Abstract
Background
Hypoglycemia and hyperglycemia during closed-loop insulin delivery based on subcutaneous (SC) glucose sensing may arise due to (1) overdosing and underdosing of insulin by control algorithm and (2) difference between plasma glucose (PG) and sensor glucose, which may be transient (kinetics origin and sensor artifacts) or persistent (calibration error [CE]). Using in silico testing, we assessed hypoglycemia and hyperglycemia incidence during over-night closed loop. Additionally, a comparison was made against incidence observed experimentally during open-loop single-night in-clinic studies in young people with type 1 diabetes mellitus (T1DM) treated by continuous SC insulin infusion.
Methods
Simulation environment comprising 18 virtual subjects with T1DM was used to simulate overnight closed-loop study with a model predictive control (MPC) algorithm. A 15 h experiment started at 17:00 and ended at 08:00 the next day. Closed loop commenced at 21:00 and continued for 11 h. At 18:00, protocol included meal (50 g carbo-hydrates) accompanied by prandial insulin. The MPC algorithm advised on insulin infusion every 15 min. Sensor glucose was obtained by combining model-calculated noise-free interstitial glucose with experimentally derived tran-sient and persistent sensor artifacts associated with FreeStyle Navigator® (FSN). Transient artifacts were obtained from FSN sensor pairs worn by 58 subjects with T1DM over 194 nighttime periods. Persistent difference due to FSN CE was quantified from 585 FSN sensor insertions, yielding 1421 calibration sessions from 248 subjects with diabetes.
Results
Episodes of severe (PG ≤ 36 mg/dl) and significant (PG ≤ 45 mg/dl) hypoglycemia and significant hy-perglycemia (PG ≥ 300 mg/dl) were extracted from 18,000 simulated closed-loop nights. Severe hypoglycemia was not observed when FSN CE was less than 45%. Hypoglycemia and hyperglycemia incidence during open loop was assessed from 21 overnight studies in 17 young subjects with T1DM (8 males; 13.5 ± 3.6 years of age; body mass index 21.0 ± 4.0 kg/m2; duration diabetes 6.4 ± 4.1 years; hemoglobin A1c 8.5% ± 1.8%; mean ± standard deviation) participating in the Artificial Pancreas Project at Cambridge. Severe and significant hypoglycemia during simulated closed loop occurred 0.75 and 17.11 times per 100 person years compared to 1739 and 3479 times per 100 person years during experimental open loop, respectively. Signifi-cant hyperglycemia during closed loop and open loop occurred 75 and 15,654 times per 100 person years, respec-tively.
Conclusions
The incidence of severe and significant hypoglycemia reduced 2300- and 200-fold, respectively, during simu-lated overnight closed loop with MPC compared to that observed during open-loop overnight clinical studies in young subjects with T1DM. Hyperglycemia was 200 times less likely. Overnight closed loop with the FSN and the MPC algorithm is expected to reduce substantially the risk of hypoglycemia and hyperglycemia.
Keywords: artificial pancreas, computer simulation, glucose regulation, risk analysis
Introduction
Since 2000, at least five continuous or semicontinuous glucose monitors have received regulatory ap-proval.1 In combination with continuous subcutaneous insulin infusion (CSII),2 these devices have promoted research toward closed-loop systems, which deliver insulin according to real-time needs, as op-posed to open-loop systems, which lack the real-time responsiveness to changing glucose levels. A closed-loop sys-tem, also called the artificial pancreas, consists of three components: a continuous glucose monitor (CGM) to measure subcutaneous (SC) glucose concentration, a titrating algorithm to compute the amount of insulin to be de-livered, and an insulin pump to deliver computed insulin doses subcutaneously. So far, only a few prototypes have been developed, and testing has been confined to clinical settings.3–8 However, an aggressive concerted effort promises accelerated progress toward home testing of closed-loop systems.
The development, evaluation, and testing of closed-loop systems are time-consuming, costly, and confounded byethical and regulatory issues. Apart from early stage testing in animals such as the dog9,10 or the swine,11 testing in the computer (virtual) environment, also termed in silico testing, is the only other alternative to evaluate and optimize control algorithms outside human studies. Chassin and colleagues has developed a simulation environment and testing methodology12 using a glucoregulatory model developed in a multitracer study13 and evaluated a glucose controller developed within the Adicol Project.14 Another simulator has been reported by Cobelli and associates,15 building on model-independent quantification of glucose fluxes occurring during a meal.16 The latter simulator has been accepted by the Food and Drug Administration to replace animal testing. Patek and coworkers provided guidelines for preclinical testing of control algorithms.17
Closed-loop systems may revolutionize management of type 1 diabetes mellitus (T1DM), but their introduction is likely to be gradual, starting from simpler applications such as hypoglycemia prevention or overnight glucose control and progressing to more complex approaches such as 24/7 glucose control.8 The main reason for gradual deployment is the uncertain risk of hypoglycemia and hyperglycemia, which may arise due to (1) intrinsic overdosing and underdosing of insulin by a control algorithm and (2) persistent and transient differences between plasma glucose (PG) and sensor glucose (SG). The transient differences could be either of physiological origin (SC glucose kinetics) or due to a temporal CGM device artifact. The persistent differences result from the CGM CE. The relatively slow absorption of subcutaneously administered “rapid-acting” insulin analogues and other system imperfections such as pump delivery errors may exacerbate the hypoglycemia and hyperglycemia risks.
As far as we are aware, no quantitative risk assessment of closed-loop insulin delivery has been made and contrasted against risks associated with the standard open-loop therapy. In the present study, we used an in silico approach to quantify incidence and duration of hypoglycemia and hyperglycemia during overnight closed-loop insulin delivery and compared these results against incidence rates observed during open-loop single-night in-clinic studies in young people with T1DM treated by CSII.
Methods
Glucose Control Algorithm
In the present study, we used a control algorithm based on the model predictive control (MPC) paradigm18 to deliver insulin in a closed-loop fashion. Every 15 min, simulated real-time SG was fed into the MPC controller, which calculated SC insulin infusion for the insulin pump. The MPC controller adopts a compartment model of glucose kinetics describing the effect of (1) SC rapid-acting insulin analogue and (2) the carbohydrate (CHO) content of meals on SG excursions.
The glucoregulatory model is initialized using subject's weight, total daily insulin dose, and the basal insulin profile.These values feed into estimates of temporal insulin sensitivity and glucose and insulin distribution volumes. Using a Kalman filter approach, real-time SG measurements are used to update two model parameters: (1) a glucose flux quantifying model misspecification and (2) CHO bioavailability. Several competing models differing in the rate of SC insulin absorption and action and the CHO absorption profile are run in parallel. A computationally efficient, stochastic-based approach is used to derive a combined model that best explains observed SG excursions.19
Following estimation of model parameters, the combined model is used to forecast PG excursions over a 2.5 h prediction horizon. A sequence of SC insulin infusion rates is determined, which approximates the desired PG trajectory, characterized by a slow decline from hyperglycemia and a rapid recovery from hypoglycemia to target glucose, which is set at minimum to 104 mg/dl but is elevated up to 132 mg/dl to take into account inaccuracies of model-based predictions. The first infusion rate from the sequence of SC insulin infusion rates is delivered by the insulin pump subject to safety checks, which can reduce the infusion rate to prevent insulin overdosing. These checks include (1) imposing a maximum infusion rate of two to five times the pre-programmed basal rate, depending on the current SG level, the time since the previous meal(s), and CHO content of meal(s); (2) shutting off insulin delivery at a SG of 77 mg/dl; (3) reducing insulin delivery when SG is decreasing rapidly; and (iv) capping the insulin infusion to the preprogrammed basal rate if a pump occlusion is inferred by the MPC.
For the purposes of the present study, MPC algorithm Version 0.02.02 was used. Earlier versions of the algorithm were used in clinical studies for overnight closed-loop insulin delivery in children and adolescents with T1DM.20–22
Simulation Environment
A simulation environment designed to support the development of closed-loop insulin delivery systems was used.12 The simulation environment is flexible and allows the following components to be defined: a model of glucose regulation, an experimental protocol, a glucose sensing model, an insulin pump model, and outcome metrics. For the present study, we adopted a model of glucose kinetics and insulin action described by Hovorka and colleagues.14,23 Other submodels include the model of SC insulin kinetics, the model of gut absorption, and the model of interstitial glucose (IG) kinetics.23,24
The simulator includes 18 synthetic subjects with T1DM defined by 18 parameter sets, representing the virtual population. A subset of parameters were estimated from experimental data collected in subjects with T1DM,14 and the remaining parameters were drawn from informed probability distributions.13,23 The intersubject variability is addressed through assigning a unique set of parameter values to each individual synthetic subject. The subjects vary, for instance, in their insulin sensitivity to glucose distribution, disposal, and endogenous glucose production.14,23 The virtual subjects are characterized by their daily insulin requirements (0.35 ± 0.14 U/day/kg), insulin-to-CHO ratio (1.7 ± 1.0 U/10 g CHO), and body weight (74.9 ± 14.4 kg). Intraindividual variability of the glucoregulatory system is represented by superimposing oscillations on selected model parameters or adding random interoccasion variability to parameter values. In the present study, sinusoidal oscillations with an amplitude of 5% and a 3 h period were superimposed on nominal values of most model parameters. Each parameter had a different phase generated randomly from a uniform distribution U[0,3 h]. Bioavailability of ingested CHO is characterized by 20% interoccasion variability.
For the purposes of the present study, the glucose measurement error model was derived from experimental data. The SG concentration was obtained as SG(t) = IG(t) × (1 + CE) + D(t), where IG(t) is noise-free IG concentration calculated by the glucoregulatory model and normalized such that, at the steady-state, it is identical to PG; CE is FreeStyle Navigator® (FSN) calibration error (see section FreeStyle Navigator Calibration Error for details); and D(t) is the dropout trace (see section FreeStyle Navigator Dropouts for details). The pump delivery error model was assumed zero mean, uncorrelated, with a constant 5% coefficient of variation for the continuous insulin infusion and the insulin bolus. The simulation environment is implemented in Matlab® (The Mathworks, Natick, MA).
FreeStyle Navigator Dropouts
The FSN CGM system with TRUstart Algorithm (Abbott Diabetes Care, Alameda, CA) was used for the present study. The FSN system occasionally exhibits a nonzero-mean signal artifact referred to here as “dropout,” where certain mechanical perturbation of the sensor results in a momentarily attenuated glucose concentration.25
Dropouts were quantified using data from a study where 58 subjects with T1DM had worn two simultaneous sensors over the course of up to 5 days.26 Values from the two sensors worn simultaneously on each subject were paired every minute. The pointwise difference betweenthe paired glucose readings was computed. To account for residual CE, a segment's pointwise difference was normalized by subtracting the median bias of the segment.
From each pair, only time segments that overlap the nighttime period were used, resulting in 285 nighttime segments. Segments with insufficient data, either due to a sensor starting or ending in the middle of the nighttime session or due to missing data, were excluded. In total, 91 segments were excluded because they contained less than 840 one-minute data points over the 900 min nighttime session span. As a result, 194 nighttime segments were available for simulation purposes.
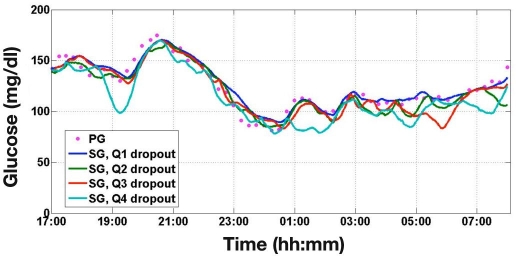
The mean absolute difference in each segment was used to quantify dropout severity, and the 194 nighttime sessions were separated into four quartiles. Ten dropout segments were chosen randomly from each quartile and used in simulation studies. The simulation environment adds the selected dropout segment onto the modeled IG concentration. Simulated CGM traces incorporating dropout data from each quartile are shown in Figure 1.
Figure 1.
Simulated SG traces from the four quartiles of dropout severity alongside the underlying PG trace. Q1 represents negligible dropouts while Q4 represents the most severe dropouts.
FreeStyle Navigator Calibration Error
FreeStyle Navigator CE is defined as CE = (SG – IG)/IG. In these simulations, therefore, a +5% CE means that the reported SG value is consistently 1.05 times higher than expected for a given IG concentration.
The FSN CGM system is designed for 5-day wear, with calibrations nominally scheduled at 1, 2, 10, 24, and 72 h after sensor insertion. For the present study, a morning CGM sensor insertion is assumed for the nighttime-only closed-loop control. Thus, each nighttime, closed-loop session is assumed not to include a scheduled calibration, allowing CE to remain constant for the duration of the night session.
The 116 insertions used to generate the dropout signals in addition to 469 insertions from other studies were used to generate a distribution of the CE. The sensor data set comprised 248 subjects with T1DM or type 2 diabetes mellitus and were a combination of general sensor wear and in-clinic wear that included periods of specific glucose and insulin challenges.
As IG and PG are assumed to be identical at the steady state, CE can be approximated using an alternative definition: CE = (SG – PG)/PG. The CE for a single calibration session was calculated from pairs of SG–reference glucose values where all the SG values were derived from a single calibration and reference glucose used for calibration were excluded from the calculations. Unlike the calculation of dropouts, only reference glucose values measured using the inbuilt blood glucose meter were used. In addition, the real-time calibration of SG values used the FSN CGM system with TRUstart algorithm.
Excluding calibration sessions containing less than ten SG–reference glucose pairs, 585 insertions yielded 1421 calibration sessions. The CE for each session was computed by comparing the median value of the relative difference between SG and reference glucose, and 1421 FSN CEs were generated using 35,200 SG–reference glucose pairs, yielding an average of 25 pairs for every calibration session.
Protocol of Simulation Studies
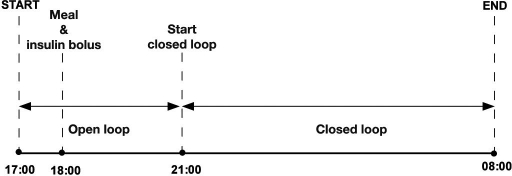
The simulated study was 15 h long, starting at 17:00 and ending at 08:00 the next day (see Figure 2). Plasma glucose at the start of the simulated study was drawn from a lognormal distribution, with a mean of 126 mg/dl constrained to a range from 72 to 180 mg/dl. A meal consisting of 50 g CHO was planned at 18:00 and was accompanied by a prandial insulin bolus. The insulin infusion rate between 17:00 and 21:00 was calculated using the simulation model of a particular virtual subject assuming steady-state conditions at the start of the experiment. At 21:00, the closed-loop glucose control algorithm took over the insulin delivery. The insulin infusion rate was calculated every 15 min on the basis of CGM values, which included the dropout and CE components. Closed-loop control continued until the end of the simulated experiment at 08:00. Rescue CHOs (15 g CHO) were administered at SG values 63 mg/dl (3.5 mmol/liter) or below when confirmed by a PG value of 63 mg/dl or below, simulating a confirmatory finger stick glucose measurement. Correction insulin boluses were not administered at hyperglycemia.
Figure 2.
Protocol of simulated overnight closed-loop study.
The simulation studies were run in batches differing by the level of FSN CE. In total, 25 levels of FSN CEs ranging from −80% to +100% were simulated. The range covering 0% to 60% error was subdivided into 5% steps. The remaining range was spaced 10% apart. Each of the 18 virtual subjects with T1DM was associated with one of 40 randomly selected CGM dropout traces (10 traces from each of the 4 quartiles of increasing severity). This resulted in 720 different combinations and formed a single simulation batch. Each batch was run with all 25 levels of FSN CE, totaling 18,000 simulated overnight studies.
Open Loop Studies
Within the Artificial Pancreas Project at Cambridge, 17 children and adolescents with T1DM treated by CSII for at least 3 months participated in APCam01 study (monitoring study) and APCam03 (exercise study) conducted at the Wellcome Trust Clinical Research Centre, Addenbrooke's Hospital, University of Cambridge, UK. Informed consent was obtained from all study participants or their caregivers. The APCam0120 and APCam0322 clinical studies were originally designed to compare overnight closed-loop control against the standard CSII treatment. In the present analysis, only results from the CSII investigations are reported. The study protocols were approved by the Cambridgeshire 3 Ethics Committee. The subjects' demographic data are shown in Table 1. Four subjects participated in both studies.
Table 1.
Demographic Data of Young Subjects with Type 1 Diabetes Mellitus Participating in APCam01 and APCam03 Stud-ies
| Study | N | Gender (m/f) | Age (years) | Body mass index (kg/m2) | Hemoglobin A1c (%) | Duration of diabetes (years) | Total daily insulin (U/kg/day) |
|---|---|---|---|---|---|---|---|
| APCam01 | 12 | 7/5 | 13.1 ± 4.2 | 21.9 ± 4.3 | 8.7 ± 2.0 | 6.7 ± 4.5 | 0.89 ± 0.27 |
| APCam03 | 9 | 3/6 | 14.4 ± 1.8 | 20.0 ± 2.2 | 7.8 ± 1.0 | 5.8 ± 3.0 | 0.93 ± 0.23 |
In APCam01, on subject's arrival at the Clinical Research Facility at 16:00, a sampling cannula was inserted in a vein of an arm and kept patent with sodium chloride. At 18:00, the subjects ate a self-selected meal (87 ± 23 g CHO) accompanied by prandial insulin (9 ± 5 U; 31% ± 9% of total daily bolus amount) calculated according to the individual insulin-to-CHO ratio and supplemented by correction dose. Plasma glucose was determined every 15 min from 17:00 to 08:00 the next day. At least two weeks before the first study night, the CSII treatment was optimized by a healthcare professional by retrospectively analyzing 72 h of nonreal-time SG data.
In APCam03, at least one week before the study, the subjects attended the Clinical Research Facility and a ramped treadmill protocol was used for the estimation of the peak VO2 as an indicator of the maximum exercise effort. Continuous recording of VO2 with breath-by-breath sampling was taken during the treadmill test and for 2 min during recovery after exercise test termination. Heart rate monitoring was maintained. On the study day, the subjects arrived at 15:00 at the Clinical Research Facility. A sampling cannula was inserted and kept patent with sodium chloride. At 16:00, subjects had a light meal chosen from a list of standardized snacks (45 ± 13g CHO, 12 ± 3 g fat, 14 ± 4 g protein) accompanied by prandial bolus (4 ± 2 U). The subject exercised at 55% VO2max on the treadmill from 18:00 until 18:45, with a rest from 18:20 to 18:25. During exercise, basal insulin was left unmodified or was reduced according to individual guidelines. During the night, the subject's standard insulin pump settings were applied. Plasma glucose was determined every 15 min from 16:00 to 08:00 the next day. If PG dropped below 36 mg/dl, GlucoGel© (BBI Healthcare, UK) was given and the study night terminated.
Data Analysis
Severe and significant hypoglycemia was declared at PG ≤ 36 mg/dl (2.0 mmol/liter) and ≤ 45 mg/dl (2.5 mmol/liter), respectively. These are levels when cognitive behavioral defenses are compromised.27 Significant hyperglycemia was declared at PG ≥ 300 mg/dl (16.7 mmol/liter).
The empirical probability distribution function of FSN CE was calculated from the 1421 calibration sessions. During simulated closed-loop studies, occurrence and duration of hypoglycemia and hyperglycemia based on the simulated PG trace were recorded from 21:00 to 08:00. The probability of hypoglycemia and hyperglycemia events occurring overnight at a given FSN CE is obtained as a product of the probability, ci, of the given FSN CE and the probability of overnight hypoglycemia and hyperglycemia, hi, at the given FSN CE. The overall event probability P is obtained as the sum of these products over the 25 levels of FSN CE, i.e., P = ∑cihi. For APCam01 and APCam03 studies, the overall event probability is obtained as the number of hypoglycemia and hyperglycemia events divided by the number of overnight stays. The overall incidence is obtained as reciprocal to the overall event probability.
During simulated closed-loop studies, mean PG, mean SG, and time-in-target 80–145 mg/dl were calculated between 20:00 and 08:00 to assess the performance of the MPC algorithm at different levels of FSN CE. Values are shown as mean ± standard deviation unless stated otherwise.
Results
Simulated Closed-Loop Studies
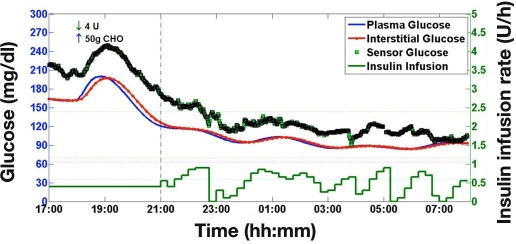
A sample simulation study with a +20% FSN CE using dropout trace from quartile two is shown in Figure 3. Overall, 18,000 simulation studies were performed; 720 simulation studies were run for each of the 25 levels of FSN CE. During simulations, the MPC algorithm was unaware of FSN CE and the extent of the CGM dropout.
Figure 3.
A sample simulation of overnight closed-loop control adopting a +20% FSN CE and a dropout trace from quartile two.
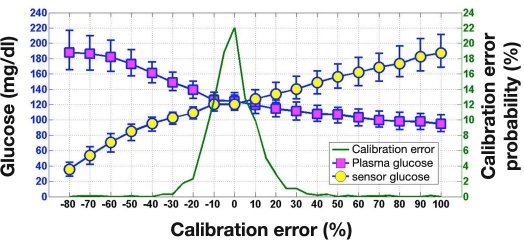
Figure 4 shows PG and SG values obtained simultaneously during simulation studies at FSN CEs ranging from −80% to +100%. As expected, increasing levels of FSN CE result in progressively lower median PG. The MPC algorithm steps up insulin delivery to limit the increase in SG, unaware of progressively increasing gap between sensor and PG. Employing the SG values, the MPC algorithm performs less efficiently at high FSN CE (see Figure 5, which plots time-in-target values). However, employing the PG values, the MPC algorithm achieves 60% or higher time-in-target for FSN CE ranging from −20% to +100%.
Figure 4.
Plasma glucose and SG (median [interquartile range]; N = 720 at each level) during simulated overnight closed-loop studies at different levels of FSN CE. The FSN CE probability distribution function is also shown.
Figure 5.
Time spent in the glucose target range (80 to 145 mg/dl) as quantified using PG and SG (median [interquartile range]; N = 720 at each level) during simulated overnight closed-loop studies at different levels of FSN CE. The FSN CE probability distribution function is also shown.
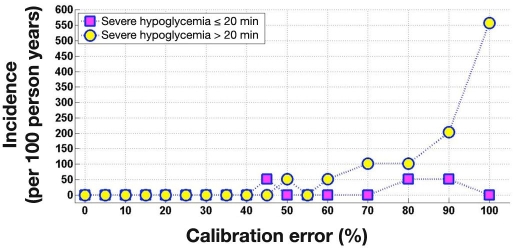
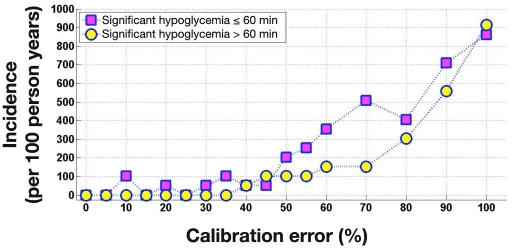
Figures 6 and 7 show the incidence of severe (PG ≤ 36 mg/dl) and significant (PG ≤ 45 mg/dl) hypoglycemia across FSN CE. Severe hypoglycemia did not occur at FSN CE of 40% or lower. Significant hypoglycemia did not occur at FSN CE of 5% or lower. Table 2 breaks down severe hypoglycemia events according to their duration, providing more detailed information. The longest duration of severe and significant hypoglycemia occurred at the highest 100% FSN CE, lasting for 79 and 178 min, respectively.
Figure 6.
Incidence of severe hypoglycemia (≤36 mg/dl) 20 min or shorter and longer than 20 min during simulated overnight closed-loop studies as a function of FSN CE. At each level of FSN CE, 720 simulations were run; occurrence of one event in 720 simulations corresponds to around 50 events per 100 person years.
Figure 7.
Incidence of significant hypoglycemia (≤45 mg/dl) 60 min or shorter and longer than 60 min during simulated overnight closed-loop studies as a function of FSN CE. At each level of FSN CE, 720 simulations were run; occurrence of one event in 720 simulations corresponds to around 50 events per 100 per-son years.
Table 2.
Incidence of Severe Hypoglycemia (PG ≤ 36 mg/dl) per 100 Person Years during Simulated Over-night Closed-Loop Studies at Increasing Levels of Free-Style Navigator Calibration Error
| PG | FSN CE (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| <45 | 45 | 50 | 55 | 60 | 70 | 80 | 90 | 100 | ||
| ≤36 mg/dl | Any duration | a | 51 | 51 | a | 51 | 101 | 152 | 254 | 558 |
| ≤20 min | a | 51 | a | a | a | a | 51 | 51 | a | |
| 20–40 min | a | a | 51 | a | 51 | a | a | 101 | 304 | |
| >40 min | a | a | a | a | a | 101 | 101 | 101 | 254 | |
Severe hypoglycemia event not observed during 720 patient-night simulations for a corresponding level of FSN CE; incidence is less than 51 events per 100 person years.
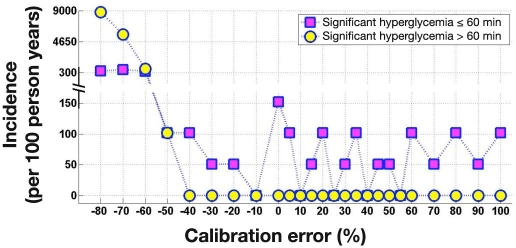
Figure 8 plots the incidence of significant hyperglycemia (PG ≥ 300 mg/dl) for the different levels of FSN CE. Significant hyperglycemia lasting 60 min or less was present at most levels of FSN CE, while events lasting more than 60 min occurred when FSN CE was below −40%. The longest duration of significant hyperglycemia occurred at the −80% FSN CE, lasting for 455 min.
Figure 8.
Incidence of significant hyperglycemia (>300 mg/dl) 60 min or shorter and longer than 60 min during simulated overnight closed-loop studies as a function of FSN CE. At each level of FSN CE, 720 simulations were run; occurrence of one event in 720 simulations corresponds to around 50 events per 100 person years.
FreeStyle Navigator Calibration Error Distribution
The probability distribution of FSN CE generated from 1421 calibration sessions is shown in Figure 4 and is replicated in Figure 5. Approximately 3/4 of the distribution resides within the −10% to +10% range of FSN CE; 35 out of 1421 (2.5%) calibration sessions had FSN CE of 30% or higher. Approximately the same number of sessions (37 out of 1421) had a CE of −30% or lower.
Open-Loop Studies
During APCam01 and APCam03 studies, PG at 20:00 was 207 ± 97 mg/dl. Average overnight PG from 20:00 to 08:00 was 146 ± 65 mg/dl. Time spent in the target glucose range from 20:00 to 08:00 was 40% (18–61%) (median [interquartile range]).
During APCam03, one severe hypoglycemic event was observed (PG ≤ 36 mg/dl). The subject was given GlucoGel, and the study night was terminated; thus the duration of the untreated severe hypoglycemic event cannot be ascertained. Two episodes of significant hypoglycemia were observed (PG ≤ 45 mg/dl): one in study APCam01 over 45 min in duration and another in APCam03 over 75 min in duration, preceding the severe hypoglycemic event above.
Overall Incidence of Hypoglycemia and Hyperglycemia
The overall incidence of hypoglycemia and hyperglycemia during closed-loop and open-loop studies is shown in Table 3.
Table 3.
Incidence of Hypoglycemia and Hyperglycemia per 100 Person Years during Simulated Overnight Closed-Loop Studies and during Overnight Open-Loop Studies APCam01 and APCam03a
| PG | Simulated closed loop (per 100 person years) | Open loop (per 100 person years) | |
|---|---|---|---|
| ≤36 mg/dl | Any duration | 0.75 | 1,739b |
| ≤20 min | 0.21 | — | |
| 20–40 min | 0.18 | — | |
| >40 min | 0.36 | — | |
| ≤45 mg/dl | Any duration | 17.11 | 3,479 |
| ≤60 min | 15.36 | 1,739 | |
| 60–90 min | 1.07 | 1,739 | |
| 90–120 min | 0.43 | c | |
| >120 min | 0.25 | c | |
| ≥300 mg/dl | Any duration | 75.38 | 15,654 |
| ≤60 min | 61.09 | 10,436 | |
| 60–180 min | 5.64 | 3,479 | |
| 180–360 min | 5.82 | 1,739 | |
| >360 min | 2.82 | c | |
Statistics for significant hypoglycemic events (≤45 mg/dl) include severe hypoglycemic events (≤36 mg/dl).
Study stopped when PG was below 36 mg/dl, and thus duration of hypogly-cemia could not be established.
Event not observed during 21 nights of APCam01 and APCam03; incidence is less than 1739 events per 100 person years.
Discussion
The present study suggests that overnight closed loop combining an MPC algorithm and the FSN CGM system is expected to reduce the risk of hypoglycemia and hyperglycemia compared to the standard CSII therapy. Overnight closed-loop insulin delivery is expected to reduce the incidence of (1) severe hypoglycemia 2300-fold, (2) significant hypoglycemia 200-fold, and (3) significant hyperglycemia 200-fold.
These reductions are indicative rather than conclusive given the differences in subject populations; the low incidence of hypoglycemic events, particularly those observed clinically during the CSII treatment; and uncertainties associated with in silico testing. It is important to stress that simulated results need to be verified with clinical data and that efforts should be made to assess true hypoglycemia incidence, which may not be indicated by SG traces alone due to the possible presence of the kinds of persistent and transient sensing errors described in this article. In addition, as average SG levels may be reduced during closed-loop insulin delivery compared to the standard CSII treatment, the presence of transient errors due to dropouts may erroneously suggest an increase in hypoglycemic events, i.e., SG may temporarily drop below the hypoglycemic threshold while PG remains above the threshold.
The incidence calculations are influenced by three main components: the persistent sensing error, the transient sensing error, and insulin misdosing by the control algorithm. In the present study, the assessment of the first two components is based on large observational data sets, providing solid foundations for the incidence calculations. The assessment of the last component is addressed by in silico testing. These simulations are the least strong part of our approach due to limitations of the glucose regulation model but facilitate a rational way to assess performance of a closed-loop system prior to its evaluation in larger clinical studies.
It is argued that the persistent sensing error poses a greater risk of hypoglycemia than the transient sensing error. When SG consistently exceeds PG levels, the risk of undetected sustained hypoglycemia increases; for example,a 100% persistent error translates a PG reading of 50 mg/dl into a SG reading of 100 mg/dl. The persistent error reflects primarily the SG CE. The present study suggests that severe hypoglycemia arises only at an FSN CE of 45% and higher with the study-specific MPC algorithm. This represents 0.845% of the calibration segments. Thus the characterization of tails of the distribution of the SG CE is essential for the correct quantification of the hypoglycemia risk, suggesting that risk calculations can only be carried out once large data sets characterizing the performance of any particular CGM system are available.
From a closed-loop control perspective, transient errors such as dropouts could trigger a momentary reduction or cessation of insulin command due to the perceived hypoglycemia event (present or near future). Such a response might increase the risk of hyperglycemia. Closed-loop systems with a strong predictive and/or derivative term might generate a momentarily exaggerated insulin command when a rapid dropout recovery occurs. If PG is already low, then this transient response could increase the risk of hypoglycemia. The effect of dropouts is illustrated in Figure 1. Four simulated SG traces with different levels of dropout severity are shown alongside the underlying PG measurements.
In the present study, the transient error was obtained by taking the difference of two SG traces and correcting them for CE. Methodologically, this approach overestimates the transient error as, by definition, when subtracting two SG traces, the variances of the two transient errors presented in the component SG traces add up. However, a visual inspection of simultaneously observed SG traces in quartiles two to four indicates that the transient error in one of the two SG traces typically dominates, justifying our pragmatic approach, which preserves important characteristics such as dropout clustering.
Prior investigation of the validity of the predictions made by in silico testing increases the confidence in the incidence calculations. We previously validated the virtual population of 18 subjects with T1DM by simulating a 15 hclinical study with an MPC algorithm.28 The protocol of the simulated study reflected the APCam01 study conducted in 12 children and adolescents with T1DM.20 Premeal PG during the simulated study was designed to match that of the real study (177 ± 56 versus 171 ± 67 mg/dl, p = not significant; unpaired t test). Sensor glucoseat the start of closed-loop control (220 ± 72 versus 191 ± 54 mg/dl, p = NS) and mean overnight SG (137 ± 22 versus 141 ± 25 mg/dl, p = not significant) were similar during simulated and real studies. Time spent in the target glucose range 80 to 145 mg/dl was not significantly different at 69% (62–78%) versus 63% (49–78%) (median [interquartile range], p = not significant]. Kovatchev and associates' low blood glucose index [0.5 (0.2–0.9) versus 0.3 (0.0–1.0), p = not significant] and high blood glucose index [3.4 (1.3–6.8) versus 3.7 (0.6–6.8), p = not significant]29 were also similar during the real and simulated studies, supporting the validity of glucose predictions at low and high glucose levels.
We further accessed the validity of in silico predictions by simulating open-loop studies. First, optimum prandial and optimum basal insulin to achieve and maintain PG at 108 mg/dl were determined for the 18 virtual subjects during a 15 h simulated study commencing at 17:00, with a 50 g CHO meal planned at 18:00. Then basal insulin was increased by 20% and an identical study design was simulated. Additional simulations were performed, with basal insulin increased by 55% and 85%. These increases in the basal insulin delivery corresponded to differences between the average delivered insulin rate and the average insulin rate preprogrammed on the insulin pump during 33 overnight closed-loop studies in young people with T1DM treated by CSII.30 In these 33 closed-loop studies, a 20% overestimation of basal insulin was observed in three studies, a 55% overestimation in four studies, and an 85% overestimation in one study.
At the 20% overestimation of basal insulin, the simulations yielded no severe hypoglycemia and one significant hypoglycemia in the 18 virtual subjects. At the 55% overestimation, five and three hypoglycemia events were observed. At the 85% overestimation, eight and two events occurred. This indicates the incidence of severe hypoglycemia during simulated studies at 1720 per 100 person years, which tallies extremely well with a corresponding incidence of 1739 per 100 person years recorded during “true” open loop studies (see Table 3). The incidence of significant hypoglycemia during simulations was 1044 per 100 person years, which is less but still comparable to that observed experimentally at 3479 per 100 person years; the difference in the incidence rates corresponds to two significant hypoglycemia events over 33 nights. Overall, these results suggest that in silico simulations provide acceptable predictions of hypoglycemia incidence during open-loop studies, supporting the validity of in silico predictions during closed-loop studies.
The MPC algorithm used in the present study has important in-built safety features. It uses the pre-programmed insulin infusion rate as an initial estimate of the insulin needed to achieve normoglycemia. If SG increases, the MPC algorithm controller steps up insulin delivery but does so cautiously and at the expense of suboptimal SG levels. This is evident in Figures 4 and 5, which demonstrate that, with increasing levels of FSN CE, the mean SG concentration increases and the time-in-target assessed with the use of SG decreases. This design feature of the MPC algorithm reduces the impact of FSN CE on the risk of hypoglycemia.
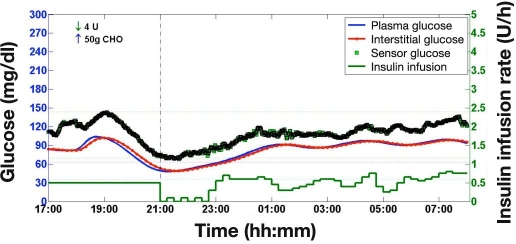
The simulation study design included a relatively small evening meal compared to the body weight of the virtual subjects. Additionally, premeal PG was constrained tolevels between 72 and 180 mg/dl. In combination, these two study design aspects limit postprandial hyperglycemia excursions, which are expected to be more pronounced after larger meal sizes and at elevated premeal PG values. Conversely, prandial insulin overdosing due to overestimation of the meal size may result in early postprandial hypoglycemia, which cannot be prevented by closed-loop insulin delivery even if insulin infusion is stopped. Some of the episodes of hypoglycemia observed in the present study were directly attributable to prandial insulin overdosing prior to the start of closed-loop control. An example is shown in Figure 9, where the insulin overdelivery is confounded by a +30% FSN CE. Hypoglycemia occurred prior to the start of the closed-loop session. Although insulin delivery virtually stopped at the start of closed loop, PG and SG continued to decrease for another 30 min. The hypoglycemia event remained undetected, as SG did not reach the hypoglycemia threshold of 63 mg/dl.
Figure 9.
A sample simulation showing hypoglycemia due to prandial insulin overdosing. Prandial insulin accompanied meal at 18:00. Closed loop started at 21:00. Sensor glucose was obtained using a +30% FSN CE and a dropout trace from quartile two. Hypoglycemia occurred before the start of the closed-loop session and continued to worsen for another 30 min after the start of closed loop although insulin delivery was virtually turned off. Hypoglycemia was undetected, as SG did not reach the hypoglycemia threshold of 63 mg/dl. FreeStyle Navigator CE at +30% or higher is estimated to occur 2.5% of the time, assuming no recalibration is performed between scheduled calibrations.
The use of CGM alone is expected to reduce the hypoglycemia and hyperglycemia risks as observed in the Juvenile Diabetes Research Foundation CGM trial.31 The observed improvements are clinically important but lack the scale offered by the overnight closed-loop approach. However, even with the overnight closed-loop approach, the risk of hypoglycemia and hyperglycemia is not eliminated. The duration of significant and severe hypoglycemia during simulation studies is limited to 1 and 3 h, which is slightly less than the 2–4 h of SG-documented hypoglycemia that has been reported prior to seizures.32
The FSN CE distribution shown in Figures 4 and 5 was constructed assuming that only the five FSN scheduled calibrations are performed. If a manual recalibration was performed to rectify excessive CEs that would have been evident when SG was compared against a finger stick reading, the risk of hypoglycemia and hyperglycemia during overnight closed loop could be further reduced.
More detailed information about transient and persistent sensing errors is required to determine if the present results may be transferable to other commercially available CGM systems.33 Transferability to other control algorithms is uncertain given the wide range of control approaches.
In conclusion, overnight closed loop using an MPC algorithm and real-time glucose sensing by the FSN system may offer a 200- to 2300-fold reduction of the hypoglycemia and hyperglycemia incidence. This suggests that ex-isting continuous glucose sensing technologies facilitate safe closed-loop insulin delivery, although confirma-tion in large clinical studies is required.
Acknowledgments
We are thankful to John Lum for his valuable input.
Abbreviations
- CE
calibration error
- CGM
continuous glucose monitor
- CHO
carbohydrate
- CSII
continuous subcutaneous insulin infusion
- FSN
FreeStyle Navigator
- IG
interstitial glucose
- MPC
model predictive control
- PG
plasma glucose
- SC
subcutaneous
- SG
sensor glucose
- T1DM
type 1 diabetes mellitus
References
- 1.Klonoff DC. Continuous glucose monitoring: roadmap for 21st century diabetes therapy. Diabetes Care. 2005;28(5):1231–1239. doi: 10.2337/diacare.28.5.1231. [DOI] [PubMed] [Google Scholar]
- 2.Pickup JC, Keen H, Parsons JA, Alberti KG. Continuous subcutaneous insulin infusion: an approach to achieving normoglycaemia. Br Med J. 1978;1(6107):204–207. doi: 10.1136/bmj.1.6107.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hovorka R. Continuous glucose monitoring and closed-loop systems. Diabet Med. 2006;23(1):1–12. doi: 10.1111/j.1464-5491.2005.01672.x. [DOI] [PubMed] [Google Scholar]
- 4.Steil GM, Rebrin K. Closed-loop insulin delivery—what lies between where we are and where we are going? Expert Opin Drug Deliv. 2005;2(2):353–362. doi: 10.1517/17425247.2.2.353. [DOI] [PubMed] [Google Scholar]
- 5.Shalitin S, Phillip M. Closing the loop: combining insulin pumps and glucose sensors in children with type 1 diabetes mellitus. Pediatr Diabetes. 2006;7(Suppl 4):45–49. doi: 10.1111/j.1399-543X.2006.00170.x. [DOI] [PubMed] [Google Scholar]
- 6.Renard E, Costalat G, Chevassus H, Bringer J. Artificial beta-cell: clinical experience toward an implantable closed-loop insulin delivery system. Diabetes Metab. 2006;32(5 Pt 2):497–502. doi: 10.1016/s1262-3636(06)72802-6. [DOI] [PubMed] [Google Scholar]
- 7.Hovorka R, Wilinska ME, Chassin LJ, Dunger DB. Roadmap to the artificial pancreas. Diabetes Res Clin Pract. 2006;74(Suppl 2):S178–S182. [Google Scholar]
- 8.Hovorka R. The future of continuous glucose monitoring: closed loop. Curr Diabetes Rev. 2008;4(3):269–279. doi: 10.2174/157339908785294479. [DOI] [PubMed] [Google Scholar]
- 9.Panteleon AE, Loutseiko M, Steil GM, Rebrin K. Evaluation of the effect of gain on the meal response of an automated closed-loop insulin delivery system. Diabetes. 2006;55(7):1995–2000. doi: 10.2337/db05-1346. [DOI] [PubMed] [Google Scholar]
- 10.Rebrin K, Fischer U, von Woedtke T, Abel P, Brunstein E. Automated feedback control of subcutaneous glucose concentration in diabetic dogs. Diabetologia. 1989;32(8):573–576. doi: 10.1007/BF00285330. [DOI] [PubMed] [Google Scholar]
- 11.El-Khatib FH, Jiang J, Damiano ER. Adaptive closed-loop control provides blood-glucose regulation using dual subcutaneous insulin and glucagon infusion in diabetic swine. J Diabetes Sci Technol. 2007;1(2):181–192. doi: 10.1177/193229680700100208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chassin LJ, Wilinska ME, Hovorka R. Evaluation of glucose controllers in virtual environment: methodology and sample application. Artif Intell Med. 2004;32(3):171–181. doi: 10.1016/j.artmed.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 13.Hovorka R, Shojaee-Moradie F, Carroll PV, Chassin LJ, Gowrie IJ, Jackson NC, Tudor RS, Umpleby AM, Jones RH. Partitioning glucose distribution/transport, disposal, and endogenous production during IVGTT. Am J Physiol Endocrinol Metab. 2002;282(5):E992–E1007. doi: 10.1152/ajpendo.00304.2001. [DOI] [PubMed] [Google Scholar]
- 14.Hovorka R, Chassin LJ, Wilinska ME, Canonico V, Akwi JA, Federici MO, Massi-Benedetti M, Hutzli I, Zaugg C, Kaufmann H, Both M, Vering T, Schaller HC, Schaupp L, Bodenlenz M, Pieber TR. Closing the loop: the Adicol experience. Diabetes Technol Ther. 2004;6(3):307–318. doi: 10.1089/152091504774197990. [DOI] [PubMed] [Google Scholar]
- 15.Dalla Man C, Raimondo DM, Rizza RA, Cobelli C. GIM, simulation software of meal glucose—insulin model. J Diabetes Sci Technol. 2007;1(3):323–330. doi: 10.1177/193229680700100303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Basu R, Dalla Man C, Campioni M, Basu A, Klee G, Toffolo G, Cobelli C, Rizza RA. Effects of age and sex on postprandial glucose metabolism: differences in glu-cose turnover, insulin secretion, insulin action, and hepatic insulin extraction. Diabetes. 2006;55(7):2001–2014. doi: 10.2337/db05-1692. [DOI] [PubMed] [Google Scholar]
- 17.Patek SD, Bequette BW, Breton M, Buckingham BA, Dassau E, Doyle FJ, III, Lum J, Magni L, Zisser H. In silico preclinical trials: methodology and engineering guide to closed-loop control in type 1 diabetes mellitus. J Diabetes Sci Technol. 2009;3(2):269–282. doi: 10.1177/193229680900300207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bequette BW. A critical assessment of algorithms and challenges in the development of a closed-loop artificial pancreas. Diabetes Technol Ther. 2005;7(1):28–47. doi: 10.1089/dia.2005.7.28. [DOI] [PubMed] [Google Scholar]
- 19.Mazor E, Averbuch A, Bar-Shalom Y, Dayan J. Interacting multiple model methods in target tracking: A survey. IEEE Trans Aerosp Electron Syst. 1998;34(1):103–123. [Google Scholar]
- 20.Hovorka R, Acerini CL, Allen J, Chassin LJ, Larsen AM, De Palma A, Wilinska ME, Dunger DB. Overnight sc-sc closed-loop control improves glucose control and reduces risk of hypoglycaemia in children and adolescents with type 1 diabetes. Diabetes. 2008;57(Suppl 1):A22. [Google Scholar]
- 21.Hovorka R, Acerini CL, Allen J, Chassin LJ, Larsen AM, Mundt D, De Palma A, Wilinska ME, Dunger DB. Good overnight closed-loop glucose control in children and adolescents with type 1 diabetes following ingestion of large, rapidly and slowly absorbed evening meat. Diabetologia. 2008;51(Suppl 1):181. [Google Scholar]
- 22.Hovorka R, Acerini CL, Allen JM, Chassin LJ, Ekelund U, Elleri D, Larsen AM, Nodale M, Wilinska ME, Dunger DB. Overnight closed-loop delivery following afternoon exercise in adolescents with type 1 diabetes (T1D) http://www.abstractserver.com/attd2009/planner/sp.php?go=abstract&action=abstract_show&absno=168&ATTD2009=kl79dn1k31jspnql6a6i5fjdt4&ATTD2009=kl79dn1k31jspnql6a6i5fjdt4. Accessed August 25 2009.
- 23.Hovorka R, Canonico V, Chassin LJ, Haueter U, Massi-Benedetti M, Orsini Federici M, Pieber TR, Schaller HC, Schaupp L, Vering T, Wilinska ME. Nonlinear model predictive control of glucose concentration in subjects with type 1 diabetes. Physiol Meas. 2004;25(4):905–920. doi: 10.1088/0967-3334/25/4/010. [DOI] [PubMed] [Google Scholar]
- 24.Wilinska ME, Chassin LJ, Schaller HC, Schaupp L, Pieber TR, Hovorka R. Insulin kinetics in type-1 diabetes: continuous and bolus delivery of rapid acting insulin. IEEE Trans Biomed Eng. 2005;52(1):3–12. doi: 10.1109/TBME.2004.839639. [DOI] [PubMed] [Google Scholar]
- 25.McGarraugh G, Bergenstal R. Detection of hypoglycemia with continuous interstitial and traditional blood glucose monitoring using the FreeStyle navigator continuous glucose monitoring system. Diabetes Technol Ther. 2009;11(3):145–150. doi: 10.1089/dia.2008.0047. [DOI] [PubMed] [Google Scholar]
- 26.Weinstein RL, Schwartz SL, Brazg RL, Bugler JR, Peyser TA, McGarraugh GV. Accuracy of the 5-day FreeStyle Navigator continuous glucose monitoring system: comparison with frequent laboratory reference measurements. Diabetes Care. 2007;30(5):1125–1130. doi: 10.2337/dc06-1602. [DOI] [PubMed] [Google Scholar]
- 27.Cryer PE. The barrier of hypoglycemia in diabetes. Diabetes. 2008;57(12):3169–3176. doi: 10.2337/db08-1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wilinska ME, Acerini CL, Allen JM, Chassin LJ, Dunger DB, Hovorka R. Validation of simulation environment utilizing clinical data collected during overnight closed-loop glucose control in children and adolescents with type 1 diabetes. J Diabetes Sci Technol. 2009;3(2):A175. [Google Scholar]
- 29.Kovatchev BP, Cox DJ, Gonder-Frederick LA, Young-Hyman D, Schlundt D, Clarke W. Assessment of risk for severe hypoglycemia among adults with IDDM: validation of the low blood glucose index. Diabetes Care. 1998;21(11):1870–1875. doi: 10.2337/diacare.21.11.1870. [DOI] [PubMed] [Google Scholar]
- 30.Hovorka R, Acerini CL, Allen JM, Chassin LJ, Kollman C, Elleri D, Harris J, Hovorka T, Larsen AMF, Nodale M, De Palma A, Wilinska ME, Xing DY, Dunger DB. Overnight closed-loop insulin delivery in children and adolescents with type 1 diabetes: towards home testing. Diabetes. 2009;58(Suppl 1):A54. [Google Scholar]
- 31.Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group. Tamborlane WV, Beck RW, Bode BW, Buckingham B, Chase HP, Clemons R, Fiallo-Scharer R, Fox LA, Gilliam LK, Hirsch IB, Huang ES, Kollman C, Kowalski AJ, Laffel L, Lawrence JM, Lee J, Mauras N, O'Grady M, Ruedy KJ, Tansey M, Tsalikian E, Weinzimer S, Wilson DM, Wolpert H, Wysocki T, Xing D. Continuous glucose monitoring and intensive treatment of type 1 diabetes. N Engl J Med. 2008;359(14):1464–1476. doi: 10.1056/NEJMoa0805017. [DOI] [PubMed] [Google Scholar]
- 32.Buckingham B, Wilson DM, Lecher T, Hanas R, Kaiserman K, Cameron F. Duration of nocturnal hypoglycemia before seizures. Diabetes Care. 2008;31(11):2110–2112. doi: 10.2337/dc08-0863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kovatchev B, Anderson S, Heinemann L, Clarke W. Comparison of the numerical and clinical accuracy of four continuous glucose monitors. Diabetes Care. 2008;31(6):1160–1164. doi: 10.2337/dc07-2401. [DOI] [PMC free article] [PubMed] [Google Scholar]