabolic effect from the dose of neutral protamine Hagedorn (NPH) insulin (25 IU at night) applied the evening before was still present. It is difficult to understand the massive increase in serum C-peptide levels after the meal as no duration of diabetes is given for the patients studied. This indicates that a considerable amount of endogenous insulin was secreted as a response to the meal stimulus. The mean starting levels of C-peptide (fasting levels) were in the range of 1.5 ng/ml, whereas in the table with the clinical characteristics of the patient presented in the paper, the mean C-peptide was declared to be 0.24 ng/ml.
In another study with a very similar study design and similar shortcomings, 21 patients with type 2 suboptimally treated with OADs (no HbA1c values are provided) were studied.31 It is of no surprise that the addition of insulin (100 U buccal insulin) on one study day led to reduced postprandial glycemic excursions in comparison to the study day with OAD (metformin and glyburide) only. High maximal postprandial C-peptide (6 ng/ml with Oral-lyn and 8.5 ng/ml) indicates high endogenous insulin secretion on both study days.
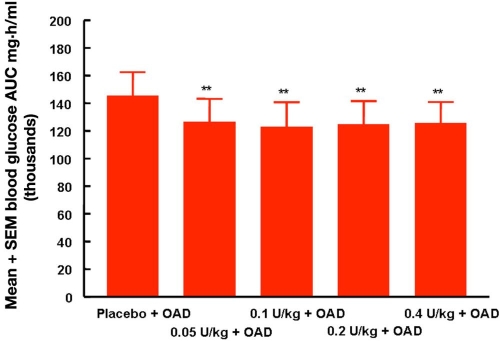
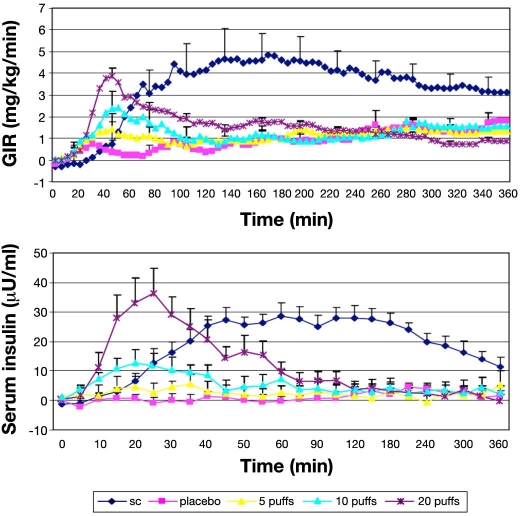
In a series of three publications, the PK and PD properties of Oral-lyn were studied by means of the glucose clamp technique by an Israel-based group of researchers.32–34 On one study day in a single-dose, open-label GCP study, the six healthy subjects in the first study received a SC injection of 0.1 U/kg regular human insulin in the umbilical region by means of a syringe (mean dose 7.6 U), and on the other study day, they received 150 U of buccal insulin.32 The manual glucose clamp technique employed (target blood glucose level 90 mg/dl; baseline intravenous insulin infusion 0.2 mU/kg/min) allows adjustment of GIR in 5 min intervals. Blood glucose was measured by means of a glucometer and not with a laboratory system. It appears as if the baseline glycemia, insulinemia, and C-peptide levels of the subjects differ considerably but nonsignificantly between the two study days. Oral-lyn showed a significantly more rapid absorption (Tmax 23 versus 83 min) to higher levels (54 versus 31 µU/ml) than SC insulin injection. The insulin levels returned to baseline levels within 90 min after application. Parallel to the PK results, the GIR reached maximal levels earlier with buccal insulin (44 versus 100 min); however, the maximal levels were comparable between the two formulations (6.8 versus 6.2 mg/kg/min). Baseline GIR was reached again after 120–150 min with Oral-lyn. The total amount of glucose infused over 360 min was significantly higher with SC insulin. The mean relative bioavailability was 2.6% using the AUC for 0–360 min, and the biopotency was 2.7%. Interestingly, four subjects complained of a strange sensation and taste in their mouths. Another small clamp study with seven healthy subjects investigated the important aspect of dose-response relationship: 10 puffs placebo spray; 5, 10, and 20 puffs of Oral-lyn; and one dose of 0.1 U/kg regular human insulin, respectively (Figure 10).33 Clearly, it would have been advantageous to have more doses of SC insulin in parallel. To suppress endogenous insulin secretion of the subjects, they received an intravenous infusion of somatostatin and insulin. Increasing doses of Oral-lyn induced a dose-response relationship with respect to maximal serum insulin levels, while time to maximal levels was similar. Within the limitations of the experimental approach used (discussed later), the intraindividual variability observed with the AUC under the serum insulin profile was in the range of 50%. This appears to be higher than with SC insulin. Unfortunately, the statistical analysis provided compared all five study days only and not the three different doses of buccal insulin separately, at least for the PK results. The PD responses were in line with the PK ones, i.e., the maximal GIR increased with higher doses. Time to maximal metabolic effect and a number of other parameters were comparable with the three doses of buccal insulin (p values are reported for these comparisons).
Figure 10.
Mean GIR and serum insulin levels over 360 min following administration of SC insulin and buccal insulin in three different doses (+placebo spray) in healthy subjects t = 0 min.33
Studying the dose-response relationship of Oral-lyn in six patients with type 1 diabetes in the third clamp study with a very similar study design showed comparable PK and PD results, resulting in a linear dose-response relationship.34 Interestingly, in this study, the variability of buccal insulin was >2 times the variability observed with SC insulin.
In two additional meal studies, the benefits of buccal insulin in patients with type 1 diabetes were investigated.35,36 In a study from Italy, 18 patients with a good metabolic control (mean HbA1c = 6.7%; body mass index = 23 kg/m2; duration of disease = 6 years) ate a standardized meal (630 kJ; 55% carbohydrates) on one study day and injected their typical dose of regular insulin (range 5–20 IU), with an injection meal interval of 20 min. On the other day they applied buccal insulin (5–7 times the SC dose applied). This is the first study in which calculation of the required sample size was described. Again, it was stated that the intraindividual variability of certain parameters was calculated (coefficient of variation of maximal serum insulin levels = 22%; maximal blood glucose = 35%); however, it is not clear how this was done. Mean preprandial glycemic values were in the range of 150–170 mg/dl. Postprandial glucose excursions were not different between the two study days. Again, late postprandial glycemia was higher with buccal insulin. The increase in insulinemia after application of buccal insulin appeared to be slower and longer lasting than in the previous studies; it was lower with Oral-lyn than with SC insulin (too small dose).
In another study from Ecuador of 10 patients with type 1 diabetes, the patients remained on their basal insulin therapy (insulin glargine twice per day; HbA1c = 7.5%). Regular insulin was injected 30 min before the meals on three study days and was replaced by two doses of buccal insulin (8 to 12 puffs each) just before and after each meal (split dose). Blood glucose was self-measured nine times per day by the patients with a conventional glucose meter. Average glucose levels in this open-label study were comparable with both types of insulin treatment; however, it is not described in this publication if and how the patients self-adjusted the insulin doses to the current needs according to the self-measured glycemia and carbohydrate content of the meals. The number of low blood glucose values (<3.3 mmol/liter) was comparable in both phases of the study, and no severe hypoglycemia was observed. The authors conclude that similar gluco-dynamic responses were seen as with prandial regular insulin and buccal insulin. It is of interest that the more rapid onset of action attributed to Oral-lyn compared to regular insulin did not result in any relevant differences in postprandial metabolic control as clearly demonstrated in this study.
In summary, the outcome of these studies (most with a small sample size and performed without an appropriate blinding, which would require a double dummy), study performance indicates that the time-action profile of Oral-lyn is characterized by a more rapid increase in insulin action compared to that of SC regular human insulin. It appears as if Oral-lyn is mainly absorbed and effective in the first 2 h after its administration. This allows repeated application of prandial insulin within a dinner with a long duration without running into the risk of adding up the metabolic effect of multiple insulin applications.
It is a pity that no appropriate head-to-head comparative study with a rapid-acting insulin analog has been performed to date. Another aspect that is essential with each type of insulin therapy and that appeared not to have been studied appropriately thus far with buccal insulin is the reproducibility of the metabolic effect induced. Studies about the intraindividual variability observed after SC injection of prandial or basal insulin documented the considerable difference in the metabolic effect observed in the same patient when the same dose of the same insulin was applied.37 It is mandatory that, in such studies, the identical dose of insulin was applied repeatedly in the same patients under identical experimental conditions. Attempts to calculate the intraindividual coefficient of variability from study days with different doses are of limited credibility. In view of the more complex application procedure of Oral-lyn, which requires more active collaboration of the patients than with SC insulin therapy, one wonders how big the variability is per se with an identical application procedure and what the variability is if patients voluntarily “misuse” the application device.
Biopotency and Safety of Buccal Insulin
Relative biopotency is stated only in one of the glucose clamp studies. It is puzzling that the reviewers of other manuscripts have not asked for this key information. It appears as if the biopotency is quite low, i.e., in the range of 1–2%. In other words, more than 95% of the applied insulin will be swallowed by the patients. It would be interesting to study if some of this swallowed insulin escapes from presystemic metabolism or if at least some of it is absorbed via the pulmonary tract. In view of the fact that this product is already on the market in some countries and most likely will become available in more countries, it appears as if costs of goods are not a big hurdle in finding an appropriate price.
With respect to the safety of this approach, no acute side effects were reported in the clinical studies with Oral-lyn. One can regard it as an advantage that the pluristratified buccal epithelium is most probably more robust than a monolayer of enterocytes in the gut toward the insulin and the excipients in the buccal insulin formulation. Incorporation of absorption enhancers over long periods of time gives rise to potential cell damage. Even if no acute side effects were observed, which has to appear massively and rapidly to be detected, long-term side effects are quite insidious. As with each new development, no definitive statement can be made about the long-term safety of the exact formulation used with this buccal insulin, even if animal studies are clean. Hopefully, the phase III study (discussed later) will provide more answers to this critical question.
Evidence for Use of Oral Insulin and Buccal Insulin
To the best knowledge of the authors, no randomized controlled trials have been performed with any of the oral insulin developments; at least, no full publication about the studies (e.g., the Emisphere phase II study) performed thus far has been made available. Currently, one 6-month phase III trial with 750 patients with type 1 diabetes in 72 centers in the United States, Canada, Russia, and Eastern Europe is being conducted with Oral-lyn. In this study, buccal insulin will be compared to prandial injections of regular insulin (not rapid-acting insulin analogs); using twice-daily NPH as basal insulin. So the comparator is a more classical insulin therapy. It is not clear how the patients were distributed between the centers, i.e., from which country the majority of the patients have been recruited. The primary endpoint is a change in HbA1c. This noninferiority study will hopefully provide clear data about long-term efficacy and tolerability of this approach. Other long-term studies with Oral-lyn, which appeared to be performed uncontrolled in some cases, were not published in detail thus far.27
One wonders how the regulatory authorities in the United States and Europe will react when the companies present the results of their studies with oral insulin in order to obtain market approval. In light of the high requirements for GCP, data quality, and good manufacturing practice of study drug formulation, one fears that at least some of these applications will fail. However, in view of the necessity to guarantee the safety of the patients (which is the essential requirement), the barriers for a successful drug development have been increased significantly for good reason. It would not be fair to blame only the respective companies if they do not fulfill all regulatory aspects because their economical resources are limited. One assumes that these companies hope to attract with their early/small studies one of the big pharmaceutical companies to take over their approach/technology. A successful clinical development will most likely need the resources and knowledge of these larger companies. However, until now, the big companies have been very reluctant to invest heavily into oral insulin development or a buccal insulin formulation. Many of these companies have a respective history, i.e., at one time, they had looked more closely into a given technology, but the outcome of the due diligence procedure was not positive. For example, for a number of years, Eli Lilly cooperated with Generex in the clinical development of their buccal insulin; however, in the end, Eli Lilly withdrew from the effort.
Conclusions
In summary, it appears as if therapeutic amounts of insulin can be delivered with oral insulin and buccal insulin; however, large amounts have to be applied in most cases. Whether this low biopotency means a high price once the given development would come to the market is not clear right now, as the costs for insulin per se is not the only factor that determines the price. We should not forget that the better compliance that is probably associated with a noninvasive insulin therapy hopefully enables a better metabolic control and hence reduces the extremely high costs associated with treatment of diabetes-related late complications.
The PK/PD properties of most developments appear to be appropriate for painless coverage of prandial insulin requirements. Unfortunately, it is not clear if “confounding” factors like meals blunt the metabolic effect in a relevant manner. Also, the reproducibility of the metabolic effect induced is not clear. Without appropriately designed and performed phase II and III trials at hand, it is not possible to make any clear statement about the benefits/risk ratio of the different oral insulin developments. However, if the regulatory authorities accept the data presented by the companies (in some cases, it appears as if the development was not systematic enough), there is a good chance that an oral insulin formulation or the more likely buccal insulin formulation currently in the end phase of its clinical development will be the next ARIA to come to the market in the United States and Europe.
Acknowledgments
We acknowledge many excellent discussions with Larry Hirsch, vice president, Global Medical Affairs, Diabetes Care, Becton Dickinson, about the topic of this review. Yves Jacques has read this manuscript very carefully and added many comments. However, his main focus was on the absorption properties of the buccal mucosa.
Abbreviations
- ARIA
alternative routes of insulin administration
- GCP
Good Clinical Practice
- GI
gastrointestinal tract
- GIR
glucose infusion rates
- HbA1c
hemoglobin A1c
- HDV-I
hepatic-directed vesicles loaded with insulin
- HTM
hepatocyte-targeting molecule
- NPH
neutral protamine Hagedorn
- OAD
oral antidiabetes drug
- PD
pharmacodynamic
- PK
pharmacokinetic
- SC
subcutaneous
References
- 1.Heinemann L. The failure of Exubera: are we beating a dead horse? J Diabetes Sci Technol. 2008;2(3):518–529. doi: 10.1177/193229680800200325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pettis RJ, Hompesch M, Kapitza C, Harvey NG, Ginsberg B, Heinemann L. Intra-dermal insulin lispro application with a new microneedle delivery system led to a substantially more rapid insulin absorption than subcutaneous injection. Diabetes. 2006;55(Suppl 1):A26. [Google Scholar]
- 3.Carino GP, Mathiowitz E. Oral insulin delivery. Adv Drug Deliv Rev. 1999;35(2-3):249–257. doi: 10.1016/s0169-409x(98)00075-1. [DOI] [PubMed] [Google Scholar]
- 4.Lassmann-Vague V, Raccah D. Alternative routes of insulin delivery. Diabetes Metab. 2006;32(5 Pt 2):513–522. doi: 10.1016/s1262-3636(06)72804-x. [DOI] [PubMed] [Google Scholar]
- 5.Owens DR, Zinman B, Bolli G. Alternative routes of insulin delivery. Diabet Med. 2003;20(11):886–898. doi: 10.1046/j.1464-5491.2003.01076.x. [DOI] [PubMed] [Google Scholar]
- 6.Sadrzadeh N, Glembourtt MJ, Stevenson CL. Peptide drug delivery strategies for the treatment of diabetes. J Pharm Sci. 2007;96(8):1925–1954. doi: 10.1002/jps.20848. [DOI] [PubMed] [Google Scholar]
- 7.Khafagy el-S, Morishita M, Onuki Y, Takayama K. Current challenges in non-invasive insulin delivery systems: a comparative review. Adv Drug Deliv Rev. 2007;59(15):1521–1546. doi: 10.1016/j.addr.2007.08.019. [DOI] [PubMed] [Google Scholar]
- 8.Berger M. Oral insulin 1922–1992: the history of continuous ambition and failure. In: Berger M, Gries FA, editors. Frontiers in insulin pharmacology. Germany: Thieme Publishing Group; 1993. pp. 144–148. [Google Scholar]
- 9.Wajcberg E, Miyazaki Y, Triplitt C, Cersosimo E, DeFronzo RA. Dose-response effect of a single administration of oral hexyl-insulin monoconjugate 2 in healthy nondiabetic subjects. Diabetes Care. 2004;27(12):2868–2873. doi: 10.2337/diacare.27.12.2868. [DOI] [PubMed] [Google Scholar]
- 10.Kidron M, Dinh S, Menachem Y, Abbas R, Variano B, Goldberg M, Arbit E, Bar-On H. A novel per-oral insulin formulation: proof of concept study in non-diabetic subjects. Diabet Med. 2004;21(4):354–357. doi: 10.1111/j.1464-5491.2004.01160.x. [DOI] [PubMed] [Google Scholar]
- 11.Kapitza C, Arbit E, Abbas R, Goldberg M, Hompesch M, Heinemann L, Heise T. Oral insulproof of concept in type 2 diabetes patients. Diabetes. 2003;52(Suppl 1):A37. [Google Scholar]
- 12.Heise T, Kapitza C, Nosek L, Becket P, Gelfand R, Goldberg M, Arbit E. Oral insulin as first-line therapy in type 2 diabetes: a randomized controlled pilot study. Diabetologia. 2004;47(Suppl 1):A5. [Google Scholar]
- 13.Heise T, Nosek L, Arbit E, Beckett P, Gelfand R, Porter KM, Goldberg M, Kapitza C. Reduction of postprandial blood glucose excursions by an optimized formulation of oral insulin. Diabetes. 2005;54(Suppl 1):A103. [Google Scholar]
- 14.Goldberg M, Dinh S, Castelli C, Majuru S, Arbit E. Improved glycemic control with oral recombinant human insulin in patients with type 2 diabetes (T2DM) inadequately controlled on metformin. Diabetes. 2007;56(Suppl 2):A121. [Google Scholar]
- 15.Clement S, Still JG, Kosutic G, McAllister RG. Oral insulin product hexyl-insulin monoconjugate 2 (HIM2) in type 1 diabetes mellitus: the glucose stabilization effects of HIM2. Diabetes Technol Ther. 2002;4(4):459–466. doi: 10.1089/152091502760306544. [DOI] [PubMed] [Google Scholar]
- 16.Kipnes M, Dandona P, Tripathy D, Still JG, Kosutic G. Control of postprandial plasma glucose by an oral insulin product (HIM2) in patients with type 2 diabetes. Diabetes Care. 2003;26(2):421–426. doi: 10.2337/diacare.26.2.421. [DOI] [PubMed] [Google Scholar]
- 17.Wajcberg E, Miyazaki Y, Triplitt C, Cersosimo E, DeFronzo RA. Dose-response effect of a single administration of oral hexyl-insulin monoconjugate 2 in healthy nondiabetic subjects. Diabetes Care. 2004;27(12):2868–2873. doi: 10.2337/diacare.27.12.2868. [DOI] [PubMed] [Google Scholar]
- 18.Phillips J, Russel-Jones DL, Wright J, Brackenridge A, New R, Bansal G. Early evaluation of a novel oral insulin delivery system in healthy volunteers. Diabetes. 2004;53(Suppl 2):A113. [Google Scholar]
- 19.Luzio S, Dunseath G, Lockett A, Broke-Smith TP, New RR, Owens D. Comparison of an oral insulin (Capsulin) and Actrapid during an isoglycemic clamp study in persons with type 2 diabetes. Diabetes. 2008;56(Suppl 1):A10. doi: 10.1111/j.1463-1326.2009.01146.x. [DOI] [PubMed] [Google Scholar]
- 20.Broke-Smith TP, Luzio S, Lockett A, New RR, Owens DR. Repeat-dosing of oral insulin (Capsulin) in persons with type 2 diabetes. Diabetologia. 2008;51(Suppl 1):S8. doi: 10.1111/j.1463-1326.2009.01146.x. [DOI] [PubMed] [Google Scholar]
- 21.Davis SN, Geho B, Tate D, Galassetti P, Lau J, Granner D, Mann S. The effects of HDV-insulin on carbohydrate metabolism in type 1 diabetic patients. J Diabetes Complications. 2001;15(5):227–233. doi: 10.1016/s1056-8727(01)00154-4. [DOI] [PubMed] [Google Scholar]
- 22.Schwartz S, Geho B, Rosenberg L. Single-blind, placebo-controlled, dose-ranging trial of oral HDV-insulin in patients with type 2 diabetes mellitus. Diabetes. 2008;57(Suppl 1):A127. doi: 10.1177/1932296814524871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schwartz S, Geho B, Rosenberg L, Lau J. A 2-week randomized active comparator study of two HDV-Insulin routes (SC and oral) and SC human insulin in patients with type 1 diabetes. Diabetes. 2008;57(Suppl 1):A124. [Google Scholar]
- 24.Kidron M, Raz I, Schruefer C, Showb H, Wolfensberger M. Pharmacokinetics (PK) and pharmacodynamics (PD) or oral insulin in healthy subjects. Diabetes. 2008;57(Suppl 1):A127. [Google Scholar]
- 25.Faber-Heinemann G, Hess E, Hess G, von Hübbenet J, Kaltheuner M, Krakow D, Lederle M, Molinski M, Nitzsche G, Reuter HM, Scheper N, Simonsohn M, Heinemann L. Realität der Insulintherapie bei Typ 2 Diabetes in Deutschland: Daten aus 41 Schwerpunktpraxen. Diabetes, Stoffwechsel, und Herz. 2008;5:357–361. [Google Scholar]
- 26.Al-Waili NS. Sublingual human insulin for hyperglycaemia in type I diabetes. JPMA. 1999;49(7):167–169. [PubMed] [Google Scholar]
- 27.Bernstein G. Delivery of insulin to the buccal mucosa utilizing the RapidMist system. Expert Opin Drug Deliv. 2008;5(9):1047–1055. doi: 10.1517/17425247.5.9.1047. [DOI] [PubMed] [Google Scholar]
- 28.Cavallo MG, Coppolino G, Romero S, Pozzilli P. Inhaled insulin in type 1 diabetes. Lancet. 2001;357(9272):1980. doi: 10.1016/s0140-6736(00)05051-0. [DOI] [PubMed] [Google Scholar]
- 29.Modi P, Mihic M, Lewin A. The evolving role of oral insulin in the treatment of diabetes using a novel RapidMist System. Diabetes Metab Res Rev. 2002;18(Suppl 1):S38–S42. doi: 10.1002/dmrr.208. [DOI] [PubMed] [Google Scholar]
- 30.Guevara-Aguirre J, Guevara M, Saavedra J, Mihic M, Modi P. Oral spray insulin in treatment of type 2 diabetes: a comparison of efficacy of the oral spray insulin (Oralin) with subcutaneous (SC) insulin injection, a proof of concept study. Diabetes Metab Res Rev. 2004;20(6):472–478. doi: 10.1002/dmrr.477. [DOI] [PubMed] [Google Scholar]
- 31.Guevara-Aguirre J, Guevara M, Saavedra J, Mihic M, Modi P. Beneficial effects of addition of oral spray insulin (Oralin) on insulin secretion and metabolic control in subjects with type 2 diabetes mellitus suboptimally controlled on oral hypoglycemic agents. Diabetes Technol Ther. 2004;6(1):1–8. doi: 10.1089/152091504322783341. [DOI] [PubMed] [Google Scholar]
- 32.Cernea S, Kidron M, Wohlgelernter J, Modi P, Raz I. Comparison of pharmacokinetic and pharmacodynamic properties of single-dose oral insulin spray and subcutaneous insulin injection in healthy subjects using the euglycemic clamp technique. Clin Ther. 2004;26(12):2084–2091. doi: 10.1016/j.clinthera.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 33.Cernea S, Kidron M, Wohlgelernter J, Modi P, Raz I. Dose-response relationship of oral insulin spray in healthy subjects. Diabetes Care. 2005;28(6):1353–1357. doi: 10.2337/diacare.28.6.1353. [DOI] [PubMed] [Google Scholar]
- 34.Cernea S, Kidron M, Wohlgelernter J, Raz I. Dose-response relationship of an oral insulin spray in six patients with type 1 diabetes: a single-center, randomized, single-blind, 5-way crossover study. Clin Ther. 2005;27(10):1562–1570. doi: 10.1016/j.clinthera.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 35.IMDIAB Group. Pozzilli P, Manfrini S, Buzzetti R, Lampeter E, Leeuw ID, Iafusco D, Prisco M, Ionescu-Tirgoviste C, Kolouskovà S, Linn T, Ludvigsson J, Madàcsy L, Mrozikiewicz AS, Mrozikiewicz PM, Podar T, Vavrinec J, Vialettes B, Visalli N, Yilmaz T, Browne PD. Glucose evaluation trial for remission (GETREM) in type 1 diabetes: a European multicentre study. Diabetes Res Clin Pract. 2005;68(3):258–264. doi: 10.1016/j.diabres.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 36.Guevara-Aguirre J, Guevara-Aguirre M, Saavedra J, Bernstein G, Rosenbloom AL. Comparison of oral insulin spray and subcutaneous regular insulin at mealtime in type 1 diabetes. Diabetes Technol Ther. 2007;9(4):372–376. doi: 10.1089/dia.2006.0019. [DOI] [PubMed] [Google Scholar]
- 37.Heinemann L. Variability of insulin absorption and insulin action. Diabetes Technol Ther. 2002;4(5):673–682. doi: 10.1089/152091502320798312. [DOI] [PubMed] [Google Scholar]
- 38.Veuillez F, Kalia YN, Jacques Y, Deshusses J, Buri P. Factors and strategies for improving buccal absorption of peptides. Eur J Pharm Biopharm. 2001;51(2):93–109. doi: 10.1016/s0939-6411(00)00144-2. [DOI] [PubMed] [Google Scholar]