Abstract
Purpose
We conducted a phase II trial of neoadjuvant paclitaxel, carboplatin and gemcitabine as well as transurethral resection of bladder tumor to evaluate the clinical T0 (cT0) rate with paclitaxel, carboplatin and gemcitabine, and to study cystoscopic surveillance or immediate cystectomy for patients with cT0 status following chemotherapy.
Materials and Methods
Patients with T2-T4a chemotherapy and radiation naive urothelial cancer were eligible. T2+ tumor had to be diagnosed by transurethral bladder tumor resection followed by a second transurethral bladder tumor resection to confirm persistent disease within 16 weeks of the first resection. Three cycles of paclitaxel, carboplatin and gemcitabine were administered within 8 weeks of the second transurethral bladder tumor resection. Patients with cT0 status after paclitaxel, carboplatin and gemcitabine therapy could elect immediate cystectomy or cystoscopic surveillance, and those with greater than cT0 status were to undergo immediate cystectomy.
Results
Of 77 patients 74 were assessable, and cT0 status after paclitaxel, carboplatin and gemcitabine was achieved in 34 of 74 patients (46%). Of the 34 patients with cT0 status 10 underwent immediate cystectomy, 6 of whom had persistent cancer. Persistent tumor at transurethral bladder tumor resection was seen in 28 patients (38%) and 21 underwent cystectomy. Thus, 35 of 74 patients underwent cystectomy. With a median followup of 22 months 2-year overall survival was 59% (95% CI 45, 72) and among cT0 cases it was 75% (95% CI 57, 93).
Conclusions
Although neoadjuvant paclitaxel, carboplatin and gemcitabine had a promising 46% cT0 rate, the study failed to meet the primary objective as there was an unacceptably high rate (60%) of persistent cancer at cystectomy in patients presumed to have pT0 status. Patients completing neoadjuvant chemotherapy should strongly consider definitive local therapy rather than cystoscopic surveillance regardless of post-chemotherapy cT0 status.
Keywords: carcinoma, transitional cell, drug therapy
Radical cystectomy with regional lymphadenectomy has remained the cornerstone of therapy for patients with muscle invasive urothelial (commonly transitional cell) carcinoma. While others have championed alternative treatments such as partial cystectomy, deep transurethral resection, radiation therapy or radiation plus chemotherapy, none of these approaches appear to offer enhanced patient survival compared to historical data for cystectomy alone. Furthermore, these alternative therapeutic approaches are often performed on highly selected patients. Thus, if incorrect selection criteria are used, patient survival may in fact be potentially disadvantaged.1
Following reports of favorable tumor response rates and prolonged overall survival with MVAC chemotherapy combination in patients with metastatic urothelial cancer,2 SWOG tested this regimen in a randomized phase III trial of neoadjuvant MVAC followed by cystectomy vs cytectomy alone in patients with T2-T4 disease (S8710).3 S8710 demonstrated that neoadjuvant MVAC prolongs survival in locally advanced urothelial cancer with a 25% improvement in survival in the MVAC arm. Interestingly 5-year survival was 88% for the 38% of patients who achieved a pathological complete response (pT0) at cystectomy.3
The results from a separate but similar study conducted by Millikan et al showed that patients with TCC treated with MVAC fell into distinct risk categories based on whether residual disease was present at surgery.4 Those with pT0 status experienced a 5-year survival of 88% vs 12% in those with positive nodes at cystectomy.
In a retrospective analysis of 432 patients with T2+ urothelial cancers Herr et al showed that in a subset of 151 patients who had less than T2 disease following neoadjuvant chemotherapy, overall survival was similar regardless of whether patients underwent immediate cystectomy or cystoscopic surveillance.5 Specifically the 10-year survival rates were reported to be 71% vs 76%, respectively. This observation led us to hypothesize that after neoadjuvant chemotherapy clinical T0 status (as a surrogate for pT0 status) would result in improved outcomes regardless of cystectomy timing (immediate or delayed).
Materials and Methods
Eligibility
Patients had to have histologically confirmed muscle invasive (T2-T4a) TCC with a negative metastatic evaluation to be included in the study. The muscle invasive nature had to be present on the first TURBT and confirmed on repeat TURBT performed within 16 weeks of the initial resection. Measurable disease was not required. Patients needed to have adequate hematological, hepatic and renal function, and a Zubrod performance status of 0 to 2 to be eligible. No prior systemic chemotherapy or radiation therapy for urothelial cancer was allowed. The protocol was approved by the institutional review boards of all participating institutions and all patients provided written informed consent.
Study Design and Statistical Considerations
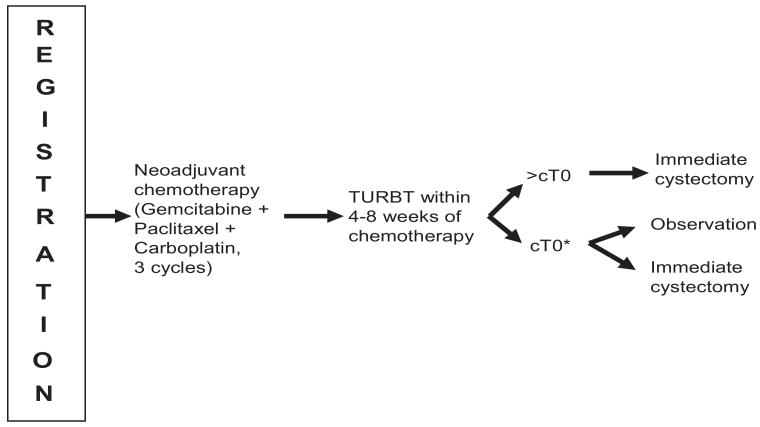
A schema of the study design is shown in figure 1. Patients received 3 cycles of paclitaxel (80 mg/m2, days 1 and 8), carboplatin-C (AUC 5 on day 1) and gemcitabine (800 mg/m2, days 1 and 8), all given intravenously. Chemotherapy dose modifications were allowed for interim hematological and nonhematological toxicities. After neoadjuvant chemotherapy was completed patients were to undergo another re-staging TURBT. Based on these findings the patients with cT0 status were given the option of proceeding with immediate cystectomy or pursuing cystoscopic surveillance following a balanced discussion with the urologist. If residual tumor was found at the post-chemotherapy TURBT the patient would undergo immediate cystectomy.
Figure 1.
Schema design of SWOG S0219
* Action decided after balance discussion with physician
Version 2.0 of the National Cancer Institute Common Toxicity Criteria was used for toxicity assessment. The primary objective was assessment of the cT0 rate of this chemotherapy regimen as assessed at the post-chemotherapy TURBT. Secondary objectives included 1) assessment of overall survival, 2) evaluation of the toxicity of the regimen and 3) assessment of feasibility of this sequential treatment approach.
An accrual range of 60 to 95 patients was determined with a higher goal set to optimize tumor specimen collection. The PCG regimen would be of considerable interest if the true cT0 rate were 40% or greater but of no further interest if it were 25% or less. The null hypothesis that this regimen is of no further interest would be rejected if a cT0 rate of 33% or greater were observed using a 1-stage phase II design. This 1-sided 0.05 level test had a power of 85% with a sample size of 70 patients and 89% with 80 patients. Overall survival was estimated by the Kaplan-Meier method.6 All analyses are based on the data available as of April 3, 2008.
Results
Patient Characteristics
The study was activated in January 2003 and closed to accrual in December 2007. There were 77 patients from 16 institutions registered to this study. Two patients were ineligible, 1 because no TCC was seen on the second TURBT, and the second because the initial and second TURBT were more than 16 weeks apart. Another patient, although eligible, did not receive any protocol treatment and, therefore, was not assessed for any end points. Baseline characteristics for the 74 assessable patients are given in table 1.
Table 1. Baseline patient characteristics.
| Median age (range) | 69 (49–83) |
| No. gender (%): | |
| M | 56 (76) |
| F | 18 (24) |
| No. performance status (%): | |
| 0 | 50 (76) |
| 1 | 16 (24) |
| Missing | 8 |
| No. race (%): | |
| White | 70 (94) |
| Black | 2 (3) |
| Other | 2 (3) |
| No. clinical T stage (%): | |
| T2 | 52 (70) |
| T3 | 17 (23) |
| T4a | 5 (7) |
| No. prior intravesical therapy (%): | |
| Yes | 11 (16) |
| No | 59 (84) |
| Missing | 4 |
| No. yrs from initial diagnosis (%): | |
| Less than 1 | 37 (61) |
| 1 + | 24 (39) |
| Missing | 1 |
Toxicity
A total of 71 patients were evaluable for toxicities with 53 (75%) experiencing a grade 3 or greater adverse event. A summary of the most common treatment related adverse events is shown in table 2. Hematological toxicities were most prevalent, with the most common grade 3 or greater toxicities being neutropenia, leukopenia and thrombocytopenia. One patient had a grade 4 gastrointestinal anastomotic leak and another experienced a grade 4 abnormal troponin I level. There was 1 treatment related death from infection with neutropenia. Grade 3 nonhematological toxicities were uncommon.
Table 2. Most common treatment related adverse events.
| No. (%) | |||
|---|---|---|---|
| Any Grade | Grade 3 | Grade 4 | |
| Fatigue | 59 (83) | 3 (4) | 0 |
| Neutropenia/granulocytopenia | 49 (69) | 26 (37) | 10 (14) |
| Anemia | 46 (65) | 5 (7) | 0 |
| Leukopenia | 44 (62) | 16 (23) | 5 (7) |
| Thrombocytopenia | 40 (56) | 10 (14) | 3 (4) |
| Alopecia | 35 (49) | 0 | 0 |
| Nausea | 28 (39) | 0 | 0 |
| Anorexia | 18 (25) | 0 | 0 |
| Diarrhea | 17 (24) | 0 | 0 |
Response
Findings based on TURBT and cystectomy are summarized in table 3. Of 74 patients 34 had cT0 status at TURBT after PCG for a response rate of 46% (95% CI 34, 58). Of these patients 10 underwent immediate cystectomy, of whom only 4 were truly pT0 status. Pathological findings for the other 6 patients showed pT2-T4, grade 3 TCC. Residual disease was reported in 28 patients (38%) at the post-PCG TURBT, of whom 21 underwent the protocol specified immediate cystectomy. Twelve patients (16%) did not undergo the protocol specified after PCG resection and are considered nonresponders. Reasons for not performing TURBT included patient death on treatment (1), progressive disease (2), patient refusal (5) and electing to go straight to cystectomy (4). Pathology results showed all of these last 4 patients to have pT2-T4, grade 3 TCC. Overall 35 patients (47%) underwent immediate cystectomy.
Table 3. Post-PCG TURBT and cystectomy results.
| No./Total No. (%) | |
|---|---|
| No residual disease (cT0) on TURBT: | 34/74 (46) |
| Immediate cystectomy: | 10/34 (29) |
| No residual disease (pT0) | 4/10 (40) |
| Residual disease (greater than pT0) | 6/10 (60) |
| Residual disease (greater than cT0) on TURBT: | 28/74 (38) |
| Immediate cystectomy: | 21/28 (75) |
| Residual disease (greater than pT0) | 21/21 (100) |
| No TURBT performed: | 12/74 (16) |
| Immediate cystectomy: | 4/12 (33) |
| Residual disease (greater than pT0) | 4/4 (100) |
Survival
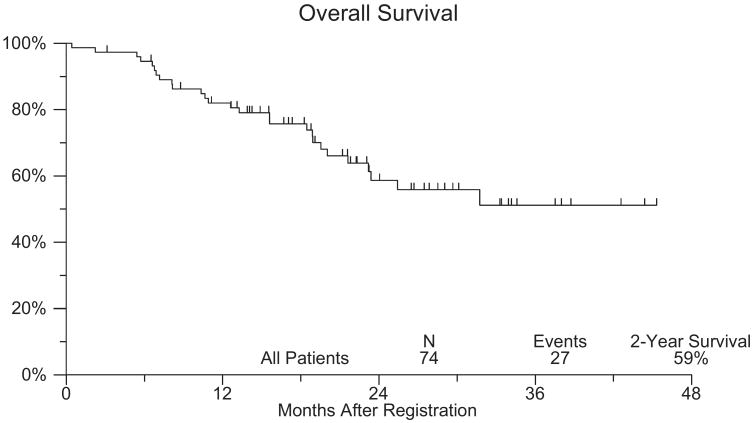
Overall survival in this patient population is shown in figure 2. With a median followup of 22 months the Kaplan-Meier estimate of 2-year survival was 59% (95% CI 45, 72). Among the 34 patients with cT0 status at post-PCG TURBT the 2-year survival estimate was 75% (95% CI 57, 93). Of the 24 patients with cT0 disease who did not undergo cystectomy 5 died and the current 2-year OS estimate is 76%. Of the 10 patients with cT0 disease who underwent cystectomy 2 died and the current 2-year OS estimate is 70%.
Figure 2.
Overall survival of patients on SWOG 0219
Discussion
While muscle invasive TCC represents only 25% of patients initially presenting with this disease, these patients account for 85% of the mortality from the disease.7 A recent investigation of outcomes for patients who had undergone cystectomy before 1985 and after 1985 (the year neoadjuvant therapy became widely available) reported no substantial improvement in survival for patients undergoing cystectomy.8 In these 2 groups of patients those who had pT3 TCC before 1985 had a 5-year survival of 33% while after 1985 the 5-year survival was 35%. Comparable figures for only pT2 disease showed a modest 7% improvement in survival in patients after 1985 (67% vs 60%).
In the last 30 years the Southwest Oncology Group has conducted a series of clinical trials focused on improving outcomes for patients with locally advanced urothelial cancer. The first study (SWOG 8221) evaluated the effects of 2,000 rad delivered before radical cystectomy. Unfortunately this approach showed no such advantage.9 Following the reports of the success of MVAC chemotherapy in combination in patients with metastatic urothelial cancer, SWOG 8710 was performed.10 The underlying hypothesis was that MVAC might also be effective in patients with micrometastasis, thus potentially improving the cure rate in addition to cystectomy alone.
In SWOG 8710 the median survival for patients who received MVAC followed by cystectomy was 6.2 years. However, in those patients who underwent immediate cystectomy it was only 3.8 years. The benefit became 3.5 years for cT3-T4 and 2.5 years for cT2. Overall patients receiving neoadjuvant MVAC had a 25% reduction in the risk of dying of urothelial cancer. Furthermore, the survival rate for the 38% of patients treated with MVAC with pT0 status was 88%.4 Thus, S0219 appeared a logical way to build on these results. The major objective of the trial was to prospectively define the complete response rate after neoadjuvant chemotherapy as determined by transuretheral resection after neoadjuvant chemotherapy.
In addition, if tissue was collected before and after chemotherapy, it would allow molecular analysis aimed at identifying molecular patterns that would predict if a patient would or would not respond to chemotherapy. Finally for those patients who did not respond it would offer an opportunity to identify targets that could be used to improve future therapy. It was hoped that this approach would assure that neoadjuvant chemotherapy would be given only to the cohort of patients with the highest likelihood of responding. It was a surprise that it took 5 years to accrue 77 patients to this study although SWOG 8710 showed positive results favoring outcomes for patients who underwent neoadjuvant chemotherapy. The accrual became of academic importance since 6 of 10 patients with cT0 status after chemotherapy who elected to undergo immediate cystectomy were found to have residual T2 or greater disease at cystectomy. Although pathological T stage is not known for the majority of the patients with cT0 disease who did not have immediate cystectomy, this rate of missed muscle invasive disease indicates that TURBT does not accurately identify those patients who are truly disease-free after chemotherapy. The 2-year OS rate for the 10 patients with cT0 disease who underwent immediate cystectomy was 70% while that for the 24 with cT0 disease who were followed was 76%. Thus, there is no evidence of a difference in overall survival between these small subgroups.
It was initially thought that PCG, a modified version of a previously piloted combination, would have less toxicity and comparable efficacy compared to MVAC and, thus, would be more acceptable to physicians and patients in a neoadjuvant setting.10 However, concerns about a noncisplatin or non-MVAC regimen in the neoadjuvant setting, as well as the availability of other combination chemotherapies, may have hampered accrual to S0219. Additional obstacles may have included uncertainty as to which patients would respond to neoadjuvant chemotherapy and concerns about the accuracy of the post-chemotherapy TURBT, both of which may have led to a preference for immediate cystectomy.
Despite these obstacles to accrual 77 patients were successfully registered. We observed a cT0 rate of 46% (95% CI 34, 58), which did meet the primary study end point. However, 6 of the 10 patients with cT0 status after neoadjuvant chemotherapy who elected to undergo immediate cystectomy were found to have residual T2 or greater disease at cystectomy. Thus, this rate of missed muscle invasive disease suggests that TURBT may not accurately identify those patients who are disease-free after chemotherapy.
Conclusions
Single-institution studies have demonstrated that patients with cT0 status after chemotherapy who forego cystectomy have a survival rate similar to those undergoing immediate cystectomy.5,11–13 However, our results clearly indicate that, while neoadjuvant chemotherapy can be given safely, and while patients who respond to chemotherapy have a favorable outcome, in a multi-institutional setting after neoadjuvant chemotherapy TURBT cannot be safely used to designate a patient as not requiring cystectomy. In single institutional settings the practice should be considered investigational. Correlative studies are ongoing and will be reported in a separate work.
Acknowledgments
Supported by Public Health Service Cooperative Agreement Grants awarded by the National Cancer Institute, Department of Health and Human Services CA32102, CA38926, CA27057, CA46441, CA14028, CA 63848, CA35192, CA45377, CA67575, CA68183, CA20319, CA35128, CA42777, CA12644, CA58882, CA35119, CA35178, and by Eli Lilly and Company.
Abbreviations and Acronyms
- MVAC
methotrexate, vinblastine, adriamycin and cisplatin
- OS
overall survival
- PCG
paclitaxel, carboplatin and gemcitabine
- SWOG
Southwest Oncology Group
- TCC
transitional cell carcinoma
- TURBT
transurethral resection of bladder tumor
References
- 1.Malkowicz SB, van Poppel H, Mickisch G, Pansadoro V, Thuroff J, Soloway MS, et al. Muscle-invasive urothelial carcinoma of the bladder. Urology. 2007;69:3. doi: 10.1016/j.urology.2006.10.040. [DOI] [PubMed] [Google Scholar]
- 2.Sternberg CN, Yagoda A, Scher HI, Watson RC, Herr HW, Morse MJ, et al. M-VAC (methotrexate, viblastine, doxorubicin and cisplatin) for advanced transitional cell carcinoma of the urothelium. J Urol. 1988;139:461. doi: 10.1016/s0022-5347(17)42494-3. [DOI] [PubMed] [Google Scholar]
- 3.Grossman HB, Natale RB, Tangen CM, Speights VO, Vogelzang NJ, Trump DL, et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med. 2003;349:859. doi: 10.1056/NEJMoa022148. [DOI] [PubMed] [Google Scholar]
- 4.Millikan R, Dinney C, Swanson D, Sweeney P, Ro JY, Smith TL, et al. Integrated therapy for locally advanced bladder cancer: final report of a randomized trial of cystectomy plus adjuvant M-VAC versus cystectomy with both preoperative and postoperative M-VAC. J Clin Oncol. 2001;19:4005. doi: 10.1200/JCO.2001.19.20.4005. [DOI] [PubMed] [Google Scholar]
- 5.Herr HW, Bajorin DF, Scher HI. Neoadjuvant chemotherapy and bladder-sparing surgery for invasive bladder cancer: ten-year outcome. J Clin Oncol. 1998;16:1298. doi: 10.1200/JCO.1998.16.4.1298. [DOI] [PubMed] [Google Scholar]
- 6.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;52:457. [Google Scholar]
- 7.de Vere White RW, Stapp E. Predicting prognosis in patients with superficial bladder cancer. Oncology. 1998;12:1717. [PubMed] [Google Scholar]
- 8.Herr HW, Dotan Z, Donat SM, Bajorin DF. Defining optimal therapy for muscle invasive bladder cancer. J Urol. 2007;177:437. doi: 10.1016/j.juro.2006.09.027. [DOI] [PubMed] [Google Scholar]
- 9.Crawford ED, Das S, Smith JA., Jr Preoperative radiation therapy in the treatment of bladder cancer. Urol Clin North Am. 1987;14:781. [PubMed] [Google Scholar]
- 10.Hussain M, Vaishampayan U, Du W, Redman B, Smith DC. Combination paclitaxel, carboplatin, and gemcitabine is an active treatment for advanced urothelial cancer. J Clin Oncol. 2001;19:2527. doi: 10.1200/JCO.2001.19.9.2527. [DOI] [PubMed] [Google Scholar]
- 11.Sternberg CN, Pansadoro V, Calabro F, Marini L, van Rijn A, Carli PD, et al. Neo-adjuvant chemotherapy and bladder preservation in locally advanced transitional cell carcinoma of the bladder. Ann Oncol. 1999;10:1301. doi: 10.1023/a:1008350518083. [DOI] [PubMed] [Google Scholar]
- 12.Thomas DJ, Roberts JT, Hall RR, Reading J. Radical transurethral resection and chemotherapy in the treatment of muscle-invasive bladder cancer: a long-term follow-up. BJU Int. 1999;83:432. doi: 10.1046/j.1464-410x.1999.00970.x. [DOI] [PubMed] [Google Scholar]
- 13.Solsona E, Iborra I, Ricos JV, Monros JL, Casanova J, Calabuig C. Feasibility of transurethral resection for muscle infiltrating carcinoma of the bladder: long-term followup of a prospective study. J Urol. 1998;159:95. doi: 10.1016/s0022-5347(01)64022-9. [DOI] [PubMed] [Google Scholar]