Abstract
New effort has been made to develop closed-loop glucose control, using subcutaneous (SC) glucose sensing and continuous subcutaneous insulin infusion (CSII) from a pump, and a control algorithm. An approach based on a model predictive control (MPC) algorithm has been utilized during closed-loop control in type 1 diabetes patients. Here we describe the preliminary clinical experience with this approach.
Six type 1 diabetes patients (three in each of two clinical investigation centers in Padova and Montpellier), using CSII, aged 36 ± 8 and 48 ± 6 years, duration of diabetes 12 ± 8 and 29 ± 4 years, hemoglobin A1c 7.4% ± 0.1% and 7.3% ± 0.3%, body mass index 23.2 ± 0.3 and 28.4 ± 2.2 kg/m2, respectively, were studied on two occasions during 22 h overnight hospital admissions 2–4 weeks apart. A Freestyle Navigator® continuous glucose monitor and an OmniPod® insulin pump were applied in each trial. Admission 1 used open-loop control, while admission 2 employed closed-loop control using our MPC algorithm.
In Padova, two out of three subjects showed better performance with the closed-loop system compared to open loop. Altogether, mean overnight plasma glucose (PG) levels were 134 versus 111 mg/dl during open loop versus closed loop, respectively. The percentage of time spent at PG > 140 mg/dl was 45% versus 12%, while postbreakfast mean PG was 165 versus 156 mg/dl during open loop versus closed loop, respectively. Also, in Montpellier, two patients out of three showed a better glucose control during closed-loop trials. Avoidance of nocturnal hypoglycemic excursions was a clear benefit during algorithm-guided insulin delivery in all cases.
This preliminary set of studies demonstrates that closed-loop control based entirely on SC glucose sensing and insulin delivery is feasible and can be applied to improve glucose control in patients with type 1 diabetes, although the algorithm needs to be further improved to achieve better glycemic control.
Keywords: continuous glucose monitoring, control algorithm, glucose sensor, insulin pump, modeling, simulation, type 1 diabetes
Introduction
Serious attempts to substitute in a fully artificial way the endocrine function of the pancreas started with the introduction of systems in which metabolic control was achieved by measuring intravenous glucose concentration and delivering the appropriate amounts of insulin intravenously.1–4
Subsequently, systems were introduced in which glucose was sampled intravenously and insulin was administered intraperitoneally.5–7 With the advent of small subcutaneous (SC) continuous glucose monitoring (CGM) devices, new effort has been made to develop SC–SC closed-loop control, using CGM coupled with an insulin infusion pump, and a control algorithm.8–10
To date, the only two clinical trials employing SC CGM and insulin delivery have been based on proportional-integrative-derivative (PID) control,9–11 an approach thus based on an algorithm that is purely reactive to blood glucose (BG) changes. This way of proceeding tends to overcompensate for postprandial BG peaks with a risk of subsequent hypoglycemia.
Moreover, PID is rather insensitive to the time delay inherent to the SC routes of glucose sensing and insulin delivery. Indeed, SC CGM measures glucose concentration in the interstitium, which is in equilibrium with BG presumably via diffusion processes.12,13 To account for the gradient between BG and interstitial glucose (IG), CGM devices are calibrated with capillary glucose, but calibration itself cannot eliminate the time lag due to BG-to-IG glucose transport and device processing time (instrument delay), which is typically of 5–15 minutes.14 Furthermore, before affecting glucose metabolism, subcutaneously injected insulin must be delivered into systemic circulation; this process can take up to one hour9 even with the rapid-acting insulin analogues.
Because of the limitations of PID, model-based18 predictive control9,15–17 (MPC) has gained favor for the following reasons: (1) a personalized model adapts better to individual glucose dynamics and may reduce the time delays inherent to SC glucose monitoring and SC insulin infusion, (2) a model-based approach allows for the use of meal or hypoglycemia detection methods,19 and (3) a model can “learn” from daily life events (e.g., timing of meals) and then optimize the response to a subsequent meal using this information.20–22
A new MPC algorithm for type 1 diabetes has been proposed, and its performance has been assessed in silico using a computer simulator based on a previously reported model of glucose metabolism,23 an approach that has been accepted by the Food and Drug Administration as a substitute to animal trials.18
However, to date, the testing of the MPC algorithm has never been performed in vivo, inside a clinical trial.
Here we present the results of pilot studies, performed at the Divisione di Malattie del Metabolismo of the University of Padova and at the Endocrinology Department of Montpellier University Hospital, on two sets of three type 1 diabetes subjects.
The main objective of this article is to present to the scientific community the experimental protocol used in this pilot study and to underline positive results but also cite some critical issues that arose during the trials. However, given the reduced number of patients studied to date, the present study does not aim to assess the performance of the MPC algorithm statistically nor to compare MPC versus other control strategy.
Methods
Subjects
In Padova, we studied three (two males, one female) type 1 diabetes patients using continuous subcutaneous insulin infusion. Their age was 36 ± 8 (mean ± standard error of the mean) years, duration of diabetes was 12 ± 8 years, hemoglobin A1c (HbA1c) at admission was 7.4% ± 0.1%, and body mass index (BMI) was 23.2 ± 0.3 kg/m2. Patients recruited at the Montpellier center had the following characteristics: three males, aged 48 ± 6 years, duration of diabetes of 29 ± 4 years, HbA1c of 7.3% ± 0.3%, and BMI of 28.4 ± 2.2 kg/m2 (Table 1). Criteria for inclusion were 21 years of age or older, have type 1 diabetes for at least 2 years, use an insulin pump, and willing to use lispro insulin (Humalog®, Eli-Lilly, Indianapolis, IN) for the duration of the inpatient study. Patients with insulin allergy, uncontrolled retinopathy, cardiovascular disease, hepatic or renal insufficiency, pregnant or lactating women and those intending to become pregnant, using a medication that significantly impacts glucose metabolism (e.g., oral steroids), or using a device that may pose electromagnetic compatibility issues and/or radiofrequency interference with the FreeStyle Navigator® CGM device were excluded.
Table 1.
Clinical Characteristics of Patients Evaluated
| Patient | Sex | Age (years) | Duration of diabetes (years) | HbA1c (%) | BMI (kg/m2) |
|---|---|---|---|---|---|
| PD-001 | F | 50 | 6 | 7.4 | 23.7 |
| PD-002 | M | 33 | 28 | 7.7 | 22.8 |
| PD-003 | M | 24 | 2 | 7.2 | 23.1 |
| MPL-001 | M | 41 | 26 | 7.6 | 31.0 |
| MPL-002 | M | 43 | 24 | 6.6 | 24.1 |
| MPL-003 | M | 59 | 38 | 7.6 | 30.1 |
Procedure
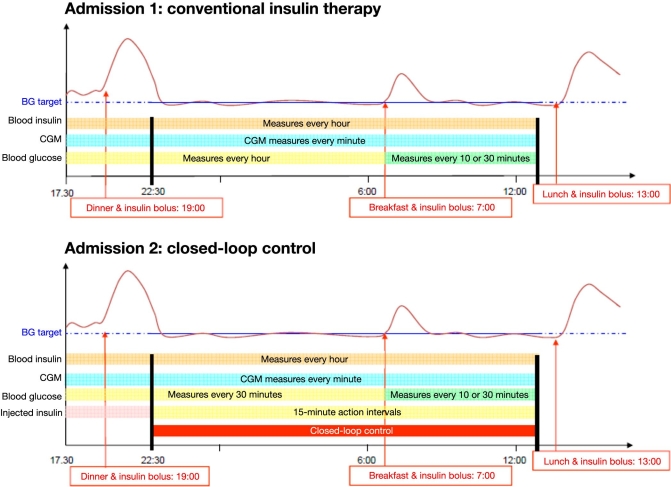
Each patient had two 22 h overnight hospital admissions separated by 2–4 weeks (see Figure 1). Each hospital admission began at 4:00 PM and ended at 1:00 PM on the following day. Subjects ate dinner (at 7:00 PM) and lunch with identical carbohydrate (CHO) content during both admissions following their usual habits and had identical morning mixed meal tests of Ensure Plus® (Abbott Nutrition) containing 50 g CHO, 11 g fat, and 13 g protein at 7:00 AM. In addition, 3 g of [6,6-2H2] glucose was added to the breakfast in order to segregate endogenous from exogenous glucose and to facilitate future modeling. Two days before each admission, two Freestyle Navigator CGM devices (Abbott Diabetes Care, Alameda, CA) were inserted. One major advantage of the device is that it can display a signal from measured IG every minute and communicate the estimated plasma glucose (PG) value in real time via a device provided by the manufacturer (“cradle”) to an external central processing unit, which can also contain the implementation of the control algorithm. The glucose sensors were inserted into fatty tissue 5 mm under the skin on the lower abdomen and remained attached until the end of the admission. The CGM signal was calibrated according to the manufacturer's guidelines with a finger stick BG using a glucometer at hours 10, 12, 24, and 72 after insertion. Additional finger sticks may be required if the initial calibration fails. At the time of admission, the patient's pump was substituted with an OmniPod® insulin pump (Insulet Corp., Boston, MA), which was used for BG control throughout the hospital studies.
Figure 1.
Design of the study.
Admission 1 used open-loop control with the subjects following their usual insulin routine. As shown in Figure 1, CGM data were collected every minute until the end of the experimental session. A 22 gauge intravenous line was placed in a forearm or antecubital vein for sampling of PG and insulin, which were measured with a Beckman glucose analyzer in Padova and a YSI Stat 2300 Plus in Montpellier, every hour from 5:30 PM to 6.30 AM, and they were measured more frequently after breakfast (at 7:00 AM, 7:10 AM, 7:20 AM, 7:30 AM, 8:00 AM, 8:30 AM, 9:00 AM, 9:30 AM, 10:00 AM, 10:30 AM, 11:00 AM, 11:30 AM, 12:00 PM, and 12:30 PM).
As for admission 1, two days before each admission, two Freestyle Navigator CGM devices were inserted. At the beginning of admission 2, the CGM system that performed better during the previous two days was designated as primary and connected to the PC using the “cradle;” this allowed us to transfer CGM data to the control algorithm every minute, running on the PC. Predinner insulin bolus (before closed loop starts) was controlled by the attending physician based on patient diary and CHO intake. The MPC closed-loop control started at 10:30 PM. Based on the CGM data automatically transferred to the PC, the control algorithm suggested insulin boluses every 15 min, which, if accepted, were programmed into the insulin pump by the attending physician. This was done for safety reasons, allowing the physician to override insulin delivery at any time if it was considered dangerous for the patient. Thus the control was not fully automated but was still a closed-loop control, even if “physician supervised.” Reference BG was measured every 30 min, using a Beckman glucose analyzer or a YSI, from 5:30 PM to 6:30 AM while they are measured more frequently after breakfast (at 7.:00 AM,7:10 AM, 7:20 AM, 7:30, 8:00 AM, 8:30 AM, 9:00 AM, 9:30 AM, 10:00 AM, 10:30 AM, 11:00 AM, 11:30 AM, 12:00 PM, and 12:30 PM); the protocol also required switching to more frequent sampling of 15 min reference BG sampling if hypoglycemia occurred or was imminent. Fast-acting CHO (usually glucose tablet) was given when reference BG fell below 70 mg/dl, regardless of the readings of the two CGM devices.
The design of the protocol, with two experimental sessions under the same experimental conditions (time and CHO content of the meals) apart from insulin delivery (standard therapy during admission 1 versus closed-loop controlled insulin delivery during admission 2), has two main advantages. First, it allows a comparison of algorithm performance against the standard therapy adopted in the same subjects, thus avoiding possible artifacts due to intersubject variability. Then the datacollected during the first admission allowed for personalizing, at least in part, the MPC algorithm (at least 2 weeks passed between the first and the second admission in order to have time to analyze the blood samples and provide measurements of glucose and insulin concentrations).
The adopted MPC algorithm is the unconstrained linear MPC proposed in Reference 17, where all the mathematical details can be found, designed, and tested preclinically using independently developed and validated computer simulation environment.18 Briefly, the MPC suggests the optimal amount of insulin to be infused in the next 15 min based on the CGM data measured in the past 45 min, the insulin administered in the past 45 min, and the predicted CGM in the future 240 min, this last element being obtained by a mean model of the system and future meal information. The use of an extended prediction horizon guarantees a better regulation even without very accurate predictions. The suggested amount of insulin is obtained by minimizing the difference between the predicted glucose and the target while penalizing the use of large amounts of insulin. The balance between the two is weighted by a parameter, q, which represents the “aggressiveness” of the control algorithm: high values of q allow the algorithm to administer more insulin in order to rapidly reach the target BG; conversely, with low values of q, large amounts of insulin are discouraged. The “aggressiveness” of the MPC algorithm is calculated using clinical parameters of the patient, such as individual body weight (kg), average total daily insulin use, CHO ratio, and premeal PG and insulin concentrations. Some advantages of the considered MPC scheme are that it is easily implementable on any hardware platform because real-time optimization is avoided and the individualization is based on a few well-known parameters of the patient.
Results
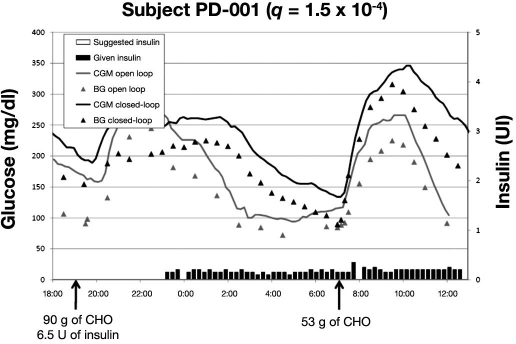
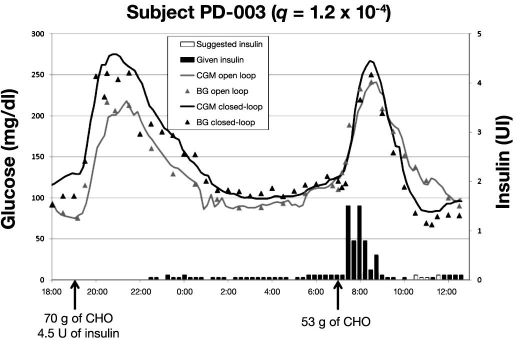
Figures 2–4 represent the typical glucose profiles during both the open-loop and the closed-loop experiments and the insulin infusions during closed-loop control in the 3 patients studied in Padova. The most remarkable finding is that, during closed-loop control, nocturnal hypoglycemia was avoided. The second important point is that, compared to open-loop, closed-loop allows for tighter glycemic control during the night. This better glycemic control during closed-loop experiment was independent from baseline insulin.
Figure 2.
Data of first patient studied in Padova; BG and CGM measured during admission 1 (open loop) and admission 2 (closed loop). Suggested and given insulin boluses relate to closed-loop admission.
Figure 4.
Data of third patient studied in Padova; BG and CGM measured during admission 1 (open loop) and admission 2 (closed loop). Suggested and given insulin boluses relate to closed-loop admission.
The mean overnight PG values were 134 mg/dl (99, 141, 163) and 111 mg/dl (97, 106, 130) during open and closed loop, respectively. The percentage of time spent at PG > 140 mg/dl was 45% during open loop and 12% during closed loop, respectively. As far as the postbreakfast control, during the open loop, the mean PG was 165 mg/dl (135, 163, 197), whereas during closed-loop, it was 156 mg/dl (103, 144, 219), respectively.
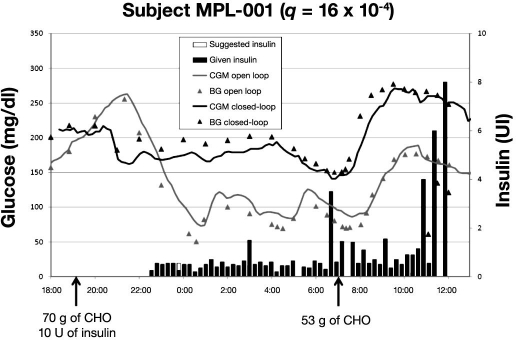
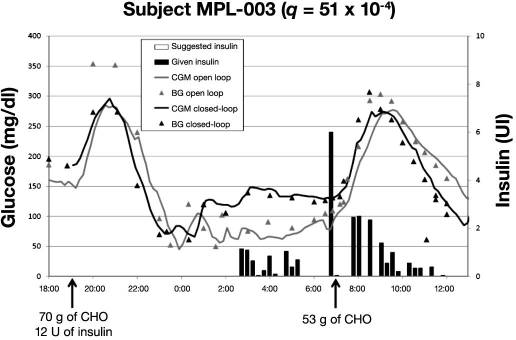
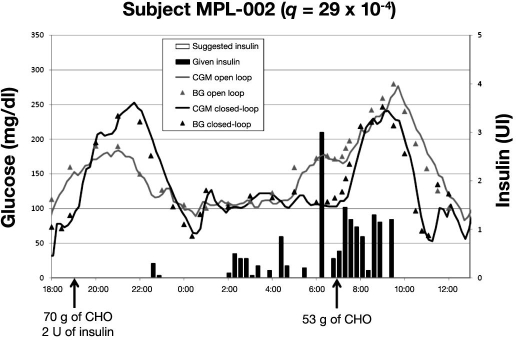
Figures 5–7 present BG profiles of the 3 patients investigated in Montpellier during open- and closed-loop trials, as well as insulin delivery during closed-loop trials. Similar to the observations in Padova, the main benefit of algorithm-guided insulin delivery was the avoidance of hypoglycemic deviations at night. However, due to a too weakly aggressive insulin delivery in patient MPL-001 (q was 16 × 10−4; in all likelihood, a higher q value would have provided a better glycemic control), mean BG was kept too high at night during the closed-loop trial. Of note, the stability of nighttime glucose levels during closed-loop trials was much better in all three cases than with usual insulin delivery rates.
Figure 5.
Data of first patient studied in Montpellier; BG and CGM measured during admission 1 (open loop) and admission 2 (closed loop). Suggested and given insulin boluses relate to closed-loop admission.
Figure 7.
Data of third patient studied in Montpellier; BG and CGM glucose measured during admission 1 (open loop) and admission 2 (closed loop). Suggested and given insulin boluses relate to closed-loop admission.
Figure 6.
Data of second patient studied in Montpellier; BG and CGM measured during admission 1 (open loop) and admission 2 (closed loop). Suggested and given insulin boluses relate to closed-loop admission.
Overnight mean BG levels were 91, 137, and 98 mg/dl versus 181, 119, and 111 mg/dl during open- versus closed-loop trials in the three cases (MPL-001, MPL-002, and MPL-003), respectively. Corresponding overnight percentage of time above 140 mg/dl was 11, 33, and 11 versus 100, 11, and 0. Postbreakfast BG levels were 128, 198, and 213 versus 236, 145, and 201 mg/dl. Altogether, glucose levels appeared better during closed-loop control in two cases out of three.
Discussion
In this article, we report preliminary studies in six type 1 diabetes patients who underwent closed-loop trials using a SC glucose sensor and a wearable insulin pump. For thispurpose, the main component of MPC, a model linking insulin infusion and meal ingestion to glucose excursions, was used. The model enables the prediction of future glucose excursions resulting from projected insulin infusion rates, thus enabling the construction of insulin infusion rates leading to a predefined target glucose excursion.
One of the main expected benefits of the model predictive approach17 over other prediction models such as previously tested PID-based systems9–11 is that premeal insulin increase is suggested by the MPC on the basis of individual tailoring of the algorithm to the patient's routine evaluated from data of a previous open-loop trial. This is possible only with an MPC-type controller, whereas control algorithms that are purely reactive to glucose changes, such as PID, would deliver the entire amount of insulin needed to cover the meal after it has occurred with the risk of postprandial hypoglycemia.
Although MPC appears, in theory, superior to PID and the ideal approach to control both fasting and postprandial PG levels, we faced several problems that can be seen in the profiles of each subject.
In subject PD-001 (Figure 2), one can observe a different reaction to dinner, even with the same amount of insulin injected. However, during closed loop, glucose was slowly brought at euglycemia before breakfast, without risk of hypoglycemic events. On the other hand, after breakfast, glucose was higher than during open loop, and this may be due to the small “aggressiveness” of the algorithm (q was 1.5 × 10−4); in all likelihood, a higher q value would have provided a better glycemic control. As a matter of fact, as indicated by the black bars, less insulin was delivered during closed loop and a prebreakfast insulin bolus was absent.
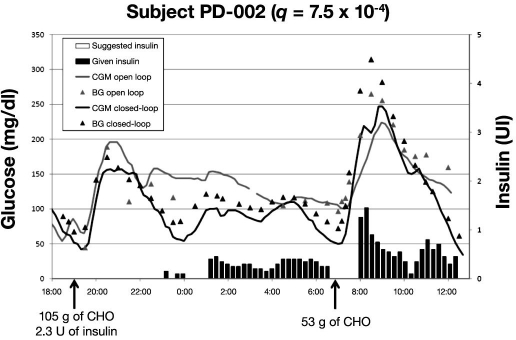
From the profile of subject PD-002 (Figure 3), one can observe that, during the night, the glycemic control was good. However, just before breakfast, PG was close to the hypoglycemic threshold. As a result, the algorithm delayed insulin delivery from the beginning of the food intake, as indicated by the absence of black bars. This resulted not only in poor glycemic control, but also an exaggerated, late insulin delivery, which produced hypoglycemia.
Figure 3.
Data of second patient studied in Padova; BG and CGM measured during admission 1 (open loop) and admission 2 (closed loop). Suggested and given insulin boluses relate to closed-loop admission.
From the profile of subject PD-003 (Figure 4), a discrepancy between suggested and given insulin is evident from 10:30 to 11:30. There, PG was around 70 mg/dl while the sensor was 15 mg/dl higher. Therefore, our clinical judgment was to refute the suggestion of injecting insulin in order to reduce the risk of hypoglycemia. In this case, the right amount of insulin delivery (judged by the attending physician) and the timed delivery of insulin before breakfast resulted in a fairly good metabolic control.
These first trials emphasize the concept that the presence of early and timed insulin release in response to the breakfast is essential to obtain a fair control of postprandial glucose excursion. The importance of this approach is particularly important also because, needless to say, the delivery of insulin takes place in the peripheral circulation.
In patient MPL-001, who was the only case of worst glucose control during the closed-loop trial, the failure comes from the lack of algorithm-guided increment of insulin delivery while the patient had his BG level stable but close to 180 mg/dl. Aggressiveness of the MPC algorithm (q = 16 × 10−4) was clearly insufficient in this case. However, it is very difficult to predict what would have been the optimal q for this patient. For sure, it would have been higher than that actually employed. It is probable that the patient's relatively high BMI (31.6) may have played a role in this issue.
Nighttime control was excellent in patient MPL-002. Of note, the algorithm allowed an effective control of the dawn phenomenon that occurred in the open-loop trial thanks to a suggested increased insulin delivery in the last period of the night. This is not surprising since, even if the control algorithm had been tuned on a simulator, which did not include the dawn phenomenon, the ability to correct for unaccounted disturbances is one of the major advantages of a closed-loop compared to an open-loop strategy. In fact, the algorithm is informed every 15 min about glucose level and its rate of change, and if an unexpected increase in glucose is detected, insulin is delivered to reduce glucose excursion. In contrast, bolus delivery at early breakfast time was not sufficient to obtain a control of the postmeal excursion that was similar to that observed with open loop. Subsequent boluses guided by the algorithm to limit postbreakfast excursion led to postmeal hypoglycemia.
Hypoglycemia at nighttime was well prevented by the insulin delivery tuned by the algorithm in patient MPL-03. A similar failure to limit postbreakfast excursion occurred as in patient MPL-002, although it was not followed by hypoglycemia.
In conclusion, this preliminary set of studies, where CGM together with insulin pumps were linked to models of the human metabolism and control algorithms, has demonstrated that closed-loop control based entirely on SC glucose sensing and insulin delivery is feasible and can be applied in the clinical settings to optimize the glucose control in patients with type 1 diabetes. In most cases, the algorithm showed a good efficiency in maintaining BG in a safe range at nighttime. In contrast, postbreakfast glucose excursions were poorly anticipated and mastered by the algorithm. The algorithm needs to be further improved to fully allow a condition of normoglycemia.
Acknowledgments
We thank Valentina Romor, R.N., and Marilisa Danieletto, R.N., in Padova and the Clinical Investigation Center team at the University Hospital Center in Montpellier for their valuable help.
Abbreviations
- BG
blood glucose
- BMI
body mass index
- CGM
continuous glucose monitoring
- CHO
carbohydrate
- CSII
continuous subcutaneous insulin infusion
- HbA1c
hemoglobin A1c
- IG
interstitial glucose
- MPC
model predictive control
- PG
plasma glucose
- PID
proportional integrative derivative
- SC
subcutaneous
References
- 1.Santiago JV, Clemens AH, Clarke WL, Kipnis DM. Closed-loop and open-loop devices for blood glucose control in normal and diabetic subjects. Diabetes. 1979;28(1):71–84. doi: 10.2337/diab.28.1.71. [DOI] [PubMed] [Google Scholar]
- 2.Parker RS, Doyle FJ, III, Peppas NA. A model-based algorithm for blood glucose control in type I diabetic patients. IEEE Trans Biomed Eng. 1999;46(2):148–157. doi: 10.1109/10.740877. [DOI] [PubMed] [Google Scholar]
- 3.Fischer U, Schenk W, Salzsieder E, Albrecht G, Abel P, Freyse EJ. Does physiological blood glucose control require an adaptive control strategy? IEEE Trans Biomed Eng. 1987;34(8):575–582. doi: 10.1109/tbme.1987.326068. [DOI] [PubMed] [Google Scholar]
- 4.Albisser AM, Leibel BS, Ewart TG, Davidovac Z, Botz CK, Zingg W. An artificial endocrine pancreas. Diabetes. 1974;23(5):389–396. doi: 10.2337/diab.23.5.389. [DOI] [PubMed] [Google Scholar]
- 5.Renard E. Implantable closed-loop glucose-sensing and insulin delivery: the future for insulin pump therapy. Curr Opin Pharmacol. 2002;2(6):708–716. doi: 10.1016/s1471-4892(02)00216-3. [DOI] [PubMed] [Google Scholar]
- 6.Renard E, Costalat G, Chevassus H, Bringer J. Closed-loop insulin delivery using implanted insulin pumps and sensors in type 1 diabetic patients. Diabetes Res Clin Pract. 2006;74(Suppl 2):S173–S177. [Google Scholar]
- 7.Bellazzi R, Nucci G, Cobelli C. The subcutaneous route to insulin-dependent diabetes therapy. IEEE Eng Med Biol Mag. 2001;20(1):54–64. doi: 10.1109/51.897828. [DOI] [PubMed] [Google Scholar]
- 8.Hovorka R. Continuous glucose monitoring and closed-loop systems. Diabet Med. 2006;23(1):1–12. doi: 10.1111/j.1464-5491.2005.01672.x. [DOI] [PubMed] [Google Scholar]
- 9.Steil GM, Rebrin K, Darwin C, Hariri F, Saad MF. Feasibility of automating insulin delivery for the treatment of type 1 diabetes. Diabetes. 2006;55(12):3344–3350. doi: 10.2337/db06-0419. [DOI] [PubMed] [Google Scholar]
- 10.Weinzimer SA, Steil GM, Swan KL, Dziura J, Kurtz N, Tamborlane WV. Fully automated closed-loop insulin delivery versus semiautomated hybrid control in pediatric patients with type 1 diabetes using an artificial pancreas. Diabetes Care. 2008;31(5):934–939. doi: 10.2337/dc07-1967. [DOI] [PubMed] [Google Scholar]
- 11.Percival MW, Zisser H, Jovanovič L, Doyle FJ., III Closed-loop control and advisory mode evaluation of an artificial pancreatic β cell: use of proportional-integrative-derivative equivalent model-based controllers. J Diabetes Sci Technol. 2008;2(4):636–644. doi: 10.1177/193229680800200415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Steil GM, Rebrin K, Hariri F, Jinagonda S, Tadros S, Darwin C, Saad MF. Interstitial fluid glucose dynamics during insulin-induced hypoglycemia. Diabetologia. 2005;48(9):1833–1840. doi: 10.1007/s00125-005-1852-x. [DOI] [PubMed] [Google Scholar]
- 13.Boyne MS, Silver DM, Kaplan J, Saudek CD. Timing of changes in interstitial and venous blood glucose measured with a continuous subcutaneous glucose sensor. Diabetes. 2003;52(11):2790–2794. doi: 10.2337/diabetes.52.11.2790. [DOI] [PubMed] [Google Scholar]
- 14.Kulcu E, Tamada JA, Reach G, Potts RO, Lesho MJ. Physiological differences between interstitial glucose and blood glucose measured in human subjects. Diabetes Care. 2003;26(8):2405–2409. doi: 10.2337/diacare.26.8.2405. [DOI] [PubMed] [Google Scholar]
- 15.Dua P, Doyle FJ, III, Pistikopoulos EN. Model-based blood glucose control for type 1 diabetes via parametric programming. IEEE Trans Biomed Eng. 2006;53(8):1478–1491. doi: 10.1109/TBME.2006.878075. [DOI] [PubMed] [Google Scholar]
- 16.Hovorka R, Canonico V, Chassin LJ, Haueter U, Massi-Benedetti M, Orsini Federici M, Pieber TR, Schaller HC, Schaupp L, Vering T, Wilinska ME. Nonlinear model predictive control of glucose concentration in subjects with type 1 diabetes. Physiol Meas. 2004;25(4):905–920. doi: 10.1088/0967-3334/25/4/010. [DOI] [PubMed] [Google Scholar]
- 17.Magni L, Raimondo DM, Bossi L, Dalla Man C, De Nicolao G, Kovatchev B, Cobelli C. Model predictive control of type 1 diabetes: an in silico trial. J Diabetes Sci Technol. 2007;1(6):804–812. doi: 10.1177/193229680700100603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kovatchev BP, Breton M, Man CD, Cobelli C. In silico preclinical trials: a proof of concept in closed-loop control of type 1 diabetes. J Diabetes Sci Technol. 2009;3(1):44–55. doi: 10.1177/193229680900300106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dassau E, Bequette BW, Buckingham BA, Doyle FJ., III Detection of a meal using continuous glucose monitoring: implications for an artificial beta-cell. Diabetes Care. 2008;31(2):295–300. doi: 10.2337/dc07-1293. [DOI] [PubMed] [Google Scholar]
- 20.Palerm CC, Zisser H, Bevier WC, Jovanovic L, Doyle FJ., III Prandial insulin dosing using run-to-run control: application of clinical data and medical expertise to define a suitable performance metric. Diabetes Care. 2007;30(5):1131–1136. doi: 10.2337/dc06-2115. [DOI] [PubMed] [Google Scholar]
- 21.Zisser H, Jovanovic L, Doyle FJ, III, Ospina P, Owens C. Run-to-run control of meal-related insulin dosing. Diabetes Technol Ther. 2005;7(1):48–57. doi: 10.1089/dia.2005.7.48. [DOI] [PubMed] [Google Scholar]
- 22.Owens C, Zisser H, Jovanovic L, Srinivasan B, Bonvin D, Doyle FJ., III Run-to-run control of blood glucose concentrations for people with type 1 diabetes mellitus. IEEE Trans Biomed Eng. 2006;53(6):996–1005. doi: 10.1109/TBME.2006.872818. [DOI] [PubMed] [Google Scholar]
- 23.Dalla Man C, Rizza RA, Cobelli C. Meal simulation model of the glucose-insulin system. IEEE Trans Biomed Eng. 2007;54(10):1740–1749. doi: 10.1109/TBME.2007.893506. [DOI] [PubMed] [Google Scholar]