Abstract
We review evidence that structural brain abnormalities are associated with abuse of amphetamines. A brief history of amphetamine use/abuse, and evidence for toxicity is followed by a summary of findings from structural magnetic resonance imaging (MRI) studies of human subjects who had abused amphetamines and children who were exposed to amphetamines in utero. Evidence comes from studies that used a variety of techniques that include manual tracing, pattern matching, voxel-based, tensor-based, or cortical thickness mapping, quantification of white matter signal hyperintensities, and diffusion tensor imaging. Ten studies compared controls to individuals who were exposed to methamphetamine. Three studies assessed individuals exposed to 3-4-methylenedioxymethamphetamine (MDMA). Brain structural abnormalities were consistently reported in amphetamine abusers, as compared to control subjects. These included lower cortical gray matter volume and higher striatal volume than control subjects. These differences might reflect brain features that could predispose to substance dependence. High striatal volumes might also reflect compensation for toxicity in the dopamine-rich basal ganglia. Prenatal exposure was associated with striatal volume that was below control values, suggesting that such compensation might not occur in utero. Several forms of white matter abnormality are also common, and may involve gliosis. Many of the limitations and inconsistencies in the literature relate to techniques and cross-sectional designs, which cannot infer causality. Potential confounding influences include effects of pre-existing risk/protective factors, development, gender, severity of amphetamine abuse, abuse of other drugs, abstinence, and differences in lifestyle. Longitudinal designs in which multimodal datasets are acquired and are subjected to multivariate analyses would enhance our ability to provide general conclusions regarding the associations between amphetamine abuse and brain structure.
Keywords: brain structure, drug abuse, amphetamine, methamphetamine, ecstasy
Amphetamines and their use
Synthesized in 1887, amphetamine (1-methyl-2-phenethylamine) was the first member of a group of compounds that have similar structures and biological properties and are collectively called “amphetamines”. The group also includes methamphetamine, synthesized six years later, and 3-4-methylenedioxymethamphetamine (MDMA), patented in 1914. Amphetamines produce their principal effects by increasing synaptic levels of the biogenic amines, dopamine, norepinephrine and serotonin, through multiple mechanisms1-3.
Amphetamine is FDA-approved for treatment of attention deficit-hyperactivity disorder (ADHD) and narcolepsy. Methamphetamine is approved for treatment of ADHD and obesity. Both drugs are classified by the Drug Enforcement Agency (DEA) as belonging in Schedule II, having accepted medical uses but being tightly controlled because of their potential for abuse that can lead to severe psychological and physiological dependence. Although once unregulated and used as an adjunct in psychotherapy, MDMA has been added to DEA Schedule I, indicating a high potential for abuse but no accepted medical use.
Amphetamines have been used illegally since the FDA limited them to prescription use in 1965. From 1971, when 30% of US college students surveyed reported using amphetamines illegally4, illicit amphetamine use declined in the US during the 1980s. However, use subsequently increased in the 1990s and has continued to rise in young adults. Although data from the 2006 US National Survey indicated that illicit use of the combined licit and illicitly-manufactured amphetamine may have peaked, illicit use of prescription medications is currently at its highest level in decades and amphetamines are the most abused prescription medications5.
In addition to abuse of pharmaceutical amphetamines, there has been substantial illicit manufacture and abuse of methamphetamine and MDMA. Substantial levels of illicit manufacture of methamphetamine began in the 1960s in the United States and elsewhere, and has accelerated in both amount and geographic distribution since the 1980s. Methamphetamine has thus become one of the world’s fastest growing illicit drug problems6. Since its criminalization in the 1980s, MDMA has been manufactured and consumed as a popular recreational drug, commonly known as “ecstasy”. Legislative changes have not effectively discouraged its use7.
In sum, abuse of amphetamines constitutes a serious public health concern. Amphetamines are the prescription drugs most commonly abused by adolescents and young adults, and illicit amphetamines are second only to marijuana as a form of illicit drug abuse in young adults5. Prevalence of problematic use of amphetamines by older adults has also been rising. Emergency department mentions of amphetamines among patients 55 years and older have increased 700% from 1995 to 20028, for example.
Amphetamine neurotoxicity
It is well known that exposure of experimental animals to acute, high doses of amphetamine and methamphetamine alters dopaminergic neurons that innervate the striatum (caudate-putamen)9,10. Exposure of experimental animals to acute, high doses of MDMA alters serotonergic neurons11,12. Dopaminergic toxicity is inferred from deficits in phenotypic markers for dopaminergic nerve terminals, including dopamine itself, its biosynthetic enzymes tyrosine hydroxylase and L-aromatic amino acid decarboxylase, and both the plasma membrane dopamine transporter (DAT) and the vesicular monoamine transporter (VMAT)13. In rats given MDMA, protracted depletion of forebrain 5-HT (serotonin), reductions in evoked 5-HT release, changes in hormone secretion, and persistent anxiety-like behaviors have also been interpreted as evidence for neurotoxicity, although this interpretation is not conclusive12.
High doses of amphetamines have produced hyperemia, hemorrhage and glial proliferation in monkeys14, and enlarged chromatolytic medullary neurons in cats15. Parenteral dosing in rodents can also produce swelling or reduction of dopaminergic axons, reduced dopaminergic terminals, and serotonin deficits. Deficits in dopaminergic nerve terminals are not always accompanied by apparent damage to the dopamine-containing cell bodies within the substantia nigra, but can persist during years of abstinence from drug exposure. The mechanisms responsible for amphetamine-induced neurotoxicity have not been fully identified. However, available evidence suggests that the high levels of cytoplasmic dopamine associated with amphetamine-mediated disruption of vesicular storage leads to accumulation of reactive oxygen species and severe oxidative stress16,17. This may be due in part to methamphetamine-associated increases in brain iron18, since iron is a well-known catalyst for oxidative reactions19. Because similar neurotoxicity has not been apparent after high-dose exposure to the non-amphetamine stimulant methylphenidate20,21, it has been speculated that damage may reflect disruption of vesicular storage of dopamine, an action of amphetamine, but not of methylphenidate.
Neurotoxicity of amphetamine used therapeutically?
It is not known whether long-term administration of prescription amphetamines at doses abused by humans produces similar alterations in the dopaminergic system22. Because evidence for amphetamine-mediated neurotoxicity in rodents derives primarily from studies in which high doses were parenterally administered23,24, and because repeated exposure of rodents to lower doses does not produce such evidence25, the relevance of these data for therapeutic use of amphetamines in humans can be questioned. However, in the most relevant animal model, adult baboons and squirrel monkeys were treated for 4 weeks with a 3:1 mixture of dextroamphetamine to levoamphetamine, a ratio similar to that found in the pharmaceutical product Adderal, at doses that mimic those used in human clinical treatment26. Plasma concentrations of amphetamine (136 +/- 21 ng/ml) matched the levels reported in human ADHD patients (120 to 140 ng/ml) after amphetamine treatments that lasted for 3 weeks27 or 6 weeks28. Both monkey species treated in this way developed 30-50% reductions in striatal dopamine, its major metabolite dihydroxyphenylacetic acid, its rate-limiting enzyme (tyrosine hydroxylase), DAT, and VMAT. Other evidence suggests that non-human primates may be more vulnerable than rodents to stimulant-induced neurotoxicity29. However, a large clinical study of clinically treated individuals with ADHD showed no impact on the developmental trajectories of brain volumes30.
The potential for amphetamine-mediated neurotoxicity may vary with age, with cumulative exposure to the drug, or even with the behavioral context of drug administration. Brain amphetamine levels at both 20 and 65 min after IP administration of 2.5 mg/kg of amphetamine were twice as high in the brains of old as compared to young rats, suggesting that aging might increase the risks for toxicity31. In fact, older rats, mice and gerbils all experienced greater methamphetamine neurotoxicity than younger animals, as manifested by striatal dopamine reduction, structural deficits and increased levels of glial fibrillary acid protein32-34. Stefanski and associates35 observed regional downregulation of dopamine D2 and D1 receptors in rats who had learned to self-administer methamphetamine. At the relatively low doses delivered, no further pathology was seen. This observation suggests that doses of methamphetamine that may be too low to be neurotoxic can induce dependence. Each of the above factors suggests that amphetamine-abusing patients examined in neuroimaging studies may represent a heterogeneous mix of individual with a combination of brain factors that might predispose to methamphetamine dependence and neurotoxic sequelae of methamphetamine use. This heterogeneity may manifest as variability in neuroimaging endpoints such as volumes of macroscopic structures within the brain.
Neurotoxicity from abuse
Most of the evidence for amphetamine-induced human brain damage comes from examination of current and former amphetamine and methamphetamine abusers. Evidence suggesting that the neurotoxicity reported in animals also occurs in methamphetamine-abusing humans has accrued from neuroimaging findings of reduced availability of dopamine D2 receptors, and transporters for dopamine, serotonin, and vesicular monoamines36. Autopsy data consistent with dopaminergic neuronal damage consist of deficits in dopamine, the dopamine transporter, and tyrosine hydroxylase15,37,38. However, autopsy data has revealed little reduction in levels of the vesicular monoamine transporter (VMAT2). These findings contrast with the profound decrements in VMAT2 expression that follow administration of high stimulant doses to rodents or nonhuman primates39-42. Because a decrease in VMAT2 is thought by some to indicate reduction of intact monoamine nerve terminals39,43-45, it has been suggested that decrements in dopamine transporter in the absence of decrements in VMAT2 may represent compensatory changes rather than degeneration of dopamine terminals38.
Proton magnetic resonance spectroscopy of cortex and basal ganglia metabolites has consistently reported reduced markers of neuronal integrity, and increased markers of glial content, suggesting that glial proliferation may follow neural damage36. Using glucose metabolism as an index of functional activity in brain, abnormalities have been observed in research subjects who were in early abstinence from chronic abuse of methamphetamine. Abnormally high relative activity was observed in amygdala, ventral striatum and lateral orbitofrontal cortex. Abnormally low activity was noted in medial prefrontal and, especially, cingulate cortex46. Continued abstinence from the drug accompanies abnormally high global and cortical glucose metabolism, particularly in the parietal lobe47,48, and relatively lower activity, when scaled to global mean activity, in striatal and thalamic regions48,49.
Some degree of recovery after protracted abstinence has been noted in studies of perfusion of the cingulate cortex50, striatal dopamine transporters51, and glucose metabolism in the thalamus but not the striatum49. When we retested 12 healthy control subjects and 10 hospitalized methamphetamine abusers one month after an initial test when the methamphetamine abusers had been abstinent for 5-9 days, glucose metabolism did not change in subcortical regions of the methamphetamine abusers but it increased in their neocortical regions 47. The increase exceeded 20% in the parietal lobes. Increased cortical activity was interpreted as reflecting effects of compensatory processes, or unmasking of damage that might be obscured by suppression of cortical glucose metabolism for at least 5 days after cessation of drug use. Alternatively, new damage might conceivably be sustained during abstinence.
The possibility of additional damage during early abstinence from amphetamine is consistent with observations that three daily exposures of rats to methamphetamine was sufficient to induce reactive gliosis that continued for over two weeks after the final exposure52. In addition, the P300 event-related potential recorded from the human scalp, which is modulated by catecholaminergic neurotransmission53,54, is reduced in amplitude during early abstinence from chronic methamphetamine abuse, and was also reduced in a rat model by 15 days of methamphetamine followed by over a week of abstinence55.
For MDMA, the evidence for human neurotoxicity is less definite than for methamphetamine, partially because of methodological problems and potential confounds in the extant studies, which will be discussed in the last half of this chapter. However, the bulk of the evidence suggests there are residual alterations of serotonergic transmission in MDMA users. There appears to be partial restitution with abstinence, but also persistent functional sequelae that persist even after long periods of abstinence56.
Structural brain abnormalities and methamphetamine
Most of the evidence for structural brain damage in human substance abusers derives from recently developed applications of magnetic resonance imaging (MRI), which can be used for quantitative morphometrics and for assessment of structural change over time. Many of the relevant studies have involved automated or semi-automated segmentation of the brain into gray and white matter components, for restricted or separate analyses. Techniques that have been used include a wide range from labor-intensive methods, such as manual slice-by-slice tracing and volume drawing of individual structures, as well as more computationally intensive approaches that include pattern matching as well as voxel-based, tensor-based and cortical thickness mapping57. This review also includes studies that assess white matter signal hyperintensities and diffusion tensor imaging (DTI) indices, since these measures help us understand the state of white matter microstructure in methamphetamine abusing individuals.
The strongest evidence for stimulant-associated differences, and possible stimulant-mediated neurotoxicity, has come from studies that relate to the use of methamphetamine (see Table 1, in which reports are presented in the approximate order of publication). We will first review studies of methamphetamine abusers and then discuss studies that assess MDMA abusers.
Table 1.
Imaging evidence for structural abnormalities in amphetamine abusers
| Groups | Male | Amphetamine use variables (MEAN ± SD) | Measures | Tesla | Regions | Assessed?* | Results | |
|---|---|---|---|---|---|---|---|---|
| Bartzokis et al. 200058 | 9 MA | 9 | MA use > 2 years (mean 6.7 ± 3.9 years) | Manual tracing of MRI image | 1.5 | Whole brain | WM,GM | ↓ temporal lobe volume |
| abstinence >2 weeks | (total, gray & white matter volumes) | frontal lobe | ↓ temporal lobe GM volume | |||||
| 16 C | 16 | temporal lobe | ||||||
| Cowan et al. 200384 | 31 polydrug with MDMA | 17 | MDMA use: 26%<11x, 48%=11-39x, 26%>40x | VBM (voxel-based morphometry) | 1.5 | Whole brain | WM,GM, | ↓ GM concentration in occipital (BA18), |
| Polydrug users, 55% used other amphetamines | gray & white matter | L. temporal (BA21), L. frontal lobe (BA45), | ||||||
| 29 polydrug without MDMA | 18 | abstinence >3 weeks | S | R/L. cerebellum, medial brainstem | ||||
| Thompson et al. 200461 | 22 MA | 15 | MA mean use 10.5±1.1 years | T1 segmentation/volume drawing | 3 | Cortical sulci & gyri | WM,GM | ↑ R. temporal occipital lobe WM & frontal horn of lateral ventricle |
| cumMA(g)=1878±126 | cortex & hippocampal pattern matching | hippocampus | ↓ R. mid-posterior cingulate GM, ↓hippocampal volume | |||||
| 21 C | 10 | used 18.9±1.8 of prior 30 days | mood, word/picture recall & recognition | ↓hippocampus correlates with ↓ word recall | ||||
| Chang et al. 200462 | 13 MA | 4 | MA exposure > 2/3 of gestation | T1 segmentation/pattern matching | 1.5 | Caudate, putamen, globus pallidus | None | ↓ striatum & hippocampus |
| age = 6.9± 3.5 | neuropsychological battery | thalamus, midbrain, | (unsegmented volume) | ↓attention, memory, visual motor integration | ||||
| 15 C | 6 | hippocampus, cerebellar vermis | ↓striatum correlated with ↓ attention & delayed verbal memory | |||||
| Chang et al. 200563 | 50 MA | 24 | MA use>2 years (mean 110± 68 months) | T1 Drawn ROIs | 1.5 | Caudate, putamen, globus pallidus | WM,GM, | ↑ striatal volumes |
| cumMA(g)=4519±5730 | neuropsychological battery | thalamus, midbrain, cerebellar vermis | A, | ↓ striatal volume correlated with ↓ cognitive performance & ↑ lifetime MA use | ||||
| 50 C | 24 | abstinence>1 week (mean 4±6 months) | Corpus Callosum (4 ROIs) | S | female MA users ↑ callosal posterior midbody | |||
| Oh et al. 200566 | 27 MA | 23 | cumMA(g)= 334±506 | MRI-modeled callosal extraction | 3 | Corpus Callosum (7 ROIs) | WM | ↑ Genu curvature |
| mean abstinence 21±35 months | automated shape analysis | ↓ width posterior midbody & isthmus | ||||||
| 18 C | 14 | callosal width in each region | (ie - frontal & parietal WM abnormalities) | |||||
| Jernigan et al. 200568 | 21 MA | 17 | MA use 6-20 years (mean 12.1±4.1 years) | T1 semi-automated morphometry | NG | Striatum, thalamus | GM, | ↑striatum & parietal cortex |
| 22 MA+HIV | 21 | cumMA(g)= 4930±94 | neuropsychological exam. (unspecified) | hippocampus, amygdala | younger MA users = ↑nucleus accumbens volume | |||
| 30 C | 17 | abstinence > 10 days (mean 94±89 days) | cortex (all lobes) | S | ↑parietal lobe correlated with cognitive impairment | |||
| Bae et al. 200669 | 33 MA | 22 | MA mean use 59±36 months | White matter hyperintensity (WMH) | 3 | Deep (all lobes + insular), | WM, | ↑ WMH (all, deep & periventricular) |
| cumMA(g)=292±247 | rated/graded on T2 MRI | periventricular (frontal and nonfrontal) | A, | Severity of deep WMH correlated with lifetime MA use | ||||
| 32 C | 21 | mean abstinence 18±29 months | S | M > Fem (No abnormality in Fem) | ||||
| Schlaepfer et al. 200674 | 16 polydrug abusers | 16 | Most (9/16) abused MA, but also abused | Semi-automated segmentation | 1.5 | Whole brain | WM,GM | ↓ frontal WM volume (both methods) |
| other drugs for 7-12 years. All used | stereological volume estimates | frontal lobe | ||||||
| 16 nonabusers | 16 | cannabis, 14 cocaine, 15 heroin. | total, gray matter, white matter | lateral ventricles | ||||
| Kim et al. 200680 | 11 short-abstinent MA | 11 | MA mean use 64±44 months | VBM | 3 | Whole Brain | WM,GM, | ↓ GM Density R. Midfrontal Cortex |
| 18 long-abstinent MA | 16 | cumMA(g)= 276±236 | WCST, Trailmaking test, STROOP | gray and white matter | A | ↓WCST was correlated with R. Midfrontal Cortex ↓ GM | ||
| 20 C | 15 | mean abstinence 20±34 months, long=>6 months | Both effects partially recover at > 6 months abstinence | |||||
| Moeller et al. 200785 | 12 polydrug with MDMA | 10 | MDMA use > 1 year (mean 6.2±5.0 years) | DTI (FA, diffusivity, longitudinal & transverse) | 1.5 | Corpus Callosum | WM | ↓ Longitudinal eigenvalues rostral corpus collosum body |
| abstinent from 0-101 days (mean 40) | Iowa Gambling Task (IGT) | (6 ROIs) | ↓IGT was correlated with rostral body longitudinal eigenvalue | |||||
| 20 C (no drugs) | 13 | |||||||
| De Win et al. 200790 | 30 MDMA users | 12 | mean MDMA use 1.8 ± 1.3 tablets | DTI (FA, apparent diffusion) | 1.5 | Thalamus, | WM, | ↑ FA centrum semiovale (trend) |
| pre + post early MDMA use | 0.9 - 29.5 months between tests (mean 8.1± 6.5) | psychological questionnaires | 3 basal ganglia ROIs, | ↑ centrum semiovale FA was correlated with number of MDMA tablets | ||||
| abstinence >3 weeks at test 2 (mean 7.7± 4.4) | MRS, PWI | centrum semiovale | S | |||||
| Chung et al. 200781 | 32 MA | 23 | MA mean use M= 75±51, Fem= 47±50 months | DTI FA | 3 | Frontal white matter ROIs | WM, | ↓ Frontal FA (WM integrity) |
| cumMA(g) M=412±543, Fem=133±150 | WCST | ↓WCST was correlated with FA | ||||||
| 30 C | 20 | >1month abstinence, mean M=24±38, Fem=43±66 | S | M >Fem | ||||
MRI=magnetic resonance imaging, MRS=magnetic resonance spectroscopy, DTI=diffusion tensor imaging, FA=fractional anisotropy, PWI= perfusion -weighted imaging, MA=methamphetamine, MDMA=methylenedioxymethamphetamine, C=control subjects, cum=cumulative, ROI=region of interest, R=right, L=left, BA= Brodmann area, NG=not given, M=male, Fem=female
WM, GM, A, & S represent assessment of white matter, gray matter, abstinence effects, and sex effects.
The first controlled study to measure brain volumes in amphetamine users compared 9 methamphetamine–dependent young men (27.9 [SD=3.9years]) with 10 cocaine-dependent men and 16 men who did not use drugs58. It involved semiautomated, histogram-driven measurements of frontal and temporal lobes on 1.5 Tesla (1.5T) T2-weighted MRI scans. Methamphetamine-dependent subjects had used the drug for more than 2 years (mean = 6.7 +/- 3.9 years), although the length of time between most recent consumption and imaging was not available. Smaller volumes of the temporal lobe, but not of the frontal lobe, were found in both methamphetamine and cocaine users, in comparisons with control men. The stimulant using groups did not differ from each other overall. However, only the cocaine group demonstrated an age-related decline in temporal lobe volume. In both groups, reductions in temporal lobe volume were localized primarily to gray matter. Smaller gray matter volume was invariably accompanied by larger white matter volume, consistent with the prolongation of complete myelination into adulthood59. The trend for increased white matter volume was especially prominent in the methamphetamine group, providing a reduced group difference for total volume. The authors speculated that age-related loss of cortical gray matter in stimulant abusers might be associated with reduced capacity to experience euphoriant effects of psychostimulants such as cocaine60, the only group to show age-related atrophic reductions. They suggested that this phenomenon might explain the well-established age-related reduction in psychostimulant addiction. However, no interpretation was made for finding stronger temporal than frontal effects. In addition, volumes of only parts of the frontal and temporal lobes were quantified.
In 2004, high-resolution surface-based computational image analysis of structural MRI (performed at 3 T) was used to map regional abnormalities in the cortex, hippocampus, white matter, and ventricles of 22 chronic methamphetamine abusers (mean use = 10.5 years) who used methamphetamine on most of the 30 days prior to entering the study, as compared with 21 age-matched control subjects who did not use amphetamines61. The methamphetamine abusers had less gray matter, averaging 11.3% below levels found in comparison subjects in the cingulate, limbic, and paralimbic cortices. The abusers displayed 7% white-matter hypertrophy and 7.8% smaller hippocampal volumes. Remarkable MRI-assessed gray matter deficits in regions of the anterior and posterior cingulate cortex of the right hemisphere overlapped regions of abnormal glucose metabolism in another sample of methamphetamine abusers that contained some of the same subjects46 (see Fig. 1). During a vigilance task, these subjects exhibited high glucose metabolism in posterior cingulate and low glucose metabolism in anterior cingulate. Hippocampal volumes, which displayed deficits in the methamphetamine abuse group, were correlated with performance on a word-recall test. The overall results were consistent with the conclusion that chronic methamphetamine abuse may produce or be associated with a selective pattern of cerebral deterioration, with prominent effects in the medial temporal lobe and limbic cortices. These data are consistent with earlier suggestions of temporal lobe deficits58 and hippocampal deficits that were postulated to contribute to impaired memory performance in methamphetamine abusers. The authors speculated that neuronal deficits and or toxicities might lead to the white-matter hypertrophy noted in both studies through developmental differences, neuroadaptation, neuropil reduction, cell death, adaptive glial proliferation or altered myelination61.
Figure 1.
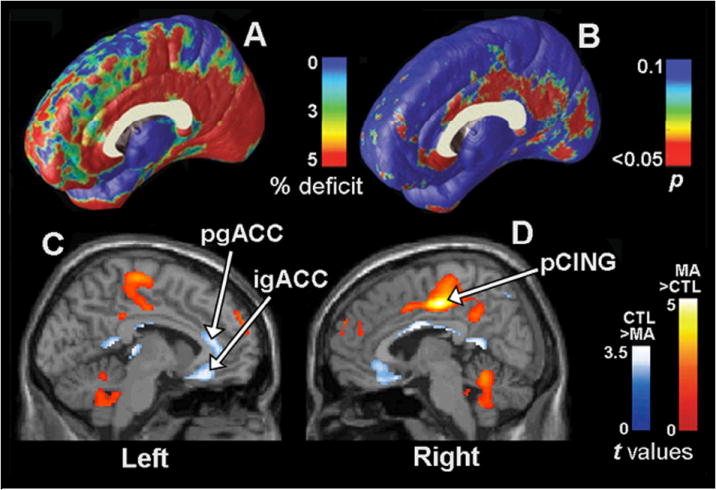
Gray matter differences and abnormalities in glucose metabolism on the medial surface of the brain (see 46,61). Group difference map (A) shows mean percentage differences in gray matter volumes in the methamphetamine (MA) group compared with the control (CTL) group, according to the color bar, and the significance of these differences (B) plotted as a map of p values. The cingulate gyrus shows gray matter deficits (red; p = 0.034, corrected), whereas other brain regions are comparatively spared (blue/green). Illustrations (C) and (D) show the locations of differences in regional cerebral glucose metabolism (relative values) assessed with PET in MA-dependent (n = 17) compared to control subjects (n = 18). The samples that produced the MRI data in (A) and (B) partially overlapped with those that produced the data in (C) and (D). In (C) and (D), statistical parametric maps reveal regions in which the MA group had higher (red) or lower (blue) relative values of glucose metabolism. Colors superimposed on a gray-scale MRI template indicate voxels where the t-test for group difference exceeds t > 1.69 (P < 0.049). A region of remarkable gray matter deficit (B) in the right hemisphere posterior cingulate cortex (pCING) also showed an apparent increase in glucose metabolism in the methamphetamine group (D). The abbreviations igACC and pgACC denote the inferior and perigenual anterior cingulate cortex, respectively.
A single study assessed effects of prenatal methamphetamine exposure on brain structure and cognitive performance62. Volumes for whole brains, cerebellum, thalamus and midbrain were similar to those of controls. However, thirteen children (mean age 6.9 years, 4 male) who were exposed to methamphetamine in utero displayed bilaterally smaller volumes of putamen (17.7%), globus pallidus (28.5%), hippocampus (19.5%), and caudate (13%), as compared to non-exposed children. Moreover, subcortical volumes in hippocampus, putamen and globus pallidus were correlated with deficits in sustained attention and delayed verbal memory, suggesting that methamphetamine exposure during gestation might be neurotoxic to these parts of the developing brain and/or that the predisposition to methamphetamine abuse inherited from the parents might also provide brain substrates for attention and mnemonic deficits.
In contrast to the smaller striatal structures in methamphetamine-exposed children, 50 adults (24 male) who were abstinent from chronic (duration > 2 years) methamphetamine use for an average of four months had larger volumes of the putamen (10%), and the globus pallidus (8%) than healthy comparison subjects (see Figure 2, reproduced from63). Female methamphetamine users also had 9.7% larger volumes of the mid-posterior corpus callosum compared to control subjects. Because those methamphetamine abusers with smaller striata had greater lifetime methamphetamine use and more impaired cognitive performance on verbal fluency and speeded motor tasks, the authors proposed that enlargement of the striatum may reflect a compensatory response to methamphetamine toxicity. They propose that the adaptation fails to maintain both function and structural integrity of the striatum after prolonged abuse. Enlargement of the striatum could be produced through inflammation and reactive gliosis, possibly abetted by glia-mediated neurotrophic effects to increase striatal sprouting, as has been shown in response to dopaminergic lesions in experimental animals64. The larger volume of the mid-posterior corpus callosum in methamphetamine-abusing women may be related to the increased glucose metabolism and perfusion that has been shown to be maximal in the parietal lobes of abstinent methamphetamine abusers47,48,65, since the parietal fibers cross in this portion of the corpus callosum.
Figure 2.
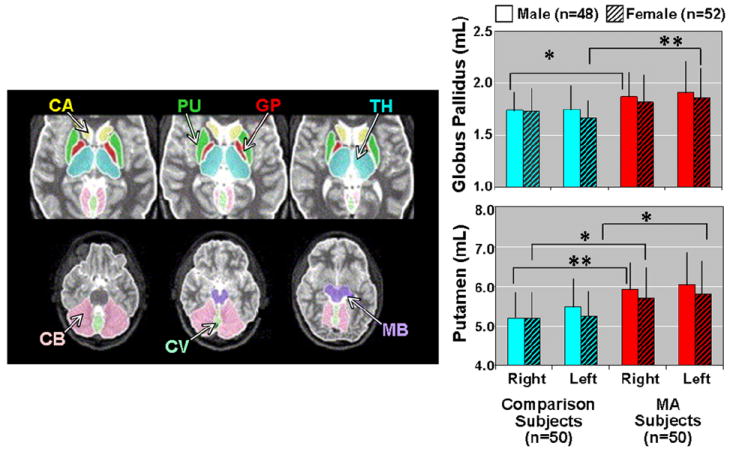
Left: Axial MRI slices showing measured brain regions. CA - caudate, PU - putamen, GP - globus pallidus, TH - thalamus, CB - cerebellum, CV - cerebellar vermis, MB - midbrain. Right: Bar graphs showing larger volumes of lentiform nuclei (putamen and globus pallidus) in methamphetamine (MA) abusers compared to healthy volunteers. Putamen MA effects: right – F1,96 = 11.74, p = .0009; left – F1,96 = 12.55, p = .0006. Globus Pallidus MA effects: right – F1,96 = 6.22, p = .01; left – F1,96 = 10.32, p = .002. *= p<.05; **= p<.005; MRI, magnetic resonance imaging 63.
Structural abnormalities in the corpus callosum were also observed in methamphetamine abusers who were abstinent for a longer period of time (mean = 21 months)66. Automated shape analysis indicated greater curvature of the genu and smaller width in both the posterior midbody and isthmus of the corpus callosum of 27 methamphetamine abusers (24 male) relative to comparison subjects. Moreover, both the genu and posterior midbody abnormalities were correlated with lifetime dose of methamphetamine. There were no group differences in total volume of the corpus callosum or of the subregional areas defined by Witelson67. Since 89% of the sample was composed of men, this observation does not directly conflict with the earlier reports of larger posterior callosal volume in female methamphetamine abusers 63. The authors suggested that the anterior and posterior shape differences in the interhemispheric white matter tracts may be related to the functional abnormalities that had been observed in frontal and parietal cortices of methamphetamine abusers.
Abnormally large volumes of the striata of former methamphetamine abusers was confirmed in a study that compared healthy individuals to subjects who were HIV+, chronic methamphetamine abusers (abstinent for an average of three months), or both68. HIV+ status without methamphetamine abuse was associated with smaller volumes than healthy control subjects in cortical and subcortical structures, including the hippocampus. In contrast, methamphetamine dependence in HIV− individuals was associated with larger volumes than control subjects within the caudate nucleus, lenticular nucleus and nucleus accumbens. Volumetric differences in the nucleus accumbens were larger for younger individuals. Because the age at which subjects were studied was highly correlated with the age of the first methamphetamine abuse, it is unclear which factor was most important. Since greater plasticity is associated with younger as compared to older adults, either finding is consistent with interpreting enlargement of the striatum as an adaptive response to amphetamine-mediated toxicity, or even as a predisposing factor to amphetamine abuse.
Although measures of neurocognitive impairment were not generally correlated with subcortical volumes, in the HIV+ methamphetamine abusers, smaller hippocampal volume was associated with more cognitive impairment68. This relationship suggests that if the lower hippocampal volumes associated with being HIV+ were caused by the HIV virus, that the functional consequences of hippocampal reduction might be exacerbated by methamphetamine abuse. Cortical volume was lower in HIV+ individuals who did not use methamphetamine, but tended to be larger in HIV- methamphetamine abusers, attaining significance in the parietal lobe. Moreover, the opposite cortical abnormalities in methamphetamine abusers and HIV+ individuals were both associated with neurocognitive impairment. This makes it less likely that the larger parietal volume in methamphetamine abusers represents an adaptive compensatory response to amphetamine-mediated toxicity, as suggested for the striatal volume increase.
Bae and associates69 assessed the prevalence, severity and location of T2-weighted MRI white matter signal hyperintensities (WMH) in 33 methamphetamine abusers who were abstinent for an average of 18 months. WMH represent patchy or diffuse changes associated with structural abnormalities that included dilated perivascular spaces, demyelination, astrocytic gliosis and arteriosclerosis70. Such findings have also been found more frequently in both cocaine abusers and opiate abusers relative to control subjects71-73. Methamphetamine abusers had greater prevalence of WMH (33%) than control subjects (3%), and greater severity of both periventricular and deep WMH, primarily in the frontal lobes. Unlike cocaine addicts71, methamphetamine abusers showed no difference in the insular region. The severity of deep WMH was correlated both with higher lifetime dose and the average daily dose of methamphetamine during abuse, but was not related to the duration of abstinence. Male methamphetamine abusers had more prevalent WMH than female abusers. While male methamphetamine abusers also had greater severity of WMH than healthy males (odds ratio = 18.9), female methamphetamine abusers did not have significantly greater severity of WMH than healthy females (odds ratio = 1.2).
One study used both semi-automated segmentation and a stereological method to assess MRI volume abnormalities in 16 males currently abusing drugs through intravenous administration. The majority (9) abused methamphetamine along with other drugs74. Intravenous drugs had been abused for between seven and twelve years. The specificity of structural abnormalities for amphetamine abuse is unclear because all intravenous drug users, but none of the control group, ingested cannabis. In addition, most drug abusers self-administered cocaine and heroin as well as methamphetamine by intravenous routes. Although these potential confounding factors complicate interpretation, the two volume assessment methods did produce highly correlated estimates (r=0.65, p<0.001). Both methods found that substance abusers had a significantly lower proportion of white matter in the frontal lobe than control subjects. In contrast, there were no group differences in the proportion of whole brain white or gray matter, frontal gray matter, or lateral ventricular volume.
Although an abnormally low proportion of frontal white matter was reported by analyses using both methods, this result is inconsistent with other studies that measured cortical gray and white matter volumes (Table 1). These contrasting reports document either no difference in white matter volumes, increased white matter volumes, or decreased gray matter volumes. The abnormally low proportion of frontal white matter may be associated with features specific to this study, such as the methods used to determine the proportion of white matter, or the confounding effects of other addictive substances. Abnormally small white matter volumes have been associated with alcohol abuse75. Cocaine abuse has been associated with impaired myelination76,77. While no subjects reported histories of or current alcohol abuse, 88% of the drug-abusing sample reported intravenous use of cocaine, as compared to the 56% who reported methamphetamine abuse. Therefore, differences from the control group are at least as likely to be related to use of cocaine as to use of methamphetamine.
Voxel-based morphometry (VBM) is a whole-brain method for assessing volume differences between tissue segmented brain images which has been developed and popularized within the Statistical Parametric Mapping (SPM) software package78,79. VBM was used to compare gray matter and white matter between healthy control subjects and former methamphetamine users who were abstinent for either less than six months (n=11) or more (n=18) than six months80. Lower “gray matter density” was reported in the right middle frontal gyrus (BA 10) of methamphetamine abusers, as compared to control subjects. Methamphetamine abusers also scored lower on a neurospsychological test of executive functioning, the Wisconsin Card Sort Test (WCST). Total WCST errors of methamphetamine abusers, but not control subjects, were correlated with the right middle frontal gray matter density within the area that provided the largest group difference. Both the gray matter and WCST scores were closer to control values in methamphetamine abusers who reported long-term abstinence, relative to those who reported only short-term abstinence. There were no significant white- matter abnormalities. Results could not be separately assessed for women.
A recent report of structural brain abnormalities in methamphetamine abusers came from authors who used diffusion tensor imaging (DTI) to assess white matter integrity81. DTI measures changes in microstructural white matter organization by providing measures of functional anisotropy (FA) and diffusivity of water flow. These measures are sensitive to disorganization and damage to axons and their myelin sheaths82-83. Decreased white-matter integrity was inferred from abnormally low FA in bilateral frontal white matter voxels at the anterior commissure-posterior commissure (AC-PC) plane and in right hemisphere prefrontal white matter 5 mm above the AC-PC plane in 32 abstinent (>1 month) methamphetamine abusers, as compared to 30 healthy comparison subjects. Frontal executive functions, as assessed by WCST total errors, were also impaired in methamphetamine abusers. WCST errors correlated with FA values in right prefrontal white matter 5 mm above the AC-PC plane. Separate gender analyses revealed that both the FA and WCST abnormalities associated with methamphetamine abuse were significant only in the male, but not in the female subjects, suggesting the possibility for a protective factor in female methamphetamine abusers. Whether such differences represent a predisposition to addiction or are secondary to stimulant toxicity needs to be clarified through prospective study designs.
Structural brain abnormalities and MDMA
Three imaging studies assessed brain structure in MDMA abusers. The first one used VBM to compare 31 polydrug users who took MDMA (26% 5-10 times, 48% 11-39x, 26% > 40x), with 29 polydrug users who had never taken MDMA84. No subject had taken MDMA for at least three weeks prior to testing. In cerebellum, occipital cortex (BA 18), left hemisphere temporal (BA21) and frontal cortices (BA45), and medial brainstem, MDMA users had lower gray matter concentration than polydrug users who had not used MDMA. There were no group differences in white matter concentration, and no differences between men and women. The specificity of the gray matter findings for MDMA is compromised because MDMA abusers who self-reported polydrug abuse also reported more severe substance abuse than polydrug users who reported never using MDMA. They thus reported significantly more episodes of use of seven additional classes of abused drugs (hallucinogens, cocaine, cannabis, opiates, PCP, sedatives, non-MDMA amphetamines). Use of non-MDMA amphetamines was reported by 55% of MDMA users but only 10% of comparison subjects. The authors reported similar VBM results when they used a less conservative statistical threshold after removal of covariation associated with individual drug categories, but they make no attempt to remove effects of overall severity of drug use. It would not be surprising, for example, if abuse of all sympathomimetic stimulants produced some effects similar to those produced by MDMA. Separate removal of covariance associated with lifetime incidence of cocaine use and non-MDMA amphetamine abuse could not rule out the possibility that use of the other sympathomimetic drugs, rather that MDMA use per se, contributed to group differences in the morphometric measures obtained in this study.
A DTI study compared healthy control subjects to 12 chronic MDMA abusers. MDMA abusers had consumed MDMA an average of 181 times. All had consumed it at least 15 times. These subjects had been abstinent from MDMA between 0 (two subjects were urine +) and 101 days (mean = 40 days). The MDMA abusers did not significantly differ from 20 comparison subjects in FA, mean diffusivity or transverse diffusivity in any of six corpus callosum sub-regions. However, longitudinal diffusivity, the diffusion in the direction of the white-matter fibers, was lower in MDMA abusers within the rostral body of the corpus callosum, suggesting the presence of damage to or differences in anterior callosal white matter 85. Moreover, rostral body longitudinal diffusivity was correlated with the proportion of advantageous choices on a measure of effective decision making, the Iowa Gambling Task, in the subset of 11 MDMA users and 15 control subjects who completed this task. Although this relationship suggests that MDMA-mediated damage to or differences in rostral body axons may impair some forms of judgment, as with the VBM study84, greater use of other drugs by the MDMA abusers provided a potential confounding influence. Because MDMA use is associated with a lifestyle that often includes polysubstance abuse, it is difficult to control for use of all other abused substances.
Beyond controlling for use of other drugs, it is of importance to determine if structural brain differences in those who have used MDMA, or have abused other substances, are truly consequences of their drug use, or antecedents that might predispose to such use. As pointed out above, it is possible that some abnormalities represent preselection or biological vulnerability markers that predate actual drug taking. Because MDMA is occasionally administered to human beings in research studies, and has been used therapeutically as a psychotherapy adjunct in disorders characterized by anxiety86-88, including post traumatic stress disorder and terminal cancer89, it is also important for ethical decision making to determine the possible negative consequences, if any, of low doses of MDMA.
A recent paper by investigators in the Netherlands demonstrates the advantages of a prospective study design in addressing these issues90. MDMA-naïve young adults who reported a high probability they would soon start using the drug, or reported that their friends already used the drug, were assessed with proton magnetic resonance spectroscopy, perfusion-weighted imaging (PWI), DTI, and psychological questionnaires. The first thirty subjects to report MDMA use were reassessed between 0.9 and 29.5 months after the initial assessment (mean 8.1 +/- 6.5 months). Most had used MDMA a single time (mean 1.8 +/- 1.3 tablets), with last use at least 3 weeks (mean 7.7 +/- 4.4 weeks) prior to retesting. The only other drug to increase in frequency of use between assessments was cocaine. Separate analyses excluded the four subjects who increased their cocaine use. PWI revealed a significant reduction in the regional relative cerebral blood volume in dorsolateral frontal grey matter after MDMA use. No differences between sessions in the spectroscopy or DTI measures survived correction for multiple comparisons. However, a marginal increase in FA within the white matter centrum semiovale was noted after MDMA use. This increase was correlated with total amount of ecstasy tablets ingested. Since reduced rather than increased functional anisotropy is thought to be associated with axon damage, the authors concluded that while the frontal grey matter finding suggested the possibility of sustained local vasoconstriction, there were no indications of structural damage to axons or neurons after initial low doses of MDMA.
Discussion: gray matter and white matter
Gray matter abnormalities
The most consistently reported specific structural abnormality in amphetamine abusers is lower cortical gray matter density or volume58,61,80,84. Reduced gray matter has been reported within all of the cortical lobes from at least one study: temporal58,61,84, frontal61,80,84, occipital61,84 and parietal61. Only one study reported an increase in cortical gray matter volume, and this was localized to the parietal lobe68. Increased parietal cortex gray matter in this study was associated with cognitive impairment, suggesting that might represent a measure of toxicity.
In contrast, both studies that assessed striatal grey matter in adult amphetamine abusers reported larger striatal volumes when compared to comparison subjects63,68. A study of children exposed to methamphetamine in utero reported striatal volumes that were 13-30% smaller than healthy children, data that was interpreted as evidence of prenatal dopaminergic neurotoxicity62. In adults, association of larger volumes of the striatum with better cognitive performance, lower lifetime methamphetamine use63, and with greater neuroplasticity68 suggested that increasing the volume of the striatum may be a compensatory response to initial neurotoxicity which might fail above a cumulative neurotoxic load.
However, similar enlargement of the striatum induced by neuroleptic treatment of schizophrenic patients is generally considered a sign of toxicity. The classic neuroleptics that are most strongly linked to enlargement of the striatum are also associated with the choreoathetoid movements of tardive dyskinesia, which are similar to choreoathetoid movements reported in amphetamine abusers91. Choreoathetoid movement disorders are thought to result from a high ratio of dopamine to acetylcholine activity in the basal ganglia, which by inhibiting GABAergic influences on thalamic neurons, leads to increased glutamate-mediated excitation of neocortex. The second generation “atypical” neuroleptics, which produce less impact on the dopaminergic system, have a much lower incidence, and may even reverse both tardive dyskinesia and enlargement of the striatum92. Therefore, the larger basal ganglia volume in methamphetamine abusers might be construed as converging evidence that direct cathecholamine effectors have a high potential for toxicity.
One possible mechanism for such toxicity is the increase in free radical production during the metabolism of catecholamines. This process may be exacerbated by the high iron levels that are present in the basal ganglia, since iron is a well known catalyst of free radical reactions. A recent primate study showed that methamphetamine use increases iron in brain, which could further exacerbate such toxicity18. Antipsychotic medications have also been shown to influence iron levels93 and to influence iron-related toxicity94 in rodent models. Thus, pharmacologic disturbances of catecholamine metabolism, especially dopamine metabolism, may be a generalized way of disturbing iron metabolism, and thus producing toxicity in the basal ganglia95. Recent demonstration of higher iron levels in males than females96 suggests this mechanism may increase the vulnerability of males to stimulant neurotoxicity, consistent with two reports of greater white-matter abnormalities in male as compared with female methamphetamine users69,81.
Can the idea that amphetamines damage the dopamine-rich basal ganglia through iron-related toxicity or a similar process be reconciled with the evidence suggesting that striatal enlargement in methamphetamine abusers can be a compensatory adaptation? Although there is evidence that several mechanisms contribute to the induction of tardive dyskinesia by typical neuroleptics, the most prominent theory implicates postsynaptic dopamine receptor hypersensitivity92. We propose that dopamine hypersensitivity may be an adaptive response to reduction of dopamine activity in the striatum mediated either through chronic dopaminergic blockade or through amphetamine-mediated loss of dopaminergic terminals. Although typical neuroleptics have been largely replaced in order to reduce the risk for development of tardive dyskinesia early in the disease process, their efficacy is comparable to any of the newer antipsychotic medications. A recent study of psychotic symptomatology and treatment-associated changes in the basal ganglia revealed significant volume increases in caudate and putamen after only four weeks of antipsychotic treatment. The increases in left striatum were not associated with drug treatment per se, but were associated with the reduction of positive symptoms97. Prenatal exposure to methamphetamine was associated with striatal deficits in children which were correlated with cognitive deficits62, suggesting that the mechanism that increases local volume to compensate for striatal damage is not available in utero.
White matter abnormalities
Overall, white matter abnormalities in amphetamine abusers have now been reported more often than gray matter abnormalities. Of the 13 studies in Table 1, 11 assessed white matter and 7 reported at least one significant white matter abnormality. In addition, among the four papers that did not describe white matter structural abnormalities, one reported that findings of low gray matter in methamphetamine abusers were invariably accompanied by trends for congruent local increases in white matter58. Another reported that initial use of MDMA produced trends for increased FA in frontoparietal white matter that were significantly correlated with the number of MDMA tablets consumed90. The specific form of white matter abnormality associated with amphetamine use is less clear, since increased volume61,63, decreased volume66,74, increased T2-hyperintensities69, and reduced DTI measures of normal white matter diffusion81,85 have all been reported. Gray matter reductions and white matter expansion are seen in healthy individuals through middle age59. Deviations from these normal developmental trajectories may result from use of psychostimulants as has been suggested in cocaine dependence77. In the dynamic background of healthy adult brain developmental trajectories, the timing of MRI studies in relation to abuse onset and age of subjects may account for some of the discrepancies.
Limitations and possible reasons for inconsistency
Multiple interpretations of structural abnormality: vulnerability factors, signs of damage, or compensatory responses?
Extant studies of the effects of amphetamines on brain structure suffer from several important limitations. Most studies employ a cross-sectional one-assessment design, which cannot determine whether volume differences in amphetamine abusers predated drug abuse (e.g., are risk or protective factors for developing addiction), directly represent brain damage, or are compensatory responses to such damage. Is having less frontal lobe grey matter a risk factor for amphetamine abuse? Do large brains indicate greater tissue resources against toxicity, thereby conferring reduced vulnerability to damage from amphetamine exposure? Investigations employing prospective longitudinal designs such as the Netherlands XTC study90 are sorely needed to determine if abnormalities in the brain structure of amphetamine abusers were actually caused by amphetamines at all.
Determination of the relationship between structural abnormalities and multiple indices of both the severity of abuse and of cognitive performance are very helpful in characterizing the functional significance of structural abnormalities, but they have been unevenly employed. For example, amphetamine abusers have exhibited deficits in tests of executive cognitive functions similar to those exhibited by patients with frontal damage98 (see also review by Robbins and colleagues, this volume). Table 1 shows that worse performance on the WCST was associated with evidence of frontal structural abnormality in methamphetamine abusers in both white81 and gray matter80, and that a frontal white matter abnormality in MDMA abusers was associated with decreased performance on the Iowa Gambling Task85. However, four other studies in which amphetamine abusers exhibited structural abnormalities in the frontal lobes did not measure executive functions66,69,74,84.
Along with exploration of risk factors for unwanted consequences of stimulant abuse, it is equally important to identify potential protective factors. For example, it has been reported that both lithium and valproate treatment protect against amphetamine-induced alterations of brain choline concentrations noted in bipolar disorder patients99. Recent animal studies have produced evidence for neuroprotection against amphetamine-mediated toxicity by several substances, including nomifensine100, methyllycaconitine101, coenzyme Q10102, baicalein103 and melatonin104. Impairment of learned place preference consolidation by amphetamine-induced neurotoxicity was ameliorated by administration of a glutathione precursor105. Evidence suggests that lower brain volume is a predisposing factor for developing alcohol dependence106. Larger premorbid brain size may protect against cocaine-related brain damage107. The mechanisms proposed for cocaine neurotoxicity are similar to those proposed for amphetamine toxicity. This suggests that having a larger brain may increase a general brain reserve for coping with neurotoxic influences. It is highly likely that both risk and neuroprotective effects are genetically modulated. Charting the relationship of amphetamine use to both a priori genetic polymorphisms and to a posteriori brain structural, brain functional, and behavioral consequences of chronic amphetamine use will lead to better understanding and treatment of amphetamine-mediated brain damage.
Developmental timecourses
During the years of greatest risk for addiction (teens to middle age) there are normal changes in gray and white matter volumes (increases in white and decreases in gray)59 which may interact with effects of amphetamine. At the far end of the lifespan, the hypothesis of amphetamine-mediated acceleration of aging in older adults could be explored by assessing chronic amphetamine abusers using multimodal functional and structural neuroimaging indices of cerebral health during normal aging. Such indices have undergone extensive development in recent years108. Cross-sectional designs cannot unambiguously characterize the influence of amphetamine abuse on normal developmental trajectories. Longitudinal assessment of amphetamine-mediated changes could provide more informative assessment of drug influences on brain structure58.
Sex differences
The importance of subject gender on amphetamine-associated structural brain abnormalities has not been well studied. Seven studies in Table 1 tested more than 4 amphetamine abusers of both sexes but only 5 reported explicit assessment of sex effects and only 2 tested more than 12 subjects of both sexes. Two studies of MDMA abusers found no sex differences84,90. One study reported increased volume of the posterior midbody of the corpus callosum in female, but not male methamphetamine abusers63. Two other studies of methamphetamine abusers reported white matter abnormalities in men but not in women69,81. It has been hypothesized that since aromatase, the enzyme that produces estrogen in astrocytes, is involved in glial repair after brain injury, estrogen might have neuroprotective effects65. In addition, female sex has been described to be “promyelinating”, and may accelerate brain myelination. Such acceleration could potentially produce different developmental timecourses of vulnerability to toxicity in men and women. These effects could explain some of the male preponderance in the epidemiology of addiction and other developmental disorders that are often comorbid with addiction109,110.
Possible nonspecificity for amphetamines
Many amphetamine abusers, particularly MDMA abusers, are polydrug abusers, and are particularly prone to additional abuse of nonamphetamine stimulants such as cocaine. In many studies reporting structural brain abnormalities in amphetamine abusers, effects of exposure to a specific amphetamine are confounded with greater use of other abused substances, classes of abused substances (i.e. CNS stimulants), or the overall severity of substance abuse, even in studies where attempts were made to compare polydrug abusers who abuse amphetamines with polydrug abusers who do not. Cognitive impairment may also be associated with structural abnormalities. Such impairment in MDMA abusers has been more closely related to use of cannabis than to use of MDMA111. Amphetamine abusers may be predisposed to abuse drugs by differences in personality or early experiences, which could also have brain structural correlates. Other potential confounds derive from lifestyle related effects, such as the circadian disruption, extended aerobic exercise, and high-volume music exposure112 that have been associated with MDMA use within the “rave” dance culture. Differences in nutritional practice could predate or be caused by amphetamine abuse.
Severity of methamphetamine abuse
Both within and across studies, the amphetamine abusers who have been assessed for structural brain abnormality vary widely in lifetime exposure to amphetamines. It can be difficult to validate exposure history variables, since almost all studies use self-report, and accept retrospective ratings over many years from subjects who may vary in the intactness of memory systems (see review article by Robbins and colleagues, this volume). Exposure is usually reported as the number of exposures or the cumulative grams of drug ingested. Both measures are imprecise in most patients due to the variable purity of nonpharmaceutical amphetamines. The duration of abuse is another important variable. Excluding studies of initial use, studies in Table 1 include minimal durations of abuse that range from 1 to 7 years, and average cumulative total use of methamphetamine that ranges from 276 to 4930g. All of these problems compromise attempts to relate severity of abuse or drug exposure to structural abnormalities. Future studies should report at least the range, a measure of central tendency, and a measure of dispersion for the cumulative dose and duration of amphetamine abuse. Studies should obtain external validation of histories from medical records, family reports, or other sources whenever possible.
Duration of abstinence
Postmortem studies of animals and humans suggest that the primary dopaminergic damage produced by amphetamines involves terminals and processes rather than cell bodies. Previous studies of functional or metabolic measures assessed during prolonged abstinence from prior chronic amphetamine abuse have reported evidence for partial abstinence-related normalization of low cingulate cortex perfusion50 and striatal dopamine transporters in methamphetamine abusers51, and low 5-HT transporter binding in MDMA abusers (summarized in88). There are substantial differences in the duration of time from participants’ last amphetamine exposure to their structural brain imaging between studies and among individuals in each study. Mean abstinence in Table 1 ranges from about 7 to 730 days. Only three studies explore these effects of abstinence on structural abnormalities in amphetamine abusers. Although assessment of abstinence was not a focus of a study of the effects of methamphetamine and subject gender on subcortical volume measures, the authors note that the primary effect of methamphetamine in their study, larger striatal volumes, was uncorrelated with duration of abstinence63. White matter abnormalities in T2-signal hyperintensities of methamphetamine abusers were also unrelated to duration of abstinence69. In contrast, a VBM study reported that low frontal lobe gray matter density and associated cognitive deficits in methamphetamine abusers were both ameliorated after at least 6 months of abstinence80.
White matter density was not related to duration of abstinence. Frontal gray matter changes produced by methamphetamine may thus be more reversible during abstinence than white matter changes. This idea is consistent with results of a recent proton magnetic resonance spectroscopy study in which cumulative lifetime exposure to methamphetamine correlated inversely with frontal concentrations of N-acetyl-aspartate in both white and gray matter. These data, suggest dose-dependent damage, with only the frontal gray matter concentration providing correlations with the duration of abstinence113. The other studies that did not find effects of abstinence did not assess frontal gray matter. The study that examined white matter abnormalities alone found no effects of abstinence using a distribution (18 +/- 29 months)69, which was similar to the VBM study (20 +/- 34 months), although the study of subcortical gray matter used more recently abstinent methamphetamine abusers (4 +/- 6 months)63. This would be expected to decrease the power to detect normalization during longer durations of abstinence. More research, preferably with longitudinal study designs, is needed to characterize both “dose-response” relationships between chronic exposure to amphetamine and the development of structural brain abnormalities, and time dependent effects on structural normalization during protracted periods of abstinence.
Different techniques
Imaging techniques for quantifying brain macro- or microstructure of amphetamine abusers include segmentation, manual tracing/drawing methods, pattern matching, MRI hyperintensity rating, DTI, and VBM. These techniques have different strengths and limitations. Gray matter reductions can be accompanied by white matter volume increases. Such changes could cancel each other out in unsegmented volume measurements. Pattern matching methods can be made sensitive to shape changes that would also be undetected by drawing methods that assess overall volume. Region of interest and automated voxel based DTI assessment of white matter microstructure114 can speak to changes in connectivity between brain regions, but yield little signal in gray matter regions.
VBM is enjoying increasing popularity because of the ease of comprehensive automated voxelwise whole-brain analysis, relative to the more labor-intensive techniques that involve drawing regions or structures onto individual brains in order to make volume measurements. However, the reliability and generalizability of VBM results remain controversial115-117. While some studies have validated the technique against manual tracing methods79,118-121, other studies have reported somewhat discordant results122-124. Measurement of total cerebrospinal fluid volume obtained with VBM are higher than those obtained with manual tracing methods125. Since the operational definition for gray matter density has not been resolved, it may be that different types of VBM measure very different things (i.e. volume vs. the statistical probability of a voxel being gray matter). Although the underlying statistical assumptions are reasonably conservative126,127, proper use of VBM requires proper preprocessing128,129, and the selected smoothing kernel may need to differ for optimal VBM of gray and white matter119,130. Understanding of the proper uses and limitations of VBM are still developing. A recent editorial summarizes core principles for conducting and reporting studies in ways that make them most useful and interpretable in the context of other techniques for structural image analysis131.
Finally, a myriad of technical issues are involved in the quantification of gray and white matter. These include differences in gray/white contrast produced by different MRI sequences and differences due to alignment of images132. As high-field strength instruments are becoming more prevalent, contrast differences caused by differences in the field strengths of MRI scanners may also contribute to inconsistent results. Such differences can result from a combination of changes in T1 relaxation-related contrast that are known to occur at different field strengths, and increased sensitivity to the effects of tissue iron on T2 relaxation-related contrast at higher field strengths. Thus, different field strengths may change the image contrast in ways that “shift” the apparent border between gray/white matter and thereby influence volumetric results. This possibility is further exacerbated for the study of structural effects of amphetamine abuse by the recent observation that methamphetamine use can directly influence brain iron levels18.
Panel A of Figure 3 shows that two groups of methamphetamine abusers and control subjects who were assessed with MRI instruments operating at 1.5 and 3 Tesla show no scanner-related differences in total brain volume. In contrast, Panel B shows a significant difference between the fraction of brain volume comprising gray matter between methamphetamine abusers and healthy control subjects scanned at the lower (1.5 Tesla), but not higher (3.0 Tesla) field strength (Welch two sample t-test p-value = 0.013). Although this observation suggests that group differences may be harder to detect at higher field strengths, there are probably not yet enough studies to assess this idea. Of 6 papers in Table 1 that probed both white and gray matter abnormalities in amphetamine abusers, both of the two studies that used a 3.0 Tesla field and 3 of 4 studies that used a 1.5 Tesla field reported gray matter abnormalities. Half of the studies at both field strengths reported white matter abnormalities.
Figure 3.
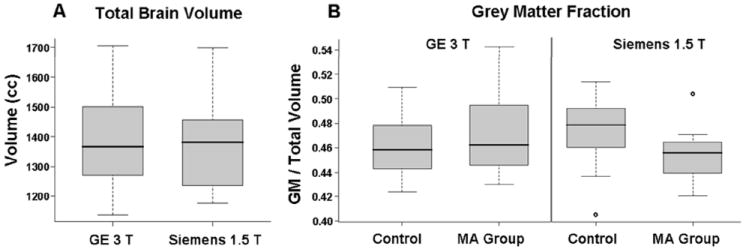
Box-and-whisker plots of structural MRI data in two scanners with different field strengths. A) Total brain volume from MRI images acquired in a 3.0 T General Electric (GE) scanner compared to images acquired from a 1.5 T Siemens Sonata scanner. There is no difference between the two samples. B) Differences in whole-brain gray matter fraction between control subjects and methamphetamine abusers scanned in the GE 3.0 T scanner and the Siemens Sonata 1.5 T scanner. There was no significant difference between the control group and methamphetamine group in the GE scanner. However, there was a significant difference between groups scanned in the Siemens scanner (Welch two sample t test p value = 0.013). Dark lines indicate the median for each group, the first and third quartile for each group are indicated by the lower and upper boundaries of the box respectively, and the dashed lines represent the range of values for each group. Outliers are shown as open circles (S. Fears, J. O’Neill, G. Bartzokis, and E. D. London, unpublished).
One approach to maximizing the multiple strengths and minimizing the weaknesses associated with different imaging techniques is to incorporate multiple types of information into one investigation. This was done in a recent study that correlated results of the same MRI dataset of patients with panic disorder analyzed with manual volumetry and with automated VBM119, and in another study that combined univariate and multivariate analysis of VBM and voxel-based relaxometry data to improve identification of the network of brain abnormalities associated with temporal lobe epilepsy130. New ways of using high resolution MRI data, such as computation of voxel-based cortical thickness (VBCT) maps133, are developing rapidly. Collecting multimodal imaging datasets and analyzing them using multiple complimentary techniques is becoming increasingly feasible. A single MRI session can incorporate several sequences that assess several tissue parameters (volumes of gray and white matter, T1, T2, DTI, iron measures, etc) and also collect functional information. Exploration of such multimodal datasets will increase our understanding of both the underlying pathophysiology and the techniques themselves. Understanding of the technical aspects of brain imaging tools that we use on clinical patients must progress towards increasing the specificity of measures or combinations of measures to further the twin goals of using brain imaging to inform both medication development and clinical decision making.
Acknowledgments
This work was supported in part by grants from the National Institute on Drug Abuse: R01DA015179, R01DA020726 and P20DA022539 (EDL); R03DA20512 and R21DA023192 (JON); and M01RR00865 (UCLA GCRC).
References
- 1.Goodman LS, Hardman JG, Limbird LE, Gilman AG. Goodman & Gilman’s The Pharmacological Basis of Therapeutics. McGraw-Hill; New York: 2001. [Google Scholar]
- 2.Madras BK, Miller GM, Fischman AJ. The dopamine transporter and attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57:1397–1409. doi: 10.1016/j.biopsych.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 3.Elliott JM, Beveridge TJ. Psychostimulants and monoamine transporters: upsetting the balance. Curr Opin Pharmacol. 2005;5:94–100. doi: 10.1016/j.coph.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 4.Executive Board, Am. Acad. of Pediatrics. Use of d-amphetamine and related central nervous system stimulants in children. Pediatrics. 1973;51:302–305. [PubMed] [Google Scholar]
- 5.Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. Monitoring the future national survey results on drug use, 1975-2006: Volume I, Secondary school students. National Institute on Drug Abuse; Bethesda, MD: 2007. [Google Scholar]
- 6.Rawson RA, Condon TP. Why do we need an Addiction supplement focused on methamphetamine? Addiction. 2007;102 Suppl 1:1–4. doi: 10.1111/j.1360-0443.2006.01781.x. [DOI] [PubMed] [Google Scholar]
- 7.Peters GJ, Kok G, Abraham C. Social cognitive determinants of ecstasy use to target in evidence-based interventions: a meta-analytical review. Addiction. 2008;103:109–118. doi: 10.1111/j.1360-0443.2007.02041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Detailed Emergency Department Tables From DAWN:2002. National Survey on Drug Use and Health, U.S.Department of Health and Human Services. U.S.Department of Health and Human Services, Substance Abuse and Mental Health Service Administration (SAMHSA), Office of Applied Studies, Drug Abuse Warning Network, 2001 (03/2002 update); 2002. 1-23-2008. [Google Scholar]
- 9.Seiden LS, Ricaurte GA. Neurotoxicity of methamphetamine and related drugs. In: Meltzer HY, editor. Psychopharmacology: The Third Generation of Progress. Raven Press; New York: 1987. pp. 359–366. [Google Scholar]
- 10.Gibb JW, Hanson GR, Johnson M. Neurochemical mechanisms of toxicity. In: Cho AK, Segal DS, editors. Amphetamine and its Analogs Psychopharmacology, Toxicology, and Abuse. Academic Press; San Diego: 1994. pp. 269–296. [Google Scholar]
- 11.Seiden LS, Sabol KE. Methamphetamine and methylenedioxymethamphetamine neurotoxicity: possible mechanisms of cell destruction. NIDA Res Monogr. 1996;163:251–276. [PubMed] [Google Scholar]
- 12.Baumann MH, Wang X, Rothman RB. 3,4-Methylenedioxymethamphetamine (MDMA) neurotoxicity in rats: a reappraisal of past and present findings. Psychopharmacology (Berl) 2007;189:407–424. doi: 10.1007/s00213-006-0322-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Truong JG, Wilkins DG, Baudys J, Crouch DJ, Johnson-Davis KL, Gibb JW, Hanson GR, Fleckenstein AE. Age-dependent methamphetamine-induced alterations in vesicular monoamine transporter-2 function: implications for neurotoxicity. J Pharmacol Exp Ther. 2005;314:1087–1092. doi: 10.1124/jpet.105.085951. [DOI] [PubMed] [Google Scholar]
- 14.Dunnick JK, Eustis SL. Decreases in spontaneous tumors in rats and mice after treatment with amphetamine. Toxicology. 1991;67:325–332. doi: 10.1016/0300-483x(91)90031-u. [DOI] [PubMed] [Google Scholar]
- 15.Kita T, Wagner GC, Nakashima T. Current research on methamphetamine-induced neurotoxicity: animal models of monoamine disruption. J Pharmacol Sci. 2003;92:178–195. doi: 10.1254/jphs.92.178. [DOI] [PubMed] [Google Scholar]
- 16.Tata DA, Yamamoto BK. Interactions between methamphetamine and environmental stress: role of oxidative stress, glutamate and mitochondrial dysfunction. Addiction. 2007;102 Suppl 1:49–60. doi: 10.1111/j.1360-0443.2007.01770.x. [DOI] [PubMed] [Google Scholar]
- 17.De Vito MJ, Wagner GC. Methamphetamine-induced neuronal damage: a possible role for free radicals. Neuropharmacology. 1989;28:1145–1150. doi: 10.1016/0028-3908(89)90130-5. [DOI] [PubMed] [Google Scholar]
- 18.Melega WP, Lacan G, Harvey DC, Way BM. Methamphetamine increases basal ganglia iron to levels observed in aging. NeuroReport. 2007;18:1741–1745. doi: 10.1097/WNR.0b013e3282f0d4f4. [DOI] [PubMed] [Google Scholar]
- 19.Halliwell B, Gutteridge JMC. IRON AS A BIOLOGICAL PRO-OXIDANT. Vol. 1. ISI Atlas of Science-Biochemistry; 1988. pp. 48–52. [Google Scholar]
- 20.Segal DS, Kuczenski R. Escalating dose-binge treatment with methylphenidate: role of serotonin in the emergent behavioral profile. J Pharmacol Exp Ther. 1999;291:19–30. [PubMed] [Google Scholar]
- 21.Yuan J, McCann U, Ricaurte G. Methylphenidate and brain dopamine neurotoxicity. Brain Res. 1997;767:172–175. doi: 10.1016/s0006-8993(97)00771-3. [DOI] [PubMed] [Google Scholar]
- National Toxicology Program. NTP-CERHR monograph on the potential human reproductive and developmental effects of amphetamines. 2005;16:vii–III1. [PubMed] [Google Scholar]
- 23.Bowyer JF, Holson RR. Methamphetamine and amphetamine neurotoxicity. In: Chang LW, Dyer RS, editors. Handbook of Neurotoxicology. Marcel Dekker, Inc.; New York: 1995. pp. 845–870. [Google Scholar]
- 24.Seiden LS, Sabol KE. Neurotoxicity of methamphetamine-related drugs and cocaine. In: Chang LW, Dyer RS, editors. Handbook of Neurotoxicology. Marcel Dekker, Inc.; New York: 1995. pp. 825–843. [Google Scholar]
- 25.Segal DS, Kuczenski R. Repeated binge exposure to amphetamine and methamphetamine: Behavioral and neurochemical characterization. J Pharmacol Exp Ther. 1997;282:561–573. [PubMed] [Google Scholar]
- 26.Ricaurte GA, Mechan AO, Yuan J, Hatzidimitriou G, Xie T, Mayne AH, McCann UD. Amphetamine treatment similar to that used in the treatment of adult attention-deficit/hyperactivity disorder damages dopaminergic nerve endings in the striatum of adult nonhuman primates. J Pharmacol Exp Ther. 2005;315:91–98. doi: 10.1124/jpet.105.087916. [DOI] [PubMed] [Google Scholar]
- 27.Borcherding BG, Keysor CS, Cooper TB, Rapoport JL. Differential effects of methylphenidate and dextroamphetamine on the motor activity level of hyperactive children. Neuropsychopharmacology. 1989;2:255–263. doi: 10.1016/0893-133x(89)90029-8. [DOI] [PubMed] [Google Scholar]
- 28.McGough JJ, Biederman J, Greenhill LL, McCracken JT, Spencer TJ, Posner K, Wigal S, Gornbein J, Tulloch S, Swanson JM. Pharmacokinetics of SLI381 (ADDERALL XR), an extended-release formulation of Adderall. J Am Acad Child Adolesc Psychiatry. 2003;42:684–691. doi: 10.1097/01.CHI.0000046850.56865.CB. [DOI] [PubMed] [Google Scholar]
- 29.Advokat C. Update on amphetamine neurotoxicity and its relevance to the treatment of ADHD. J Atten Disord. 2007;11:8–16. doi: 10.1177/1087054706295605. [DOI] [PubMed] [Google Scholar]
- 30.Castellanos FX, Lee PP, Sharp W, Jeffries NO, Greenstein DK, Clasen LS, Blumenthal JD, James RS, Ebens CL, Walter JM, Zijdenbos A, Evans AC, Giedd JN, Rapoport JL. Developmental trajectories of brain volume abnormalities in children and adolescents with attention-deficit/hyperactivity disorder. Journal of the American Medical Association. 2002;288:1740–8. doi: 10.1001/jama.288.14.1740. [DOI] [PubMed] [Google Scholar]
- 31.Truex LL, Schmidt MJ. 3H-amphetamine concentrations in the brains of young and aged rats: implications for assessment of drug effects in aged animals. Neurobiol Aging. 1980;1:93–95. doi: 10.1016/0197-4580(80)90029-9. [DOI] [PubMed] [Google Scholar]
- 32.Bowyer JF, Gough B, Slikker W, Jr, Lipe GW, Newport GD, Holson RR. Effects of a cold environment or age on methamphetamine-induced dopamine release in the caudate putamen of female rats. Pharmacol Biochem Behav. 1993;44:87–98. doi: 10.1016/0091-3057(93)90284-z. [DOI] [PubMed] [Google Scholar]
- 33.Miller DB, O’Callaghan JP, Ali SF. Age as a susceptibility factor in the striatal dopaminergic neurotoxicity observed in the mouse following substituted amphetamine exposure. Ann N Y Acad Sci. 2000;914:194–207. doi: 10.1111/j.1749-6632.2000.tb05196.x. [DOI] [PubMed] [Google Scholar]
- 34.Teuchert-Noodt G, Dawirs RR. Age-related toxicity in prefrontal cortex and caudate-putamen complex of gerbils (Meriones unguiculatus) after a single dose of methamphetamine. Neuropharmacology. 1991;30:733–743. doi: 10.1016/0028-3908(91)90181-a. [DOI] [PubMed] [Google Scholar]
- 35.Stefanski R, Ladenheim B, Lee SH, Cadet JL, Goldberg SR. Neuroadaptations in the dopaminergic system after active self-administration but not after passive administration of methamphetamine. Eur J Pharmacol. 1999;371:123–135. doi: 10.1016/s0014-2999(99)00094-1. [DOI] [PubMed] [Google Scholar]
- 36.Chang L, Alicata D, Ernst T, Volkow N. Structural and metabolic brain changes in the striatum associated with methamphetamine abuse. Addiction. 2007;102 Suppl 1:16–32. doi: 10.1111/j.1360-0443.2006.01782.x. [DOI] [PubMed] [Google Scholar]
- 37.Moszczynska A, Fitzmaurice P, Ang L, Kalasinsky KS, Schmunk GA, Peretti FJ, Aiken SS, Wickham DJ, Kish SJ. Why is parkinsonism not a feature of human methamphetamine users? Brain. 2004;127:363–370. doi: 10.1093/brain/awh046. [DOI] [PubMed] [Google Scholar]
- 38.Wilson JM, Kalasinsky KS, Levey AI, Bergeron C, Reiber G, Anthony RM, Schmunk GA, Shannak K, Haycock JW, Kish SJ. Striatal dopamine nerve terminal markers in human, chronic methamphetamine users. Nat Med. 1996;2:699–703. doi: 10.1038/nm0696-699. [DOI] [PubMed] [Google Scholar]
- 39.Frey K, Kilbourn M, Robinson T. Reduced striatal vesicular monoamine transporters after neurotoxic but not after behaviorally-sensitizing doses of methamphetamine. Eur J Pharmacol. 1997;334:273–279. doi: 10.1016/s0014-2999(97)01152-7. [DOI] [PubMed] [Google Scholar]
- 40.Fumagalli F, Gainetdinov RR, Wang YM, Valenzano KJ, Miller GW, Caron MG. Increased methamphetamine neurotoxicity in heterozygous vesicular monoamine transporter 2 knock-out mice. J Neurosci. 1999;19:2424–2431. doi: 10.1523/JNEUROSCI.19-07-02424.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Harvey DC, Lacan G, Tanious SP, Melega WP. Recovery from methamphetamine induced long-term nigrostriatal dopaminergic deficits without substantia nigra cell loss. Brain Res. 2000;871:259–270. doi: 10.1016/s0006-8993(00)02439-2. [DOI] [PubMed] [Google Scholar]
- 42.Hogan KA, Staal RG, Sonsalla PK. Analysis of VMAT2 binding after methamphetamine or MPTP treatment: disparity between homogenates and vesicle preparations. J Neurochem. 2000;74:2217–2220. doi: 10.1046/j.1471-4159.2000.0742217.x. [DOI] [PubMed] [Google Scholar]
- 43.Kilbourn MR, Frey KA, Vander BT, Sherman PS. Effects of dopaminergic drug treatments on in vivo radioligand binding to brain vesicular monoamine transporters. Nucl Med Biol. 1996;23:467–471. doi: 10.1016/0969-8051(96)00023-6. [DOI] [PubMed] [Google Scholar]
- 44.Miller GW, Gainetdinov RR, Levey AI, Caron MG. Dopamine transporters and neuronal injury. Trends Pharmacol Sci. 1999;20:424–429. doi: 10.1016/s0165-6147(99)01379-6. [DOI] [PubMed] [Google Scholar]
- 45.Vander BT, Kilbourn M, Desmond T, Kuhl D, Frey K. The vesicular monoamine transporter is not regulated by dopaminergic drug treatments. Eur J Pharmacol. 1995;294:577–583. doi: 10.1016/0014-2999(95)00594-3. [DOI] [PubMed] [Google Scholar]
- 46.London ED, Simon SL, Berman SM, Mandelkern MA, Lichtman AM, Bramen J, Shinn AK, Miotto K, Learn J, Dong Y, Matochik JA, Kurian V, Newton T, Woods R, Rawson R, Ling W. Mood disturbances and regional cerebral metabolic abnormalities in recently abstinent methamphetamine abusers. Arch Gen Psychiatry. 2004;61:73–84. doi: 10.1001/archpsyc.61.1.73. [DOI] [PubMed] [Google Scholar]
- 47.Berman SM, Voytek B, Mandelkern MA, Hassid BD, Isaacson A, Monterosso J, Miotto K, Ling W, London ED. Changes in cerebral glucose metabolism during early abstinence from chronic methamphetamine abuse. Mol Psychiatry. 2008;13 doi: 10.1038/sj.mp.4002107. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Volkow ND, Chang L, Wang GJ, Fowler JS, Franceschi D, Sedler MJ, Gatley SJ, Hitzemann R, Ding YS, Wong C, Logan J. Higher cortical and lower subcortical metabolism in detoxified methamphetamine abusers. Am J Psychiatry. 2001;158:383–389. doi: 10.1176/appi.ajp.158.3.383. [DOI] [PubMed] [Google Scholar]
- 49.Wang G-J, Volkow ND, Chang L, Miller E, Sedler M, Hitzemann R, Zhu W, Logan J, Ma Y, Fowler JS. Partial recovery of brain metabolism in methamphetamine abusers after protracted abstinence. Am J Psychiatry. 2004;161:242–248. doi: 10.1176/appi.ajp.161.2.242. [DOI] [PubMed] [Google Scholar]
- 50.Hwang J, Lyoo IK, Kim SJ, Sung YH, Bae S, Cho SN, Lee HY, Lee DS, Renshaw PF. Decreased cerebral blood flow of the right anterior cingulate cortex in long-term and short-term abstinent methamphetamine users. Drug Alcohol Depend. 2006;82:177–181. doi: 10.1016/j.drugalcdep.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 51.Volkow ND, Chang L, Wang G-J, Fowler JS, Franceschi D, Sedler M, Gatley SJ, Miller E, Hitzemann R, Ding YS, Logan J. Loss of dopamine transporters in methamphetamine abusers recovers with protracted abstinence. J Neurosci. 2001;21:9414–9418. doi: 10.1523/JNEUROSCI.21-23-09414.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pennypacker KR, Kassed CA, Eidizadeh S, O’Callaghan JP. Brain injury: prolonged induction of transcription factors. Acta Neurobiol Exp (Wars) 2000;60:515–530. doi: 10.55782/ane-2000-1373. [DOI] [PubMed] [Google Scholar]
- 53.Nieuwenhuis S, Aston-Jones G, Cohen JD. Decision making, the P3, and the locus coeruleus-norepinephrine system. Psychol Bull. 2005;131:510–532. doi: 10.1037/0033-2909.131.4.510. [DOI] [PubMed] [Google Scholar]
- 54.Polich J, Criado JR. Neuropsychology and neuropharmacology of P3a and P3b. Int J Psychophysiol. 2006;60:172–185. doi: 10.1016/j.ijpsycho.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 55.Takeuchi S, Jodo E, Suzuki Y, Matsuki T, Niwa S, Kayama Y. Effects of Repeated Administration of Methamphetamine on P3-like Potentials in Rats. Int J Psychophysiol. 1999;32:183–192. doi: 10.1016/s0167-8760(99)00016-1. [DOI] [PubMed] [Google Scholar]
- 56.Gouzoulis-Mayfrank E, Daumann J. Neurotoxicity of methylenedioxyamphetamines (MDMA; ecstasy) in humans: how strong is the evidence for persistent brain damage? Addiction. 2006;101:348–361. doi: 10.1111/j.1360-0443.2006.01314.x. [DOI] [PubMed] [Google Scholar]
- 57.Apostolova LG, Thompson PM. Brain mapping as a tool to study neurodegeneration. Neurotherapeutics. 2007;4:387–400. doi: 10.1016/j.nurt.2007.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bartzokis G, Beckson M, Lu PH, Edwards N, Rapoport R, Wiseman E, Bridge P. Age-related brain volume reductions in amphetamine and cocaine addicts and normal controls: implications for addiction research. Psychiatry Research: Neuroimaging. 2000;98:93–102. doi: 10.1016/s0925-4927(99)00052-9. [DOI] [PubMed] [Google Scholar]
- 59.Bartzokis G, Beckson M, Lu PH, Nuechterlein KH, Edwards N, Mintz J. Age-related changes in frontal and temporal lobe volumes in men: a magnetic resonance imaging study. Arch Gen Psychiatry. 2001;58:461–465. doi: 10.1001/archpsyc.58.5.461. [DOI] [PubMed] [Google Scholar]
- 60.Morgan MJ, Cascella NG, Stapleton JM, Phillips RL, Yung BCK, Wong DF, Shaya EK, London ED. Sensitivity to subjective effects of cocaine in drug abusers: Relation to cerebral ventricular size. Am J Psychiatry. 1993;150:1712–1717. doi: 10.1176/ajp.150.11.1712. [DOI] [PubMed] [Google Scholar]
- 61.Thompson PM, Hayashi K, Simon SL, Geaga JA, Hong MS, Sui Y, Lee JY, Toga AW, Ling W, London ED. Structural abnormalities in the brains of human subjects who use methamphetamine. J Neurosci. 2004;24:6028–6036. doi: 10.1523/JNEUROSCI.0713-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chang L, Smith LM, LoPresti C, Yonekura ML, Kuo J, Walot I, Ernst T. Smaller subcortical volumes and cognitive deficits in children with prenatal methamphetamine exposure. Psychiatry Res. 2004;132:95–106. doi: 10.1016/j.pscychresns.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 63.Chang L, Cloak C, Patterson K, Grob C, Miller EN, Ernst T. Enlarged striatum in abstinent methamphetamine abusers: a possible compensatory response. Biol Psychiatry. 2005;57:967–974. doi: 10.1016/j.biopsych.2005.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Song DD, Haber SN. Striatal responses to partial dopaminergic lesion: evidence for compensatory sprouting. J Neurosci. 2000;20:5102–5114. doi: 10.1523/JNEUROSCI.20-13-05102.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chang L, Ernst T, Speck O, Patel H, DeSilva M, Leonido-Yee M, Miller EN. Perfusion MRI and computerized cognitive test abnormalities in abstinent methamphetamine users. Psychiatry Research: Neuroimaging. 2002;114:65–79. doi: 10.1016/s0925-4927(02)00004-5. [DOI] [PubMed] [Google Scholar]
- 66.Oh JS, Lyoo IK, Sung YH, Hwang J, Kim J, Chung A, Park KS, Kim SJ, Renshaw PF, Song IC. Shape changes of the corpus callosum in abstinent methamphetamine users. Neurosci Lett. 2005;384:76–81. doi: 10.1016/j.neulet.2005.04.082. [DOI] [PubMed] [Google Scholar]
- 67.Witelson SF. Hand and sex differences in the isthmus and genu of the human corpus callosum. A postmortem morphological study. Brain. 1989;112(Pt 3):799–835. doi: 10.1093/brain/112.3.799. [DOI] [PubMed] [Google Scholar]
- 68.Jernigan TL, Gamst AC, Archibald SL, Fennema-Notestine C, Mindt MR, Marcotte TD, Heaton RK, Ellis RJ, Grant I. Effects of methamphetamine dependence and HIV infection on cerebral morphology. Am J Psychiatry. 2005;162:1461–1472. doi: 10.1176/appi.ajp.162.8.1461. [DOI] [PubMed] [Google Scholar]
- 69.Bae SC, Lyoo IK, Sung YH, Yoo J, Chung A, Yoon SJ, Kim DJ, Hwang J, Kim SJ, Renshaw PF. Increased white matter hyperintensities in male methamphetamine abusers. Drug Alcohol Depend. 2006;81:83–88. doi: 10.1016/j.drugalcdep.2005.05.016. [DOI] [PubMed] [Google Scholar]
- 70.Awad IA, Johnson PC, Spetzler RF, Hodak JA. Incidental subcortical lesions identified on magnetic resonance imaging in the elderly. II Postmortem pathological correlations. Stroke. 1986;17:1090–1097. doi: 10.1161/01.str.17.6.1090. [DOI] [PubMed] [Google Scholar]
- 71.Bartzokis G, Goldstein IB, Hance DB, Beckson M, Shapiro D, Lu PH, Edwards N, Mintz J, Bridge P. The incidence of T2-weighted MR imaging signal abnormalities in the brain of cocaine-dependent patients is age-related and region-specific. AJNR. 1999;20:1628–1635. [PMC free article] [PubMed] [Google Scholar]
- 72.Lyoo IK, Streeter CC, Ahn KH, Lee HK, Pollack MH, Silveri MM, Nassar L, Levin JM, Sarid-Segal O, Ciraulo DA, Renshaw PF, Kaufman MJ. White matter hyperintensities in subjects with cocaine and opiate dependence and healthy comparison subjects. Psychiatry Res. 2004;131:135–145. doi: 10.1016/j.pscychresns.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 73.Volkow ND, Valentine A, Kulkarni M. Radiological and neurological changes in the drug abuse patient: a study with MRI. J Neuroradiol. 1988;15:288–293. [PubMed] [Google Scholar]
- 74.Schlaepfer TE, Lancaster E, Heidbreder R, Strain EC, Kosel M, Fisch HU, Pearlson GD. Decreased frontal white-matter volume in chronic substance abuse. Int J Neuropsychopharmacol. 2006;9:147–153. doi: 10.1017/S1461145705005705. [DOI] [PubMed] [Google Scholar]
- 75.Harper C, Matsumoto I. Ethanol and brain damage. Curr Opin Pharmacol. 2005;5:73–78. doi: 10.1016/j.coph.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 76.Albertson DN, Pruetz B, Schmidt CJ, Kuhn DM, Kapatos G, Bannon MJ. Gene expression profile of the nucleus accumbens of human cocaine abusers: evidence for dysregulation of myelin. J Neurochem. 2004;88:1211–1219. doi: 10.1046/j.1471-4159.2003.02247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bartzokis G, Beckson M, Lu PH, Edwards N, Bridge P, Mintz J. Brain maturation may be arrested in chronic cocaine addicts. Biol Psychiatry. 2002;51:605–611. doi: 10.1016/s0006-3223(02)01315-x. [DOI] [PubMed] [Google Scholar]
- 78.Ashburner J, Friston KJ. Voxel-Based Morphometry -- The Methods. Neuroimage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- 79.Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14:21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- 80.Kim SJ, Lyoo IK, Hwang J, Chung A, Hoon SY, Kim J, Kwon DH, Chang KH, Renshaw PF. Prefrontal grey-matter changes in short-term and long-term abstinent methamphetamine abusers. Int J Neuropsychopharmacol. 2006;9:221–228. doi: 10.1017/S1461145705005699. [DOI] [PubMed] [Google Scholar]
- 81.Chung A, Lyoo IK, Kim SJ, Hwang J, Bae SC, Sung YH, Sim ME, Song IC, Kim J, Chang KH, Renshaw PF. Decreased frontal white-matter integrity in abstinent methamphetamine abusers. Int J Neuropsychopharmacol. 2007;10:765–775. doi: 10.1017/S1461145706007395. [DOI] [PubMed] [Google Scholar]
- 82.Rykhlevskaia E, Gratton G, Fabiani M. Combining structural and functional neuroimaging data for studying brain connectivity: A review. Psychophysiology. 2008;45:173–87. doi: 10.1111/j.1469-8986.2007.00621.x. [DOI] [PubMed] [Google Scholar]
- 83.Song SK, Sun SW, Ju WK, Lin SJ, Cross AH, Neufeld AH. Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. Neuroimage. 2003;20:1714–1722. doi: 10.1016/j.neuroimage.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 84.Cowan RL, Lyoo IK, Sung SM, Ahn KH, Kim MJ, Hwang J, Haga E, Vimal RLP, Lukas SE, Renshaw PF. Reduced cortical gray matter density in human MDMA (ecstasy) users: a voxel-based morphometry study. Drug Alcohol Depend. 2003;72:225–235. doi: 10.1016/j.drugalcdep.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 85.Moeller FG, Steinberg JL, Lane SD, Buzby M, Swann AC, Hasan KM, Kramer LA, Narayana PA. Diffusion Tensor Imaging in MDMA Users and Controls: Association with Decision Making. Am J Drug Alcohol Abuse. 2007;33:777–789. doi: 10.1080/00952990701651564. [DOI] [PubMed] [Google Scholar]
- 86.Doblin R. A clinical plan for MDMA (Ecstasy) in the treatment of posttraumatic stress disorder (PTSD): partnering with the FDA. J Psychoactive Drugs. 2002;34:185–194. doi: 10.1080/02791072.2002.10399952. [DOI] [PubMed] [Google Scholar]
- 87.Check E. Psychedelic drugs: the ups and downs of ecstasy. Nature. 2004;429:126–128. doi: 10.1038/429126a. [DOI] [PubMed] [Google Scholar]
- 88.Cole JC, Sumnall HR. Altered states: the clinical effects of Ecstasy. Pharmacol Ther. 2003;98:35–58. doi: 10.1016/s0163-7258(03)00003-2. [DOI] [PubMed] [Google Scholar]
- 89.Bender E. FDA approves study of ecstasy in some terminally ill cancer patients. Psychiatric News. 2005;40:46. [Google Scholar]
- 90.de Win MM, Reneman L, Jager G, Vlieger EJ, Olabarriaga SD, Lavini C, Bisschops I, Majoie CB, Booij J, den Heeten GJ, van den BW. A prospective cohort study on sustained effects of low-dose ecstasy use on the brain in new ecstasy users. Neuropsychopharmacology. 2007;32:458–470. doi: 10.1038/sj.npp.1301225. [DOI] [PubMed] [Google Scholar]
- 91.Downes MA, Whyte IM. Amphetamine-induced movement disorder. Emerg Med Australas. 2005;17:277–280. doi: 10.1111/j.1742-6723.2005.00735.x. [DOI] [PubMed] [Google Scholar]
- 92.Margolese HC, Chouinard G, Kolivakis TT, Beauclair L, Miller R. Tardive dyskinesia in the era of typical and atypical antipsychotics. Part 1: pathophysiology and mechanisms of induction. Can J Psychiatry. 2005;50:541–547. doi: 10.1177/070674370505000907. [DOI] [PubMed] [Google Scholar]
- 93.Isaac G, Fredriksson A, Danielsson R, Eriksson P, Bergquist J. Brain lipid composition in postnatal iron-induced motor behavior alterations following chronic neuroleptic administration in mice. FEBS J. 2006;273:2232–2243. doi: 10.1111/j.1742-4658.2006.05236.x. [DOI] [PubMed] [Google Scholar]
- 94.Double KL, Halliday GM, Henderson J, Griffiths FM, Heinemann T, Riederer P, Gerlach M. The dopamine receptor agonist lisuride attenuates iron-mediated dopaminergic neurodegeneration. Exp Neurol. 2003;184:530–535. doi: 10.1016/j.expneurol.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 95.Wirshing DA, Bartzokis G, Pierre JM, Wirshing WC, Sun A, Tishler TA, Marder SR. Tardive dyskinesia and serum iron indices. Biol Psychiatry. 1998;44:493–498. doi: 10.1016/s0006-3223(97)00453-8. [DOI] [PubMed] [Google Scholar]
- 96.Bartzokis G, Tishler TA, Lu PH, Villablanca P, Altshuler LL, Carter M, Huang D, Edwards N, Mintz J. Brain ferritin iron may influence age- and gender-related risks of neurodegeneration. Neurobiol Aging. 2007;28:414–423. doi: 10.1016/j.neurobiolaging.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 97.Taylor S, Christensen JD, Holcomb JM, Garver DL. Volume increases in striatum associated with positive symptom reduction in schizophrenia: a preliminary observation. Psychiatry Res. 2005;140:85–89. doi: 10.1016/j.pscychresns.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 98.Rogers RD, Everitt BJ, Baldacchino A, Blackshaw AJ, Swainson R, Wynne K, Baker NB, Hunter J, Carthy T, Booker E, London M, Deakin JF, Sahakian BJ, Robbins TW. Dissociable deficits in the decision-making cognition of chronic amphetamine abusers, opiate abusers, patients with focal damage to prefrontal cortex, and tryptophan-depleted normal volunteers: Evidence for monoaminergic mechanisms. Neuropsychopharmacology. 1999;20:322–339. doi: 10.1016/S0893-133X(98)00091-8. [DOI] [PubMed] [Google Scholar]
- 99.Silverstone PH, Asghar SJ, O’Donnell T, Ulrich M, Hanstock CC. Lithium and valproate protect against dextro-amphetamine induced brain choline concentration changes in bipolar disorder patients. World J Biol Psychiatry. 2004;5:38–44. doi: 10.1080/15622970410029906. [DOI] [PubMed] [Google Scholar]
- 100.Wan FJ, Shiah IS, Lin HC, Huang SY, Tung CS. Nomifensine attenuates d-amphetamine-induced dopamine terminal neurotoxicity in the striatum of rats. Chin J Physiol. 2000;43:69–74. [PubMed] [Google Scholar]
- 101.Escubedo E, Chipana C, Perez-Sanchez M, Camarasa J, Pubill D. Methyllycaconitine prevents methamphetamine-induced effects in mouse striatum: involvement of alpha7 nicotinic receptors. J Pharmacol Exp Ther. 2005;315:658–667. doi: 10.1124/jpet.105.089748. [DOI] [PubMed] [Google Scholar]
- 102.Klongpanichapak S, Govitrapong P, Sharma SK, Ebadi M. Attenuation of cocaine and methamphetamine neurotoxicity by coenzyme Q10. Neurochem Res. 2006;31:303–311. doi: 10.1007/s11064-005-9025-3. [DOI] [PubMed] [Google Scholar]
- 103.Wu PH, Shen YC, Wang YH, Chi CW, Yen JC. Baicalein attenuates methamphetamine-induced loss of dopamine transporter in mouse striatum. Toxicology. 2006;226:238–245. doi: 10.1016/j.tox.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 104.Klongpanichapak S, Phansuwan-Pujito P, Ebadi M, Govitrapong P. Melatonin protects SK-N-SH neuroblastoma cells from amphetamine-induced neurotoxicity. J Pineal Res. 2007;43:65–73. doi: 10.1111/j.1600-079X.2007.00444.x. [DOI] [PubMed] [Google Scholar]
- 105.Achat-Mendes C, Anderson KL, Itzhak Y. Impairment in consolidation of learned place preference following dopaminergic neurotoxicity in mice is ameliorated by N-acetylcysteine but not D1 and D2 dopamine receptor agonists. Neuropsychopharmacology. 2007;32:531–541. doi: 10.1038/sj.npp.1301119. [DOI] [PubMed] [Google Scholar]
- 106.Benegal V, Antony G, Venkatasubramanian G, Jayakumar PN. Gray matter volume abnormalities and externalizing symptoms in subjects at high risk for alcohol dependence. Addict Biol. 2007;12:122–132. doi: 10.1111/j.1369-1600.2006.00043.x. [DOI] [PubMed] [Google Scholar]
- 107.Di Sclafani V, Clark HW, Tolou-Shams M, Bloomer CW, Salas GA, Norman D, Fein G. Premorbid brain size is a determinant of functional reserve in abstinent crack-cocaine and crack-cocaine-alcohol-dependent adults. J Int Neuropsychol Soc. 1998;4:559–565. doi: 10.1017/s1355617798466049. [DOI] [PubMed] [Google Scholar]
- 108.Kochunov P, Thompson PM, Coyle TR, Lancaster JL, Kochunov V, Royall D, Mangin JF, Riviere D, Fox PT. Relationship among neuroimaging indices of cerebral health during normal aging. Hum Brain Mapp. 2008;29:36–45. doi: 10.1002/hbm.20369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Benes FM, Turtle M, Khan Y, Farol P. Myelination of a key relay zone in the hippocampal formation occurs in the human brain during childhood, adolescence, and adulthood. Arch Gen Psychiatry. 1994;51:477–484. doi: 10.1001/archpsyc.1994.03950060041004. [DOI] [PubMed] [Google Scholar]
- 110.Bartzokis G. Brain myelination in prevalent neuropsychiatric developmental disorders: Primary and comorbid addiction. In: Flaherty Lois T., editor. Adolescent Psychiatry. Vol. 29. The Annals of the American Society for Adolescent Psychiatry; Routledge: 2005. pp. 55–96. [PMC free article] [PubMed] [Google Scholar]
- 111.Croft RJ, Mackay AJ, Mills ATD, Gruzelier JGH. The relative contributions of ecstasy and cannabis to cognitive impairment. Psychopharmacology (Berl) 2001;153:373–379. doi: 10.1007/s002130000591. [DOI] [PubMed] [Google Scholar]
- 112.Morton AJ, Hickey MA, Dean LC. Methamphetamine toxicity in mice is potentiated by exposure to loud music. NeuroReport. 2001;12:3277–3281. doi: 10.1097/00001756-200110290-00026. [DOI] [PubMed] [Google Scholar]
- 113.Sung YH, Cho SC, Hwang J, Kim SJ, Kim H, Bae S, Kim N, Chang KH, Daniels M, Renshaw PF, Lyoo IK. Relationship between N-acetyl-aspartate in gray and white matter of abstinent methamphetamine abusers and their history of drug abuse: A proton magnetic resonance spectroscopy study. Drug Alcohol Depend. 2007;88:28–35. doi: 10.1016/j.drugalcdep.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 114.Snook L, Plewes C, Beaulieu C. Voxel based versus region of interest analysis in diffusion tensor imaging of neurodevelopment. Neuroimage. 2007;34:243–252. doi: 10.1016/j.neuroimage.2006.07.021. [DOI] [PubMed] [Google Scholar]
- 115.Ashburner J, Friston KJ. Why voxel-based morphometry should be used. Neuroimage. 2001;14:1238–1243. doi: 10.1006/nimg.2001.0961. [DOI] [PubMed] [Google Scholar]
- 116.Bookstein FL. “Voxel-based morphometry” should not be used with imperfectly registered images. Neuroimage. 2001;14:1454–1462. doi: 10.1006/nimg.2001.0770. [DOI] [PubMed] [Google Scholar]
- 117.Davatzikos C. Why voxel-based morphometric analysis should be used with great caution when characterizing group differences. Neuroimage. 2004;23:17–20. doi: 10.1016/j.neuroimage.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 118.Maguire EA, Gadian DG, Johnsrude IS, Good CD, Ashburner J, Frackowiak RSJ, Frith CD. Navigation-related structural change in the hippocampi of taxi drivers. Proc Natl Acad Sci USA. 2000;97:4398–4403. doi: 10.1073/pnas.070039597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Uchida RR, Del-Ben CM, Araujo D, Busatto-Filho G, Duran FL, Crippa JA, Graeff FG. Correlation between voxel based morphometry and manual volumetry in magnetic resonance images of the human brain. An Acad Bras Cienc. 2008;80:149–156. doi: 10.1590/s0001-37652008000100010. [DOI] [PubMed] [Google Scholar]
- 120.Keller SS, Mackay CE, Barrick TR, Wieshmann UC, Howard MA, Roberts N. Voxel-based morphometric comparison of hippocampal and extrahippocampal abnormalities in patients with left and right hippocampal atrophy. Neuroimage. 2002;16:23–31. doi: 10.1006/nimg.2001.1072. [DOI] [PubMed] [Google Scholar]
- 121.Kubicki M, Shenton ME, Salisbury DF, Hirayasu Y, Kasai K, Kikinis R, Jolesz FA, McCarley RW. Voxel-based morphometric analysis of gray matter in first episode schizophrenia. Neuroimage. 2002;17:1711–1719. doi: 10.1006/nimg.2002.1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Good CD, Scahill RI, Fox NC, Ashburner J, Friston KJ, Chan D, Crum WR, Rossor MN, Frackowiak RS. Automatic differentiation of anatomical patterns in the human brain: validation with studies of degenerative dementias. Neuroimage. 2002;17:29–46. doi: 10.1006/nimg.2002.1202. [DOI] [PubMed] [Google Scholar]
- 123.Job DE, Whalley HC, McConnell S, Glabus M, Johnstone EC, Lawrie SM. Voxel-based morphometry of grey matter densities in subjects at high risk of schizophrenia. Schizophr Res. 2003;64:1–13. doi: 10.1016/s0920-9964(03)00158-0. [DOI] [PubMed] [Google Scholar]
- 124.Job DE, Whalley HC, McConnell S, Glabus M, Johnstone EC, Lawrie SM. Structural gray matter differences between first-episode schizophrenics and normal controls using voxel-based morphometry. Neuroimage. 2002;17:880–889. [PubMed] [Google Scholar]
- 125.Thacker NA. University of Manchester; 2005. Tutorial: A Critical Analysis of Voxel Based Morphometry (VBM) http://www.tina-vision.net/docs/memos/2003-011.pdf. 3-14-2008. [Google Scholar]
- 126.Salmond CH, Ashburner J, Vargha-Khadem A, Connelly A, Gadian DG, Friston KJ. Distributional assumptions in voxel-based morphometry. Neuroimage. 2002;17:1027–1030. [PubMed] [Google Scholar]
- 127.Meyer-Lindenberg A, Nicodemus KK, Egan MF, Callicott JH, Mattay V, Weinberger DR. False positives in imaging genetics. Neuroimage. 2008;40:655–661. doi: 10.1016/j.neuroimage.2007.11.058. [DOI] [PubMed] [Google Scholar]
- 128.Fein G, Landman B, Tran H, Barakos J, Moon K, Di S, Shumway V, Shumway R. Statistical parametric mapping of brain morphology: sensitivity is dramatically increased by using brain-extracted images as inputs. Neuroimage. 2006;30:1187–1195. doi: 10.1016/j.neuroimage.2005.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Keller SS, Wilke M, Wieshmann UC, Sluming VA, Roberts N. Comparison of standard and optimized voxel-based morphometry for analysis of brain changes associated with temporal lobe epilepsy. Neuroimage. 2004;23:860–868. doi: 10.1016/j.neuroimage.2004.07.030. [DOI] [PubMed] [Google Scholar]
- 130.Pell GS, Briellmann RS, Pardoe H, Abbott DF, Jackson GD. Composite voxel-based analysis of volume and T2 relaxometry in temporal lobe epilepsy. Neuroimage. 2008;39:1151–1161. doi: 10.1016/j.neuroimage.2007.09.061. [DOI] [PubMed] [Google Scholar]
- 131.Ridgway GR, Henley SM, Rohrer JD, Scahill RI, Warren JD, Fox NC. Ten simple rules for reporting voxel-based morphometry studies. Neuroimage. 2008;39 doi: 10.1016/j.neuroimage.2008.01.003. in press. [DOI] [PubMed] [Google Scholar]
- 132.Bartzokis G, Lu PH. Brain Volume: Age-Related Changes. In: Squire LR, editor. Encyclopedia of Neuroscience. Oxford: Academic Press; 2008. [Google Scholar]
- 133.Hutton C, De VE, Ashburner J, Deichmann R, Turner R. Voxel-based cortical thickness measurements in MRI. Neuroimage. 2008;39 doi: 10.1016/j.neuroimage.2008.01.027. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]


