Abstract
Background
The importance of near-normal blood glucose in the immediate postoperative period is generally accepted and is best achieved in the perioperative period with a constant intravenous (IV) infusion of insulin. This requires intensive nursing only achievable in an intensive care unit (ICU) setting. Glucose management after transfer to a regular nursing floor (RNF) has not been studied systematically. In August 2006, the Cleveland Clinic began using long-acting insulin glargine as the insulin infusion was terminated in the ICU.
Methods
This prospective analysis examined all patients receiving IV insulin infusion after cardiothoracic surgery in a 1 month period. The analyses evaluated the safety and efficacy of a protocol using a transition to subcutaneous insulin glargine of 50% of the calculated 24 h requirement at the end of the ICU insulin infusion protocol in preparation for transfer to the RNF.
Results
Only 1 patient in 99 developed hypoglycemia, and no patient suffered severe hypoglycemia (glucose < 40 mg/dl), while the majority (70%) had euglycemia (glucose between 70 and 150 mg/dl).
Conclusions
This approach was both safe—as there was very little hypoglycemia (1 patient in 99)—and effective, as blood sugar was well controlled in most subjects. Efficacy for achieving euglycemia was 70%. Efficacy was likely reduced because of the upper limit of insulin glargine dosage imposed by some providers as a safety consideration. Although there was a physician option to override, the maximum protocol dose of 30 U was rarely exceeded, leading to inadequate dosing in some subjects who required high insulin infusion rates in the ICU.
Keywords: coronary artery bypass graft, euglycemia, hyperglycemia, hypoglycemia, insulin glargine, perioperative glucose, transition, valve surgery
Background
Several lines of evidence suggest that glycemic control in the hospital setting may be associated with improved outcomes. Funary and colleagues1–9 have reported that outcomes in coronary artery bypass graft (CABG) recipients are affected by glycemic control, especially for the 3 days following the procedure. Specifically, 72 h of glycemic control is associated with reduced sternal wound infections and hospital mortality by more than half and length of stay by approximately 30% compared to historic controls, essentially eliminating the increased risk for mortality previously seen in patients with diabetes mellitus compared to those without diabetes mellitus. In the current hospital environment, most patients admitted to a cardiothoracic intensive care unit (ICU) do not stay for this critical 72 h. The average length of stay at the Cleveland Clinic cardiovascular intensive care unit (CVICU) is between 24 and 26 h before patients are transferred to a regular nursing floor.
There are several published and unpublished regimens for intensive glycemic control in the ICU. The Cleveland Clinic has had an insulin infusion protocol in the CVICU for more than 10 years. This protocol was developed in conjunction with CV anesthesiology (who are responsible for the metabolic and physiologic aspects of postoperative care), endocrinology, and nursing. However, approaches for transition of insulin orders as part of patient care to a regular nursing floor (RNF) have been less well characterized. Schmeltz and associates10 and Bode and coworkers11 each suggested 80% of the daily calculated requirement (based on the final 6 h of the infusion rate). This clearly is an improvement over earlier approaches of subcutaneous (SC) “coverage” insulin or “sliding scale.” The sliding scale insulin approach fails to consider the need for basal insulin in most patients. Thus coverage is associated with wide variations in blood glucose as well as risks for hyperglycemia and hypoglycemia. Concerns for risk of hypoglycemia during the transition of care (i.e., the change in care from an ICU team to an RNF team) have led to the coverage insulin approach even in many hospitals where intravenous (IV) insulin is used in the ICU. One of the significant concerns about the use of basal insulin is the perceived risk for hypoglycemia. In an effort to facilitate one step in this transition from the ICU to the RNF, we recently implemented an insulin conversion protocol for postoperative cardiac surgical patients on IV insulin in the ICU to transition to basal SC insulin at the time of transfer to the RNF.
The purpose of this evaluation was to determine the safety and efficacy of the insulin glargine protocol in postoperative cardiac surgical patients transitioning from the CVICU to the RNF. Ninety-nine patients who received insulin infusions in the CVICU after cardiac surgery and were transferred to RNF in a timely fashion (within 72 h of surgery) between December 1 and December 31, 2007, provided the basis for this analysis.
Methods
Insulin Infusion and Subcutaneous Insulin Transition Protocols
Surgical patients undergoing a CABG, valve replacement/repairs, or aortic repair/replacement were given lactated Ringer's solution for maintenance fluids intraoperatively and D5-0.2NS in the CVICU. If blood glucose levels are ≥150 mg/dl on two assessments within 1 h during surgery, an IV infusion of regular insulin is initiated and continued in the CVICU. Hyperglycemia is identified by hourly assessments of glucose as well as electrolytes and arterial blood gases intraoperatively. Similar assessments are made at least every 4 h during the CVICU stay and more frequently if glucose exceeds 110 mg/dl. If an insulin infusion is active, glucose is assessed hourly until stable and then assessed every 2 h. For some patients, the insulin infusion protocol is implemented postoperatively, using the same criteria. If blood glucose reaches a level of 150 mg/dl, a nurse-driven insulin infusion protocol (see Appendix) similar to that described by Osburne and colleagues12 is initiated with a blood glucose target at or below 120 mg/dl. If the blood glucose reaches 80 mg/dl, the infusion is halted and restarted only if the threshold of 150 mg/dl is again reached. On the day of transfer from the CVICU to the RNF, an insulin glargine dose is calculated, extrapolating from the average IV regular insulin dose infused during the previous 8 h. The SC glargine dose (U) is calculated as the total amount of IV regular insulin received in the previous 8 h (U) multiplied by 3 and then divided by 2. If the patient received less than 8 h of IV regular insulin prior to planned transfer from the ICU, the single glargine dose would be half of the calculated amortized dose and is given 2 h before discontinuing the insulin infusion. In addition to the SC injection of insulin glargine, a sliding scale of rapid-acting insulin is used in a supplemental fashion that was generally proportional to the glargine dose. Most patients were not eating much at this time, so meal coverage was not prescribed.
Insulin glargine is administered in the CVICU 2 h prior to discontinuation of IV insulin infusion in anticipation of transfer. The original protocol specified a maximal protocol dose of 30 U of insulin glargine, unless higher doses were approved by the treating physician. Override of this upper dose limit varied with providers as did the comfort in ordering more insulin for those patients who needed higher insulin infusion rates. Generally, when ordered by the endocrinology service, the full calculated dose was administered, but this was less likely when ordered by nonendocrine providers. Upon arrival to the RNF, 4 point of care (POC) blood glucose measurements are obtained at 6 h intervals until the patient begins eating, and the endocrinology service is consulted, if not already on the case, for further management. Once a patient begins to eat, the timing of POC testing was changed to before each meal and at bedtime, and short-acting insulin was prescribed to address meal coverage as well as a supplemental scale to address inadequate coverage.
Study Design
The Cleveland Clinic Foundation institutional review board approved a prospective design to examine data from all postoperative patients ≥18 years old who had undergone a CABG, aortic surgery, or valve replacement/repair requiring insulin infusion in the CVICU (five separate ICUs for a 1 month period, December 1 through December 31, 2007). Data were collected using concurrent noninterventional medical record review using medication administration records and electronic medication record system pharmacy records. Patient selection included diabetes and nondiabetes patients but excluded patients who were readmitted from RNFs for complications or patients who required CVICU stays longer than 7 days. Data collection involved de-identified information on demographics, diabetes mellitus history (if known, whether type 1 or type 2, and if type 2, whether or not previously treated with insulin), hemoglobin A1c, date and type of cardiac surgery (CABG initial or redo, aortic or valvular surgery or combination), catecholamine use in the CVICU (epinephrine, norepinephrine, or none) from a CV anesthesia database. Data were collected for the total amount of regular insulin received by IV infusion in the previous 8 h as well as the date and time insulin infusion started and discontinued from pharmacy records. Providers ordered the glargine dose (U), but the pharmacy calculated dose based on a review of the actual IV insulin required, and these were compared. Point of care blood testing date, time, and value (mg/dl) were collected. Primary assessment was the incidence of hypoglycemia (≤70 mg/dl) and hyperglycemia (≥150 mg/dl) with the insulin transfer protocol with a single value for either leading to those classifications. Secondary assessments were performed to assess blood glucose control using the calculated dose appropriate for diabetes patients as well as those without diabetes and postoperative hyperglycemic patients and to evaluate whether infusion with epinephrine or norepinephrine was administered during the 8 h insulin infusion period used to calculate the glargine dose influenced the appropriateness of the dosing.
Analysis
Data were evaluated using descriptive analysis for patient demographics, baseline characteristics, and the percentage of hypoglycemic and hyperglycemic patients on the insulin glargine protocol. Descriptive analysis was also utilized to analyze data on the patients who received pressors during the glargine dose calculation and those who received no pressor. Also analyzed was a comparison of appropriateness of the insulin glargine protocol in patients with a history of diabetes mellitus and those without known diabetes mellitus.
Statistics
Chi square were calculated for evidence of independence using the online calculator. In the situation of low numbers in a single cell, a Fisher's exact test was used.
Patient Subjects
All patients treated with IV insulin protocol post CV surgery in the CVICU in the month of December 2007 were included in this assessment of the transition protocol that had been used for at least 1 year prior. The subjects were identified by a confidential study number, and patient identifiers were not entered into the database system. The data were stored and secured in a nonshared hard drive database with a password-protected system that was only accessible to the principal and coprincipal investigators. Data collection forms were kept in a locked, secured cabinet, in which access to the data was limited to the investigators during the data collection period. At the conclusion of data analysis, data collection sheets were destroyed and de-identified information will be maintained in the database system for approximately 6 years per Health Insurance Portability and Accountability Act regulations.
Results
There were 99 consecutive patients who were treated with this protocol. The demographic and surgical features of the patients are summarized in Table 1. Mean (± standard deviation) age was 64 (+ 13), and 67% were male. Most patients did not have diabetes mellitus (n = 76). Of the 23 with previous history of diabetes, 21 had type 2 and 2 had type 1. The types of surgery are summarized in Table 1, with the majority involving valve-related surgery (61), followed by valve only and valve surgery in conjunction with coronary bypass (20) and CABGs alone (18). In the majority (76 of 99 patients in the study period), this was begun intraoperatively, while in 23, the insulin infusion was begun in the ICU after transfer from the operating room.
Table 1.
Characteristics of 99 Patients Treated with Subcutaneous Insulin Transition Protocol
| Age, mean ± standard deviation (years) | 64 ± 13 |
| Gender (male) | 67% |
| Weight median (kg) | 85 (43–192) |
| No known diabetes mellitus Known diabetes mellitus: | 76 |
| Type 1 | 2 |
| Type 2 | 21 |
| Location IV insulin infusion started: | |
| Operating room | 76 |
| ICU | 23 |
| Type of cardiac surgery: | |
| CABG only | 18 |
| Valve only | 61 |
| Valve plus CABG | 20 |
The insulin glargine doses divided by 20 U ranges are summarized in Table 2. Insulin glargine doses for diabetes and nondiabetes subjects are shown in Table 3. While 50% (12 of 24) of the diabetes patients required >20 U, only 23% (17 of 76) of the nondiabetes patients required >20 U (x2 = 7.573, p < .01, Table 3). Evaluation of several factors associated with the glycemia status after the insulin glargine transition demonstrated that there was no relationship between transition in insulin dose and the risk for hypoglycemia, hyperglycemia, or euglycemia. Only one patient without a history of diabetes developed hypoglycemia (blood glucose 64 mg/dl) after receiving a moderate dose (24 U) of SC insulin. Figure 1 shows the relationship among in the actual dose administered (y axis) and the calculated dose that would have been administered if the protocol dose had been given exactly according to the formula (x axis). This figure shows that calculated doses were often higher than administered doses, reflecting the upper limit of 30 U that required physician override to be administered. Patients whose preoperative status was “no diabetes mellitus” were not statistically more likely to be euglycemic after the insulin glargine transition protocol than those with a preoperative diagnosis of “diabetes mellitus” (x2 = 6.92, p > .1). Vasopressor use did not affect transition insulin dose (x2 = 1.40, p > .1, Table 4) or the risk for hyperglycemia (x2 = 0.1487, p > .5, Table 5).
Table 2.
Outcomes in Glycemia Related to Subcutaneous Insulin Dose at Transition
| Transition insulin (glargine) dose ranges (U) | 0–20 | 21–40 | >41 |
| n | 70 | 22 | 7 |
| Hypoglycemia (<70 mg/dl) | 0 | 1 | 0 |
| Hyperglycemia (>150 mg/dl) | 17 | 10 | 2 |
| Euglycemia (70–150 mg/dl) | 53 | 11 | 5 |
Table 3.
Subcutaneous Insulin Dose Ranges in the Transition Protocol by Preoperative Diabetes Mellitus Statusa
| 0–20 U | 21–40 U | >41 U | |
|---|---|---|---|
| Diabetes n = 23 | 11 | 9 | 3 |
| No diabetes n = 76 | 59 | 13 | 4 |
Previous history of diabetes more likely to need greater than 20 U of glargine at transition (x2 = 7.573, p < .01).
Figure 1.
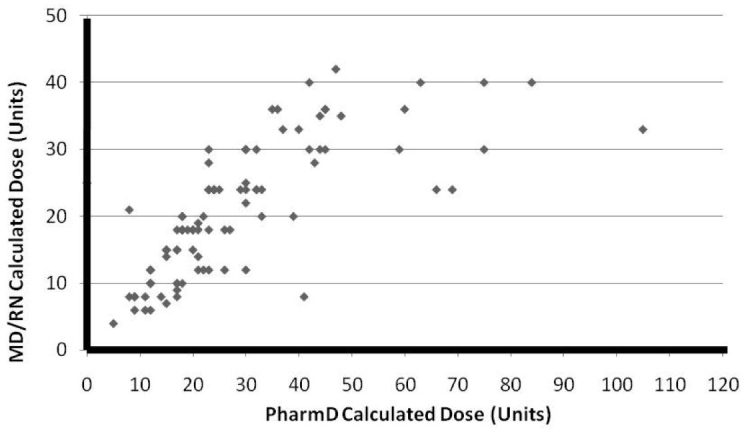
This reflects close agreement of the administered dose to the dose calculated from the insulin infusion rate up to 35 or 40 U but a reluctance to give the calculated dose much above 40 U, which accounts for most, if not all, of the patients who were hyperglycemic on transition to the RNF.
Table 4.
Effects of Vasopressor Use in Subcutaneous Insulin Dose Ranges in 99 Patients Treated with an Insulin (Glargine) Transition Dosea
| Insulin dose range (U) | 0–20 | 21–40 | >41 |
| (+) Vasopressor | 14 | 5 | 4 |
| (−) Vasopressor | 56 | 17 | 3 |
Patients who had received pressors were not more likely to require more than 20 U glargine than those who had not (x2 = 1.40, p > .1)
Table 5.
Glycemic Status (Hypoglycemia, Hyperglycemia, or Euglycemia) after Insulin Transition Dose (Glargine) in 99 Patients Characterized by Insulin Dose Range, Presence or Absence of Diabetes Mellitus, and Use or No Use of Pressors
| Preoperative diabetes status | Diabetes | No diabetes |
|---|---|---|
| n | 23 | 76 |
| Hypoglycemia (<70 mg/dl) | 0 | 1 |
| Hyperglycemia (>150 mg/dl) | 16 | 13 |
| Euglycemia (70–150 mg/dl) | 7 | 63 |
| Vasopressor use | Yes | No |
| n | 23 | 76 |
| Hypoglycemia (<70 mg/dl) | 0 | 1 |
| Hyperglycemia (>150 mg/dl) | 6 | 23 |
| Euglycemia (70–150 mg/dl) | 17 | 52 |
Discussion
The information obtained from this observational study is notable for the following features. First, in spite of moderate doses of SC insulin at the time of transition, we found only 1 episode of hypoglycemia, which was mild and with no critical hypoglycemia values (>40 mg/dl). Second, relatively high doses of insulin, in many cases above the 30 U originally specified in the protocol, were required to achieve euglycemia. Hyperglycemia occurred most frequently in subjects whose proposed doses based on the protocol had lower doses given because of the ceiling standard protocol dose of 30 U being used because of safety concerns. We suspect that hyperglycemia would have been less frequent had higher doses based on the transition formula actually been allowed as reflected in Figure 1. Third, most of the patients who received the transition protocol did not have previously diagnosed diabetes. Thus this treatment strategy should not be limited to patients with diabetes and can be given safely to patients without diabetes. Euglycemia was achieved more commonly in patients without a history of diabetes. Finally, the use of pressors in the CVICU did not have a significant effect on the transition insulin dose or the subsequent risk for hyperglycemia. Thus the transition protocol can be implemented equally effectively in patients who did or did not require pressors.
Unpublished observational data from the Cleveland Clinic obtained in the mid 1990s suggested that hyperglycemia in the immediate postoperative period was associated with increased risk for sternal wound infections in both diabetes and nondiabetes patients. On that basis, insulin infusion protocols were implemented as a quality measure. The seminal observations of Furnary and colleagues1–8 have demonstrated that the prevention of hyperglycemia during the first 72 h following cardiac surgery is associated with a 63% reduction in deep sternal wound infections, a 65% reduction in hospital mortality, and a 30% reduction in hospital length of stay. Other lines of evidence supporting the concept that glycemic control and/or insulin therapy to improve outcomes in ICU patients include the large trial by Van den Berghe and associates13–17 in Europe. She confirmed that strict glycemic control improved morbidity outcomes in diabetes and nondiabetes patients with elevated glucose in an surgical intensive care unit (SICU) population that included a large percentage of cardiac surgery patients.
Postoperative hyperglycemia, defined as plasma glucose levels ≥200 mg/dl, is a common metabolic manifestation of operative and postoperative patient stress.1 High blood glucose levels caused by this stress have been shown to be a risk factor for increased morbidity and mortality among surgical patients, resulting in complications such as impaired wound healing, severe infections, polyneuropathy, and multiple organ failure. Postoperative hyperglycemia characterized by increased glucose production and suppressed insulin secretion is felt to be caused by the excessive release of counter-regulatory hormones such as glucagon, epinephrine, norepinephrine, and glucocorticoids, as well as the overproduction of the cytokines (inflammatory mediators) TNF-α, IL-1, and IL-6.18 While the meta-analyses by Weiner and coworkers19 and Pittas and colleagues20 bring into question the most appropriate glycemic target, the risk for infectious complications in surgical patients is a confirmed benefit of tighter glucose control.
The treatment for hyperglycemia has encompassed various types of insulin regimens based on individual pharmacodynamic characteristics. Historically, treatment for postoperative hyperglycemia was SC sliding scale regular insulin. However, Gearhart and colleagues21 demonstrated ineffective glycemic control with the short-acting sliding scale resulting in wide fluctuations in blood glucose values, high glucose concentrations despite treatment, and longer treatment periods. Although regular insulin may have a relatively rapid onset of action with a peak concentration at 2 to 4 h, it requires frequent dosing with a short duration of action of only 6 to 8 h.22
Insulin neutral protamine Hagedorn (NPH) and insulin glargine have each been utilized in the treatment of postoperative hyperglycemia. Insulin NPH, an intermediate-acting insulin, exhibits a duration of action longer than regular insulin but shorter than glargine. In comparison to four times daily dosing with regular insulin, NPH can be dosed twice daily with a peak effect at 4–6 h and a duration of 12–16 h.21–23 It is the peak that makes this insulin less than ideal for the purpose of transition from an insulin infusion.
Insulin glargine is a long-acting insulin analogue that has biologic activity similar to human insulin in stimulating glucose uptake by the adipose and skeletal muscle tissues and importantly in inhibiting hepatic glucose production.24 The pharmacokinetic profile of insulin glargine that distinguishes it from other types of insulin is its average duration of about 24 h, and its relative lack of a peak produces a profile ideal for basal insulin that provides the anticatabolic actions of basal insulin. Insulin glargine exists as an acidic solution with a pH of 4 that is neutralized in the SC tissue upon injection, promoting the development of microprecipitates. The microprecipitates slowly release small amounts of insulin into the circulation, exhibiting a constant concentration of insulin over a 24 h period, mimicking a continuous infusion of regular insulin.22–24 With little peak in concentration and a 24 h duration of action, insulin glargine offers a convenient, once-daily dosing.22–24
A study conducted by Yeldandi and colleagues23 compared once-daily insulin glargine to twice-daily NPH/regular insulin in postoperative CV surgical patients undergoing a CABG or valve replacement/repair transitioning from the SICU to the RNF. All patients with postoperative glucose values above the goal of 100 to 140 mg/dl were initiated on an IV insulin infusion. Prior to transfer from the SICU to the RNF, patients were randomized to receive either once-daily insulin glargine or twice-daily insulin NPH/regular. The initial glargine and NPH/regular insulin doses were calculated based on the last stable IV infusion rate, and the IV and SC insulin regimens were overlapped for 1 to 2 h prior to discontinuing the IV infusion. On the RNF, insulin glargine demonstrated similar glycemic control in the nondiabetes group (p = .065) but significantly higher mean blood glucose levels in the diabetes group (p = .016) compared to the NPH/regular regimen. However, the incidence of hypoglycemia was significantly lower with insulin glargine than NPH/regular in both subjects with diabetes and those without (p = .036).
The importance of avoidance of hypoglycemia seen in the Cleveland Clinic approach should not be overlooked. The findings of Gandhi and associates25 show no benefit and greater mortality with intensively treated cardiac surgery patients. The SUGAR-NICE study,26 managing a more heterogeneous group of critically ill patients, failed to show benefit of more intensive glucose control. Each of these may be explained by the higher incidence of hypoglycemia seen in the intensive groups of each study. The volume of patients submitted to this protocol is seen in as few as 100 or more studies, where such subjects are managed for hyperglycemia after CV surgery each month. That is more than the total subjects in the trial of Gandhi and coworkers.25 Only Van den Berghe13–17 has a comparable volume of subjects managed in a uniform fashion at a single center.
In summary, we offer a protocol for conversion from IV regular insulin infusion given in the ICU to a SC basal insulin regime that is quite safe and effective. Our study shows that the approach to substituting 50% of the previous 24 h calculated daily need of insulin glargine is not associated with a significant risk for hypoglycemia and should allay such fears. The use of the full calculated dose would likely have improved the efficacy further and would unlikely have reduced the safety of this protocol.
Abbreviations
- CABG
coronary artery bypass graft
- CV
cardiovascular
- CVICU
cardiovascular intensive care unit
- ICU
intensive care unit
- IV
intravenous
- NPH
neutral protamine Hagedorn
- POC
point of care
- RNF
regular nursing floor
- SC
subcutaneous
- SICU
surgical intensive care unit
Appendix: Cleveland Clinic Intensive Care Unit Insulin Protocol Do Not Use for Diabetic Ketoacidosis (DKA)
Goal Blood Glucose
For CICU, MICU, SICU, Neuro ICU, Heart Failure ICU, H21, HTU, H62, H63: 80 – 120 mg/dl
For CVICU: Day of surgery (until Midnight) Blood Glucose Goal: 80 – 150 mg/dl; Post-op day #1 (midnight) until discharge from CVICU Blood
Glucose Goal: 80 – 120 mg/dl. If BG is within target range upon arrival to the CVICU, reduce the infusion by 50% upon admission unless the rate has already been reduced by 50% in the OR.
Insulin Continuous Infusion – Do Not Use for Diabetic Ketoacidosis (DKA)
-
Regular Insulin 100 units/100 ml in 0.9% normal saline; concentration 1 unit/ml
-
If blood glucose (BG) > 150 mg/dl for 4 consecutive measurements
Bolus dose: 0.05 units/kg (maximum bolus is 5 units)
Then initiate continuous infusion: initial rate 0.05 units/kg/hr (maximum initial rate is 5 units/hr)
See table 1 (Insulin Infusion Adjustment) for adjustment of insulin rate
-
Blood Glucose Monitoring
Monitor BG every 2 hours (exceptions noted below)
Hypoglycemia Protocol
If BG ≤ 60 mg/dL – stop insulin infusion, give 25 – 50 mL of 50% dextrose solution, notify ICU resident, obtain BG level every 30 minutes until BG > 80 mg/dL for three consecutive levels, and then check blood glucose every 2 hours
If BG 61 – 70 mg/dL – stop insulin infusion, obtain BG level every 1 hour until BG > 80 mg/dL for three consecutive measurements, then check blood glucose every 2 hours
If BG DECREASES ≥ 30 mg/dL since last level Tand BG = 71 – 85 mg/dL T- stop infusion, obtain BG level every 1 hour until BG > 80 mg/dL for three consecutive levels, then check BG every 2 hours
If enteral nutrition or total parenteral nutrition is stopped, decrease insulin infusion rate by 50% and monitor blood glucose levels every 1 hour until BG > 80 mg/dl for three consecutive levels, then check blood glucose every 2 hours
Resuming Insulin Infusion
Restart insulin infusion when first blood glucose value is ≥ 150 mg/dl. Do not bolus. Restart insulin infusion at half the previous rate. Obtain blood glucose in 1 hour and reevaluate.
Table 1:
Insulin Infusion Adjustment: ***DO NOT ADJUST INSULIN RATE EVERY HOUR – ONLY MAKE ADJUSTMENTS TO THE INSULIN RATE EVERY TWO HOURS***
| Blood Glucose | If BG DECREASES ≥ 30 mg/dl since last level | If BG is STABLE (change in BG < 30 mg/dl) since last level | If BG INCREASES ≥ 30 mg/dl since last level |
|---|---|---|---|
| ≤ 60 | Stop insulin infusion See Hypoglycemia Protocol | Stop insulin infusion See Hypoglycemia Protocol | — |
| 61 – 70 | Stop insulin infusion See Hypoglycemia Protocol | Stop insulin infusion/See Hypoglycemia Protocol | — |
| 71-85 | Stop insulin infusion See Hypoglycemia Protocol | Decrease rate by 50% | — |
| 86 – 100 | Decrease rate by 50% | Decrease rate by 50% | — |
| 101 – 115 | Decrease rate by 50% | Continue current rate | — |
| 116 – 150 | Decrease rate by by 50% | Increase rate by 25% | Increase rate by 25% |
| 151 – 200 | Decrease rate by by 25% | Increase rate by 25% | Bolus 2 units/Increase rate by 25% |
| 201 – 250 | Continue current rate | Bolus 2 units/Increase rate by 25% | Bolus 4 units/Increase rate by 25% |
| 251 – 300 | Continue current rate | Bolus 4 units/Increase rate by 50% | Bolus 6 units/Increase rate by 50% |
| 351 – 400 | Continue current rate | Bolus 8 units/Increase rate by 50% | Bolus 10 units/Increase rate by 50% |
| > 400 | Notify ICU Resident | Notify ICU Resident | Notify ICU Resident |
(Note: If Note: if insulin rate is ≥ 30 units/hr, notify ICU resident)
Important Notes
Insulin sensitivity will usually improve over time in the critically ill patient. Because of this, the need for insulin may decrease throughout the ICU stay.
Insulin requirements will usually increase when starting glucocorticoid therapy
Prior to discharge from the ICU, patients should be evaluated for transition to the standardized insulin order set.
Calculation of Insulin Infusions
Table 2:
Table 2: Calculation of INFUSION RATE adjustments
| Current Rate | Increase by 25% | Increase by 50% | Decrease by 25% | Decrease by 50% |
|---|---|---|---|---|
| 1 | 1.2 | 1.5 | 0.8 | 0.5 |
| 2 | 2.5 | 3 | 1.5 | 1 |
| 3 | 3.8 | 4.5 | 2.2 | 1.5 |
| 4 | 5 | 6 | 3 | 2 |
| 5 | 6.2 | 7.5 | 3.8 | 2.5 |
| 6 | 7.5 | 9 | 4.5 | 3 |
| 7 | 8.8 | 10.5 | 5.2 | 3.5 |
| 8 | 10 | 12 | 6 | 4 |
| 9 | 11.2 | 13.5 | 6.8 | 4.5 |
| 10 | 12.5 | 15 | 7.5 | 5 |
| 11 | 13.8 | 16.5 | 8.2 | 5.5 |
| 12 | 15 | 18 | 9 | 6 |
| 13 | 16.2 | 19.5 | 9.8 | 6.5 |
| 14 | 17.5 | 21 | 10.5 | 7 |
| 15 | 18.8 | 22.5 | 11.2 | 7.5 |
| 16 | 20 | 24 | 12 | 8 |
| 17 | 21.2 | 25.5 | 12.8 | 8.5 |
| 18 | 22.5 | 27 | 13.5 | 9 |
| 19 | 23.8 | 28.5 | 14.2 | 9.5 |
| 20 | 25 | 30 | 15 | 10 |
| 21 | 26.5 | 31.5 | 15.8 | 10.5 |
| 22 | 27.5 | 33 | 16.5 | 11 |
| 23 | 28.8 | 34.5 | 17.2 | 11.5 |
| 24 | 30 | 36 | 18 | 12 |
| 25 | 31.2 | 37.5 | 18.8 | 12.5 |
| 26 | 32.5 | 39 | 19.5 | 13 |
| 27 | 33.8 | 40.5 | 20.2 | 13.5 |
| 28 | 35 | 42 | 21 | 14 |
| 29 | 36.2 | 43.5 | 21.8 | 14.5 |
| 30 | 37.5 | 45 | 22.5 | 15 |
| 31 | 38.8 | 46.5 | 23.2 | 15.5 |
| 32 | 40 | 48 | 24 | 16 |
| 33 | 41.2 | 49.5 | 24.8 | 16.5 |
| 34 | 42.5 | 25.50 | 17 | |
| 35 | 43.8 | 26.2 | 17.5 | |
| 36 | 45 | 27 | 18 | |
| 37 | 46.2 | 27.8 | 18.5 | |
| 38 | 47.5 | 28.5 | 19 | |
| 39 | 48.8 | 29.2 | 19.5 | |
| 40 | 50 | 30 | 20 | |
| 41 | 30.8 | 20.5 | ||
| 42 | 31.5 | 21 | ||
| 43 | 32.2 | 21.5 | ||
| 44 | 33 | 22 | ||
| 45 | 33.8 | 22.5 | ||
| 46 | 34.5 | 23 | ||
| 47 | 35.2 | 23.5 | ||
| 48 | 36 | 24 | ||
| 49 | 36.8 | 24.5 | ||
| 50 | 37.5 | 25 |
References
- 1.Furnary AP, Zerr KJ, Grunkemeier GL, Starr A. Continuous intravenous insulin infusion reduces the incidence of deep sternal wound infection in diabetic patients after cardiac surgical procedures. Ann Thorac Surg. 1999;67(2):352–360. doi: 10.1016/s0003-4975(99)00014-4. [DOI] [PubMed] [Google Scholar]
- 2.Furnary AP, Gao G, Grunkemeier GL, Wu Y, Zerr KJ, Bookin SO, Floten HS, Starr A. Continuous insulin infusion reduces mortality in patients with diabetes undergoing coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2003;125(5):1007–1021. doi: 10.1067/mtc.2003.181. [DOI] [PubMed] [Google Scholar]
- 3.Furnary AP, Wu Y, Bookin SO. Effect of hyperglycemia and continuous intravenous insulin infusions on outcomes of cardiac surgical procedures: the Portland Diabetic Project. Endocr Pract. 2004;10(Suppl 2):21–33. doi: 10.4158/EP.10.S2.21. [DOI] [PubMed] [Google Scholar]
- 4.Furnary AP, Cheek DB, Holmes SC, Howell WL, Kelly SP. Achieving tight glycemic control in the operating room: lessons learned from 12 years in the trenches of a paradigm shift in anesthetic care. Semin Thorac Cardiovasc Surg. 2006;18(4):339–345. doi: 10.1053/j.semtcvs.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 5.Furnary AP, Wu Y. Eliminating the diabetic disadvantage: the Portland Diabetic Project. Semin Thorac Cardiovasc Surg. 2006;18(4):302–308. doi: 10.1053/j.semtcvs.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 6.Furnary AP. Diabetes, hyperglycemia, and the cardiac surgery patient: introduction. Semin Thorac Cardiovasc Surg. 2006;18(4):278–280. doi: 10.1053/j.semtcvs.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 7.Furnary AP, Wu Y. Clinical effects of hyperglycemia in the cardiac surgery population: the Portland Diabetic Project. Endocr Pract. 2006;12(Suppl 3):22–26. doi: 10.4158/EP.12.S3.22. [DOI] [PubMed] [Google Scholar]
- 8.Furnary AP, Braithwaite SS. Effects of outcome on in-hospital transition from intravenous insulin infusion to subcutaneous therapy. Am J Cardiol. 2006;98(4):557–564. doi: 10.1016/j.amjcard.2006.02.065. [DOI] [PubMed] [Google Scholar]
- 9.Zerr KJ, Furnary AP, Grunkemeier GL, Bookin S, Kanhere V, Starr A. Glucose control lowers the risk of wound infection in diabetics after open heart operations. Ann Thorac Surg. 1997;63(2):356–361. doi: 10.1016/s0003-4975(96)01044-2. [DOI] [PubMed] [Google Scholar]
- 10.Schmeltz LR, DeSantis AJ, Schmidt K, O'Shea-Mahler E, Rhee C, Brandt S, Peterson S, Molitch ME. Conversion of intravenous insulin infusions to subcutaneously administered insulin glargine in patients hyperglycemia. Endocr Pract. 2006;12(6):641–650. doi: 10.4158/EP.12.6.641. [DOI] [PubMed] [Google Scholar]
- 11.Bode BW, Braithwaite SS, Steed RD, Davidson PC. Intravenous insulin infusion therapy: indications, methods, and transition to subcutaneous insulin therapy. Endocr Pract. 2004;10(Supp 2):71–80. doi: 10.4158/EP.10.S2.71. [DOI] [PubMed] [Google Scholar]
- 12.Osburne RC, Cook CB, Stockton L, Baird M, Harmon V, Keddo A, Pounds T, Lowey L, Reid J, McGowan KA, Davidson PC. Improving hyperglycemia management in the intensive care unit: preliminary report of a nurse-driven quality improvement project using a redesigned insulin infusion algorithm. Diabetes Educ. 2006;32(3):394–403. doi: 10.1177/0145721706288072. [DOI] [PubMed] [Google Scholar]
- 13.Van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, Vlasselaers D, Ferdinande P, Lauwers P, Bouillon R. Intensive insulin therapy in the critically ill patients. N Engl J Med. 2001;345(19):1359–1367. doi: 10.1056/NEJMoa011300. [DOI] [PubMed] [Google Scholar]
- 14.Van den Berghe G. How does blood glucose control with insulin save lives in intensive care? J Clin Invest. 2004;114(9):1187–1195. doi: 10.1172/JCI23506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van den Berghe G, Schoonheydt K, Becx P, Bruyninckx F, Wouters PJ. Insulin therapy protects the central and peripheral nervous system of intensive care patients. Neurology. 2005;64(8):1348–1353. doi: 10.1212/01.WNL.0000158442.08857.FC. [DOI] [PubMed] [Google Scholar]
- 16.Van den Berghe G, Wilmer A, Milants I, Wouters PJ, Bouckaert B, Bruyninckx F, Bouillon R, Schetz M. Intensive insulin therapy in mixed medical/surgical intensive care units: benefit versus harm. Diabetes. 2006;55(11):3151–3159. doi: 10.2337/db06-0855. [DOI] [PubMed] [Google Scholar]
- 17.Van den Berghe G, Wilmer A, Hermans G, Meersseman W, Wouters PJ, Milants I, Van Wijngaerden E, Bobbaers H, Bouillon R. Intensive insulin therapy in the medical ICU. N Engl J Med. 2006;354(5):449–461. doi: 10.1056/NEJMoa052521. [DOI] [PubMed] [Google Scholar]
- 18.McCowen KC, Malhotra A, Bistrian BR. Stress-induced hyperglycemia. Crit Care Clin. 2001;17(1):107–124. doi: 10.1016/s0749-0704(05)70154-8. [DOI] [PubMed] [Google Scholar]
- 19.Wiener RS, Wiener DC, Larson RJ. Benefits and risks of tight glucose control in critically ill adults: a meta-analysis. JAMA. 2008;300(8):933–944. doi: 10.1001/jama.300.8.933. [DOI] [PubMed] [Google Scholar]
- 20.Pittas AG, Siegel RD, Lau J. Insulin therapy and in-hospital mortality in critically ill patients: systematic review and meta-analysis of randomized controlled trials. JPEN J Parenter Enteral Nutr. 2006;30(2):164–172. doi: 10.1177/0148607106030002164. [DOI] [PubMed] [Google Scholar]
- 21.Gearhart JG, Duncan JL, III, Replogle WH, Forbes RC, Walley EJ. Efficacy of sliding-scale insulin therapy: a comparison with prospective regimens. Fam Pract Res J. 1994;14(4):313–322. [PubMed] [Google Scholar]
- 22.Hirsch IB. Insulin analogues. N Engl J Med. 2005;352(2):174–183. doi: 10.1056/NEJMra040832. [DOI] [PubMed] [Google Scholar]
- 23.Yeldandi RR, Lurie A, Baldwin D. Comparison of once-daily glargine insulin with twice-daily NPH/regular insulin for control of hyperglycemia in inpatients after cardiovascular surgery. Diabetes Technol Ther. 2006;8(6):609–616. doi: 10.1089/dia.2006.8.609. [DOI] [PubMed] [Google Scholar]
- 24.Lantus® package insert. Kansas City: Aventis Pharmaceuticals; 2005. [Google Scholar]
- 25.Gandhi GY, Nuttall GA, Abel MD, Mullany CJ, Schaff HV, O'Brien PC, Johnson mg, Williams AR, Cutshall SM, Mundy LM, Rizza RA, McMahon MM. Intensive intraoperative insulin therapy versus conventional glucose management during cardiac surgery: a randomized trial. Ann Intern Med. 2007;146(4):233–243. doi: 10.7326/0003-4819-146-4-200702200-00002. [DOI] [PubMed] [Google Scholar]
- 26.NICE-SUGAR Study Investigators. Finfer S, Chittock DR, Su SY, Blair D, Foster D, Dhingra V, Bellomo R, Cook D, Dodek P, Henderson WR, Hébert PC, Heritier S, Heyland DK, McArthur C, McDonald E, Mitchell I, Myburgh JA, Norton R, Potter J, Robinson BG, Ronco JJ. Intensive versus conventional glucose control in critically ill patients. N Engl J Med. 2009;360(13):1283–1297. doi: 10.1056/NEJMoa0810625. [DOI] [PubMed] [Google Scholar]


