Abstract
Through the use of enzymatic sensors—inserted subcutaneously in the abdomen or ex vivo by means of microdialysis fluid extraction—real-time minimally invasive continuous glucose monitoring (CGM) devices estimate blood glucose by measuring a patient's interstitial fluid (ISF) glucose concentration. Signals ac-quired from the interstitial space are subsequently calibrated with capillary blood glucose samples, a method that has raised certain questions regarding the effects of physiological time lags and of the duration of processing delays built into these devices. The time delay between a blood glucose reading and the value displayed by a continuous glucose monitor consists of the sum of the time lag between ISF and plasma glucose, in addition to the inherent electro-chemical sensor delay due to the reaction process and any front-end signal-processing delays required to produce smooth traces. Presented is a review of commercially available, minimally invasive continuous glucose monitors with manufacturer-reported device delays. The data acquisition process for the Medtronic MiniMed (Northridge, CA) continuous glucose monitoring system—CGMS® Gold—and the Guardian® RT monitor is described with associated delays incurred for each processing step. Filter responses for each algorithm are examined using in vitro hypoglycemic and hyperglycemic clamps, as well as with an analysis of fast glucose excursions from a typical meal response. Results demonstrate that the digital filters used by each algorithm do not cause adverse effects to fast physiol-ogic glucose excursions, although nonphysiologic signal characteristics can produce greater delays.
Keywords: blood glucose, continuous glucose monitoring, insulin, interstitial fluid, sensor
Introduction
Patients with type 1 diabetes can reduce hyperglycemia by intensive insulin therapy and by agressive monitoring of blood glucose (BG) levels. These steps can lead to a vast reduction in microvascular disease, a decrease in mor-bidity, and a slowing of the onset, or progression, of severe complications.1 However, more aggressive management of BG levels to attain tighter control is hindered by fear of hypoglycemia. Daily self-monitoring of blood glucose (SMBG) levels can improve control, but glucometers require an inconvenient—and, for some, painful—finger stick, which for some may limit the number of daily measurements. Several noninvasive and minimally invasive continuous glucose monitoring (CGM) devices frequently acquire glucose measurements without patient intervention.
The first Food and Drug Administration (FDA)-approved CGM system was Medtronic MiniMed's continuous glucose monitoring system (CGMS),2 which performs a retrospective analysis over 3 days. Studies have demonstrated the efficacy of this device in its ability to reduce hemoglobin A1c (HbA1c) levels when utilized under clinical supervision.3,4 Medtronic MiniMed's Guardian RT5 was the first real-time commercially available CGM device. The monitor calculates and displays glucose values every 5 minutes. In a 12-week multicenter study6 consisting of 156 stable type 1 patients with HbA1c levels ≥8.1%, it was demonstrated that by monitoring glucose levels continuously and by setting hyperglycemic and hypoglycemic alarm thresholds, patients decreased their HbA1c levels by an average of 1.1 HbA1c percentage points—a 0.7% improvement over the control group taking blood glucose measurements using a traditional finger stick and glucometer. HbA1c levels were reduced by 2% HbA1c points in over 26% of patients. Furthermore, hypoglycemia (BG ≤70 mg/dl) occurred once in each study arm and only occurred in one case while the patient was wearing the device. The hypoglycemic episode was confirmed by finger stick.
The next real-time CGM device approved for the U.S. market was DexCom's (San Diego, CA) STS™ system. In a similar study7 that included 91 type 1 and type 2 diabetes patients (of which a small percentage of patients had type 2 diabetes), subjects were monitored with the STS device for a period of 72 hours. Patients were assigned either to a control group—which excluded display and alarms, allowing only data storage—or to a treatment arm, which consisted of three periods, the first without display and the second and third with display and alarms. Compared to the control group, the treatment arm spent 21% less time hypoglycemic, 23% less time hyperglycemic, and 26% more time in the target range. More recently, DexCom upgraded the STS system to the SEVEN® Plus, which is the first CGM device approved for use of up to 7 days.
Abbott Diabetes was the next medical device manufacturer to gain FDA regulatory approval for their real-time CGM device, the Freestyle Navigator®, which requires only four finger stick calibrations over a 5-day period and calculates glucose concentration every minute. In a 137-patient multicenter study8 that included insulin-dependent type 1 and 2 diabetes patients, comparisons in glycemic control were made for 20 days of masked use to 21 days of unmasked use with real-time glucose sensor readings every minute with threshold alarms. In the type 1 population, a 55% time reduction below a threshold of 55 mg/dl was observed during unmasked periods, in contrast to masked periods, with the mean hypoglycemic rate decreasing from 1.1 to 0.8 per day.
Minimally invasive CGM devices measure glucose concentration from interstitial fluid (ISF) through the use of electrochemical sensors,2 which are inserted subcutaneously in the abdominal interstitial space, or by utilizing microdialysis techniques,9 which are applied in order to extract ISF with a fiber generally inserted in the adipose tissue of the abdominal region. Microdialysis techniques also require diffusion of ISF, as well as a perfusion solution for extraction and ex vivo analysis. Menarini Diagnostics' (Florence, Italy) GlucoDay® device10,11 was the first system to use a micropump to deliver a perfusion solution, which undergoes diffusional exchange with ISF. The solution is then removed for analysis.
The effectiveness of CGM in detecting hypoglycemic events when compared to SMBG was demonstrated further in a study12 with the microdialysis system from Roche Diagnostics (Indianapolis, IN), the subcutaneous continuous glucose monitoring system (SCGM1). The study showed that in contrast to CGM, performing SMBG with a customary BG meter and four finger sticks daily could miss up to 71% of hypoglycemic events, and testing up to seven times daily could result in 58% missed events. The occurrence of hypoglycemia was validated with 75 blood glucose points per subject.
More novel approaches that are considered noninvasive perform transdermal fluid extraction by compromising the epidermis with energy, such as ultrasound,13 or by reverse iontophoresis.14 Sontra Medical Corporation (Franklin, MA), now Echo Therapeutics, developed SonoPrep®—a glucose patch that extracts ISF by applying ultrasonic energy to the skin and, in turn, measures glucose concentration in the ISF ex vivo through their glucose flux biosensor and transmits this value to Symphony™, a CGM device codeveloped with Bayer Diagnostics (Tarrytown, NY). Animas Corporation's (West Chester, PA) GlucoWatch®15 measures ISF glucose by reverse iontophoresis. Unfortunately, widespread acceptance of the device was limited by its expense, as well as by skin irritation experienced by a large percentage of users.16,17
Continuous glucose monitoring devices measuring ISF glucose exhibit time delays when compared to capillary blood glucose. Short delays are due to transit time effects due to diffusion through a glucose membrane, where the delay is often dependent on membrane thickness. Sensors measuring ISF glucose will lag blood glucose by the time it takes for glucose to diffuse from the capillary to the interstitial space adjacent to the sensor or sampling device. Microdialysis-based approaches undergo an additional delay to extract ISF. Furthermore, sensor signals require filtering to smooth any electronic noise and artifacts that can be present, creating a delay that is proportional to the amount of smoothing required. This article discusses the various sources of delay between instantaneous blood glucose measures and the glucose values reported in real time by CGM devices. In addition, preprocessing algorithms used by the CGMS Gold and Guardian RT monitors are described, with delays reported for each processing step.
Interstitial Fluid–Plasma Glucose Dynamics
The relationship between ISF glucose and plasma glucose has been under debate for some time. A popular theory is referred to as the “push–pull phenomenon,”18 whereby glucose first enters the ISF from plasma and leaves by insulin-induced cellular uptake. Under the principles of this theory, ISF glucose leads plasma glucose when glucose is falling and lags during increasing concentrations. Several studies, how-ever, contradict this hypothesis by showing that ISF glucose consistently lags plasma glucose.19–21
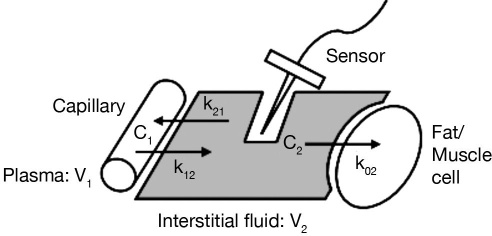
A widely accepted explanation of the dynamic relationship among ISF, fat/muscle, and plasma glucose is illustrated by Figure 1. This model20 assumes that the capillary separating plasma and ISF compartments creates a resistance to the diffusion of glucose into the interstitial space. Glucose is cleared from the interstitial space by a rate proportional to the concentration of glucose in that compartment, and the rate of glucose uptake from the subcutaneous tissue is assumed constant, as are glucose diffusion rates between plasma and subcutaneous tissue. Steady-state glucose concentration in the ISF compartment (C2) is dependent on the rate of glucose clearance from this compartment and the rate of glucose diffusion to the compartment. As rate parameters are assumed constant, the time lag between ISF and plasma glucose concentration is constant, as is the gradient. Steil and colleagues21 established this consistency with rate parameters unaffected by rising and falling glucose levels, which holds true for different insulin concentrations.
Figure 1.
A two-compartment model of the plasma–ISF glucose relationship.
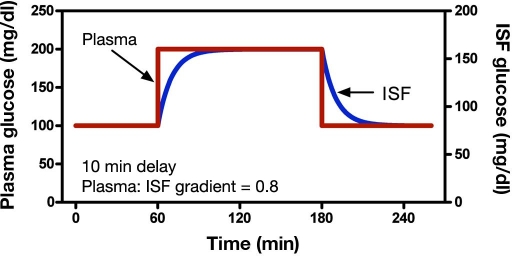
A theoretical plasma glucose clamp response is illustrated in Figure 2, with the resulting ISF glucose concentration superimposed with a fixed 0.8 gradient where glucose diffuses from plasma to ISF with a first-order time lag of 10 minutes. The gradient was chosen arbitrarily to demonstrate how glucose transverses the capillary membrane, mainly by diffusion. The model holds true for any gradient less than 1, as a gradient must ex-ist for glucose to be transported by the mechanism. In this example, it takes approximately 50 minutes (equiva-lent to five time constants) for the transient response from the ISF glucose concentration to equilibrate com-pletely. One time constant represents 63% of the distance a function has moved to reaching its final value. This final value is typically reached following five time constants. As plasma glucose is estimated from a measurement of ISF glucose using an electrochemical sensor, a low current in the nanoampere range is generated through the electrochemical reaction, which is considered to be proportional to ISF glucose. Subsequently, sensor measurements are calibrated with a capillary BG measurement by a finger stick and glucometer.
Figure 2.
A theoretical ISF glucose response to a plasma glucose step function.
Delays in CGM Devices
Manufacturer-Reported Time Delays
Table 1 presents manufacturer-reported system delays of CGM devices measuring ISF glucose. The GlucoWatch time delay is 10 minutes, chosen to equal half of its original sampling interval. The device required 3 minutes to collect an adequate amount of glucose after activating the sensing electrodes for 7 minutes. The manufacturer estimates that the overall delay between blood glucose and GlucoWatch-reported glucose values is somewhere in the region of 18 ± 10 minutes.14 However, this time lag was determined using cross-correlation.22 While this is a common approach to determining signal time lag, it is only applicable to delays that are time shifts and not to time lags that have first- or second-order characteristics that likely exist between ISF and plasma glucose.
The time delay originally reported for Abbot's Freestyle Navigator23 was 8 minutes, with approximately 3 minutes allocated to processing and 5 minutes attributed to physiology. In a more recent study,24 the manufacturer reported an overall delay of 12.6 minutes, which was determined empirically by time-shifting sensor signals to be optimally aligned with reference venous measurements. In this study (n = 20,362), sensor signals were shifted and calibrated with capillary finger stick measurements and then compared with venous blood glucose samples for the given time shift. The mean difference between sensor readings and blood glucose was measured for each time shift until the minimum error was achieved. The investigation included 58 type 1 subjects. Venous blood samples were captured every 15 minutes over 50 hours duration for each subject, with fast glucose excursions induced by insulin and glucose challenges.
Roche reported an inherent physical time lag of 31 ± 2 minutes for its microdialysis SCGM1 device.25 Physical lag was calculated in vitro (n = 10) and is defined as the time it takes the sensor to reach 95% of the glucose concentration in a beaker following normalization or settling to a stable current. The physical lag encompassed the transport lag with a flow rate of 0.3 μl/min, which was the time duration for the perfusion solution to travel from the microdialysis catheter to the ex vivo enzymatic sensors. The sensor response time was estimated to be less than 1 minute due to fast enzymatic reaction time.
Menarini Diagnostics reported a much shorter delay for its microdialysis-based device, GlucoDay.10 The manufacturer reported that it takes 53 seconds for the dialysate to reach the enzymatic biosensor from the filament in subcutaneous tissue. Further delays are incurred for the reaction process and data acquisition system, enabling detection of glucose changes every 1 minute, 4 seconds. However, a more recent study19 reported greater delays for this device, stating that the delay for the dialysate to travel through the microdialysis catheter is 6 minutes. This is likely due to a different flow rate setting in the programmable micropump, where 10 μl/minute was used for the latter study. Greater flow rates will decrease time delay but will likely decrease accuracy. DexCom, for its part, has not published any information regarding delays for its STS device, but the company notified users that there is an approximate 10-minute delay between blood glucose and its monitor value. However, with the upgrade to the SEVEN system, an average delay of 5 minutesis reported.
CGMS Gold
The CGMS Gold device acquires a current (nanoampere range) proportional to ISF glucose that is amplified, converted to a voltage, and digitized to a 12-bit signal at a rate of 0.1 Hz. This signal is processed by three filters. The first is a moving average filter that performs noise reduction and decimation to a 1-minute sample interval. A second nonlinear rate-limiting filter restricts the rate of change of the sen-sor signal during segments when the sensor output is changing at a nonphysiologic rate and, therefore, behaves with an artifact-filtering action. A third filter performs data smoothing and decimation to produce the 5-minute sensor signal ready for calibration. The total system delay during steady-state operation is 3 minutes, where the delay associated with the nonlinear filter increases at a rate proportional to the rate of rising and falling transient effects.
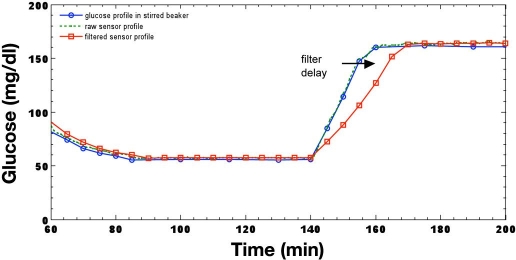
Figure 3 illustrates the results of an in vitro investigation26 that recreated a clamp study27 to illustrate recovery from a hypoglycemic clamp produced by a step-like response using a well-stirred beaker and a glucose infusion pump. One glucose point is used to calibrate the sensor following preprocessing, which includes filtering sensor current before it is used to calculate glucose. Clearly, glucose rises at a nonphysiologic rate from approximately 60 to 160 mg/dl in around 20 minutes, which equates to an average rate of 5 mg/dl/minute. The nonlinear filter of the CGMS algorithm extends the overall delay at this rate, from a 3-minute delay to approximately 10 minutes, as a result of the fast glucose excursion, where the preceding filters have total delays of 30 seconds and 2.5 minutes.
Figure 3.
In vitro reproduction of a hypoglycemic glucose profile processed by the CGMS.
Guardian RT
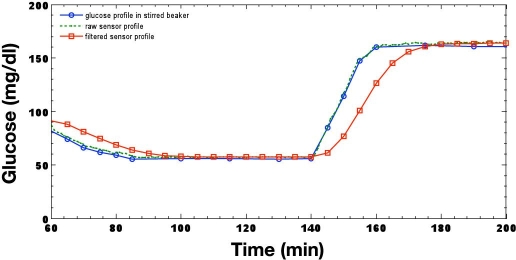
The Guardian RT system converts sensor current to a proportional frequency with a current-to-frequency-measuring transducer. The resultant pulse train, which are pulses output at a rate proportional to sensor current, is counted and accumulated over a 1-minute interval. This 1-minute signal is processed by a seventh-order finite impulse response filter,22 which is a moving average filter, and decimated to a 5-minute sample time interval. The 5-minute signal undergoes further smoothing by a moving average filter, producing a net delay of 8.25 minutes. The Guardian RT algorithm output is illustrated in Figure 4 for the same glucose step function applied previously to the CGMS algorithm, with sensor signals calibrated with a single reference point. Unlike the nonlinear filter described previously for the CGMS device, this filter is linear; therefore, delay is not a function of frequency. The delay, however, is no longer the group delay, or half the time required for a sample to transverse through the filter weights, but is determined by the step response of the filter, which is the integral of its impulse response.22 The delay, thus, is not constant and in this example exceeds the group delay of 8.25 minutes when reaching steady state. The step, or edge, response is nonphysiologic and, although rare, will occur in some circumstances—for example, during filter initialization or when a significant artifact is present in the sensor signal.
Figure 4.
In vitro reproduction of a hypoglycemic glucose profile processed by the Guardian RT.
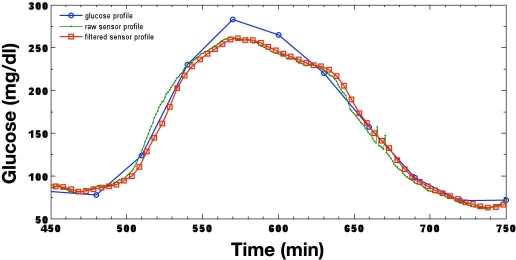
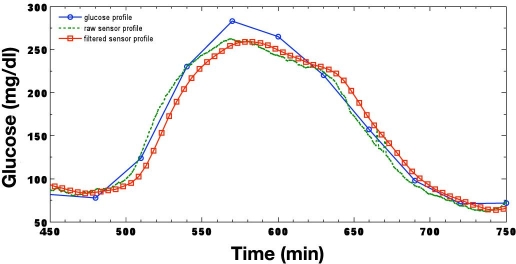
The output of the CGMS and Guardian RT algorithms following the processing of a typical meal–response curve is illustrated in Figure 5 and Figure 6, respectively. Pre- and postprandial glucose excursions from approximately 75 mg/dl at 480 minutes to a peak exceeding 275 mg/dl at 570 minutes produce an average glucose rate of change of over 2.2 mg/dl/minute. Glucose reaches its nadir of 75 mg/dl at 720 minutes, falling at an average rate of greater than 1.3 mg/dl/minute. It is evident from each figure that the CGMS nonlinear filter has very little effect as an artifact has not been detected; likewise, the Guardian RT maintains a constant group delay. The inherit sensor lag time is approximately 1–2 minutes, with a processing filter delay of 5 minutes for the CGMS monitor and 8.5 minutes for the Guardian RT.
Figure 5.
Typical fast meal response when processed by the CGMS.
Figure 6.
Typical fast meal response when processed by the Guardian RT.
Discussion
Noninvasive glucose monitors that interrogate blood vessels to estimate glucose concentration have the potential to one day acquire instantaneous blood glucose measurements in real time with a satisfactory degree of accuracy. This development would remove any ambi-guity associated with the ISF and plasma glucose time delay. As of this publication, there are three manufacturers marketing CGM products in the United States: Medtronic (CGMS Gold, Guardian RT, and Guardian REAL-Time with real-time display), Abbott Diabetes Care (FreeStyle Navigator system), and DexCom (SEVEN), all of which are based on a minimally invasive percutaneously inserted sensor lasting 3–7 days. Completely noninvasive technologies have been slow to come to market but are clearly more desir-able. Each of these devices on the U.S. market employs an enzymatic sensor inserted subcutaneously in the abdominal area. Differences in reported delays for each device can most likely be attributed to the degree of smoothing performed in the data acquisition hardware and calibration algorithm. The approximate total latency for these devices in reporting blood glucose is around 8–15 minutes, which includes sensor reaction time, signal-processing delays, and ISF-to-plasma glucose equilibration time. The ISF glucose time lag is in the region of 5–10 minutes, which is an acceptable delay for real-time monitoring. Additionally, each monitor calculates and displays a glucose rate-of-change indicator, allowing patients to understand how quickly their glucose level is digressing. This rate-of-change parameter also enables the use of predictive alarms, by which patients are alerted of impending hyper- or hypoglycemic events and can, thus, take the appropriate action.
Presented examples include the CGMS Gold and Guardian RT algorithms' filter responses to a transition from an in vitro hypoglycemic clamp to a hyperglycemic clamp, as well as responses to quick glucose excursions following a rapidly absorbed meal. In each algorithm described, the sensor output includes the preprocessed sensor signal, which has a degree of smoothing by digital filtering and, therefore, has an additive filter group delay. This signal is calibrated and the resultant sensor glucose can be downloaded. Digital filters have a particular impulse response and corresponding group delay. This group delay can be seen during normal operation. However, when subjected to edge or step responses, a much larger delay is created. The filter step response function—the integral of the filter impulse response—demonstrates greater delays, which are often seen during signal transitions. This response is also seen during filter initialization and is observed in some circumstances when nonphysiologic characteristics exist, such as those created through movement artifact. Nonlinear filters incorporated in the CGMS Gold device are intended to filter artifacts and only switch on when signal characteristics are beyond what is physiologically possible. This feature was demonstrated by a fast meal response and a nonphysiologic glucose step function. The total delay of each system is unaffected by a reasonably fast meal response. However, caution is advised when using the sensor in nonphysiologic conditions, such as a fast transition from one steady state to another. Furthermore, investigations performed in vitro are limited and do not replicate the varying dynamics seen in vivo and, therefore, can only demonstrate sensor delay.
Conclusion
The overall physiologic lag can be ∼3–12 minutes, whereas the intrinsic electrochemical sensor lag is 1–2 minutes. Delays are due to the diffusion of glucose across the capillary endothelial barrier and the glucose rate-limiting membrane and are constant. Underlying this conclusion is the fact that diffusion is a linear process—meaning that the response to a 10-mg/dl change in glucose will have the identical shape as the response to a 100-mg/dlchange in glucose, the time to reach 67% of the maximal response being identical in both cases. Sensor device delays are partitioned into three components: the sensor per se, the device filters, and the physiologic delay. Software programs reduce unwanted noise in sensor signals, which can be the more prominent delay. However, the delay may or may not be constant.
Sensor signals are noisy and require digital filtering. This noise is generally physiologic in origin, with an insig-nificant level of correlated white Gaussian noise likely generated from electronics. Lower noise levels require less filtering and, therefore, present smaller time delays. Although filtering is often necessary, predictive alerts can be used to overcome signal-processing delays and physiological time lags. However, in closed-loop systems, it is desirable to reduce delays due to slow subcutaneous insulin kinetics. To achieve this, a type of inverse, or adaptive, filter is required that could attempt to compensate for the physiological time lag while reducing noise.
Abbreviations
- BG
blood glucose
- CGM
continuous glucose monitoring
- CGMS
continuous glucose monitoring system
- FDA
Food and Drug Administration
- HbA1c
hemoglobin A1c
- ISF
interstitial fluid
- SCGM1
subcutaneous continuous glucose monitoring system
- SMBG
self-monitoring of blood glucose
References
- 1.The Diabetes Control and Complications Trials Research Group. The effect of intensive treatment of diabetes on the development and progression of long term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329(14):977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 2.Mastrototaro JJ. The MiniMed continuous glucose monitoring system. J Pediatr Endoncrinol Metab. 1999;12:751–758. [PubMed] [Google Scholar]
- 3.Bode BW, Gross TM, Thornton KR, Mastrototaro JJ. Continuous glucose monitoring used to adjust diabetes therapy improves glycosylated hemoglobin: a pilot study. Diabetes Res Clin Pract. 1999;46(3):183–189. doi: 10.1016/s0168-8227(99)00113-8. [DOI] [PubMed] [Google Scholar]
- 4.Gross TM, Bode BW, Einhorn D, Kayne DM, Reed JH, White NH, Mastrototaro JJ. Performance evaluation of the MiniMed continuous glucose monitoring system dur-ing patient home use. Diabetes Technol Ther. 2000;2(1):49–56. doi: 10.1089/152091500316737. [DOI] [PubMed] [Google Scholar]
- 5.Bode B, Gross K, Rikalo N, Schwartz S, Wahl T, Page C, Gross T, Mastrototaro J. Alarms based on real-time sensor glucose values alert patients to hypo- and hypergly-cemia: the guardian continuous monitoring system. Diabetes Technol Ther. 2004;6(2):105–113. doi: 10.1089/152091504773731285. [DOI] [PubMed] [Google Scholar]
- 6.Deiss D, Bolinder J, Riveline JP, Battelino T, Bosi E, Tubiana-Rufi N, Kerr D, Phillip M. Improved glycemic control in poorly controlled patients with type 1 diabetes using real-time continuous glucose monitoring. Diabetes Care. 2006;29(12):2730–2732. doi: 10.2337/dc06-1134. [DOI] [PubMed] [Google Scholar]
- 7.Garg S, Zisser H, Schwartz S, Bailey T, Kaplan R, Ellis S, Jovanovic L. Improvement in glycemic excursions with a transcutaneous, real-time continuous glucose sen-sor: a randomized controlled trial. Diabetes Care. 2006;29(1):44–50. doi: 10.2337/diacare.29.01.06.dc05-1686. [DOI] [PubMed] [Google Scholar]
- 8.Bode B, Silver M, Weiss R, Martin K. Evaluation of a continuous glucose monitoring system for home-use conditions. Managed Care. 2008;17(8):40–45. [PubMed] [Google Scholar]
- 9.Heinemann L. Glucose Monitoring Study Group. Continuous glucose monitoring by means of the microdialysis technique: underlying fundamental aspects. Diabetes Technol Ther. 2003;5(4):545–561. doi: 10.1089/152091503322250578. [DOI] [PubMed] [Google Scholar]
- 10.Poscia A, Mascini M, Moscone D, Luzzana M, Caramenti G, Cremonesi P, Valgimigli F, Bongiovanni C, Varalli M. A microdialysis technique for continuous subcuta-neous glucose monitoring in diabetic patients (part 1) Biosens Bioelectron. 2003;18(7):891–898. doi: 10.1016/s0956-5663(02)00216-6. [DOI] [PubMed] [Google Scholar]
- 11.Varalli M, Marelli G, Maran A, Bistoni S, Luzzana M, Cremonesi P, Caramenti G, Valgimigli F, Poscia A. A microdialysis technique for continuous subcutaneous glucose moni-toring in diabetic patients (part 2) Biosens Bioelectron. 2003;18(7):899–905. doi: 10.1016/s0956-5663(02)00215-4. [DOI] [PubMed] [Google Scholar]
- 12.Jungheim K, Wientjes KJ, Heinemann L, Lodwig V, Koschinsky T, Schoonen AJ. Glucose Monitoring Study Group. Subcutaneous continuous glucose monitoring: feasi-bility of a new microdialysis-based glucose sensor system. Diabetes Care. 2001;24(9):1696–1697. doi: 10.2337/diacare.24.9.1696. [DOI] [PubMed] [Google Scholar]
- 13.Chuang H, Taylor E, Davison TW. Clinical evaluation of a continuous minimally invasive glucose flux sensor placed over ultrasonically permeated skin. Diabetes Technol Ther. 2004;6(1):21–30. doi: 10.1089/152091504322783378. [DOI] [PubMed] [Google Scholar]
- 14.Tamada JA, Garg S, Jovanovic L, Pitzer KR, Fermi S, Potts RO. Noninvasive glucose monitoring: comprehensive clinical results. Cygnus Research Team. JAMA. 1999;282(19):1839–1844. doi: 10.1001/jama.282.19.1839. [DOI] [PubMed] [Google Scholar]
- 15.Tierney MJ, Tamada JA, Potts RO, Eastman RC, Pitzer K, Ackerman NR, Fermi SJ. The GlucoWatch biographer: a frequent automatic and noninvasive glucose monitor. Ann Med. 2000;32(9):632–641. doi: 10.3109/07853890009002034. [DOI] [PubMed] [Google Scholar]
- 16.Chase HP, Beck R, Tamborlane W, Buckingham B, Mauras N, Tsalikian E, Wysocki T, Weinzimer S, Kollman C, Ruedy K, Xing DA. Randomized multicenter trial comparing the GlucoWatch Biographer with standard glucose monitoring in children with type 1 diabetes. Diabetes Care. 2005;28(5):1101–1106. doi: 10.2337/diacare.28.5.1101. [DOI] [PubMed] [Google Scholar]
- 17.Tamada JA, Davis TL, Leptien AD, Lee J, Wang B, Lopatin M, Wei C, Wilson D, Comyns K, Eastman RC. The effect of preapplication of corticosteroids on skin irrita-tion and performance of the GlucoWatch G2 Biographer. Diabetes Technol Ther. 2004;6(3):357–367. doi: 10.1089/152091504774198052. [DOI] [PubMed] [Google Scholar]
- 18.Thome-Duret V, Reach G, Gangnerau MN, Lemonnier F, Klein JC, Zhang Y, Hu Y, Wilson GS. Use of a subcutaneous glucose sensor to detect decreases in glucose concentration prior to observation in blood. Anal Chem. 1996;68(21):3822–3826. doi: 10.1021/ac960069i. [DOI] [PubMed] [Google Scholar]
- 19.Wentholt IM, Hart AA, Hoekstra JB, Devries JH. Relationship between interstitial and blood glucose in type 1 diabetes patients: delay and the push-pull phenomenon revis-ited. Diabetes Technol Ther. 2007;9(2):169–175. doi: 10.1089/dia.2006.0007. [DOI] [PubMed] [Google Scholar]
- 20.Rebrin K, Steil GM. Can interstitial glucose assessment replace blood glucose measurements? Diabetes Technol Ther. 2000;2(3):461–472. doi: 10.1089/15209150050194332. [DOI] [PubMed] [Google Scholar]
- 21.Steil GM, Rebrin K, Hariri F, Jinagonda S, Tadros S, Darwin C, Saad MF. Interstitial fluid glucose dynamics during insulin-induced hypoglycemia. Diabetologia. 2005;48(9):1833–1840. doi: 10.1007/s00125-005-1852-x. [DOI] [PubMed] [Google Scholar]
- 22.Smith SW. 2nd ed. CA: California Technical Publishing; 2003. The scientist and engineer's guide to digital signal processing. [Google Scholar]
- 23.Feldman B, Brazg R, Schwartz S, Weinstein R. A continuous glucose sensor based on wired enzyme technology–results from a 3-day trial in patients with type 1 diabe-tes. Diabetes Technol Ther. 2003;5(5):769–779. doi: 10.1089/152091503322526978. [DOI] [PubMed] [Google Scholar]
- 24.Weinstein RL, Schwartz SL, Brazg RL, Bugler JR, Peyser TA, McGarraugh GV. Accuracy of the 5-day FreeStyle Navigator Continuous Glucose Monitoring System: comparison with frequent laboratory reference measurements. Diabetes Care. 2007;30(5):1125–1130. doi: 10.2337/dc06-1602. [DOI] [PubMed] [Google Scholar]
- 25.Schoemaker M, Andreis E, Roper J, Kotulla R, Lodwig V, Obermaier K, Stephan P, Reuschling W, Rutschmann M, Schwaninger R, Wittmann U, Rinne H, Kontschieder H, Strohmeier W. The SCGM1 System: subcutaneous continuous glucose monitoring based on microdialysis technique. Diabetes Technol Ther. 2003;5(4):599–608. doi: 10.1089/152091503322250613. [DOI] [PubMed] [Google Scholar]
- 26.Voskanyan G, Keenan DB, Mastrototaro JJ, Steil GM. Putative delays in interstitial fluid (ISF) glucose kinetics can be attributed to the glucose sensing systems used to measure them rather than the delay in ISF glucose itself. J Diabetes Sci Technol. 2007;1(5):639–644. doi: 10.1177/193229680700100507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Monsod TP, Flanagan DE, Rife F, Saenz R, Caprio S, Sherwin RS, Tamborlane WV. Do sensor glucose levels accurately predict plasma glucose concentrations during hypoglycemia and hyperinsulinemia? Diabetes Care. 2002;25(5):889–893. doi: 10.2337/diacare.25.5.889. [DOI] [PubMed] [Google Scholar]