Abstract
The corticospinal system (CS), critical for controlling skilled movements, develops during the late prenatal and early postnatal periods in all species examined. In the cat, there is a sequence of development of the mature pattern of terminations of corticospinal tract axons in the spinal gray matter, followed by motor map development of the primary motor cortex. Skilled limb movements begin to be expressed as the map develops. Development of the proper connections between CS axons and spinal neurons in cats depends on CS neural activity and motor behavioral experience during a critical postnatal period. Reversible CS inactivation or preventing limb use produces an aberrant distribution of CS axon terminations and impairs visually guided movements. This altered pattern of CS connections after inactivation in cats resembles the aberrant pattern of motor responses evoked by transcranial magnetic stimulation in hemiplegic cerebral palsy patients. Left untreated, these impairments do not resolve. We have found that activity-dependent processes can be harnessed in cats to reestablish normal CS connections and function. This finding suggests that CS connectivity and function might some day be restored in hemiplegic cerebral palsy.
Keywords: Motor cortex, spinal cord, postnatal, cat, human, motor control, reaching, grasping, visually-guided locomotion, cerebral palsy, hemiplegia
Introduction
A key question is the extent to which motor development reflects a sequence of events that is largely independent of an individual’s particular experiences. Early-developing brain stem motor tracts (Martin et al. 1980) and spinal circuits, the latter of which have been shown to depend on transcriptional codes (Jessell, 2000; Lee and Pfaff, 2001), could mediate the simple postural responses and rhythmic behaviors that comprise most of a neonate’s motor repitoire. The late-developing corticospinal tracts (Martin et al. 1980; Martin, 2005), also dependent on a transcriptional code for corticospinal neuron differentiation (Arlotta et al. 2005; Molyneaux et al. 2005), could mediate the more complex and integrated movements that are expressed in older infants. Genetically-timed sequences of motor pathway and circuit development could set the stage for an experience independent elaboration of controlled movements that humans, and animals alike, show during early postnatal life.
Alternatively, does early motor development depend on motor experience and other activity-dependent processes? Neurons in the developing central nervous system are active (Shatz, 1990), including motor control centers (Wenner and O’Donovan, 2001), thereby opening the possibility for important activity-dependent developmental processes. We know that the molecular guidance cues governing the formation of topographic sensory maps in the brain function in cooperation with the activity of sensory neurons (McLaughlin and O’Leary, 2005). Moreover, learning of new skills is a life-long function of the motor systems. Early motor system development may rely on active learning and experience to help direct the myriad of structural changes taking place, especially after motor pathway axons grow to their targets.
Surprisingly, little is known of the importance of motor experience and activity-dependent processes in shaping development of the motor system and the behaviors they control. We have made this complex question tractable by focusing on the corticospinal (CS) system and development of some of the movements it controls. The CS system is the principal system in humans for controlling skilled limb movement (Porter and Lemon, 1993). This system largely develops postnatally as human infants and many animal species begin to express adaptive and visually-guided movements. As the last motor system to develop, and the one that develops in parallel with a neonate’s expanding motor repertoire, the CS system is an excellent candidate for examining the influence of activity-dependent processes.
In this review, we focus on our research in the cat, a species that, like humans, has motor skills that improve during a protracted postnatal period (for more comparative discussions on development of the CS system in monkeys and rodents, see (Terashima, 1995; Olivier et al. 1997; Martin, 2005)). We begin with a brief consideration of the normal development of CS axon terminals in the spinal cord and then consider activity- and use-dependent processes shaping CS development and skilled motor behavior. Next, we discuss our recent work showing how activity-dependent processes can be harnessed to restore CS connections and motor function after the system becomes dysfunctional early in development. Finally, we close by considering how findings in the animal can provide a substrate upon which to plan rehabilitative strategies in patients with cerebral palsy.
Normal development of CS terminations in the spinal cord
Work in rodents has examined differentiation of CS neurons (Arlotta et al. 2005; Molyneaux et al. 2005) and guidance of their axons to the spinal cord (Terashima, 1995; Dottori et al. 1998; Joosten and Bar, 1999; Coonan et al. 2001; Liu et al. 2005). Evidence shows that these processes are driven by transcriptional codes and intrinsic factors in the developing nervous system. CS axons within the white matter send collateral branches into the spinal gray matter, which may be under the control of EphA4-Ephrin signaling (Canty et al. 2006). The pattern of terminations of CS axons in the spinal gray matter established initially during early postnatal development bares little resemblance to the pattern later in development and maturity (Li and Martin, 2000, 2001). In the cat, individual collateral branches have a broad dorsoventral (i.e., laminar) distribution until 6-7 weeks of age. Figure 1A shows an example of CS terminal labeling after anterograde tracer injection into the primary motor cortex (M1) in an immature cat (5 weeks of age). This injection was centered in the rostral M1 subregion, which preferentially projects ventrally in the spinal gray matter (Li and Martin, 2000). CS axon terminations extend bilaterally throughout the intermediate zone and ventral horn. The inset in part A show terminations after an injection into the caudal M1 subregion, which preferentially projects dorsally. The combined projections from rostral and caudal M1 cover most of the contralateral and a substantial portion of the ipsilateral gray matter. By 7-8 weeks of age, the pattern of CS terminations is refined. While the two subregions maintain preferential dorsal and ventral distributions in maturity (Figure 1B and inset), the overall projection is restricted to the deeper dorsal horn layers and the upper ventral horn. The sustaining ipsilateral terminations after week 8 are located ventromedially (lamina 8). Transient terminations, present in a particular area early in development but subsequently eliminated, extend into the ipsilateral gray matter (Cabana and Martin, 1985; Theriault and Tatton, 1989; Alisky et al. 1992), and in the rodent, well into the white matter (Curfs et al. 1994, 1995), where they may be synapsing on distal dendrites of motoneurons and interneurons. Note that in rats and cats there are transient terminations in the motor nuclei during development but few are maintained in maturity (Li and Martin, 2000; Salimi and Martin, 2004; Kamiyama et al. 2006). In vitro studies in the rat have shown that pruning of CS connections in the cord is NMDA receptor-dependent (Takuma et al. 2002; Ohno et al. 2004).
Figure 1.
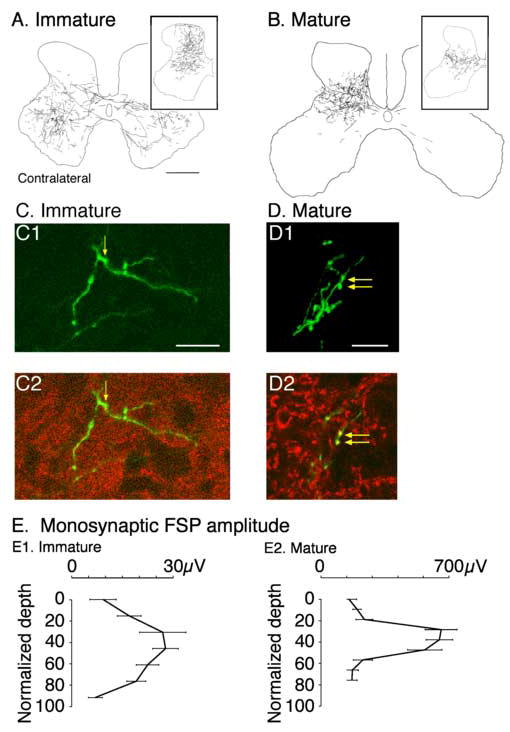
Development of the CS tract axon spinal terminations in the cervical cord of the cat. A. Terminations in 5 week animal, showing a bilateral pattern. Tracer was injected into the rostral M1 subregion, which results in a ventral bias to the termination field. The inset shows an example of terminations from the caudal M1, which has a dorsal bias. B. Distribution of terminations in the adult. The main figure shows terminations from caudal M1 and the inset, from rostral M1 (Li and Martin, 2000). C. Confocal micrographs of immature (C) and mature (D) CS terminal. Part 1 of each panel is a projection image of the labeled terminal. The labeled terminal is green. Part 2 shows 1 μm optical slices within the projection image. Red corresponds to synaptophysin immuno-label and green, anterograde axonal label. Double labeled presynaptic boutons are indicated by the yellow arrows; yellow corresponds to overlay of green and red labeling (Meng et al. 2004). E. Dorsoventral distribution of the CS monosynaptic focal synaptic potential (FSP). Note that the potential is smaller and dorsoventrally more extensive in the immature animal (Meng and Martin, 2003). Calibrations. A, B. 1 mm; C. 25 μm; D. 50 μm.
As transient branches are eliminated, there is a parallel increase in local branching and presynaptic sites in the terminals that survive (Figure 1 C, D) (Li and Martin, 2002; Meng and Martin, 2003). In the cat, further local CS axon terminal growth and synapse formation occurs between weeks 7 and 11, and probably continues later (Li and Martin, 2001). In the monkey, late outgrowth continues for several years postnatally (Armand et al. 1997). This late CS axon terminal growth, together with late maturation of conduction along the CS tract (Koh and Eyre, 1988) and elaboration of the cortical motor map (Chakrabarty and Martin, 2000), underlies the highly protracted period of CS system development.
Development of local branching and presynaptic sites leads to stronger connections with spinal target neurons. The strength of connections between CS axon terminals and spinal neurons in the cat has been revealed by stimulating CS axons in the pyramidal tract and recording focal monosynaptic potentials in the spinal gray matter. In immature animals, stimulation of CS axons evokes responses throughout the dorsoventral gray matter, although the amplitude of the postsynaptic responses is largest in the middle layers (Figure 1E1). By contrast, in mature animals (Figure 1E2) responses are largely limited to the middle layers only. Moreover, there is a remarkable increase in the amplitude of responses in maturity.
It is misleading to think that the presence of postsynaptic responses in the young animal after pyramidal stimulation or the human after TMS indicates that the CS system is functional at a very young age. Although electrical stimulation of the CS system in immature cats can evoke spinal responses (Meng and Martin, 2003), the responses are small and insufficient to evoke muscle responses unless the electrical stimulation currents are very high (Meng and Martin, 2003). What can account for the subsequent development of strong functional connections between CS terminals and spinal neurons? Not only do CS axon terminals develop more branches and presynaptic sites during early postnatal life, but there is also an enhanced capacity to summate descending control signals. Using pairs of electrical stimuli to CS axons in the pyramid or trains of stimuli of varying duration, we recorded the evoked spinal and muscle responses (Meng et al. 2004). We found that the spinal postsynaptic response to the second of a pair of stimuli was larger at all ages, termed facilitation, and that facilitation increases with age. With stronger facilitation as the corticospinal system matures, M1 neurons can activate spinal motor circuits with lower levels of neural activity.
Activity- and use-dependent development of CS connections
As an animal matures, some CS axon branches are pruned, which leads to more focused activation of spinal circuits. Other axon branches grow denser terminals and develop more presynaptic sites, which leads to stronger connections with spinal motor circuits and, together with stronger facilitation, a greater capacity of the CS system to regulate spinal motor circuit functions. This is development of connectional specificity, the process determining the particular circuits a corticospinal neuron engages, and thus the neuron’s motor control functions. What drives this refinement process? Does this reflect the playing out of an activity-independent genetic or transcriptional program or is there an important dependence on neural activity in the developing motor systems and the animal’s particular motor experiences? We have begun to study the role of neural activity and experience in shaping CS system development. To examine the generalized role of activity, we have conducted experiments in which the level of CS activity was either decreased or increased during early postnatal development.
We decreased CS system activity between weeks 5 and 7, the period when the distribution of CS axons in the gray matter normally is refined, by infusion of the GABAA agonist Muscimol into M1. This inactivation is reversible in 2-3 days after cessation of the infusion. This inactivation impairs the contact placing reaction but does not noticeably impair generalized use of the limbs contralateral to the inactivation. We examined the effect of this inactivation on development of the distribution of CS axon terminations in the spinal cord (Martin et al. 1999). Unilateral inactivation changes the distribution of CS axon terminals from both the silenced as well the contralateral active side. Figure 2 contrasts the differences in projections from the silenced cortex (A1; contralateral) and the active cortex (A4; bilateral) in mature animals after reversible inactivation from 5-7 weeks. Early silenced corticospinal axons fail to maintain axons within territories normally occupied by CS axons, which is consistent with activity-dependent competition between the silenced and active CS projections. As we shall see below, this aberrant pattern correlates with motor control impairments. These CS terminals also have fewer branches and presynaptic boutons (Figure 2A2, A3) (Friel and Martin, 2005). In contrast, the contralateral active system develops an extensive contralateral projection and also maintains significant ipsilateral terminations in the intermediate zone and dorsal horn (Figure 2A4). The sparse ipsilateral CS terminations that are normally present in the ventromedial gray matter are thought to serve axial muscle control (Armand and Kuypers, 1980). After inactivation, ipsilateral terminations from the active side may have additional functions because they terminate in lateral zones for controlling limb muscles. Thus, the reduction in terminations of the silenced side is balanced on that side by maintenance of ipsilateral terminations of the active system (Martin et al. 1999). Importantly, left untreated, these topographic changes persist into maturity, as do the motor control impairments produced by the inactivation (see below).
Figure 2.
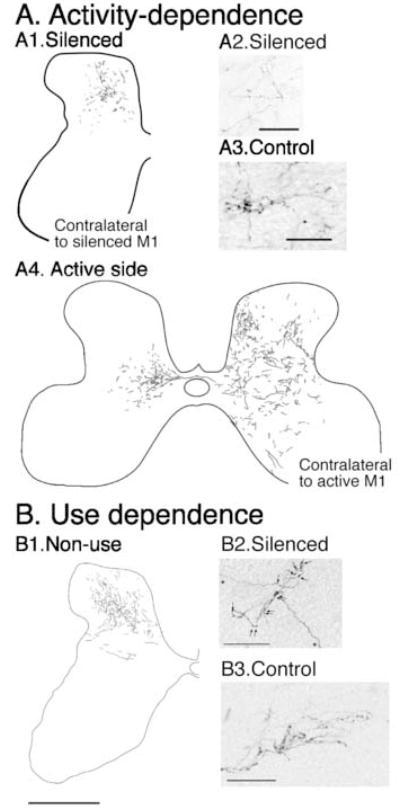
Activity (A)- and use-dependent (B) development of CS terminals in the cervical cord. A1. Terminations from the silenced M1. A2, 3. Morphology of silenced and control CS terminations. A4. Terminations from the active M1. (Friel and Martin, 2005) B1. Terminations on the non-used side of the cervical cord. B2, 3. Morphology of non-used and control CS terminations. (Martin et al. 2004) Calibrations. A1. 1 mm (bar in B1); A2, A3. 50 μm; B1. 1 mm; B2, B3. 50 μm.
The anatomical changes we see after inactivation parallel changes in CS system organization in cerebral palsy patients with spastic hemiplegia. In this condition, transcranial magnetic stimulation (TMS) of the motor strip contralateral to the hemiparetic limb produces minimal effects at elevated thresholds (Carr et al. 1993), suggestive of a functionally reduced contralateral CS tract projection. By contrast, TMS of the undamaged cortex produces bilateral motor effects at normal thresholds, which suggests development of strong contralateral projections as well as the maintenance of ipsilateral projections (Farmer et al. 1991; Carr et al. 1993; Eyre et al. 2001).
When the motor cortices on the two sides are both inactivated a contralateral pattern of terminations develops but the overall density of terminations is less than expected (Martin and Lee, 1999). This suggests that changes in the CS terminal distribution caused by activity blockade are due to activity-dependent competition between developing CS terminals and other spinal neural systems. We propose that refinement of the CS termination pattern depends, in part, on competitive synaptic interactions among CS terminations and between CS and other spinal terminals. We envisage these activity-dependent interactions to stem from tonic levels of spontaneous M1 activity, phasic activity reflecting motor control signaling, and sensory input reflecting limb movement.
Our findings show that the active side maintains bilateral terminations at the expense of the silenced side. Another way to examine the role of activity on the two sides in shaping the pattern of terminations is to electrically stimulate CS axons to augment activity unilaterally. CS axons collect in the medullary pyramid before projecting into the spinal cord; thus, electrical stimulation at this site is an efficient way to promote activity of the system. We stimulated CS axons in the pyramid during the refinement period (weeks 5-7) for two hours each day, using a stimulus pattern that would phasically activate spinal motor circuits every two seconds (Salimi and Martin, 2004). This stimulation resulted in maintenance of transient ipsilateral (and contralateral) terminations at 8 weeks (Figure 3A). Normally, there are predominantly contralateral terminations at this age (Figure 3B). We also found that projections from the non-stimulated side were displaced dorsally as a consequence of this stimulation into a territory with less dense ipsilateral CS terminations during early development (Figure 3C). Maintenance of transient terminations on the stimulated side and dorsal displacement of non-stimulated axons are both consistent with the activity-dependent competition model: the stimulated axons are more competitive at maintaining and securing new synaptic space at the expense of the non-stimulated axons.
Figure 3.
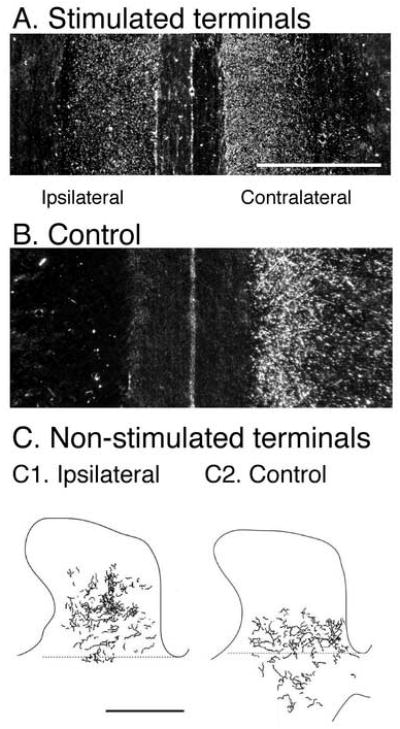
Effects of CS system electrical stimulation on developing CS axon terminations. A, B. Horizontal sections through the deep dorsal horn. CS axon terminals are labeled using horseradish peroxidase conjugated to wheat germ agglutinin (white dots). Stimulation (A) results in a bilateral distribution of terminations while only contralateral terminations are in the control (B). C. Effect of unilateral stimulation on the non-stimulated CS system (C1) showing a dorsal shift in the terminations compared with the control (C2). BDA was used to label the non-stimulated axons. (Salimi and Martin, 2004) Calibrations. A, B, C. 1 mm.
These findings show the importance of the level of neural activity—and in the case of the stimulation experiments, possibly the particular pattern—in shaping the early postnatal refinement of CS terminations. An important question not resolved by these experiments is how the pattern of limb use affects CS axon refinement. We prevented limb use in kittens by injecting Botulinum toxin A (BTX) into several forelimb muscles (Martin et al. 2004). Muscle weakening and limb disuse were maintained between weeks 3 and 7 by weekly BTX injections. We found that, like M1 activity blockade, preventing limb use prevents the late developmental growth of CS axon terminals and presynaptic sites (Figure 2B). As with activity blockade, when limb use is regained later in development CS axon terminals do not recoup lost connections (Martin et al. 2004). Preventing limb use produces the same aberrant pattern of contralateral CS terminations as M1 activity blockade suggesting that animals do not activate CS neurons controlling the weakened limb.
Early postnatal activity blockade and preventing limb use, which both lead to aberrant CS connections in the spinal cord, also lead to motor control impairments. When M1 neural activity returned after the blockade, animals expressed two impairments. One impairment is over-reaching the target during reaching and the second impairment is that animals are not able to coordinate the grasp effectively once the target is contacted (Martin et al. 2000). During visually-guided locomotion an overstepping is produced by M1 inactivation that is analogous to over-reaching (discussed further below)(Friel et al. 2004). In the case of preventing limb use, once the effects of BTX wore off, animals showed a grasping impairment only (Martin et al. 2004). Without intervention, these motor control impairments—similar to the aberrant CS connectivity—do not resolve. While aberrant CS connectivity in the spinal gray matter correlates with the motor control impairments, this does not preclude a contribution by other components of the motor systems. For example, over-reaching after M1 inactivation is similar to the classic sign of hypermetria after cerebellar dysfunction. It is plausible that this impairment also reflects aberrant development of cerebellar circuitry, as a consequence of aberrant CS system development.
Normal and Use Development of the M1 map
A characteristic feature of M1 is the representation of body muscles, or the motor map (Porter and Lemon, 1993). While transcranial magnetic stimulation has been used to study development of the CS projection to the cord in humans and monkeys (Eyre et al. 1991; Nezu et al. 1997; Olivier et al. 1997), development of the motor representation has only been studied in the cat (Chakrabarty and Martin, 2000). The motor map can be assessed using microstimulation, whereby a microelectrode is used to excite a small population of cortical neurons.
In the cat the motor representation is first detected at about 2 months in the cat (Bruce and Tatton, 1980; Chakrabarty and Martin, 2000). Prior to this time, M1 stimulation does not evoke motor responses (Bruce and Tatton, 1980). This corresponds to the early period of axon growth into the gray matter and refinement of connections. During the following month, there is an increase in the percentage of sites from which stimulation evokes a motor response with a concomitant decrease in the current threshold ((Chakrabarty and Martin, 2000); Figure 4A). There is also an elaboration of the motor map, from initially representing only proximal muscles to one representing all forelimb joints. As kittens reach maturity, there is also a higher percentage of stimulation sites from which effects at multiple joints are produced. These multijoint sites could play a role in encoding interjoint synergies. The proximal to distal progression in motor map development is reminiscent to the proximal to distal control strategy of human infants during arm movement development (Berthier et al. 1999).
Figure 4.
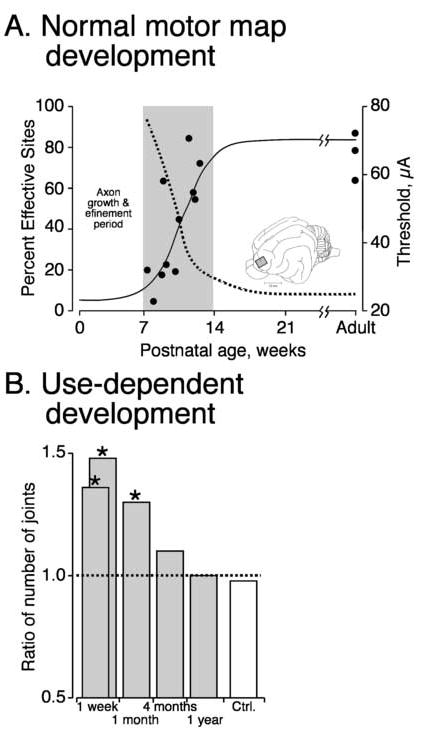
Development of the motor map in the M1. A. Reduction in threshold for evoking movement by microstimulation (dotted line) and the increase in effective sites (solid line) with age.(Chakrabarty and Martin, 2000) B. effect of prehension training between weeks 7 and 11 on the incidence of sites where stimulation produced muscle contraction around multiple contralateral forelimb joints (Martin et al. 2005).
Many features of motor map development in the cat can be modified by early motor experiences during the period of map formation (between weeks 7 and 14). By promoting early experiences with prehension training, the electrical threshold for evoking responses decreased, while the percentages of effective sites and multi-joint sites (Figure 4B; 1 week) both increased (Martin et al. 2005). In contrast, preventing limb experience during development of the motor map increased the threshold for evoking responses and decreased the percentages of effective sites and multi-joint sites. These changes in map organization revert to control values several months after normal experience returns (Figure 4B; note reduction in bar height from 1 month to 1 year)(Martin et al. 2005). This return back to control levels reflects plasticity that persists throughout life, as suggested by studies in the rat (Kleim et al. 2003).
Harnessing CS system activity to promote function
Activity- and use-dependent growth of CS axon terminals during an early postnatal period sets the stage for further growth and synapse formation later in development. This late growth is necessary for establishing strong connections between M1 and spinal motor circuits and for development of skilled limb control. Without CS neural activity or limb use during early development, CS tract terminations to the spinal cord gray matter are altered, axon terminals and synapses are reduced, and there are significant limb control impairments. That these defects do not significantly ameliorate later in development is consistent with the synaptic competition hypothesis proposed earlier that, once disadvantaged, CS neurons are unable to regain lost connections and functions.
Is there no way to improve connections after an early period of reduced activity or limb disuse or can activity- and use-dependent competition be harnessed later in development so that disadvantaged CS axons regain lost connectivity to restore function? To examine this question we focused on the anatomical and behavioral defects that are produced by inactivation of M1 between weeks 5-7. This is a clinically relevant experimental model because, as discussed earlier, the anatomical changes after this inactivation closely parallel changes in CS system organization in humans with hemiplegic cerebral palsy. The goal of our experiments is to augment the connections and functions of the previously silenced CS system. In the context of the CS synaptic competition hypothesis, augmenting the competitive advantage of the silenced CS connections could be achieved in either of two ways. First, stimulation of the previously silenced CS system will increase the activity of its spinal terminations, which, we propose, would enhance their competitive advantage in securing and maintaining synaptic contacts. Second, inactivation of the contralateral CS system, which was active between weeks 5-7, would silence both its aberrant ipsilateral connections as well as the contralateral terminals. We propose that this late inactivation reduces the competitive advantage of the previously active system in maintaining existing synaptic connections, as well as competing for new synaptic space. While inactivation or stimulation would affect the CS system at all levels, the spinal gray matter is potentially a critical locus for synaptic interactions between the previously silenced side and the aberrant ipsilateral terminations of the active side since it is a site for bilateral terminations (Figure 2A1, A4). We have chosen the second approach to examine the question of reducing the competitive capacity of the contralateral active CS system. We infused muscimol into the previously active M1 (Friel et al. 2006). We specifically targeted the inactivation to occur during the period of late CS axonal growth in the spinal cord, weeks 8-11. Earlier we had shown that there is substantial local axon terminal outgrowth during this late developmental period (Li and Martin, 2001).
M1 in the left hemisphere was reversibly inactivated during the topographic refinement period, weeks 5-7. This produces the anatomical and behavioral defects described above; this CS system is termed the impaired CS system. Next, we inactivated the right M1 during the late growth period, weeks 8-11. The right side is termed the alternate inactivated system. Figures 5A and B show the distribution of CS terminations in 15 week old animals with different M1 activity manipulations. The CS terminations of fifteen week old untreated animals (Figure 5C) appear mature in distribution and morphology. Part A shows the dorsal distribution of terminations of the impaired CS system in the contralateral spinal cord after the 5-7 week inactivation only (also see Figure 1A1 for another example). After the alternate inactivation (B) there is a remarkable redistribution of axons from dorsal laminae to ventral laminae (note density of labeling in the dashed box). CS axon distribution after the alternate inactivation is similar to control (C). Quantification of these results (not shown) indicated a significant reduction in dorsal label and a concomitant increase in ventral label after the alternate inactivation. Importantly, this topographic redistribution was accompanied by an increase in presynaptic bouton density on CS axons within the deeper laminae that was not significantly different from age-matched controls. An increased bouton density is likely to lead to stronger connections.
Figure 5.
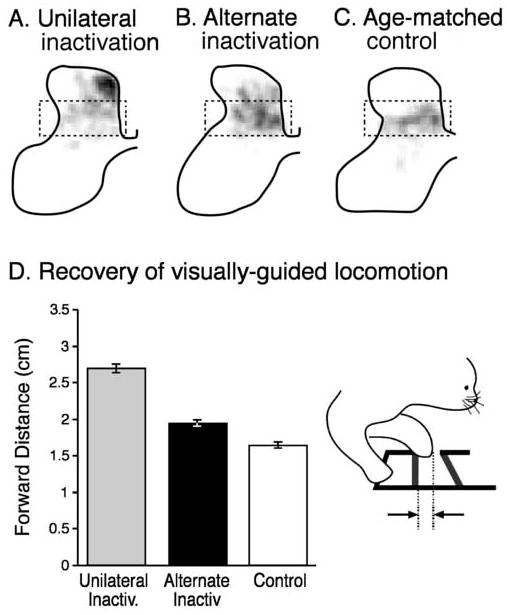
Alternate inactivation restores connectivity and function. A, B, C. Distribution of contralateral CS axon terminal label in 15 week old animals shown as a gray scale, where white is no label and black is maximal label per square micrometer. The same gray scale was used for each part. The normal distribution is highlighted by the dashed box, which corresponds to the control (C). Unilateral inactivation results in dorsal label (A) while after the alternate inactivation (B), label is more ventral, like the control. D. Effect of alternate inactivation on forward step distance. Inset illustrates the step measurement. (Friel et al. 2006).
In addition to normalizing the dorsoventral distribution of CS axon terminal labeling, the alternate inactivation resulted in a remarkable amount of restoration of function. We studied visually-guided locomotion, which has been shown in cats to depend on an intact CS tract (Drew et al. 1996; Drew et al. 2002). Moreover, neurons in M1 modulate their activity in association with an animal’s guided responses (Drew, 1993). Cats were trained to walk along a horizontal ladder that required precise placement of the paw on the rungs. We used high-speed video to measure paw placement on the ladder rung (see inset to Figure 5D). After unilateral inactivation of M1 between weeks 5 and 7, there is a permanent impairment in placement of the forepaw contralateral to the inactivation (Figure 5D; light gray bar). Animals consistently overstep the rungs of the horizontal ladder. Overstepping can be sufficiently great to cause the animals to slip off the rung. After the period of alternate inactivation, the amount of overstep was significantly reduced (i.e., closer to normal) (Figure 5D; black bar). These findings show that activity-dependent processes later in development can be harnessed to restore a more normal pattern of CS connectivity and function.
While the mechanism underlying the motor recovery is not yet known, reestablishing CS terminations into the intermediate zone likely allows for greater expression of M1 function. Motor circuit interneurons are located here, including premotor interneurons (Baldissera et al. 1981). Normally, dense, and presumably strong, CS terminations in this region may enable descending CS control signals to specify the pattern of activation of particular forelimb muscles (i.e., motor synergy) necessary for determining and stabilizing the endpoint of the movement. Sparse connections to these motor circuits could impede this function; restored dense connections to these circuits could lead to recovery.
Overall conclusions
Role of activity-dependent processes in development of the CS system
Activity independent processes, such as combinatorial transcriptional codes for CS neuronal specification (Lee and Pfaff, 2001; Arlotta et al. 2005; Molyneaux et al. 2005) and guidance cues for axon pathfinding (Tessier-Lavigne and Goodman, 1996), play key roles in the initial development of the CS system. The role activity-independent processes in development of the CS projection to the spinal cord is only beginning to be understood (Dottori et al. 1998; Joosten and Bar, 1999; Coonan et al. 2001; Liu et al. 2005). After CS axon terminals contact their spinal (and brain stem) targets, activity-dependent processes are key to refining connections and establishing the mature pattern of topographic and connectional specificity. In the developing retino-tectal (superior colliculus) system, activity-independent processes driven by multiple gradients of guidance molecules establish a coarse topographic patterns of connections that is refined by spontaneous waves of activity of retinal ganglion neurons (McLaughlin and O’Leary, 2005). Presumably, a similar mechanism operates in the developing CS system. Activity- and experience-dependent mechanisms assure that neural events at the time of motor circuit formation play a critical role in the long-term function of the system.
While in the visual system there are topographic maps in the target fields, there is no evidence for such mapping between the CS tract and spinal motor circuits. Moreover, the primary visual cortex and the visual midbrain serve a relatively limited set of visual and visuomotor functions. The spinal target field of the CS projection is functionally diverse, including somatic sensory processing circuits, precerebellar networks, motor circuit interneurons and, in some species, motoneurons (Porter and Lemon, 1993). Thus, establishing connectional specificity between M1 and the spinal cord is apt to be more complicated than in vision, because of the diversity of targets.
Relationship between the cat CS developmental model and human CS system impairments
The pattern of bilateral CS projections from the active side after unilateral M1 inactivation in the cat is remarkably similar to changes in the laterality of TMS-evoked motor responses in cerebral palsy patients with spastic hemiplegia after perinatal brain trauma. TMS of the less impaired side in patients evokes bilateral responses (Farmer et al. 1991; Carr et al. 1993; Eyre et al. 2001). By contrast, strokes in adults that produce hemiparesis do not augment the ipsilateral response, showing that the effect in cerebral palsy is linked to damage during early development, possibly at a time when the CS system has bilateral connections with the cord (Eyre et al. 2001). These findings are consistent with the hypothesis that the impaired side is rendered much less competitive in securing and maintaining spinal synaptic space than the normal or less impaired side. Cerebral palsy is a condition in which the child “grows into” the impairment because spastic hemiplegia is not expressed immediately after the perinatal traumatic event (Bouza et al. 1994). Eyre and colleagues propose that the damaged side progressively looses capacity to control motor circuits during development (Eyre, 2003). Thus, cerebral palsy may be progressive during early development as CS terminations are competing for synaptic targets and the hemiplegic signs are becoming expressed. Later in development when the competitive balance between the two sides stabilizes, in favor of the undamaged side, the motor signs stabilize.
Prospects for harnessing activity to restore CS connections and function in cerebral palsy
We showed that alternate M1 inactivation significantly augmented the density of ventral CS terminations on the affected side of the cord (i.e., inactivated during the critical refinement period) and significantly restored more accurate visually-guided locomotion. Bilateral CS synaptic interactions onto common neural circuits, combined with a protracted period of development of local connectivity, establish conditions conducive to connectional plasticity and rehabilitation. The period of alternative inactivation in our experiments was timed to coincide with the late period of local CS axonal growth in the spinal gray matter (Li and Martin, 2001). We propose that the spinal intermediate zone is a key site of convergence of contralateral projections from the affected side and aberrant ipsilateral terminations from the unaffected side. However, other sites where ipsilateral CS projections develop after inactivation, such as the red nucleus and reticular formation (Martin et al. 1999), could be affected by the alternate inactivation treatment.
An important issue that is not yet resolved is the extent to which the alternate inactivation, in addition to promoting contralateral projections, also reduces the efficacy of the aberrant ipsilateral terminations from the unaffected side. From a clinical perspective, is it sufficient to promote a normal topography of CS terminations or must the aberrant ipsilateral cortical terminations additionally be diminished? These ipsilateral fibers terminate both laterally and medially in the gray matter, where they could contact premotor circuits for limb and axial control, respectively. The aberrant ipsilateral projection—especially the lateral one—is likely to be limited in control and flexibility. In the cat, the ipsilateral projection after inactivation is predominantly comprised of branches of CS axons projecting contralaterally (Martin et al. 1999); and this may be the case in hemiplegic cerebral palsy (Farmer et al. 1991; Carr et al. 1993; Eyre et al. 2001). Thus, the control signals carried by these branches are likely to be adaptive for contralateral control and maladaptive for ipsilateral control. Indeed, motor unit activity in cerebral palsy patients can be strongly correlated between the two sides and mirror movements are common (Farmer et al. 1991; Carr et al. 1993). Whether maladaptive or not, the aberrant ipsilateral projection may be the dominant CS input to the affected side of the cord. And this may be better than not having any projections. We showed that the aberrant ipsilateral terminations help to mediate more effective control (Martin et al. 2000). Earlier, we found that when the aberrant ipsilateral terminations are inactivated during a reaching and grasping task, performance—which is already dysmetric—deteriorates further. Nevertheless, a reasonable working hypothesis is that it is first essential to restore the normal topographic pattern in the hopes of restoring appropriate connectivity. And the cat model shows that one way this can be done is to block activity on the other side. Preventing use of the limb on the unimpaired side may be similar to M1 activity reduction. Preliminary experiments suggest that electrical stimulation of the affected side can also lead to a re-distribution of connections from dorsal to more ventral laminae (Salimi et al. 2006). Importantly, our study shows that an intervention is needed to restore function; one that is specifically targeted to enhance the ability of the affected CS projections to compete with other terminations to secure synaptic space on spinal neurons. Without such an intervention, the disadvantaged CS system is unable to catch up and regain lost connections and function.
Our findings in the cat lead to two considerations for devising rehabilitation strategies in patients with cerebral palsy or other conditions that affect development of CS system connectivity. First, it is essential to synchronize an activity-based therapy with CS developmental periods. The refinement period for CS axon topography in humans may be within the first year of life when the ipsilateral motor potentials evoked by TMS diminish to their smallest values (Eyre et al. 2001). Intervention during this period could have the most robust effects. However, it is important to recognize that immature CS neurons are very plastic and preventing activity on the unimpaired side may impede motor development on that side, similar to what we showed in kittens that were prevented from using one forelimb (Figure 2B). Here, lessons have been learned from the treatment of children with strabismus, where controlled daily monocular deprivation leads to improved performance of the impaired eye without deterioration of the well-sighted eye (Mitchell et al. 2003). A human analog to the later period of local refinement—characterized in animals by laminar augmentation of the density and strength of connections (Armand et al. 1997; Li and Martin, 2001)—may extend until early to mid adolescence. This is because mature TMS response parameters are not observed until these older ages (Eyre et al. 1991; Nezu et al. 1997; Eyre et al. 2000). Intervention during the late developmental period may be akin to the period we examined with the alternate inactivation paradigm in the cat (Figure 5). Second, constraint induce movement therapy (CIMT) may be necessary to permit sufficient plasticity on the affected side. In CIMT, the less-affected arm is restrained to encourage the patient to use the affected arm in tasks. Many CIMT protocols combine constraint with training of the affected side (Gordon et al. 2005). Manipulations of CS neural activity can be achieved non-invasively in humans. Repetitive TMS, at lower frequencies than the electrical stimulation used in the cat, could augment activity levels. Moreover, particular TMS parameters have been shown to suppress the contralateral M1 (Ferbert et al. 1992; Kujirai et al. 1993). Thus, repetitive TMS to one side of M1 would balance activity between the two sides by concurrently activating the stimulated side and inhibiting the contralateral side.
Our studies show, for the first time, the importance of CS neural activity and limb use in determining the normal pattern of connections between the M1 and the spinal cord and, in turn, normal skilled motor development. By developing a mechanistic understanding of CS system development, striking parallels between aberrant development in the cat and aberrant development in the human emerge. While the activity-dependence hypothesis is remarkably effective in explaining aberrant connectivity, it does not exclude important interactions between activity-dependent and activity-independent mechanisms, which have not been explored in the developing CS system. Our results in animals suggest that, with a deeper understanding of CS development, some day an effective treatment can be achieved for cerebral palsy patients.
Acknowledgments
Supported by grants from the NIH, March of Dimes Birth Defects Foundation, United Cerebral Palsy Foundation, Christopher Reeve Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- Alisky JM, Swink TD, Tolbert DL. The postnatal spatial and temporal development of corticospinal projections in cats. Exp Brain Res. 1992;88:265–276. doi: 10.1007/BF02259101. [DOI] [PubMed] [Google Scholar]
- Arlotta P, Molyneaux BJ, Chen J, Inoue J, Kominami R, Macklis JD. Neuronal subtype-specific genes that control corticospinal motor neuron development in vivo. Neuron. 2005;45:207–221. doi: 10.1016/j.neuron.2004.12.036. [DOI] [PubMed] [Google Scholar]
- Armand J, Kuypers HGJM. Cells of origin of crossed and uncrossed corticospinal fibers in the cat. A quantitative horseradish peroxidase study. Exp Brain Res. 1980;40:23–34. doi: 10.1007/BF00236659. [DOI] [PubMed] [Google Scholar]
- Armand J, Olivier E, Edgley SA, Lemon RN. Postnatal development of corticospinal projections from motor cortex to the cervical enlargement in the macaque monkey. J Neurosci. 1997;17:251–266. doi: 10.1523/JNEUROSCI.17-01-00251.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldissera F, Hultborn H, Illert M. Integration in spinal neuronal systems. In: Brooks VB, editor. Handbook of Physiology, Section I: The Nervous System, Vol II, Motor Control. American Physiological Society; Bethesda: 1981. pp. 509–596. [Google Scholar]
- Berthier NE, Clifton RK, McCall DD, Robin DJ. Proximodistal structure of early reaching in human infants. Exp Brain Res. 1999;127:259–269. doi: 10.1007/s002210050795. [DOI] [PubMed] [Google Scholar]
- Bouza H, Dubowitz LM, Rutherford M, Pennock JM. Prediction of outcome in children with congenital hemiplegia: a magnetic resonance imaging study. Neuropediatrics. 1994;25:60–66. doi: 10.1055/s-2008-1071587. [DOI] [PubMed] [Google Scholar]
- Bruce IC, Tatton WG. Synchronous development of motor cortical output to different muscles in the kitten. Exp Brain Res. 1980;40:349–353. doi: 10.1007/BF00237802. [DOI] [PubMed] [Google Scholar]
- Cabana T, Martin GF. Corticospinal development in the North-American opossum: Evidence for a sequence in the growth of cortical axons in the spinal cord and for transient projections. Dev Brain Res. 1985;23:69–80. doi: 10.1016/0165-3806(85)90007-0. [DOI] [PubMed] [Google Scholar]
- Canty AJ, Greferath U, Turnley AM, Murphy M. Eph tyrosine kinase receptor EphA4 is required for the topographic mapping of the corticospinal tract. Proc Natl Acad Sci U S A. 2006;103:15629–15634. doi: 10.1073/pnas.0607350103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr LJ, Harrison LM, Evans AL, Stephens JA. Patterns of central motor reorganization in hemiplegic cerebral palsy. Brain. 1993;116:1223–1247. doi: 10.1093/brain/116.5.1223. [DOI] [PubMed] [Google Scholar]
- Chakrabarty S, Martin JH. Postnatal development of the motor representation in primary motor cortex. J Neurophysiol. 2000;84:2582–2594. doi: 10.1152/jn.2000.84.5.2582. [DOI] [PubMed] [Google Scholar]
- Coonan JR, Greferath U, Messenger J, Hartley L, Murphy M, Boyd AW, Dottori M, Galea MP, Bartlett PF. Development and reorganization of corticospinal projections in EphA4 deficient mice. J Comp Neurol. 2001;436:248–262. [PubMed] [Google Scholar]
- Curfs MHJM, Gribnau AAM, Dederen PJWC. Selective elimination of transient corticospinal projections in the rat cervical spinal cord gray matter. Dev Brain Res. 1994;78:182–190. doi: 10.1016/0165-3806(94)90025-6. [DOI] [PubMed] [Google Scholar]
- Curfs MHJM, Gribnau AAM, Dederen PJWC, Bergervoet-Vernooij HWM. Transient functional connections between the developing corticospinal tract and cervical spinal interneurons as demonstrated by c-fos immunohistochemistry. Dev Brain Res. 1995;87:214–219. doi: 10.1016/0165-3806(95)00058-l. [DOI] [PubMed] [Google Scholar]
- Dottori M, Hartley L, Galea M, Paxinos G, Polizzotto M, Kilpatrick T, Bartlett PF, Murphy M, Kontgen F, Boyd AW. EphA4 (Sek1) receptor tyrosine kinase is required for the development of the corticospinal tract. Proc Natl Acad Sci U S A. 1998;95:13248–13253. doi: 10.1073/pnas.95.22.13248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew T. Motor Cortical activity during voluntary gait modfications in the cat 1 cell related to the forelimbs. J Neurophysiol. 1993;70:179–199. doi: 10.1152/jn.1993.70.1.179. [DOI] [PubMed] [Google Scholar]
- Drew T, Jiang W, Widajewicz W. Contributions of the motor cortex to the control of the hindlimbs during locomotion in the cat. Brain Res Brain Res Rev. 2002;40:178–191. doi: 10.1016/s0165-0173(02)00200-x. [DOI] [PubMed] [Google Scholar]
- Drew T, Jiang W, Kably B, Lavoie S. Role of the motor cortex in the control of visually triggered gait modifications. Can J Physiol Pharmacol. 1996;74:426–442. [PubMed] [Google Scholar]
- Eyre JA. Development and plasticity of the corticospinal system in man. Neural Plast. 2003;10:93–106. doi: 10.1155/NP.2003.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyre JA, Miller S, Ramesh V. Constancy of central conduction delays during development in man: investigation of motor and somatosensory pathways. J Physiol (Lond) 1991;434:441–452. doi: 10.1113/jphysiol.1991.sp018479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyre JA, Miller S, Clowry GJ, Conway EA, Watts C. Functional corticospinal projections are established prenatally in the human foetus permitting involvement in the development of spinal motor centres. Brain. 2000;123:51–64. doi: 10.1093/brain/123.1.51. [DOI] [PubMed] [Google Scholar]
- Eyre JA, Taylor JP, Villagra F, Smith M, Miller S. Evidence of activity-dependent withdrawal of corticospinal projections during human development. Neurology. 2001;57:1543–1554. doi: 10.1212/wnl.57.9.1543. [DOI] [PubMed] [Google Scholar]
- Farmer SF, Harrison LM, Ingram DA, Stephens JA. Plasticity of central motor pathways in children with hemiplegic cerebral palsy. Neurology. 1991;41:1505–1510. doi: 10.1212/wnl.41.9.1505. [DOI] [PubMed] [Google Scholar]
- Ferbert A, Priori A, Rothwell JC, Day BL, Colebatch JG, Marsden CD. Interhemispheric inhibition of the human motor cortex. J Physiol (Lond) 1992;453:525–546. doi: 10.1113/jphysiol.1992.sp019243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friel K, Martin JH. Role of sensory-motor cortex activity in postnatal development of corticospinal axon terminals in the cat. J Comp Neurol. 2005;485:43–56. doi: 10.1002/cne.20483. [DOI] [PubMed] [Google Scholar]
- Friel K, Drew T, Martin JH. Sensorimotor cortex inactivation during a brief early postnatal period in cats produces persistent ladder-walking deficits. Society Neuroscience Abstracts 2004 [Google Scholar]
- Friel K, Sist B, Martin JH. Rebalancing corticospinal activity promotes recovery of motor skill and anatomical integrity after inactivation during a critical period. Society Neuroscience Abstracts 559.515 2006 [Google Scholar]
- Gordon AM, Charles J, Wolf SL. Methods of constraint-induced movement therapy for children with hemiplegic cerebral palsy: development of a child-friendly intervention for improving upper-extremity function. Arch Phys Med Rehabil. 2005;86:837–844. doi: 10.1016/j.apmr.2004.10.008. [DOI] [PubMed] [Google Scholar]
- Jessell TM. Neuronal specification in the spinal cord: inductive signals and transcriptional codes. Nat Rev Genet. 2000;1:20–29. doi: 10.1038/35049541. [DOI] [PubMed] [Google Scholar]
- Joosten EA, Bar DP. Axon guidance of outgrowing corticospinal fibres in the rat. J Anat. 1999;194(Pt 1):15–32. doi: 10.1046/j.1469-7580.1999.19410015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiyama T, Yoshioka N, Sakurai M. Synapse elimination in the corticospinal projection during the early postnatal period. J Neurophysiol. 2006;95:2304–2313. doi: 10.1152/jn.00295.2005. [DOI] [PubMed] [Google Scholar]
- Kleim JA, Bruneau R, Calder K, Pocock D, VandenBerg PM, MacDonald E, Monfils MH, Sutherland RJ, Nader K. Functional organization of adult motor cortex is dependent upon continued protein synthesis. Neuron. 2003;40:167–176. doi: 10.1016/s0896-6273(03)00592-0. [DOI] [PubMed] [Google Scholar]
- Koh TH, Eyre JA. Maturation of corticospinal tracts assessed by electromagnetic stimulation of the motor cortex. Arch Dis Child. 1988;63:1347–1352. doi: 10.1136/adc.63.11.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujirai T, Caramia MD, Rothwell JC, Day BL, Thompson PD, Ferbert A, Wroe S, Asselman P, Marsden CD. Corticocortical inhibition in human motor cortex. J Physiol (Lond) 1993;471:501–519. doi: 10.1113/jphysiol.1993.sp019912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SK, Pfaff SL. Transcriptional networks regulating neuronal identity in the developing spinal cord. Nat Neurosci. 2001;4(Suppl):1183–1191. doi: 10.1038/nn750. [DOI] [PubMed] [Google Scholar]
- Li Q, Martin JH. Postnatal development of differential projections from the caudal and rostral motor cortex subregions. Exp Brain Res. 2000;134:187–198. doi: 10.1007/s002210000454. [DOI] [PubMed] [Google Scholar]
- Li Q, Martin JH. Postnatal development of corticospinal axon terminal morphology in the cat. J Comp Neurol. 2001;435:127–141. doi: 10.1002/cne.1197. [DOI] [PubMed] [Google Scholar]
- Li Q, Martin JH. Postnatal development of connectional specificity of corticospinal terminals in the cat. J Comp Neurol. 2002;447:57–71. doi: 10.1002/cne.10203. [DOI] [PubMed] [Google Scholar]
- Liu Y, Shi J, Lu CC, Wang ZB, Lyuksyutova AI, Song XJ, Zou Y. Ryk-mediated Wnt repulsion regulates posterior-directed growth of corticospinal tract. Nat Neurosci. 2005;8:1151–1159. doi: 10.1038/nn1520. [DOI] [PubMed] [Google Scholar]
- Martin GF, Cabana T, Culberson JL, Curry JJ, Tschismadia I. The early development of corticobulbar and corticospinal systems. Studies using the North American opossum. Anat Embryol. 1980;161:197–213. doi: 10.1007/BF00305344. [DOI] [PubMed] [Google Scholar]
- Martin JH. The corticospinal system: From development to motor control. The Neuroscientist. 2005;11:161–173. doi: 10.1177/1073858404270843. [DOI] [PubMed] [Google Scholar]
- Martin JH, Lee S. Activity-dependent competition between developing corticospinal terminations. NeuroReport. 1999;10:2277–2282. doi: 10.1097/00001756-199908020-00010. [DOI] [PubMed] [Google Scholar]
- Martin JH, Kably B, Hacking A. Activity-dependent development of cortical axon terminations in the spinal cord and brain stem. Exp Brain Res. 1999;125:184–199. doi: 10.1007/s002210050673. [DOI] [PubMed] [Google Scholar]
- Martin JH, Hacking A, Donarummo L. Impairments in prehension produced by early postnatal sensorimotor cortex activity blockade. J Neurophysiol. 2000;83:895–906. doi: 10.1152/jn.2000.83.2.895. [DOI] [PubMed] [Google Scholar]
- Martin JH, Engber D, Meng Z. Effect of forelimb use on postnatal development of the forelimb motor representation in primary motor cortex of the cat. J Neurophysol. 2005;93:2822–2831. doi: 10.1152/jn.01060.2004. [DOI] [PubMed] [Google Scholar]
- Martin JH, Choy M, Pullman S, Meng Z. Corticospinal development depends on experience. J Neurosci. 2004;24:2122–2132. doi: 10.1523/JNEUROSCI.4616-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin T, O’Leary DD. Molecular gradients and development of retinotopic maps. Annu Rev Neurosci. 2005;28:327–355. doi: 10.1146/annurev.neuro.28.061604.135714. [DOI] [PubMed] [Google Scholar]
- Meng Z, Martin JH. Postnatal development of corticospinal synaptic actions. J Neurophysol. 2003;90:683–692. doi: 10.1152/jn.00152.2003. [DOI] [PubMed] [Google Scholar]
- Meng Z, Li Q, Martin JH. The transition from development to motor control function in the corticospinal system. J Neurosci. 2004;24:605–614. doi: 10.1523/JNEUROSCI.4313-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell DE, Kind PC, Sengpiel F, Murphy K. Brief daily periods of binocular vision prevent deprivation-induced acuity loss. Curr Biol. 2003;13:1704–1708. doi: 10.1016/j.cub.2003.09.026. [DOI] [PubMed] [Google Scholar]
- Molyneaux BJ, Arlotta P, Hirata T, Hibi M, Macklis JD. Fezl is required for the birth and specification of corticospinal motor neurons. Neuron. 2005;47:817–831. doi: 10.1016/j.neuron.2005.08.030. [DOI] [PubMed] [Google Scholar]
- Nezu A, Kimura S, Uehara S, Kobayashi T, Tanaka M, Saito K. Magnetic stimulation of motor cortex in children: Maturity of corticospinal pathway and problem of clinical application. Brain Dev. 1997;19:176–180. doi: 10.1016/s0387-7604(96)00552-9. [DOI] [PubMed] [Google Scholar]
- Ohno T, Maeda H, Sakurai M. Regionally specific distribution of corticospinal synapses because of activity-dependent synapse elimination in vitro. J Neurosci. 2004;24:1377–1384. doi: 10.1523/JNEUROSCI.3903-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivier E, Edgley SA, Armand J, Lemon RN. An electrophysiological study of the postnatal development of the corticospinal system in the macaque monkey. J Neurosci. 1997;17:267–276. doi: 10.1523/JNEUROSCI.17-01-00267.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter R, Lemon R. Corticospinal function and voluntary movement. Oxford Science; Oxford: 1993. [Google Scholar]
- Salimi I, Martin JH. Rescuing transient corticospinal terminations and promoting growth with corticospinal stimulation in kittens. J Neurosci. 2004;24:4952–4961. doi: 10.1523/JNEUROSCI.0004-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salimi I, Friel K, Sist B, Martin JH. Electrical stimulation of the intact corticospinal tract during development improves visuomotor deficits in kittens. Society Neuroscience Abstracts 2006 [Google Scholar]
- Shatz CJ. Impulse activity and the patterning of connections during CNS development. Neuron. 1990;5:745–756. doi: 10.1016/0896-6273(90)90333-b. [DOI] [PubMed] [Google Scholar]
- Takuma H, Sakurai M, Kanazawa I. In vitro formation of corticospinal synapses in an organotypic slice co-culture. Neuroscience. 2002;109:359–370. doi: 10.1016/s0306-4522(01)00472-9. [DOI] [PubMed] [Google Scholar]
- Terashima T. Anatomy, development and lesion-induced plasticity of rodent corticospinal tract. Neurosci Res. 1995;22:139–161. doi: 10.1016/0168-0102(95)00895-9. [DOI] [PubMed] [Google Scholar]
- Tessier-Lavigne M, Goodman CS. The molecular biology of axon guidance. Science. 1996;274:1123–1133. doi: 10.1126/science.274.5290.1123. [DOI] [PubMed] [Google Scholar]
- Theriault E, Tatton WG. Postnatal redistribution of pericruciate motor cortical projections within the kitten spinal cord. Dev Brain Res. 1989;45:219–237. doi: 10.1016/0165-3806(89)90041-2. [DOI] [PubMed] [Google Scholar]
- Wenner P, O’Donovan MJ. Mechanisms that initiate spontaneous network activity in the developing chick spinal cord. J Neurophysiol. 2001;86:1481–1498. doi: 10.1152/jn.2001.86.3.1481. [DOI] [PubMed] [Google Scholar]


