Abstract
Background
Self-monitoring of blood glucose empowers diabetes patients to effectively control their blood glucose (BG) levels. A potential barrier to frequent BG controls is lancing pain, intrinsically linked to pricking the finger several times a day. In this study, we compared different state-of-the-art lancing devices from leading manufacturers regarding lancing pain, and we intended to identify lancing devices that are less painful.
Methods
First, 165 subjects compared 6 different BG monitoring systems—consisting of a lancing device and a BG meter—at home for 36 days and at least 3 BG tests per day. Second, the subjects directly compared 6 different lancing devices—independent from a BG meter—in a laboratory setting. The test results were collected in questionnaires, and lancing pain was rated on a numerical rating scale.
Results
One hundred fifty-seven subjects were included in the analysis. Accu-Chek BG monitoring systems were significantly (p ≤ .006) preferred to competitor BG monitoring systems and were rated by >50% of the subjects as “less painful” than competitor BG monitoring systems. Accu-Chek lancing devices were significantly (p < .001) preferred to competitor lancing devices and were rated by >60% of the subjects as “less painful” than competitor lancing devices.
Conclusions
We found significant differences in lancing pain between lancing devices. Diabetes patients clearly preferred lancing devices that cause less lancing pain. In order to improve patient compliance with respect to an adequate glycemic control, the medical staff should preferentially prescribe lancing devices that cause less lancing pain.
Keywords: lancing device, lancing pain, pain rating, self-monitoring of blood glucose
Introduction
Several studies have demonstrated the importance of tight blood glucose (BG) control for diabetes patients, e.g., the Diabetes Control and Complications Trial1 and the United Kingdom Prospective Diabetes Study.2 Self-monitoring of blood glucose (SMBG) enables diabetes patients to effectively control their BG levels. The clinical benefits of SMBG in type 1 diabetes patients are widely accepted.3 In type 2 diabetes patients, clinical, epidemio-logical, and economical evidence supporting SMBG is accumulating steadily.4–9
Diabetes patients and health professionals can choose from a wide range of dedicated medical devices for SMBG. The first concrete step in SMBG is the collection of a few microliters of capillary blood. For diabetes patients, this means pricking the finger—or an alternative site—with a lancing device. Current BG meters require only very small amounts of blood, and the extraction of blood from the skin capillary bed is generally accepted by diabetes patients. However, the lancing pain associated with lancing into the skin is one of the obstacles to ensure good patient compliance. Burge showed that lancing pain and finger soreness are leading reasons for self-reported patient noncompliance with physician recommendations for SMBG.10
In our observational field study, we evaluated differences in lancing pain associated with using Accu Chek BG monitoring systems and lancing devices compared to three competitor brands. The choice of competitor brands was based on market shares. Our aim was (1) to detect differences in lancing pain between lancing devices and (2) to identify lancing devices that are less painful for diabetes patients.
Methods
The study protocol was approved by the Concentrics Institutional Review Board (Indianapolis, IN). All the study subjects gave written informed consent. The studywas performed in the areas of Louisville, KY, and Indianapolis, IN, in 2007. In experiment 1, the subjects compared different BG monitoring systems—consisting of a lancing device and a BG meter—in their activities of daily life over 36 days. In experiment 2, the subjects directly compared different lancing devices at a testing laboratory. The results of the comparisons were documented in questionnaires.
Materials
In this comparative study, BG monitoring systems and lancing devices from Roche and three competitors (Table 1) were evaluated.
Blood glucose monitoring systems (consisting of BG monitor and lancing device): Accu Chek Aviva and Accu Chek Multiclix (Roche, Indianapolis, IN); Accu Chek Compact Plus and Accu Chek Softclix Plus (Roche); OneTouch Ultra2 and OneTouch UltraSoft (LifeScan, Milpitas, CA); FreeStyle Freedom and TheraSense FreeStyle (Abbott, Abbott Park, IL); and Ascensia Contour and Ascensia Microlet/Vaculance (Bayer, Tarrytown, NY).
Lancing devices: Accu Chek Softclix (Roche), Accu Chek Softclix Plus (Roche), Accu Chek Multiclix (Roche), OneTouch UltraSoft (LifeScan), TheraSense FreeStyle (Abbott), Ascensia Microlet/Vaculance (Bayer).
Table 1.
Comparison of Blood Glucose Monitoring Systems and Lancing Devices
| Accu-Chek | Competitors | ||
|---|---|---|---|
| BG monitoring systems | Accu-Chek Aviva | versus | OneTouch Ultra2 |
| Accu-Chek Compact Plus | FreeStyle Freedom | ||
| Ascensia Contour | |||
| Lancing devices | Accu-Chek Softclix | versus | OneTouch UltraSoft |
| Accu-Chek Softclix Plus | TheraSense FreeStyle | ||
| Accu-Chek Multiclix | Ascensia Microlet / Vaculancea |
Accu-Chek Softclix was compared to Ascensia Vaculance, and Accu-Chek Softclix Plus and Accu-Chek Multiclix were compared to Ascensia Microlet.
Study Subjects
We recruited 165 study subjects in the areas of Louisville, KY, and Indianapolis, IN. The same subject pool was used for experiments 1 and 2. The target sample size for both experiments was 150 subjects; 165 subjects were recruited to account for attrition. In experiment 1, the subject pool was divided into three groups of 55 subjects each. In experiment 2, the subjects were regrouped into two groups of 82 subjects each. Inclusion criteria were (1) diabetes mellitus type 1 or 2, (2) experience with BG testing ≥1 year, (3) average BG testing frequency ≥3 times a day, and (4) BG meter normally used (supporting alternate site testing): Accu Chek Active (Roche), Accu Chek Aviva (Roche), Accu Chek Compact (Roche), Advance (Hypoguard), Ascensia Breeze (Bayer), Ascensia Contour (Bayer), Ascensia Dex 2 (Bayer), Ascensia Elite (Bayer), BD Latitude (Becton Dickinson), BD Logic (Becton Dickinson), FreeStyle (Abbott), FreeStyle Flash (Abbott), FreeStyle Freedom (Abbott), Liberty iTest (WaveSense), OneTouch FastTake (Lifescan), OneTouch Ultra (Lifescan), OneTouch UltraSmart (Lifescan), Precision Easy (Abbott), Precision Xtra (Abbott), Sidekick (Home Diagnostics), Sof-Tact Softsense (Abbott), or TrueTrack Smart (Home Diagnostics). Exclusion criteria were (1) illiteracy, (2) significant visual impairment, (3) participation in a study in the past 30 days, and (4) employment at a market research firm or close relation to somebody employed at a market research firm.
Study Design
The study was divided in two experiments. In experiment 1,the BG monitoring systems, Accu Chek Aviva and Accu Chek Compact Plus, and the lancing device, Accu Chek Softclix, were tested against three competitors: OneTouch Ultra2, FreeStyle Freedom, and Ascensia Contour. In experiment 2, the lancing devices, Accu Chek Softclix Plus and Accu Chek Multiclix, were tested against three competitors: OneTouch UltraSoft, TheraSense FreeStyle, and Ascensia Microlet/Vaculance. The purpose of dividing the study in two experiments was to avoid the confounding effect of a “BG monitoring system” and a “lancing device” in subject responses. Both BG monitoring system and lancing device effects for Accu Chek Aviva/Multiclix and Accu Chek Compact Plus/Softclix Plus were of interest. However, it is unlikely subjects can separate the impact of the BG monitoring system from the lancing device on perceived lancing pain when varying both simultaneously. Hence, for Accu Chek Aviva/Multiclix and Accu Chek Compact Plus/Softclix Plus, the BG monitoring system was tested in experiment 1 while the associated lancing device was tested in experiment 2, where the BG monitor was held constant and only the lancing device was varied. Perceived lancing pain was evaluated by questionnaires in which subjects made (1) comparative evaluations between the different BG monitoring systems/lancing devices, and (2) rated the different BG monitoring systems/lancing devices independently.
Procedure
The study started with an orientation meeting between the experimenter and subjects in groups of approximately six subjects each. During this meeting, the study protocol was explained, the subject demographic data were collected, consent forms were signed, and operation of the BG monitoring systems/lancing devices was demonstrated. Subjects received the testing protocol packet including all data collection instruments in an easy-to-follow format and all necessary devices and accessories to perform the testing. After the first 4 to 5 days of testing, a facilitator phoned each subject to confirm protocol compliance and answer possible questions.
In experiment 1, subjects were assigned to one of three groups: (1) Accu Chek Aviva and three competitors, (2) Accu Chek Compact Plus and three competitors, or (3) Accu Chek Softclix and three competitors. At home, subjects performed BG testing ≥3 times per day for a period of 36 days. The BG testing period of 36 days was divided into three 12-day blocks, corresponding to three lancing sites: finger, palm, and forearm. Using each device for 3 consecutive days, subjects used each of the 4 devices for ≥3 times per day, such that, over the 12-day block, all devices are used on each lancing site. Subjects tested according to a predetermined randomization scheme for both lancing devices and lancing sites. Subjects used a new lancet for every lancing event and individually adjusted the lancing depth to allow for a valid BG test. At the end of each 12-day block, subjects completed a questionnaire rating the devices against one another with regard to lancing pain on the specified lancing site.
In experiment 2, subjects were assigned to one of two groups: (1) Accu Chek Softclix Plus and three competitors or (2) Accu Chek Multiclix and three competitors. Subjects from the Accu Chek Compact Plus group from experiment 1 were assigned to the Accu Chek Softclix Plus group, and subjects from the Accu Chek Aviva group from experiment 1 were assigned to the Accu Chek Multiclix group. Subjects from the Accu Chek Softclix group from experiment 1 were evenly divided and assigned to join either the Accu Chek Softclix Plus group or Accu Chek Multiclix group. This resulted in a target of 75 observations per each combined group in experiment 2. In each group, all subjects were asked to perform a finger stick with the Accu Chek lancing device and each of the three competitor devices in randomized order. Subjects used a new lancet for every lancing event and individually adjusted the lancing depth to allow for a valid BG test. They completed questionnaires to assess lancing pain with each device and performed a paired comparison between devices. Half of the subjects in each group then performed a palm stick and the other half performed an arm stick. Measures of lancing pain were assessed for these lancing sites as well.
Questionnaires
Entry Questionnaire: Sociodemographic characteristics—age, gender, job, job experience, ethnic group, education level, SMBG frequency, SMBG experience, insulin therapy, BG monitoring system, lancing device, lancing site, impaired vision, color blindness.
Twelve-Day Questionnaires: The subjects (i) rated every possible combination of the four lancing devices (A versus B) they had used for 3 days each on a numerical rating scale (NRS) ranging from −3 (lancing device A is less painful than device B), over 0(no difference between lancing device A and lancing device B), to +3 (lancing device A is more painful than lancing device B) and (ii) answered the following question for each of the four lancing devices: “Do you believe that testing was virtually pain-free with this device: yes/no.”
Exit Questionnaire: The subjects (i) rated every possible combination of the four lancing devices (A versus B) they had used on a NRS ranging from −3 (lancing device A is less painful than device B), over 0(no difference between lancing device A and lancing device B), to +3 (lancing device A is more painful thanlancing device B) and (ii) answered the following question for each of the four lancing devices: “Do you believe that testing was virtually pain-free with this device: yes/no.”
Statistics
Statistical analysis was performed using the software SPSS 14.0 (SPSS, Chicago, IL). Comparison of groups of categorical variables was performed by chi squares statistics, comparison of binary variables with Fisher's exact test, and comparison of quantitative variables with t-test. All tests were performed two-sided at a test level of <0.05. Continuous variables were resumed as means ± standard deviation. Categorical variables were described as counts (n) or relative frequency (%). In order to identify possible factors influencing the lancing device preferences of the study subjects, we analyzed their sociodemographic characteristics with analysis of variances. The results of the pain rating on the 12-day questionnaires and the exit questionnaire (NRS ranging from −3 to +3) were grouped as follows: pain ratings from −3 to −1 = “less painful,” pain rating 0 = “equally painful,” and pain ratings from +1 to +3 = “more painful.” The data collected in “daily questionnaires” were incomplete and did not allow for a thorough statistical analysis.
Results
Sociodemographic Characteristics of the Study Subjects
From the 165 subjects enrolled in the study, 157 subjects were considered in the analysis. The data sets of eight subjects were not complete and therefore were not included in the analysis. Of all the subjects, 59.2% were female, 76.4% were older than 45 years, and the mean age of the study population was 53 ± 13 years. The study subjects were frequent SMBG users, although not all the subjects had an average BG testing frequency ≥3 times a day as initially planned: 47.1% performed SMBG on average more than 3 times a day, 56.1% performed SMBG more than 20 times per week in the week just before the study. Among the subjects, 66.9% had more than 5 years of experience with SMBG, and 52.9% had an insulin therapy. The BG monitoring systems, lancing devices, and lancing sites normally used by the subjects are given in Table 2. Out of all subjects, 96.8% normally used the finger as a lancing site.
Table 2.
Sociodemographic Characteristics of the Study Subjects
| Characteristics | Study subjectsan = 157 |
|---|---|
| Gender | |
| female | 93 (59.2%) |
| male | 64 (40.8%) |
| Age (years) | |
| ≤45 | 37 (23.6%) |
| >45 | 120 (76.4%) |
| Job type | |
| manual worker | 37 (25.9%) |
| office worker | 66 (46.2%) |
| other | 40 (28.0%) |
| Job experience (years) | |
| ≤15 | 48 (38.1%) |
| >15 | 78 (61.9%) |
| Ethnic group | |
| African American | 37 (23.6%) |
| Caucasian | 108 (68.8%) |
| Asian, Native, other | 12 (7.6%) |
| Education level | |
| high school or less | 117 (74.5%) |
| college, university | 40 (25.5%) |
| SMBG frequency (times/last week) | |
| ≤20 | 69 (43.9%) |
| >20 | 88 (56.1%) |
| Average SMBG frequency (times/day) | |
| ≤3 | 83 (52.9%) |
| >3 | 74 (47.1%) |
| SMBG experience (years) | |
| ≤5 | 52 (33.1%) |
| >5 | 105 (66.9%) |
| BG monitoring system normally used | |
| Accu-Chek (Roche) | 23 (18.0%) |
| Ascensia (Bayer) | 11 (8.6%) |
| FreeStyle (Abbott) | 21 (16.4%) |
| OneTouch (LifeScan) | 51 (39.8%) |
| other | 22 (17.2%) |
| Lancing device normally used | |
| Accu-Chek (Roche) | 17 (18.5%) |
| Ascensia (Bayer) | 8 (8.7%) |
| FreeStyle (Abbott) | 16 (17.4%) |
| OneTouch (LifeScan) | 35 (38.0%) |
| other | 16 (17.4%) |
| Lancing site normally used | |
| finger | 151 (96.8%) |
| arm or leg | 5 (3.2%) |
| Insulin therapy | 83 (52.9%) |
| Corrective eye glasses or contact lenses | 120 (76.9%) |
Data are valid n (%). Data are from the entry questionnaires.
Preference Scores and Comparison of Blood Glucose Monitoring Systems
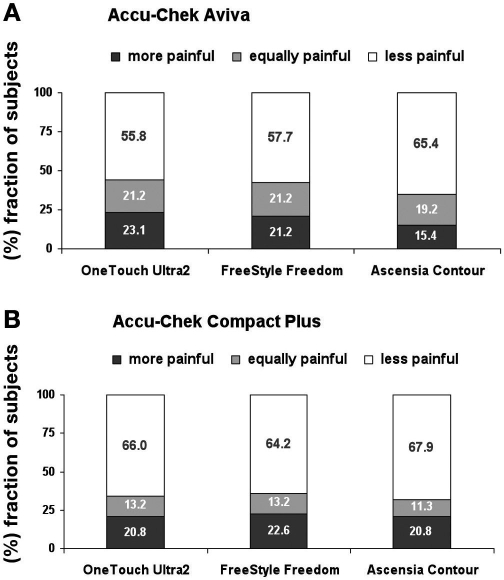
On a NRS ranging from −3 to +3, the BG monitoring systems Accu-Chek Aviva and Accu-Chek Compact Plus were significantly preferred to OneTouch Ultra2, Freestyle Freedom, and Ascensia Contour (Table 3). The preference scores from −3 to +3 are grouped into three categories: “more painful,” “equally painful,” and “less painful” (Figure 1).
Table 3.
Preference Scores of Blood Glucose Monitoring Systems
| OneTouch Ultra2 | FreeStyle Freedom | Ascensia Contour | ||||
|---|---|---|---|---|---|---|
| Scorea | p valueb | Scorea | p valueb | Scorea | p valueb | |
| Accu-Chek Aviva | 0.67 ± 1.18 | n = 52<.001 | 0.45 ± 1.12 | n = 52.006 | 0.74 ± 1.06 | n = 52<.001 |
| Accu-Chek Compact Plus | 0.62 ± 1.20 | n = 53<.001 | 0.49 ± 1.25 | n = 53.006 | 0.64 ± 1.10 | n = 53<.001 |
The subjects rated pairs of BG monitoring systems (Accu-Chek versus competitor) on a NRS ranging from −3 (Accu-Chek less painful than competitor), over 0 (no difference between Accu-Chek and competitor), to +3 (Accu-Chek more painful than competitor). Data are means ± standard deviation. Data are from the 12-day questionnaires.
Difference from 0.
Figure 1.
Pairwise comparison of Accu-Chek BG monitoring systems versus competitors with respect to lancing pain (data are from the 12-day questionnaires). E.g., Accu-Chek Aviva was rated by 23.1% of the subjects as “more painful,” by 21.2% as “equally painful,” and by 55.8% as “less painful” than OneTouch Ultra2.
Preference Scores and Comparison of Lancing Devices
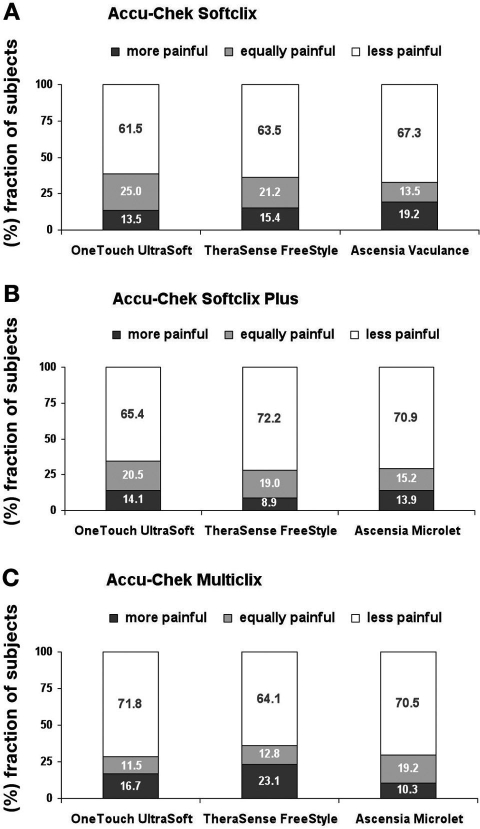
On a NRS ranging from −3 to +3, the lancing devices Accu-Chek Softclix, Accu-Chek Softclix Plus and Accu-Chek Multiclix were significantly preferred to OneTouch UltraSoft, TheraSense FreeStyle, and Ascensia Microlet /Vaculance (Table 4). The preference scores from −3 to +3 are grouped into three categories: “more painful,” “equally painful,” and “less painful” (Figure 2).
Table 4.
Preference Scores of Lancing Devices
| OneTouch UltraSoft | TheraSense FreeStyle | Ascensia Microlet / Vaculancec | ||||
|---|---|---|---|---|---|---|
| Scorea | p valueb | Scorea | p valueb | Scorea | p valueb | |
| Accu-Chek Softclix | 0.60 ± 1.17 | n = 52<.001 | 0.52 ± 1.27 | n = 52.005 | 0.70 ± 1.41 | n = 52<.001 |
| Accu-Chek Softclix Plus | 0.78 ± 1.06 | n = 78<.001 | 1.00 ± 1.12 | n = 78<.001 | 0.97 ± 1.14 | n = 78<.001 |
| Accu-Chek Multiclix | 0.88 ± 1.27 | n = 78<.001 | 0.59 ± 1.26 | n = 78<.001 | 1.03 ± 1.16 | n = 78<.001 |
The subjects rated pairs of lancing devices (Accu-Chek versus competitor) on a NRS ranging from −3 (Accu-Chek less painful than competitor), over 0 (no difference between Accu-Chek and competitor), to +3 (Accu-Chek more painful than competitor). Data are means ± standard deviation. Data are from the 12-day and exit questionnaires.
Difference from 0.
Accu-Chek Softclix was compared to Ascensia Vaculance, and Accu-Chek Softclix Plus and Accu-Chek Multiclix were compared to Ascensia Microlet.
Figure 2.
Pairwise comparison of Accu-Chek lancing devices versus competitors with respect to lancing pain (data are from the 12-day and exit questionnaires). E.g., Accu-Chek Softclix was rated by 13.5% of the subjects as “more painful,” by 25.0% as “equally painful,” and by 61.5% as “less painful” than OneTouch UltraSoft.
Pain Rating of Blood Glucose Monitoring Systems and Lancing Devices
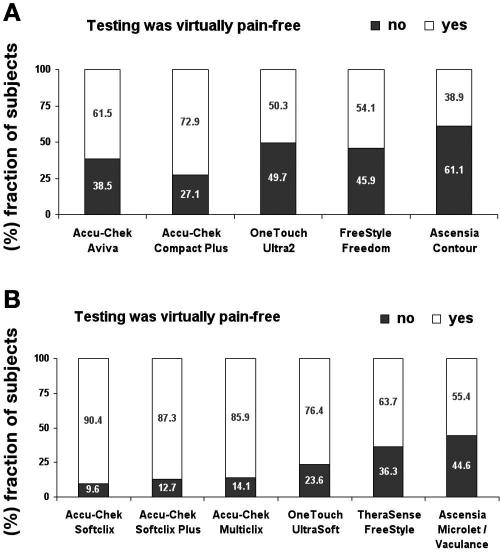
The percentage of subjects that experienced testing with the Accu-Chek BG monitoring systems or lancing systems as “virtually pain-free” was bigger than the competitor devices (Figure 3). Overall, the lancing devices were more often rated as “virtually pain-free” than the BG monitoring systems. This effect might be due to the study design: BG monitoring systems were rated retrospectively upon completion of a 12-day block, and lancing devices were rated immediately after the lancing event.
Figure 3.
Pain rating of BG monitoring systems (upper panel) and lancing devices (lower panel) (data are from the 12-day and exit questionnaires). E.g., testing with Accu-Chek Aviva was experienced as “virtually pain-free” by 61.5% of the subjects and as “not virtually pain-free” by 38.5% of the subjects.
Discussion
Our study describes the perceived lancing pain differences between lancing devices and thus makes a relative statement on individual lancing pain. In everyday life, diabetes patients do not use lancing devices on their own, but in combination with a BG monitor. Therefore, we tested not only lancing devices, but also BG monitoring systems consisting of a BG meter and a lancing device. The analysis of variances did not reveal statistically significant influences of sociodemographic characteristics on the lancing device preferences of the study subjects.
First, we found that there are significant differences in lancing pain between different state-of-the-art lancing devices from leading manufacturers. What are the factors influencing lancing pain with respect to the lancing device?
Lancet penetration depth: Lancing pain is closely related to lancet penetration depth. Smaller lancet penetration depth causes less injury to the tissue and therefore less lancing pain.11–13 Most lancing devices have an adjustable lancet penetration depth to adapt to individual differences in epidermis thickness.
Lancet speed: The mechanical pain receptors of the tissue are activated by tissue movement. Higher lancet speed minimizes tissue movement and therefore minimizes lancing pain.
Lancet shape: Lancets or “lanceolates” are manufactured by grinding facets into the tip of a metal rod, forming a pointed tip and three surfaces with two cutting edges (Figure 4). A very sharp front end of the lancet facilitates tissue penetration.
Lancet surface: A smooth lancet surface avoids friction of the tissue, further reducing lancing pain.
Lancet movement: In order to avoid painful vibrations or jolts of the lancet in the tissue, state-of-the-art lancing devices use a combination of rail-guided and cam-driven lancets, enabling a smooth lancet movement.
Skin fixation: The interface of the lancing device and the patient skin determines the quality of skin fixation during lancing. Better skin fixation means less lancing pain.
Figure 4.

Typical lancet shape with pointed tip, three surfaces, and two cutting edges.
Second, we found that the Accu-Chek devices were significantly more often rated by the study subjects as “virtually pain-free” than the competitor devices. In 1995, Fruhstorfer and Lange12 showed that Accu Chek Softclix lancing devices are significantly less painful than comparable lancing devices at a lancing depth necessary to obtain at least 20 μl of blood. More than ten years later, when BG meters require distinctly less blood for a test, we again find that Softclix lancing devices are less painful than state-of-the-art competitors.
The importance of tight glycemic control for avoiding complications of diabetes have been shown in several studies (the Diabetes Control and Complications Trial1 and the United Kingdom Prospective Diabetes Study2). From a diabetes patient perspective, the hurdles for routinely performing SMBG have to be as little as possible, one hurdle being lancing pain. In other words, reducing lancing pain has the potential to improve patient compliance with respect to an adequate glycemic control and will definitely improve the quality of life of diabetes patients. In order to lower the psychological barrier for SMBG as much as possible, the medical staff should prescribe “virtually pain-free” lancing devices to their diabetes patients.
Abbreviations
- BG
blood glucose
- NRS
numerical rating scale
- SMBG
self-monitoring of blood glucose
References
- 1.The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329(14):977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 2.U.K. Prospective Diabetes Study Group. U.K. Prospective Diabetes Study 16. Overview of 6 years' therapy of type II diabetes: a progressive disease. Diabetes. 1995;44(11):1249–1258. [PubMed] [Google Scholar]
- 3.Blonde L, Karter AJ. Current evidence regarding the value of self-monitored blood glucose testing. Am J Med. 2005;118(Suppl 9A):20S–26S. doi: 10.1016/j.amjmed.2005.07.053. [DOI] [PubMed] [Google Scholar]
- 4.Welschen LM, Bloemendal E, Nijpels G, Dekker JM, Heine RJ, Stalman WA, Bouter LM. Self-monitoring of blood glucose in patients with type 2 diabetes who are not using insulin: a systematic review. Diabetes Care. 2005;28(6):1510–1517. doi: 10.2337/diacare.28.6.1510. [DOI] [PubMed] [Google Scholar]
- 5.Sarol JN, Jr, Nicodemus NA, Jr, Tan KM, Grava MB. Self-monitoring of blood glucose as part of a multi-component therapy among non-insulin requiring type 2 diabetes patients: a meta-analysis (1966–2004) Curr Med Res Opin. 2005;21(2):173–184. doi: 10.1185/030079904X20286. [DOI] [PubMed] [Google Scholar]
- 6.Jansen JP. Self-monitoring of glucose in type 2 diabetes mellitus: a Bayesian meta-analysis of direct and indirect comparisons. Curr Med Res Opin. 2006;22(4):671–681. doi: 10.1185/030079906X96308. [DOI] [PubMed] [Google Scholar]
- 7.McGeoch G, Derry S, Moore RA. Self-monitoring of blood glucose in type-2 diabetes: what is the evidence? Diabetes Metab Res Rev. 2007;23(6):423–440. doi: 10.1002/dmrr.749. [DOI] [PubMed] [Google Scholar]
- 8.Palmer AJ, Dinneen S, Gavin JR, III, Gray A, Herman WH, Karter AJ. Cost-utility analysis in a UK setting of self-monitoring of blood glucose in patients with type 2 diabetes. Curr Med Res Opin. 2006;22(5):861–872. doi: 10.1185/030079906X104669. [DOI] [PubMed] [Google Scholar]
- 9.Schnell O, Hummel M, Weber C. Economic and clinical aspects of diabetes regarding self-monitoring of blood glucose. Diabetes Technol Ther. 2008;10(Suppl 1):S72–S81. [Google Scholar]
- 10.Burge MR. Lack of compliance with home blood glucose monitoring predicts hospitalization in diabetes. Diabetes Care. 2001;24(8):1502–1503. doi: 10.2337/diacare.24.8.1502. [DOI] [PubMed] [Google Scholar]
- 11.Fruhstorfer H, Müller T, Scheer E. Capillary blood sampling: how much pain is necessary? Part 2: relation between penetration depth and puncture pain. Pract Diab Int. 1995;12(4):184–185. [Google Scholar]
- 12.Fruhstorfer H, Lange H. Capillary blood sampling: how much pain is necessary? Part 3: pricking the finger can be less painful. Pract Diab Int. 1995;12(6):253–254. [Google Scholar]
- 13.Garvey K, Batki AD, Thomason HL, Holder R, Thorpe GH. Blood lancing systems for skin puncture. Prof Nurse. 1999;14(9):643–651. [PubMed] [Google Scholar]