Abstract
A theatrically-based intervention was given to 122 older adults who took acting lessons twice a week for 4 weeks. The training consisted of multi-modal activities (cognitive-affective-physiological) typically employed in college acting classes. Comparison groups consisted of no-treatment controls and participants instructed in a different performing art, singing. Assessment of effectiveness was performed using a battery of 11 cognitive/affective test measures that included word recall, prose comprehension/recall, word generation, digit-span ability, and problem-solving. It was found that the acting group improved significantly from pretest to posttest over both other groups. Digit-span was the only measure that failed to improve. No aspects of the intervention supplied specific training or practice on the test measures. Previous versions of the intervention with community-dwelling adults had produced similar findings but the current participants were older, less well-educated, and lived in subsidized, primarily low-income, retirement homes.
The maturing of the baby boom generation has prompted increased research into strategies to promote healthy cognitive aging or ameliorate cognitive decline. Two strands of inquiry have been particularly prominent. The first consists of studies in which the participants are trained on a specific cognitive skill such as verbal episodic memory, reasoning, or speed of processing (e.g., Willis, Tennstedt, Marsiske, Ball, Elias, Koepke, et al., 2006). The second strand of research (using both prospective and retrospective paradigms) involves identifying activities that are associated with healthy cognitive aging (for a review, see Small, Hughes, Hultsch & Dixon, 2006; see also, Kramer, Bherer, Colcombe, Dong & Greenough, 2004).
In terms of training studies, many of the early ones focused on mnemonic techniques. For example, using the method of loci, Robertson-Tchabo, Hausman and Arenberg (1976) demonstrated remarkable gains of 79% in list learning ability after five days of intensive training. However, such targeted techniques do not appear to generalize to other memory tasks. In a meta-analysis, Verhaeghen, Marcoen and Goossens (1992) examined an array of studies showing improvements in experimental groups after memory instruction compared to both control and placebo groups, but concluded that, “ . . . the plasticity associated with mnemonic training appears to be largely specific to that training” (p. 249).
Baltes, Willis, Schaie and their colleagues, (e.g., Baltes, Kliegl, & Dittmann-Kohli, 1988; Saczynski, Willis, & Schaie, 2002; Schaie & Willis, 1986a) focused primarily on fluid intelligence because crystallized intelligence has been shown to be less susceptible to aging effects (Schaie & Willis, 1986b). Many of their experiments concerned figural relations and inductive reasoning. In one study, performed as part of Penn State’s Adult Development and Enrichment Project (ADEPT), Baltes and Willis (1982) found that by providing older adults with five one-hour training sessions in either one of these abilities, performance was improved. Furthermore, these improvements were maintained for up to 6 months. An advantage of this approach is that the targeted cognitive abilities are highly specific, allowing the researchers to investigate transfer to near and far measures of cognitive ability. However, Willis (1990) reported that very little far transfer had been observed.
In a large-scale study known as ACTIVE (Advanced Cognitive Training for Independent and Vital Elderly), Ball, Berch, Helmer, Jobe, Leveck, Marsiske, et al. (2002) randomly assigned participants to one of three intervention groups or a no-contact control group. Those in the intervention groups were given instruction designed to enhance one specific cognitive ability (verbal memory, reasoning ability, or speed of processing). Participants were assessed six weeks later and improvements were found in all three areas. Two years later, the researchers reported that, although these improvements had persisted, this cognitive intervention “. . . helped normal elderly individuals to perform better on multiple measures of the specific cognitive ability for which they were trained. It did not, however, demonstrate the generalization of such interventions to everyday performance, at least in the first two years” (p. 2278). Furthermore, training in one ability (e.g., memory) did not increase performance on a different ability (e.g., speed of processing). A follow-up examination of these same participants 5 years later found that the improvements in the trained abilities were still present, especially in those participants who had been given booster training one and three years after the original intervention (Willis et al., 2006).
At least one prominent aging researcher has offered evidence that, despite the positive changes documented in the large number of interventions, the actual overall rate of cognitive decline in older adults does not appear to be reduced (Salthouse, 2006a). On the other hand, Salthouse has also pointed out that interventions may have salutary effects because a temporary boost in cognitive functioning sets a new, higher point from which the slope of decline resumes. Therefore, if there is a point in time at which an older adult can no longer function on his or her own, delaying that point is certainly worthwhile (Salthouse, 2006b). Willis eloquently captured this type of advantage in her statement about the original and the long-term follow-up study of the ACTIVE participants, “The improvements in memory, problem-solving, and concentration after training roughly counteracted the degree of cognitive decline that older people without dementia may experience over a 7 to 14 year period” (Willis, cited in Pease, 2006, p. 2).
The other promising approach to the problem of cognitive decline concerns the relationship between stimulating activities and healthy cognitive aging. For example, Wilson, Mendes De Leon, Barnes, Schneider, Bienias, Evans, et al. (2002) recruited 801 Catholic nuns, priests, and brothers who were over 65 years of age. Before starting, each participant filled out a questionnaire about the frequency of intellectually stimulating leisure pursuits in which they generally engaged (e.g., reading, doing crossword puzzles). Frequency was rated on a 5-point scale with 5 being highest. After five years, it was found that for each additional point on the stimulating activity scale, there was a 33% decrease in the risk of developing Alzheimer’s disease. Indeed, a very wide variety of studies have shown positive relationships between mentally stimulating activities and intellectual functioning (e.g., Christensen & Mackinnon, 1993; Hultsch, Hertzog, Small & Dixon, 1999; Schooler & Malatu, 2001); between social activity and survival (e.g., Glass, Mendes de Leon, Marotolli, & Berkman, 1999; Lennartsson & Silverstein, 2001) and between high levels of mental activity and lower risk of dementia (Friedland, Fritsch, Smyth, Koss, Lerner, Chen et al., 2001; Scarmeas, Levy, Tang, Manly & Stern, 2001; Stones, Dornan, & Kozma, 1989).
Additional indications of the benefits of stimulating lifestyles were found in the Bronx Aging study (Verghese, LeValley, Derby, Kulansky, Katz., Hall, et al., 2006); the Swedish Twins Study (Crowe, Andel, Pedersen, Johansson & Gatz, 2003) and the Chongqing Aging Study (Wang, Zhou, Li, Zhang, Deng, Tang, et al., 2006). (For reviews of the relationship between activity level and successful aging, see Small et al., 2006, see also Salthouse, 2006a).
Some studies have pointed to two specific elements of engagement that might be responsible for cognitive gains: novelty and multi-modal stimulation. Hultsch et al. (1999) presented evidence that novel endeavors (e.g., learning a foreign language) might be responsible for improved performance on such tasks as fact recall, word recall, and story comprehension. Glass, Freedman, Carlson, Hill, Frick, Ialongo, et al., (2004) suggested that multi-modal (i.e., cognitive and affective) stimulation was responsible for the enhanced cognitive performance observed in seniors when they gave math and literacy tutoring to inner-city school children. These studies would seem particularly relevant to the intervention described here, which involves activities that are highly novel and necessarily multi-modal.
Overview of the Intervention
This short (4-week) program of instruction in a specific type of novel, stimulating, multi-modal activity has been repeatedly tested (Noice & Noice, 2006b; Noice, Noice, Perrig-Chiello, & Perrig, 1999; Noice, Noice, & Staines, 2004). The participants perform mental-physical-emotional exercises similar to those given to beginning acting students in college and university theatre programs. The intervention grew directly out of the authors’ long-standing research interest: the cognitive processes of professional actors who must repeatedly learn extremely long roles with absolute precision and retrieve them in real time, night after night, with complete spontaneity (for reviews, see Noice & Noice, 2002, 2006a, 2006c).
The first demonstration that this approach could result in cognitive benefits took place in Basel, Switzerland (Noice et al., 1999). It was one component of a longitudinal study involving retired employees of a large chemical corporation. Over a period of many years, these participants had undergone various types of interventions including vitamin regimes (Perrig, Stähelin, & Perrig-Chiello 1999); weight lifting (Perrig-Chiello, Stähelin & Ehrsam, 1999); and reattribution training (Kaiser, Perrig-Chiello & Perrig, 1999), but theatre training produced the largest gains on standard measures of memory (Noice et al., 1999).
A far larger study in the United States used three groups: theatre, art appreciation (to control for non-content-specific effects) and no-treatment controls (Noice et al., 2004). After four weeks of instruction, those participants studying acting techniques showed significantly greater gains on both cognitive and affective measures compared to no-treatment controls. (The art appreciation group also improved but made fewer significant gains in fewer areas.) The participants in that study were fairly well educated, healthy for their age, lived in their own homes, and were able to travel by themselves to the training sites in large hospital wellness centers. Their average age was 73.7 years.
Therefore, that study left open a number of questions that the present experiment was designed to answer. Are the benefits of theatre training restricted to those relatively healthy older adults who still function well in their own homes, or could seniors (primarily low-income seniors) living in subsidized facilities make similar gains? Would participants almost a full decade older benefit? Would physical problems that inhibited mobility lessen or eliminate the positive effects? Would the fact that the majority of the current participants were less well educated reduce the benefits? Finally, would training in another form of artistic performance (singing) produce similar results, or was it the specific nature of acting training that was responsible for the gains found in our previous interventions? Indeed, the reason the singing group was added to this study was that our previous research had used an art appreciation course as a control to rule out the notion that any stimulating course in a social situation would produce the benefits that acting did. That study left open the question of whether the stimulation offered by another hands-on performing arts program would produce similar benefits. In other words, could the activation inherent in performing in front of ones peers have been responsible for the increase in test scores rather that the specific content of the acting course? To answer this, we chose singing as the performing arts control course because of its practicality; dancing would eliminate many potential participants due to physical requirements, and learning to play a musical instrument involved both a longer time commitment plus the availability of often expensive instruments. However, our voice teacher had a great deal of experience training beginners and verified that noticeable vocal improvement can occur within four weeks. Furthermore, the almost universal appeal of music is undeniable, so singing would seem to offer a pleasurable activity and keep the seniors interested and motivated.
Despite these advantages of singing, many years of research into the acting process has led us to believe that acting contains a unique combination of various elements found in a number of successful aging studies. It is novel, effortful, enjoyable, multi-modal/multi-factorial, and mentally and physically stimulating. It requires participants to truthfully react to fictional situations, an experience that is at the core of the process, Moreover, it encourages bonding in a social situation and is emotionally activating. We are not aware of any other form of leisure activity that encompasses all these elements in such concentrated form. Consequently, we expected to find significant gains in the test scores of the acting group vs. the two control groups.
Method
Participants
Participants were recruited through talks and newsletter announcements in each of four senior housing complexes in the Western suburbs of Chicago. In each venue, participants were randomly assigned to condition. Qualifications for the study consisted of being over 65 years of age, possessing sufficiently good vision (with or without glasses) to read instructional materials that were printed in very large bold type (20 pt.), and being able to move about the training area (although approximately half of the participants used canes, walkers, wheelchairs, or motorized chairs). Minorities were representative of the area population as a whole, but, due to the geographical location, the sample was predominantly Caucasian (95%).
Design
After attending the recruitment talk, interested participants filled out an enrollment form. They were subsequently given the telephone version of the Short Portable Mental Status Questionnaire (Pfeiffer, 1975) to check for existing dementia. The cutoff score for eligibility was that used by Pfeiffer as indicating intact intellectual functioning (up to 2 errors for those with some high school education; only one error allowed for those with education beyond high school). Mean age of the total sample was 81.7 years (Range: 68 - 93; SD = 5.82). Their mean educational level was 12.7 years (Range: 8 - 20; SD = 2.04). The majority (62%) had only a high school education or less.
The entire study protocol had been approved by the Elmhurst College IRB and, before starting, all participants signed informed consent forms. In each venue, participants were randomly assigned to one of three conditions: acting instruction, singing instruction, or no-treatment. (The no-treatment controls were given a complimentary course at a later date for ethical reasons.) Unlike the previous intervention in which a comparison group (art appreciation) was included to control for non content-specific effects, the current comparison group studied a different performing art (singing), to assess whether the specific nature of acting lessons conferred the observed benefits, or whether instruction and practice of other kinds of public performance would achieve similar results. Two qualified instructors with extensive professional and academic credits taught the courses in their respective fields.
Intervention Procedures
The interventions involved a total of 8 twice-weekly, one-hour classes, plus pre- and posttests, making 10 sessions in all. Homework consisted of watching or listening to professional performers (actors or singers) on TV or recordings. To minimize attrition, the participants were told that they would receive $40 at the end of the course if they had attended all sessions. No-treatment controls were tested at the same times, but with no intermediate contact. They were told that they would engage in two testing sessions, four weeks apart, and then receive training at a later date. They were also promised $40 to encourage their return for the posttest.
Theatre course
The theatre instruction consisted of increasingly demanding exercises designed to have participants experience the essence of acting (i.e., to become engrossed in communicating the meaning of the dialogue so that obvious situation-specific cognitive/affective/physiological alterations occurred in their demeanor). Other aspects of acting such as role memorization were not addressed, so the entire intervention could be devoted to practicing the core process. That is, all exercises and scenes used in the course consisted of short, easily learned phrases and were performed with the written scripts in the participants’ hands. Thus the theatre training did not demand any actual memorization.
It was constantly stressed that every individual is unique so that no one could be “better” at doing these exercises than another as long as he or she was honestly trying to obtain the goals inherent in the dramatic situation. As the instructor put it, “Nobody can be as good a you as you.” In this way, a supportive, non-competitive atmosphere was maintained.
Music course
The singing instruction encouraged the same atmosphere and consisted of teaching proper breathing techniques, supervising vocal exercises, and giving out song sheets to refresh participants’ memories for the lyrics of songs known to most Americans (e.g., “Row, Row, Row Your Boat” and “America the Beautiful”) followed by their performance. Each session was more demanding. For example, the preliminary breathing exercises started with counts of four and worked up to counts of twelve. The songs were increasingly longer and more complex, until different groups sang different songs simultaneously, keeping their minds on their own melodies and lyrics while making sure they were maintaining the same rhythm as the other group. In addition, the instructor gave the participants basic information on those aspects of music theory that had direct application to singing. It must be emphasized that no attempt was made to force the same elements into the acting and singing courses. On the contrary, in each case, the content was designed to teach the most important fundamental concepts of that particular discipline, based on the experience of highly qualified instructors.
Main Outcome Measures
Participants were assessed both at pretest and posttest. The test battery consisted of 8 cognitive instruments, plus an affective instrument, a memory controllability index (MCI), and an activity scale. All tests were administered individually, and were experimenter-timed or self-timed, according to the standard instructions for each particular test. The cognitive battery was arranged so that a test of a different type was given between any original task and the delayed version of that task. Our rationale for the choice of instruments was threefold:
each tested ability was important for healthy, independent, living.
the instruments were standard in the field, and previously observed declines in these measures had been shown to have implications for dementia (Bennett, Schneider, Arvanitakis, Kelly, Aggarwal, Shah, et al, 2006)
the tests could be administered in a single session of less than 90 minutes to avoid exhausting the participants or subjecting them to repeated testing sessions.
Word List Recall
This measure consists of 10 words, each belonging to a different category (Morris, Heyman, Mohs, Hughes, van Belle, Fillenbaum, et al., 1989). The experimenter read each word out aloud at the rate of 2 seconds each; after the full list was read, participants performed an immediate recall test. The identical procedure was repeated two more times for a total of three trials, each followed by an immediate recall test. The total number of words correctly recalled on all three trials was the dependent measure.
Delayed Word List Recall
After a time lapse of 4 minutes, a surprise recall test of the previous word list was given.
Category Fluency
The participant was asked to generate as many exemplars as possible of a given category (e.g., animals or fruits/vegetables) in a 60 second time limit (Morris et al., 1989). The total number of unique words was the dependent measure.
Digit Span
(forward and backward - Wechsler, 1987) A sequence of digits of increasing length was read aloud at the rate of one per second. After two failures to correctly reproduce a sequence of the same length, the procedure was discontinued. The total number of digit sequences correctly recalled was the performance measure. The test was repeated with different numbers, but this time the participants had to mentally reverse the digits before answering.
Story Recall Task
The East Boston Memory Test (Albert, Smith, Scherr, Taylor, Evans, & Funkenstein, 1991) is a measure of episodic memory. A short story was read containing 12 key elements. An immediate recall test was given plus a surprise delayed test after 3 minutes.
Problem solving
The instrument used was the Means-End Problem-Solving Procedure (MEPS) by Platt and Spivack (1975). Participants read a series of story stems in which a problem was stated at the beginning and an outcome at the end. The participant’s task was to fill in events that might have taken place between the discovery of the problem and its eventual solution. Participants were given two stories during each untimed test, with order being counterbalanced.
Self-reported Personal Growth
Perception of personal growth was measured using a scale developed by Ryff (1989). Participants rated each of 14 items on a 6-point Likert Scale ranging from “Strongly Disagree” to “Strongly Agree.” We chose this scale because the complete description of the underlying concept provided by the developer suggested that it precisely mirrored the kind of outcome the intervention was designed to foster.
To avoid fatiguing the participants, two additional instruments (one on memory beliefs and one on daily activities) were distributed following the pretest with instructions to fill them out and return them within two days to the front desk of the facility. Sealable envelopes were included to preserve anonymity. These instruments were used only at the pre-intervention stage because the issues of interest were whether current beliefs about memory or currently performed activities could predict improved performance for one or more of the treatment conditions.
Memory Controllability Inventory
This instrument (Lachman, Bandura, Weaver, & Elliott, 1995) contains a number of subscales such as Present Ability; Effort Utility; Inevitable Decrement. An additional question asks the participants to rate the likelihood of their developing Alzheimer’s disease
Lifestyle Activities Questionnaire
(see Wilson et al., 2002). This questionnaire assesses the frequency with which participants engage in information processing activities such as reading books, watching TV, or doing crossword puzzles. It uses a 5-point scale (1 = every day; 5 = once a year or less).
Results
Attrition
A total of 137 participants initially signed up and took the pretest. Although everyone had been informed that the training was available only to those who could attend all eight sessions, individual scheduling problems (sickness, visits from relatives, changed doctor’s appointments, last-minute invitations, etc.) resulted in 8 dropouts from the training: 4 in acting and 4 in singing. Also, 7 no-treatment controls failed to appear for the posttest, although they had been left reminder messages. Thus, over 89% of those who signed up completed the entire intervention, resulting in valid data on both pre- and posttest from 122 participants. (Table 1 presents a sociodemographic breakdown.)
Table 1. Sociodem ographic Characteristics by Group.
| Variables | Theatre N = 42 |
Voice N = 40 |
Control N = 40 |
p-value* |
|---|---|---|---|---|
| Age, y, mean (SD) | 80.24 (6.47) | 82.65 (4.67) | 81.60 (5.96) | .17 |
| Education, y, mean (SD) | ||||
| Years in School | 12.27 (1.42) | 12.78 (2.24) | 12.70 (2.52) | .61 |
| Social Support, mean (SD) | ||||
| Number of Close Relatives | 9.21 (9.41) | 9.47 (8.51) | 11.11 (13.45) | .63 |
| Number of Close Friends | 6.17 (3.20) | 5.79 (4.14) | 7.33 (6.71) | .35 |
| Health, mean (SD) | ||||
| Number of Prescription Drugs Taken Daily |
4.33 (3.67) | 5.00 (4.27) | 4.85 (2.68) | .71 |
| Marital Status | .67 | |||
| Married % | 16.7 | 15.0 | 17.5 | |
| Widowed % | 66.7 | 70.0 | 70.0 | |
| Single/Divorced % | 16.6 | 15.0 | 10.0 | |
| Gender | .48 | |||
| Females % | 81.0 | 82.5 | 90.0 |
Based on one-way ANOVAs and nonparametric tests as appropriate.
Separate analyses of variances (ANOVAs) revealed no significant differences between dropouts and completers in terms of age, F(1,135) = 1.64, p = .20, and educational level, F(1,135) = 2.08, p = .15. Also, a MANOVA was performed on the cognitive pretest measures (immediate and delayed word recall, verbal fluency, forward and backward digit span, immediate and delayed prose comprehension, and problem solving). Once again, no significant differences were found between dropouts and completers, Wilks’ Λ = .96, F(8,128) < 1.0. Of course, with the small number of dropouts (N = 15), these analyses had low power. However, even if the pattern of dropouts had been similar to that seen in other interventions (i.e., that those who felt the training was too difficult had dropped out) it would have tended to inflate the scores of the controls, thus making any bias conservative.
Pretest Differences
The subsequent analyses are based on the scores obtained from the 122 participants who completed both pre- and posttests. In order to assess whether the 3 groups differed in significant ways at pretest, we performed a multivariate analysis of variance (MANOVA) on the 8 cognitive tests. (The intercorrelations between these tests are presented in Table 2.) There was no significant main effect of group, Wilks’ Δ = 0.80, F(16, 224) = 1.62, p > .05. (Pretest means and standard deviations are shown in Table 3.) A second MANOVA was performed to assess pretest differences between groups in memory beliefs (present ability, effort utility, inevitable decrement, Alzheimer’s likelihood); but no significant group differences emerged, F < 1.0. Separate one-way analyses of variances were conducted on personal growth, age, education, number of prescription drugs, number of close friends, number of close relatives, activity scores, and personal growth at pretest, but no group differences were found (ps > .05).
Table 2. Intercorrelations between Cognitive Measures.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
|---|---|---|---|---|---|---|---|---|
| 1. Word Recall (Immediate) |
-- | .72** | .03 | .15 | .43** | .27** | .28** | .13 |
| 2. Word Recall (Delayed) |
.10 | .12 | .44** | .17 | .24** | .16 | ||
| 3. Digit Span (Fwd) | .46** | .12 | .03 | .02 | .24** | |||
| 4. Digit Span (backwd) | .14 | -.08 | -.13 | .21* | ||||
| 5. Verbal Fluency | .23* | .21 | .27** | |||||
| 6. EBM (Immediate) | .69** | .13 | ||||||
| 7. EBM (Delayed) | .10 | |||||||
| 8. Problem Solving | -- | |||||||
N = 122
p < .05
p < .01
Table 3. Pre- and Posttest ANOVA Results for Cognitive Measures by Treatment Condition.
| Pretest | Posttest | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Theatre N = 42 |
Voice N = 40 |
Control N = 40 |
Theatre N = 42 |
Voice N = 40 |
Control N = 40 |
|||||||
| Tests | M | (SD) | M | (SD) | M | (SD) | M | (SD) | M | (SD) | M | (SD) |
| Digit Span Forward | 7.50 | (1.77) | 7.28 | (1.83) | 7.48 | (2.01) | 7.76 | (1.89) | 7.25 | (2.05) | 7.23 | (1.94) |
| Digit Span Backward | 5.31 | (1.63) | 5.63 | (2.17) | 5.23 | (1.70) | 5.62 | (1.87) | 5.43 | (1.63) | 5.53 | (1.83) |
| EBM (immediate) | 7.40 | (2.00) | 7.85 | (2.07) | 8.00 | (2.23) | 9.71 | (1.95) | 9.05 | (1.92) | 8.80~ | (2.17) |
| EBM (delayed) | 7.21 | (1.14) | 8.00 | (2.00) | 7.90 | (2.63) | 9.86 | (1.75) | 8.50* | (2.62) | 8.33* | (2.35) |
| Problem Solving | 6.57 | (3.23) | 6.93 | (3.00) | 7.25 | (2.51) | 9.98 | (2.68) | 7.45** | (2.55) | 6.78** | (2.65) |
| Verbal Fluency | 33.02 | (7.47) | 29.58 | (6.57) | 30.98 | (7.32) | 37.02 | (9.20) | 29.68** | (7.08) | 30.25** | (5.88) |
| Word Recall (immediate) | 20.38 | (3.53) | 18.63 | (3.79) | 18.45 | (3.35) | 24.31 | (3.95) | 20.48** | (4.41) | 19.63** | (3.70) |
| Word Recall (delayed) | 6.52 | (1.94) | 5.45 | (2.52) | 5.38 | (2.22) | 7.83 | (2.19) | 6.08** | (2.73) | 6.13** | (2.12) |
p < .05
p < .01
p = .06
Treatment Effects
To assess treatment effects, we conducted a multivariate analysis of covariance (MANCOVA), with group membership as the independent variable (acting, singing, no-treatment), the 8 cognitive variables at posttest as the dependent variables, and age, education and pretest scores as covariates. Because our participants ranged in age from 68 to 93, the rationale for including age was that is has been identified as a risk factor; we also included age as a covariate in all subsequent analyses. Also, because education has been shown to be a strong predictor of cognitive performance (e.g., Dore, Elias, Robbins, Elias & Brennan, 2007), it was included as a second covariate. MANCOVA results revealed significant differences between the three treatment conditions on the combined dependent variables, Wilks’ Λ = .52, F(16, 204) = 4.86, p < .001, ηp2 = .28. The covariate (education) was significant, Wilks’ Λ = .86, F(8, 102) = 2.02, p = .05, ηp2 = .14 but age was not (p = .81). Follow-up univariate ANCOVAs on each of the dependent measures yielded five significant effects: immediate recall scores, F(2, 109) = 14.59, p <.001, ηp2 = .21; verbal fluency, F(2, 109) = 11.56, p <.001, ηp2 = .18; problem solving, F(2, 109) = 20.20, p <.001, ηp2 = .27; delayed recall scores, F (2, 109) = 4.14, p <.05, ηp2 2 = .07; and the delayed East Boston Memory Test scores, F (2, 109) = 3.98, p <.05, ηp2 = .07. However, results of the immediate East Boston Memory test and digit span tests (forward and backward) were not significant (p > .05).
Planned comparisons (i.e., simple contrasts with acting as the reference category) revealed that, at posttest, the acting group performed significantly better than the no-treatment controls on immediate word recall, problem solving and verbal fluency (p values < .001); the delayed East Boston Memory Test (p < .01); delayed word recall (p < .05); and (marginally) the immediate East Boston Memory Test (p = .06). When comparing the acting group to the singing group, acting also scored significantly higher on the same dependent variables The only exception was the immediate East Boston Memory test, which indicated no differences. (See Table 3.)
Additional Outcome Measures
Personal Growth
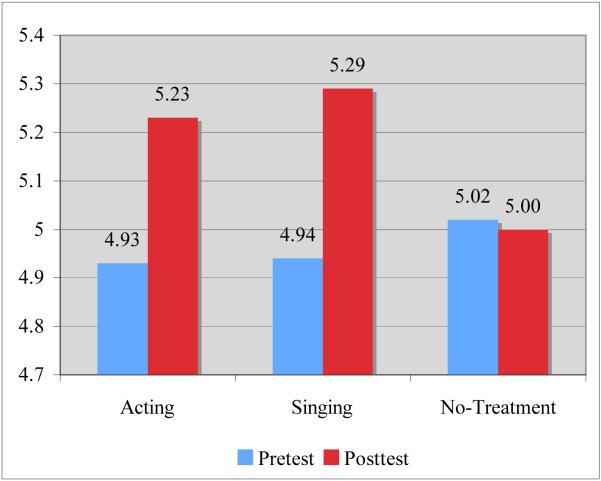
To examine whether participants’ perception of personal growth had increased after the training, we performed a 2 occasion (pretest, posttest) x 3 group membership (acting, singing, no-treatment) mixed design ANOVA. (Means and standard deviations are displayed in Table 4). There was a significant main effect of occasion, F(1, 119) = 13.46, p <.001, ηp2 = .10 indicating that scores increased from pre- to posttest. Importantly, the group by occasion interaction was also significant, F(2,119) = 4.04, p = .02, η2 = .06. To further examine this interaction, three paired sample t-tests (corrected for familywise Type I error) were performed (see Figure 1). Results indicated that participants who received either the acting or singing training showed higher pre- to posttest ratings in personal growth (ps < .01) compared to the no-treatment control participants (p = .86).
Table 4. Improvement in Perso nal Growth from Pre- to Posttest as a Function of Condition.
| Pretest | Posttest | ||||
|---|---|---|---|---|---|
| Group | Mean | SD | Mean | SD | p |
| Theatre | 4.93 | .60 | 5.23 | .61 | .003 |
| Voice | 4.94 | .74 | 5.29 | .59 | .001 |
| Control | 5.02 | .71 | 5.00 | .74 | .859 |
Note: Ratings were made on a 6-point Likert Scale ranging from strongly disagree to strongly agree.
Figure 1.
Increase in perception of personal growth from pre- to posttest as a function of group membership
MCI and Activity Scales
In a series of regressions for the acting group, the 8 cognitive outcome measures were each regressed on memory belief scores (MCI), controlling for age and education as well as the relevant pretest cognitive score. (Descriptive statistics are presented in Table 5.) However, none of the MCI predictors reached significance (data not shown). In a parallel set of 8 regressions, in which total activity score replaced MCI as the predictor, again no significant effects of activity levels on any of the cognitive outcome measures were obtained (all p-values > .20). Descriptive statistics on participation in various activities appear in Table 6 but regression data are not shown.
Table 5. Means and Standard Deviations for Beliefs about Memory Controllability.
| Theatre (N = 42) |
Voice (N = 39) |
Control (N = 39) |
||||
|---|---|---|---|---|---|---|
| Subscales | M | SD | M | SD | M | SD |
| Present Ability | 5.02 | 1.17 | 4.78 | 1.22 | 4.73 | 1.21 |
| Effort Utility | 5.71 | 1.14 | 5.44 | 1.42 | 5.52 | 1.24 |
| Inevitable Decrement | 3.25 | 1.46 | 3.12 | 1.53 | 3.31 | 1.40 |
| Alzheimer’s Likelihood | 3.70 | 1.41 | 3.66 | 1.56 | 3.67 | 1.43 |
Note: Ratings were made on a 7-point Likert Scale ranging from strongly disagree to strongly agree.
Table 6. Activity Scores for Participants by Treatment Conditions.
| Theatre N = 42 |
Voice N = 39 |
Control N = 40 |
||||
|---|---|---|---|---|---|---|
| Type of Activity | M | SD | M | SD | M | SD |
| Listen to the Radio | 4.12 | 1.40 | 3.46 | 1.55 | 3.48 | 1.62 |
| Reading Newspapers | 4.21 | 1.24 | 4.18 | 1.21 | 3.58 | 1.47 |
| Reading Magazines | 3.50 | 0.92 | 3.41 | 1.21 | 3.43 | 1.06 |
| Reading Books | 3.43 | 1.43 | 3.51 | 1.37 | 3.25 | 1.61 |
| Playing Games | 3.64 | 1.16 | 3.77 | 1.16 | 3.13 | 1.57 |
| Visiting Museums | 1.22 | 0.47 | 1.36 | 0.79 | 1.28 | 0.51 |
Note: Activity frequency ranged from 1 (least frequent) to 5 (most frequent).
Discussion
This study set out to extend a successful intervention for community-dwelling older adults to residents of subsidized, primarily low-income, housing. A previous study (Noice et al., 2004) had produced cognitive and affective gains in healthy, fairly well-to-do, community-dwelling seniors. The participants in the current study were older (81.7 yrs. vs. 73.7 yrs.) and less well educated (only 38% of the current participants had one or more years of college compared to 70% in the previous study). Furthermore, approximately half of the current participants used walkers, canes, wheelchairs or motorized chairs, whereas the participants of the previous intervention exhibited 100% unaided mobility. Despite these differences, the intervention produced significant cognitive and affective gains in the acting group. Of the 8 cognitive measures, only the digit-span tests failed to demonstrate improvement. This finding is in keeping with our previous research, and is not totally unexpected because age differences have been shown to be very slight with digit-span tasks (e.g., Craik & Jennings, 1992). In terms of our overall results, it is particularly encouraging that Bennett, Schneider, Arvanitakis, Kelly, Aggarwal, Shah, et al. (2006) found that a decline in certain episodic measures was correlated with the likelihood of Alzheimer’s, yet these were exactly the same measures that increased in this study.
Additionally, with the exception of one measure (Immediate East Boston Memory Test) the pre-posttest improvements found in comparing the acting group to the no-treatment controls were also found when comparing the acting group to the singing group. However, our affective measure, the Ryff Personal Growth scale (which has been shown to generally decline with age; Ryff and Singer, in press) revealed significant increases for both the acting and singing groups. This may possibly be explained by the nature of the singing instruction. The participants appeared to love the course and the new information it offered. For example, during one session, the instructor showed a short video clip on the human vocal apparatus, graphically illustrating how breath pressure causes the vocal cords to vibrate at various pitches. Many participants volunteered the information that they found this look into the anatomy of our vocal system fascinating. Thus, the sense of acquiring new information from a respected professional singer and professor may have resulted in self-perceived personal growth despite the lack of any demonstrable increase in cognitive performance as observed in the acting group.
Although many studies have shown a relationship between frequency of performing stimulating activities and improved cognitive performance, the Activity Scale we administered (Wilson et al., 2002) failed to show a relationship between currently performed activities and posttest improvement. However, this is far from surprising because the scale revealed the participants to be quite homogeneous in terms of the number of stimulating activities performed daily before the intervention.
In the case of the Memory Controllability Index results, no news seems to be good news. That is, memory beliefs, no matter how positive or negative, did not predict the improvements found in the acting group at posttest. Therefore, the acting intervention appeared to boost participants’ cognitive scores, independent of their memory beliefs before taking the course. Lachman, Weaver, Bandura, Elliott and Lewkowicz (1992) found a dissimilar result; however they used completely different measures and methodology. They administered the MCI before and after training, and compared cognitive restructuring treatment (instructing participants that memory can be improved through personal effort, and leading participants in group discussions about memory) with a memory skills program (providing practice in making associations between to-be-learned items, teaching visualization and attentional skills), and a combination of both types of training. The greatest increase in memory beliefs was seen in the combined treatment group. However, there was no significant increase in memory performance compared to controls. In a somewhat similar vein, Rapp, Brenes and Marsch (2002) demonstrated that participants’ memory appraisals can be modified following training in stress-reduction and memory skills (mnemonics). After training, participants in the experimental group were less likely to view memory decline as inevitable and rated their present ability more favorably. Once again, no improvement in memory performance was observed. No comparison can be made between these studies and our intervention inasmuch as we used the MCI only at pretest because our interest was whether memory beliefs would affect participants’ ability to benefit from acting instruction.
In a critique of studies showing that improved cognition accompanied the performance of stimulating activities, Salthouse, Berish, and Miles (2002) mentioned that the amount of stimulation was rarely controlled for. Using TV as an example, they pointed out that watching television could either be a completely passive activity or a very active one in which the participants attend closely to the background events, analyze the actors’ styles, or try to anticipate the next incident. A standard feature of our acting intervention is that participants are required to fill out special TV questionnaires. In them, they report their analyses of television performances seen since the last session, basing their judgments on their newly acquired knowledge of acting1. This emphasis on active involvement is characteristic of all elements of our course. Indeed, we believe that one reason the intervention is so effective is that it is structured so that passivity is never an option. Each participant had to do each acting exercise or scene in front of all the other members of the group. If he or she seemed to holding back in any way and not committing fully to the exercise, the instructor coached the participant until the desired degree of involvement was apparent. This individual attention is characteristic of almost all college arts instruction.
It must be emphasized that acting, whether by a raw beginner or experienced professional, is a unitary process. The actor does what the character would do. If the dramatic situation calls for character A to patronize character B, the actor playing character A patronizes the actor playing character B. The basic process is that simple. When actor A genuinely patronizes actor B, certain changes (body language, degree of arousal, situation-specific thoughts, accompanying affect state) will occur within the actor as a result of the involvement in the act of patronizing. There is no way to subdivide this process. One can’t tell the actor to really patronize the other actor but not to feel anything while doing it, nor can one prevent the performance situation from influencing a participant’s physiological arousal. For example, if the participant is coached to become deeply involved in the goal to demand attention before uttering the words, “Don’t talk, just listen”, the participant will experience the utterance in at least three modes:
Cognitively (the thoughts that occur while speaking the literal words)
Emotionally (the feelings that arise during the confrontation)
Physiologically (facial expressions, tones of voice, and body language that are concomitants of actively pursuing the goal)
However, the nature of the acting process prevents it from being experimentally manipulated to determine which component of the process benefits which aspect of cognitive functioning because the process is necessarily simultaneous and indivisible. Despite this inability to manipulate separate aspects of the process without compromising its essential wholeness, one can nevertheless identify principles within the whole that might mediate any observed improvement.
For example, processing factors such as causality, effort, and distinctiveness are inevitably involved in acting training because actors must know the cause (i.e. the impetus for them to speak) in order to become fully immersed in the transaction (an effortful task), and communicate their intentions in a highly specific way (distinctiveness). In addition, an intervention using theatre instruction would teach the participant to simultaneously hold in mind the goals of the characters and the accompanying movement, while experiencing the affect states and heightened arousal involved in the performance of these tasks in front of one’s peers. Obviously, all of these factors involve deep processing, and if such depth of processing becomes habitual due to the training, cognitive test performance might improve.
Furthermore, almost all dramatic situations involve some sort of problem to be solved. That is, one character wants something and the other character stands in the way. Therefore, in every dramatic transaction, the actor is trying to overcome an obstacle to achieving his or her objective, requiring the actor to stay alert to all input from the other characters that could affect achieving that objective. Once again, this effort requires a high degree of mental-emotional-physiological involvement. Also, this involvement must occur in any acting performance, whether in the short exercises and scenes we employ to avoid the need for memorization, or in full-length plays performed from memory by professional actors. Therefore, because these cognitive and affective elements are indispensable components of acting but do not appear to exist in such concentrated form in other types of performing arts, we expected to find greater gains in the acting group than in the singing group or the no-treatment controls. Thus, although the evidence supports the effectiveness of the intervention, it is not possible to tease out the specific elements responsible for the observed improvements in specific cognitive abilities due to the indivisible nature of the process. To artificially separate the cognitive-emotive-physiological activities that actors necessarily perform simultaneously would falsify the nature of acting performance. However, many investigators have speculated on the mechanisms underlying the relationship between other stimulating activities and healthy cognitive aging. Wilson and Bennett (2003) suggested that such activities exert their influence by “ . . . affecting the development or maintenance of the interconnected neural systems that underlie different forms of cognitive processing ” (p. 89). Using fMRI imaging, Park (2002) demonstrated that when participants were asked to think about meaning, there was a strong increase in activation in the meaning elaboration area of the brain (Brodmann areas 45/47). Needless to say, understanding and communicating meaning is at the heart of all acting. Other physiological studies have found a reduction in regional cerebral blood flow in older adults (e.g., MacInnes, Paull, & Quaife, 1990), but Morris, Ahmed, Syed and Toone (1993) found an increase in regional cerebral blood flow during planning operations, particularly in the left frontal cortex. Many of the activities in the current study revolved around determining and executing the plans of the characters.
Based on the above physiological research, we suggest that engaging in demanding, multimodal activities such as acting might also result in an increase of cerebral activation which, in turn, would contribute to improved cognitive performance. That is, if mental activities can change the structure of the brain in such a way as to make it more difficult for disease to take hold (as proposed by Wilson & Bennett, 2003), it might be possible to render neural systems more efficient through novel, effortful programs such as acting training that involve a number of sensory modalities.
A recent study (Vance & Crowe, 2006), reviewed evidence that cognitive stimulation, cognitive training, and other means of modifying behavior, may preserve or increase cognitive reserve. The authors cite animal studies (e.g., Diamond, Johnson, Protti, Ott & Kajisa, 1985) that show that stimulation results in morphological brain change even in very old rats. The authors also cite studies with both humans and animals (e.g., Mattson, Duan, Lee & Guo, 2001) that demonstrate that novel, stimulating activities are associated with delayed onset of dementia. Vance and Crow (2006) then present a model of neuroplasticity and cognitive reserve that would appear to have some explanatory power in terms of our own intervention. The model lists a number of mitigating factors (e.g., stimulating activities/novel experiences) that, because of the neuroplasticity of the brain, would tend to increase cognitive reserve. The majority of these factors are inherent in the acting process. A number of other researchers (e.g., Milgram, Siwak-Tapp, Araujo & Head, 2006; Stern, 2006) have invoked the concept of cognitive reserve to explain how education and life-style can protect against cognitive decline. Many of those studies have shown that an enriched cognitive environment early in life (and continuing throughout the lifespan) seems to produce this reserve. However, the current study suggests the possibility that adding a highly enriched multi-modal environment over a short period late in life can also produce the kinds of benefits associated with the protections offered by cognitive reserve.
An important difference between our acting intervention and many short-term training programs is that our test measures are never targeted to the training activities. That is, nothing in the intervention involves providing strategies for improving test performance in the same way that mnemonics instruction is designed to improve list learning. This is not to say that the acting training does not indirectly affect memory strategy. The essence of acting consists of devoting all of one’s awareness to whatever one is trying to accomplish at the present moment. At almost every session, the instructor tells the participants, “Remember, stage time is always now!” Engaging in this process repeatedly over a four week period may well make deep processing habitual, resulting in increased efficiency on all episodic memory tasks.
Recently, Denise Park and her associates (e.g., Meade & Park, 2005) performed interventions that had some parallels with ours, especially in the sense that the tests were not targeted to the training. The authors presented preliminary results showing that engaging in novel activities could produce gains on cognitive tests unrelated to the trained abilities. Older adults were taught to either quilt or operate a digital camera. Pre- post test comparison between the experimental groups and the waiting-list controls revealed selective improvement: The quilting group improved on speed of processing and the camera group on long term memory. However, our acting participants improved on almost all measures compared to both the singing group and the no-treatment controls. We believe that the differences between Meade and Park’s results and our own may be due to the multiple and highly varied sources of stimulation involved in the acting process.
The wide-ranging improvement we found raises a question. If the gains are not a result of applying a targeted strategy, might they extend to activities of daily living? It seems logical that abilities such as improved recall, text comprehension, and problem solving would also aid everyday functioning. The ACTIVE study (Ball et al., 2002) showed no transfer of the gains derived from targeted training to improvement on the Instrumental Activities of Daily Living (IADL). However, some other studies have been more encouraging. Willis et al. (2006), found that five years after the training phase in the ACTIVE study, those participants who had received reasoning training (instruction in understanding serial relationships and predicting the next item in the sequence) reported significantly less difficulty in carrying out every-day activities compared to the control group. A similar pattern was seen with the memory and speed of processing groups, but the results were not significant. Also, Edwards, Wadley, Myers, Roenker, Cissell and Ball (2002) showed that, after just 6 weeks of instruction in speed of processing, participants demonstrated significantly greater improvement from pre- to posttest on the Timed IADL compared to control groups; however, this result was not observed for the two other training groups in that study.
As of this writing, the IADL instruments used in the above mentioned studies have not been made available to researchers in general. It remains for future studies to see if the acting intervention would produce improvement on this important measure of cognitive health. Additional areas to be explored include determining the long-term effects of the intervention. The budget in this particular study did not allow for follow-up activities, but our previous research on this intervention (Noice et al., 2004) showed no decline on any of the measures for up to four months. Also, all the acting interventions to date have been run by the same highly experienced actor and theatre professor. An important area of further investigation from an applied standpoint concerns the feasibility of training in-house activity directors in senior residences to administer the intervention, thus vastly widening the potential reach of the program.
Acknowledgments
This work was supported by Grant 1 R15 AG026306-01 from the National Institute on Aging. We are indebted to Aaron Johnson for teaching the voice course and to the following students who assisted in the collection of data for this project: John Comprado, Kristin Enger, Jennifer Forster, Janette Krzyzewski, Shaira Rock, Sarah Rouleau, Shraddha Mahadevia, and Karolina Wanielista. We also thank Robert Wilson, Graham Staines and Timothy Johnson and two anonymous reviewers for their helpful comments.
Footnotes
The singing group was also assigned homework. The participants were asked to listen to singers on radio, CDs, or TV, and observe their breath control, interpretation and other elements taught in class.
Portions of this research were presented at the Joint Conference of the American Society on Aging and the National Council on Aging, Chicago, IL, March, 2007.
References
- Albert MS, Smith L, Scherr P, Taylor J, Evans DA, Funkenstein H. Use of brief cognitive tests to identify individuals in the community with clinically diagnosed Alzheimer’s disease. International Journal of Neuroscience. 1991;57:167–178. doi: 10.3109/00207459109150691. [DOI] [PubMed] [Google Scholar]
- Alley D, Suthers K, Crimmins E. Education and cognitive decline in older Americans. Research on Aging. 2007;29(1):73–94. doi: 10.1177/0164027506294245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball K, Berch DB, Helmer KF, Jobe JB, Leveck MD, Marsiske M, et al. Effects of cognitive training interventions with older adults: A randomized controlled trial. Journal of the American Medical Association. 2002;288:2271–2281. doi: 10.1001/jama.288.18.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltes PB, Kliegl R, Dittmann-Kohli F. On the locus of training gains in research on the plasticity of fluid intelligence in old age. Journal of Educational Psychology. 1988;80(3):392–400. [Google Scholar]
- Baltes PB, Willis SL. Plasticity and enhancement of intellectual functioning in old age: Penn State’s Adult Development and Enrichment Project ADEPT. In: Craik FIM, Trehub SE, editors. Aging and Cognitive Processes. Plenum Press; New York: 1982. pp. 353–389. [Google Scholar]
- Bennett DA, Schneider JA, Arvanitakis Z, Kelly JF, Aggarwal NT, Shah RC, et al. Neuropathology of older persons without cognitive impairment from two community-based studies. Neurology. 2006;66:1837–1844. doi: 10.1212/01.wnl.0000219668.47116.e6. [DOI] [PubMed] [Google Scholar]
- Christensen H, Mackinnon A. The association between mental, social and physical activity and cognitive performance in young and old subjects. Age and Ageing. 1993;22:175–182. doi: 10.1093/ageing/22.3.175. [DOI] [PubMed] [Google Scholar]
- Crowe M, Andel R, Pedersen NL, Johansson B, Gatz M. Does participation in leisure activities lead to reduced risk of Alzheimer’s disease? A prospective study of Swedish twins. Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 2003;58:P249–P255. doi: 10.1093/geronb/58.5.p249. [DOI] [PubMed] [Google Scholar]
- Diamond MC, Johnson RE, Protti AM, Ott C, Kajisa L. Plasticity in the 904-day-old rat. Experimental Neurology. 1985;87:309–317. doi: 10.1016/0014-4886(85)90221-3. [DOI] [PubMed] [Google Scholar]
- Dore GA, Elias MF, Robbins MA, Elias PK, Brennan SL. Cognitive performance and age: Norms from the Maine-Syracuse study. Experimental Aging Research. 2007;33(3):205–271. doi: 10.1080/03610730701319087. [DOI] [PubMed] [Google Scholar]
- Edwards JD, Wadley VG, Myers RS, Roenker DL, Cissell GM, Ball KK. Transfer of a speed of processing intervention to near and far cognitive functions. Gerontology. 2002;48:329–340. doi: 10.1159/000065259. [DOI] [PubMed] [Google Scholar]
- Friedland RP, Fritsch T, Smyth KA, Koss E, Lerner AJ, Chen CH, et al. Patients with Alzheimer’s disease have reduced activities in midlife compared with healthy control-group members. PNAS. 2001;98(6):3440–3445. doi: 10.1073/pnas.061002998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass TA, Freedman M, Carlson MC, Hill J, Frick KD, Ialongo N, et al. Experience Corps: Design of an intergenerational program to boost social capital and promote the health of an aging society. Journal of Urban Health. 2004;81:94–105. doi: 10.1093/jurban/jth096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass TA, Mendes de Leon C, Marotolli RA, Berkman LF. Population based study of social and productive activities as predictors of survival among elderly Americans. British Medical Journal. 1999;319:478–483. doi: 10.1136/bmj.319.7208.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultsch DF, Hertzog C, Small BJ, Dixon RA. Use it or lose it: Engaged lifestyle as a buffer of cognitive decline in aging? Psychology and Aging. 1999;14:245–263. doi: 10.1037//0882-7974.14.2.245. [DOI] [PubMed] [Google Scholar]
- Kaiser A, Perrig-Chiello P, Perrig WJ. Gedächtnis- und Reattributionstraining. In: Perrig-Chiello P, Stähelin HB, Perrig WJ, editors. Wohlbefinden, Gesundheit und cognitive Kompetenz im Alter. Verlag Haupt; Bern, Switzerland: 1999. [Google Scholar]
- Kramer AF, Bherer L, Colcombe SJ, Dong W, Greenough WT. Environmental influences on cognitive and brain plasticity during aging. Journal of Gerontology: Medical Sciences. 2004;59A(9):940–957. doi: 10.1093/gerona/59.9.m940. [DOI] [PubMed] [Google Scholar]
- Lachman ME, Bandura M, Weaver SL, Elliott E. Assessing memory control beliefs: The Memory Controllability Inventory. Aging and Cognition. 1995;2(1):67–84. [Google Scholar]
- Lachman ME, Weaver SL, Bandura M, Elliott E, Lewkowicz CJ. Improving memory and control beliefs through cognitive restructuring and self-generated strategies. Journal of Gerontology: Psychological Sciences. 1992;42(5):P293–P299. doi: 10.1093/geronj/47.5.p293. [DOI] [PubMed] [Google Scholar]
- Lennartsson C, Silverstein M. Does engagement with life enhance survival of elderly people in Sweden? The role of social and leisure activities. Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 2001;56B(6):S335–342. doi: 10.1093/geronb/56.6.s335. [DOI] [PubMed] [Google Scholar]
- MacInnes WD, Paull D, Quaife M. Longitudinal changes in regional cerebral blood flow in a normal elderly group. Archives of Clinical Neuropsychology. 1990;4(3):217–226. doi: 10.1093/arclin/4.3.217. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Duan W, Lee J, Guo Z. Suppression of brain aging and neurodegenerative disorders by dietary restriction and environment enrichment: Molecular mechanism. Mechanism of Ageing & Development. 2001;122(7):757–778. doi: 10.1016/s0047-6374(01)00226-3. [DOI] [PubMed] [Google Scholar]
- Meade ML, Park DC. In: Chodzko-Zajko W, Kramer AF, editors. Enhancing cognitive function in older adults; Enhancing Cognitive and Brain Plasticity in Older Adults: 2004 Illinois Physical Activity and Cognitive Functioning Conference; Human Kinetics Publishers. 2005. [Google Scholar]
- Milgram NW, Siwak-Tapp CT, Araujo J, Head E. Neuroprotective effects of cognitive enrichment. Ageing Research Reviews. 2006;5:354–369. doi: 10.1016/j.arr.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Morris RG, Ahmed S, Syed GMS, Toone BK. Neural correlates of planning ability: Frontal lobe activation during the Tower of London test. Neuropsychologia. 1993;21(12):1367–1378. doi: 10.1016/0028-3932(93)90104-8. [DOI] [PubMed] [Google Scholar]
- Morris J, Heyman A, Mohs R, Hughes JP, van Belle G, Fillenbaum G, et al. The consortium to establish a registry for Alzheimer’s disease (CERAD). Part I. Clinical and neuro-psychological assessment of Alzheimer’s disease. Neurology. 1989;39:1159–1165. doi: 10.1212/wnl.39.9.1159. [DOI] [PubMed] [Google Scholar]
- Noice T, Noice H. A review of recent research on the expertise of professional actors. High Ability Studies. 2002;13(1):7–19. [Google Scholar]
- Noice H, Noice T. What studies of actors and acting can tell us about memory and cognitive functioning. Current Directions in Psychological Science. 2006a;15(1):14–18. [Google Scholar]
- Noice T, Noice H. A theatrical intervention to improve cognition in intact residents of long term care facilities. Clinical Gerontologist Journal. 2006b;29(3):59–75. [Google Scholar]
- Noice H, Noice T. Artistic performance: Acting, ballet, and contemporary dance. In: Ericsson A, Charness N, Feltovich P, Hoffman R, editors. Handbook on Expertise and Expert Performance. Cambridge University Press; New York: NY: 2006c. pp. 489–504. [Google Scholar]
- Noice H, Noice T, Perrig-Chiello P, Perrig W. Improving memory in older adults by instructing them in professional actors’ learning strategies. Applied Cognitive Psychology. 1999;13:315–328. [Google Scholar]
- Noice H, Noice T, Staines G. A short-term intervention to enhance cognitive and affective functioning in older adults. Journal of Aging and Health. 2004;16(4):1–24. doi: 10.1177/0898264304265819. [DOI] [PubMed] [Google Scholar]
- Park DD. Judging meaning improves function in the aging brain. Trends in Cognitive Sciences. 2002;6(6):227–229. doi: 10.1016/s1364-6613(02)01912-5. [DOI] [PubMed] [Google Scholar]
- Pease J. University of Florida Health Science Center News & Communications. University of Florida; Gainesville, FL: Sep, 2006. Mental exercise has long-term benefits for seniors. [Google Scholar]
- Perrig WJ, Stähelin HB, Perrig-Chiello P. Biologische Determinanten der verschiedenen Gedächtnisfunktionen. In: Perrig-Chiello P, Stähelin HB, Perrig WJ, editors. Wohlbefinden, Gesundheit und cognitive Kompetenz im Alter. Verlag Haupt; Bern, Switzerland: 1999. [Google Scholar]
- Perrig-Chiello P, Stähelin HB, Ehrsam . Gesundheitsverhalten. In: Perrig-Chiello P, Stähelin HB, Perrig WJ, editors. Wohlbefinden, Gesundheit und cognitive Kompetenz im Alter. Verlag Haupt; Bern, Switzerland: 1999. [Google Scholar]
- Pfeiffer E. A short portable mental status questionnaire for the assessment of organic brain deficit in elderly patients. American Geriatrics Society. 1975;23(10):433–441. doi: 10.1111/j.1532-5415.1975.tb00927.x. [DOI] [PubMed] [Google Scholar]
- Platt J, Spivack G. The MEPS procedure manual. Community Mental Health/Mental Retardation Center, Hahnemann Medical College and Hospital; Philadelphia: 1975. [Google Scholar]
- Rapp S, Brenes G, Marsh P. Memory enhancement training for older adults with mild cognitive impairment: a preliminary study. Aging 7& Mental Health. 2002;6(1):5–11. doi: 10.1080/13607860120101077. [DOI] [PubMed] [Google Scholar]
- Robertson-Tchabo EA, Hausman CP, Arenberg D. A classical mnemonic for older learners: A trip that works. Educational Gerontology. 1976;1:215–26. [Google Scholar]
- Ryff CD. Happiness is everything, or is it? Explorations on the meaning of psychological well-being. Journal of Personality and Social Psychology. 1989;57:1069–1081. [Google Scholar]
- Ryff CD, Singer BH. Know thyself and become what you are: A eudaimonic approach to psychological well-being. Journal of Happiness Studies. in-press. [Google Scholar]
- Salthouse TA. Mental Exercise and mental aging: Evaluating the validity of the use it or lose it hypothesis. Perspectives on Psychological Science. 2006a;1:68–87. doi: 10.1111/j.1745-6916.2006.00005.x. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. Mental exercise and cognitive training; Paper presented at the 2006 Friedman Conference on Modifiers of Cognitive Aging; St. Louis, MO. 2006b, May. [Google Scholar]
- Salthouse TA, Berish DE, Miles JJ. The role of cognitive stimulation on the relations between age and cognitive functioning. Psychology and Aging. 2002;17(4):548–557. doi: 10.1037//0882-7974.17.4.548. [DOI] [PubMed] [Google Scholar]
- Saczynski JS, Willis SL, Schaie KW. Strategy use in reasoning training with older adults. Aging, Neuropsychology and Cognition. 2002;9(1):48–60. [Google Scholar]
- Scarmeas N, Levy G, Tang M-X, Manly J, Stern Y. Influence of leisure activity on the incidence of Alzheimer’s disease. Neurology. 2001;57(12):2236–2242. doi: 10.1212/wnl.57.12.2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaie KW, Willis SL. Can decline in adult intellectual functioning be reversed? Developmental Psychology. 1986a;22:223–232. [Google Scholar]
- Schaie KW, Willis SL. Adult development and aging. Little, Brown; Boston: 1986b. [Google Scholar]
- Schooler C, Malatu MS. The reciprocal effects of leisure time activities and intellectual functioning in older people: A longitudinal analysis. Psychology and Aging. 2001;16:466–483. doi: 10.1037//0882-7974.16.3.466. [DOI] [PubMed] [Google Scholar]
- Small BJ, Hughes TF, Hultsch DV, Dixon RA. Lifestyle activities and late-life changes in cognitive performance. In: Stern Y, editor. Cognitive reserve. Psychology Press; New York: 2006. pp. 173–186. [Google Scholar]
- Stern Y. Cognitive reserve and Alzheimer disease. Alzheimer Disease & Associated Disorders. 2006;20(3):S69–S74. doi: 10.1097/00002093-200607001-00010. [DOI] [PubMed] [Google Scholar]
- Stones MJ, Dornan B, Kozma A. The prediction of mortality in elderly institution residents. Journals of Gerontology. 1989;44(3):P72–P79. doi: 10.1093/geronj/44.3.p72. [DOI] [PubMed] [Google Scholar]
- Vance DR, Crowe M. A proposed model of neuroplasticity and cognitive reserve in older adults. Activities, Adaptation & Aging. 2006;30(3):61–79. [Google Scholar]
- Verghese J, LeValley A, Derby CA, Kulansky G, Katz M, et al. Leisure activities and the risk of dementia in the elderly. Neurology. 2006;66:821–827. doi: 10.1212/01.wnl.0000202520.68987.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhaeghen P, Marcoen A, Goossens L. Improving memory performance in the aged through mnemonic training: A meta-analytic study. Psychology and Aging. 1992;7:242–251. doi: 10.1037//0882-7974.7.2.242. [DOI] [PubMed] [Google Scholar]
- Wang JYJ, Zhou DHD, Li J, Zhang M, Deng J, Tang M, et al. Leisure activity and risk of cognitive impairment: the Chongqing aging study. Neurology. 2006;66:911–913. doi: 10.1212/01.wnl.0000192165.99963.2a. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Memory Scale -- Revised Manual. Psychological Corporation; San Antonio, TX: 1987. [Google Scholar]
- Willis SL. Current issues in cognitive training research. In: Lovelace EL, editor. Aging and cognition: Mental processes, self-awareness and interventions. Elsevier; Amsterdam: 1990. pp. 263–280. [Google Scholar]
- Willis SL, Tennstedt SL, Marsiske M, Ball K, Elias J, Koepke KM, et al. Long-term effects of cognitive training on everyday functional outcomes in older adults. JAMA. 2006;296(23):2805–2814. doi: 10.1001/jama.296.23.2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RS, Bennett DA. Cognitive activity and risk of Alzheimer’s disease. Current Directions in Psychological Science. 2003;12(3):87–91. [Google Scholar]
- Wilson RS, Mendes de Leon CF, Barnes LL, Schneider JA, Bienias JL, Evans DA, et al. Participation in cognitively stimulating activities and risk of incident Alzheimer disease. JAMA. 2002;287(6):742–748. doi: 10.1001/jama.287.6.742. [DOI] [PubMed] [Google Scholar]