Abstract
Purpose
The study aims to evaluate if human keratinocyte growth factor (hKGF), secreted after transduction of murine salivary glands with adenoviral vectors, can prevent oral mucositis resulting from radiation.
Experimental Design
Two serotype 5 adenoviral vectors encoding hKGF were constructed: AdEF1α-hKGF and AdLTR2EF1α-hKGF. Female C3H mice, 8 weeks old, were irradiated by single (22.5 Gy) or fractionated (5 × 8 Gy for 5 days) doses to induce oral mucositis (ulcers on tongue). One day before irradiation, the above viral vectors or an empty vector, Adcontrol, was given (1010 particles per gland) to both submandibular glands by retrograde ductal instillation. Each experiment included five groups: no irradiation and irradiation (± Adcontrol, AdEF1α-hKGF, or AdLTR2EF1α-hKGF). Blood, saliva, submandibular glands, and tongue were collected on day 7 for single-dose studies orday10 for fractionated dosing. HKGF levels were measured by ELISA.
Results
In three separate single-dose irradiation experiments, lingual ulcers were dramatically reduced after either KGF-expressing vector. Similarly, in two separate fractionated irradiation experiments, the hKGF-expressing vectors completely prevented ulcer formation. QPCR data indicated that ~ 107 to 108 particles of each vector remained in the targeted submandibular glands at the terminal time. Transgenic hKGF protein was found at high levels in saliva, serum, and submandibular gland extracts.
Conclusions
hKGF gene transfer to salivary glands prevented radiation-induced oral mucositis in mice. This proof of concept study suggests that transgenic hKGF secreted from transduced salivary glands may be useful clinically to prevent oral mucositis caused by radiation.
Mucositis is a significant and painful side effect for patients with head and neck cancer who receive radiotherapy ± chemotherapy. Mucositis can affect the ability to eat, swallow, and speak; can lead to secondary infection and weight loss; and can limit cancer treatment (1, 2). It is an acute injury to the mucosal lining and histologically characterized by ulceration and pseudomembranous formation (3). About 40,000 patients per year are treated for head and neck cancer in the United States (4, 5). Mucositis has become more widely problematic over the last 5 to 10 years, as intensive chemoradiation regimens have become more commonplace (6).
Chemotherapy-induced mucositis is typically less severe and of shorter duration (3-12 days) than that induced with radiotherapy (3-12 weeks; ref. 6). To date, there is no efficient way to prevent or reduce the severity and duration of radiation-induced oral mucositis. Palliative regimens, e.g., basic oral care, oral rinses, and analgesics, as well as antibiotics, cryotherapy, and topical anesthetics, have been used to treat radiation-induced oral mucositis (6, 7). These, however, are neither specific nor efficient at preventing or treating this condition (8 – 11).
The fibroblast growth factor (FGF) super family includes 22 polypeptide growth factors that are involved in regulating cell proliferation, migration, and differentiation during development, homeostasis, and response to injury and tissue repair (12, 13). Keratinocyte growth factor (KGF) is FGF7 and is produced by cells of mesenchymal origin (14, 15). KGF is an epithelial cell – specific growth and a differentiation factor acting exclusively through a subset of FGF receptors, FGFR2B (16). These characteristics suggest that KGF is a paracrine mediator of mesenchymal-epithelial communication (17, 18). KGF functions to stimulate epithelial cell proliferation, migration, differentiation, survival, and DNA repair (17). Many studies have shown that human KGF (hKGF) is a potentially useful agent to protect and regenerate damaged epithelial cells (17, 19 – 24). Indeed, recombinant hKGF can decrease the acute and chronic mucositis caused by chemoradiotherapy in animal models (17, 19 – 23).
Currently, recombinant hKGF (Palifermin), delivered by i.v. injection, is used clinically to treat chemotherapy-induced mucositis. Frequent injections are required and are expensive, inconvenient, and uncomfortable. This administration is also nonphysiologic, i.e., in a large bolus. Theoretically, gene transfer offers many advantages when compared with treatment with recombinant proteins. Previously, we showed that salivary glands can serve as valuable target site for applications of gene therapeutics (25, 26). Herein, we used murine submandibular glands as a tissue target and tested two serotype 5 adenoviral (Ad5) vectors encoding hKGF, AdLTR2EF1α-hKGF (a hybrid adenoretroviral vector, with potentially a longer term of trans-gene expression; ref. 27) and AdEF1α-hKGF (conventional first generation Ad5 vector), for prevention of oral mucositis after single or fractionated radiation doses. The data show that after salivary gland transduction by either vector, hKGF is secreted into both saliva and serum, and the oral mucositis caused by radiation is either reduced or prevented.
Materials and Methods
Construction of recombinant vectors
The vectors used were based on the Ad5 genome. E1 deletion was achieved by recombination of the pACCMV-pLpA shuttle plasmid with pJM17 (Microbix Biosystems, Inc.). pACLTR2EF1α and pACEF1α were modified from pACCMV-pLpA (27), with the human EF1α promoter in place of the cytomegalovirus promoter. pACLTR2EF1α also contained two DNA fragments from Moloney murine leukemia virus (27). To construct pACLTR2EF1α-hKGF and pACEF1α-hKGF, the plasmids pACLTR2EF1α and pACEF1α were digested with BamHI, filled in with T4 DNA polymerase, and then digested with SalI to ligate hKGF. pBLAST49-hFGF7 (InvivoGen) was digested with NheI and filled in with T4 DNA polymerase, and SalI linker was ligated to both ends, digested with AgeI, filled in with T4 DNA polymerase, and then digested with SalI to obtain the hKGF cDNA. The hKGF cDNA was ligated into pACLTR2EF1α and pACEF1α to get pACLTR2EF1α-hKGF and pACEF1α-hKGF, respectively. The recombinant vectors AdLTR2EF1α-hKGF and AdEF1α-hKGF were generated by homologous recombination of either pACLTR2EF1α-hKGF or pACEF1α-hKGF with pJM17 in C7 cells. Adcontrol was used as a vector control. It is an Ad5 vector without any transgene encoded (28). The titers (particles/mL) of purified vectors were determined by QPCR using primers from the E2 region (28).
Cell culture
The 293 cell line (Microbix Biosystems, Inc; ref. 29) was grown in Eagle’s MEM (Invitrogen). C7 cells, derived from 293 cells that stably express the Ad5 preterminal protein and DNA polymerase (30), were grown in high glucose DMEM (Invitrogen). A5 cells, derived from rat submandibular gland (31), were grown in McCoy’s 5A medium (Invitrogen). For all cell lines, the following supplements (Invitrogen) were included: 10% fetal bovine serum, 100 units/mL penicillin G, and 100 μg/mL streptomycin. Cells were incubated at 37°C in humidified 5% CO2.
In vitro experiments
AdLTR2EF1α-hKGF and AdEF1α-hKGF initially were tested in 293 and A5 cells. Cells were transduced at a multiplicity of infection of 100 particles per cell. Serum-free media of transduced 293 cells were collected for ELISA measurements and from transduced A5 cells for Western blot analysis (see below).
Experimental animals
Female C3H mice (National Cancer Institute Animal Production Area) were used for this study. Mice were 8 wk of age at the time of experimentation. All experiments were under the aegis of a protocol approved by National Cancer Institute Animal Care and Use Committee and were in compliance with the Guide for the Care and Use of Laboratory Animal Resources (1996) National Research Council.
In vivo viral vector delivery, blood, saliva, and tissue collection
Mice were anesthetized with ketamine (60 mg/kg) and xylazine (8 mg/kg) i.m. Vectors generally were given to both submandibular glands by retrograde ductal instillation (25, 32). Groups of mice (n = 4 or 5 per experiment) received 1 × 1010 particles per gland of either Adcontrol, AdLTR2EF1α-hKGF, or AdEF1α-hKGF. Animals that were not irradiated also served as a negative control group. Blood, saliva, and tissue were collected either on day 7 (single-dose irradiation) or day 10 (fractionated irradiation). For saliva collections, anesthetized mice were stimulated using 1 μL/g body weight of a pilocarpine solution s.c. (0.05 mg/mL). Whole saliva was collected with a 75-mm hematocrit tube (Drummond) into 1.5 mL Eppendorf tubes for 10 min and frozen immediately. Blood samples were collected from the retro-orbital sinus after the saliva collection. At the terminal time point, anesthetized animals were sacrificed in a carbon monoxide chamber and the submandibular glands and tongue were removed. Soluble extracts of submandibular glands were prepared by CelLytic M Cell Lysis reagent (Sigma).
Animal radiation
The head and neck area was irradiated by placing each animal in a specially built Lucite jig so the animal could be immobilized without the use of anesthetics (33). Additionally, the jig was fitted with a Lucite cone surrounding the head and preventing head movement during radiation. Single-dose irradiation at 22.5 Gy and fractionated irradiation at 5 × 8 Gy (8 Gy/d for 5 d) were delivered 24 h after vector delivery by a Therapax DXT300 X-ray irradiator (Pantak) using 2.0 mm Al filtration (300 kVp) at a dose rate of 1.9 Gy/min. After irradiation, animals were removed from the jig, housed (four or five animals per cage) in a climate- and light-controlled environment, and allowed free access to food and water.
Demonstration of mucositis
Excised tongues were stained in a solution of 1% toluidine blue in 10% acetic acid, rinsed with 1% acetic acid, and then rinsed with the tap water. Any erosion of epithelium or frank ulceration is visible as a deep blue color after staining. Next, tongues were fixed in 10% formalin and embedded in paraffin. Three micrometer sections were stained with H&E and examined microscopically. Macroscopic pictures were taken; using the software NIH Image J, stained ulcers were delimited, and the area was measured in pixels. To measure epithelial thickness, three microscopic pictures were taken at the top, middle, and bottom of the tongue section. For each picture, 20 measurements were made using NIH Image J software. The measurements were averaged, and this average considered the epithelial thickness.
Measurement of hKGF levels
The hKGF levels in 293 cell media (after 24 h), serum, saliva, and gland extracts, were determined by an ELISA using human KGF/FGF-7 ELISA kits from R&D Systems.
QPCR assays
Genomic DNA from submandibular glands was extracted with the Wizard Genomic DNA Purification kit (Promega). DNA (100 ng) was used per QPCR reaction. The primers E3Taq1 (5′-GAGTTGGCACCCCTATTCGA-3′) and E3Taq2 (5′-ATGCCACATCCGTT-GACTTG-3′) and probe E3Taqprobe (5′-/56-FAM/CCACCCGTGTGTACCTGGTGGACA/36-TSMTSp/-3′) for the adenoviral E3 region were used to measure vector copy number. These sequences were selected using Primer Express Primer Design software (PE Applied Biosystems), and assay was done in an ABI Prism 7700 Sequence Detector. The conditions used were as follows: 95°C for 2 min, 95°C for 8 min, 95°C for 15 s, and 60°C for 1 min for 40 cycles.
Western blot analysis
Saliva and culture medium were mixed with Tris-glycine SDS sample buffer (2 × ; Invitrogen) and loaded onto gels for Western blots, and the monoclonal antihuman KGF/FGF-7 antibody (R&D Systems) was used for detection.
Statistical analysis
Data analyses used SigmaStat version 2.0 (SPSS, Inc.) and Excel (Microsoft) software. Results herein are presented as mean values ± SE. One-way ANOVAs, followed by a Tukey test or a paired t test, were used as appropriate (see figure legends) to determine the statistical significance of differences observed.
Results
Models of oral mucositis
Previously, irradiated female C3H mice at 0, 15, 22.5, and 25 Gy, i.e., single dose and after 7 days, saw lingual ulcers at the following frequencies: 0%, 15 Gy; 75%, 22.5 Gy; and 100%, 25 Gy (33). Herein, a 22.5-Gy single irradiation dose was used to create oral mucositis, and ulcers occurred on the base of tongue in ~ 85% of mice (Fig. 1; Table 1). To establish oral mucositis with a fractionated irradiation dose scheme in mice, 6 or 8 Gy were used daily for 5 days. Nine days after starting irradiation, lingual ulcers were found in 100% of the tongues with the 8-Gy dose (Fig. 2; Table 1).
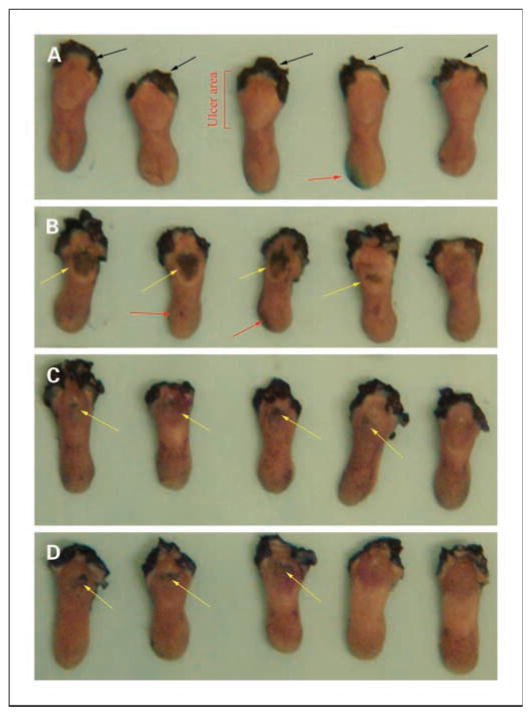
Fig. 1.
Toluidine blue staining in a single-dose irradiation experiment at day 7. A, no irradiation control. B, Adcontrol plus irradiation (22.5 Gy). C, AdEF1a-hKGF plus irradiation. D, AdLTR2EF1α-hKGF plus irradiation. Black arrows, staining at the site of incision to remove tongue; yellow arrows, ulcers (mucositis); red arrows, indicate artificial positive spots caused by trauma during harvesting of the tongue. Ulcers due to irradiation are on the dorsal surface at the base of the tongue. These results are representative of three experiments.
Table 1.
Summary of results in experiments using either a single 22.5-Gy or fractionated 8 Gy × 5 d irradiation dose
| Vector group | Presence of mucositis (%) | hKGF |
Vector copy number/100 ng DNA (× 104) | ||
|---|---|---|---|---|---|
| Saliva (pg, total) | Serum (pg, total) | Gland extract (pg/mg protein) | |||
| Single 22.5-Gy irradiation | |||||
| Adcontrol (n = 13) | 85* | 0 | 0 | 0 | 3.4 ± 0.6 |
| EF1α-hKGF (n = 13) | 50† | 10.2 ± 2.3 | 242.2 ± 37.8 | 144.3 ± 50 | 2.7 ± 0.7 |
| LTR-hKGF (n = 13) | 38‡ | 36 ± 5.2 | 640.4 ± 117.2 | 4286.7 ± 1294 | 3.2 ± 0.6 |
| Fractionated 8 Gy × 5 d irradiation | |||||
| Adcontrol (n = 8) | 100§ | 0 | 0 | 0 | 3.6 ± 0.5 |
| EF1α-hKGF (n = 8) | 0 | 2.1 ± 1.1 | 153.2 ± 64.2 | 472.8 ± 160 | 2.3 ± 0.3 |
| LTR-hKGF (n = 8) | 0 | 6.3 ± 3.1 | 746.6 ± 196.8 | 6408 ± 1731.5 | 5.6 ± 0.8 |
NOTE: For single 22.5-Gy irradiation, all data are from the day 7 time point.
Eight tongues had severe ulcers, three tongues had small ulcers, and two tongues had no ulcers in this group.
One tongue had a severe ulcer, six tongues had small ulcers, and six tongues had no ulcers in this group.
Two tongues had severe ulcers, three tongues had small ulcers, and eight tongues had no ulcers in this group. Total salivary hKGF was calculated based on 100 μL saliva/mouse. Total serum hKGF was calculated based on 2 mL serum/mouse. Data shown for hKGF levels and vector copy number are mean values ± SE from three separate experiments. For fractionated 8 Gy × 5 d irradiation, all data are from the day 10 time point.
Six tongues had severe ulcers, and two tongues had small ulcers in this group. Total salivary and serum hKGF were calculated as above. Data shown for hKGF levels and vector copy number are mean values ± SE from two separate experiments. The number of mice studied for each vector group in both radiation schemes is shown in parentheses. Note that the vector copy numbers shown represent ~ 0.1% to 1% of the total dose given.
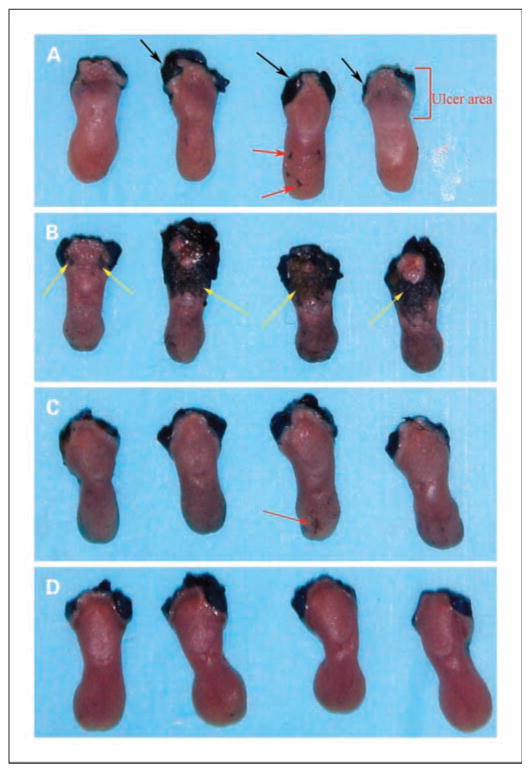
Fig. 2.
Toluidine blue staining in a fractionated dose irradiation experiment at day10. A, no irradiation control. B, Adcontrol plus irradiation (8 Gy for 5 d). C, AdEF1a-hKGF plus irradiation. D, AdLTR2EF1α-hKGF plus irradiation. Black arrows, staining at the site of incision to remove tongue; yellow arrows, ulcers (mucositis); red arrows, artificial positive spots caused by trauma during harvesting of the tongue. Ulcers due to irradiation are on the dorsal surface at the base of the tongue. These results are representative of two experiments.
Characteristics of adenoviral vectors
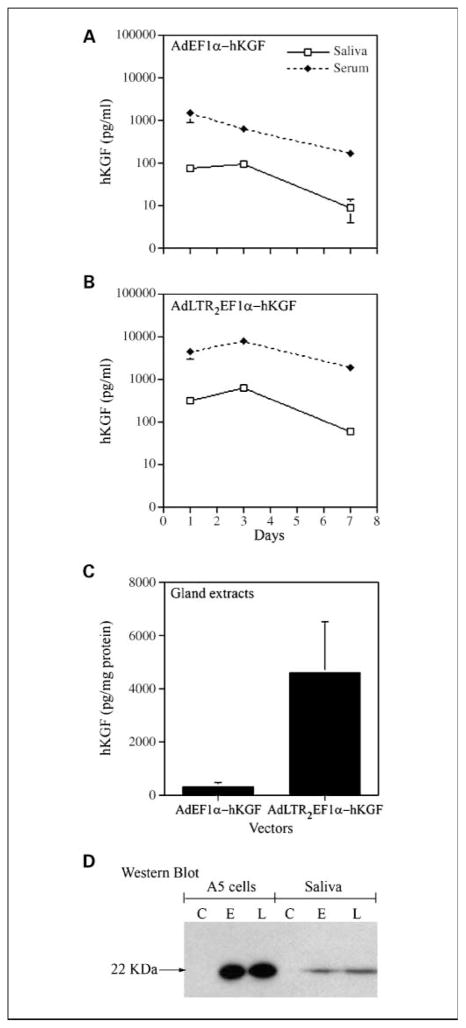
Vectors were first compared in vitro, and hKGF expression was as follows: AdLTR2EF1α-hKGF (~ 2.5 μg/mL) > AdEF1a-hKGF (~ 1.6 μg/mL). In vivo we initially tested both vectors in mice at 108, 109, or 1010 particles per gland to both submandibular glands. Expression was dose dependent with maximal levels at 1010 particles per gland (data not shown). This dose was used in all subsequent in vivo experiments. Next, we examined the time course of hKGF expression after vector administration in nonirradiated mice (Fig. 3). Peak hKGF secretion was between days 1 and 3 and then decreased for both vector groups. The AdLTR2EF1α-hKGF transduced glands secreted more hKGF into both saliva and serum and exhibited more in gland extracts than theAdEF1α-hKGF-transduced glands (Fig. 3A – C). This general finding was consistent with our previous studies using similar vectors expressing erythropoietin (27). Interestingly, there was considerable hKGF remaining in glands (~ 60 – 80%; Fig. 3C) after salivary stimulation. The concentration of hKGF in the serum was always much higher than that in the saliva (Fig. 3A and B). Western blots showed that the both vector-transduced A5 cells in vitro and submandibular glands in vivo secreted an immunoreactive protein band of the expected size (22 kDa) into medium and saliva, respectively (Fig. 3D).
Fig. 3.
Time course of hKGF secretion from nonirradiated AdEF1a-hKGF and AdLTR2EF1α-hKGF transduced mice and hKGF Western blot analysis. A, time course of hKGF appearance in saliva and serum from the AdEF1a-hKGF – transduced mice. B, time course of hKGF appearance in saliva and serum from the AdLTR2EF1α-hKGF – transduced mice. C, comparison of the hKGF levels in extracts of submandibular glands from the same mice shown in A and Bon day7. D, Western blot from conditioned serum-freemediaofA5 cells transduced with eitherAdEF1a-hKGFor AdLTR2EF1α-hKGF in vitro and saliva collected on day 2 from the AdEF1a-hKGF – or AdLTR2EF1α-hKGF – transduced mice. Lanes for Western blot are as follows: C, no vector control; E, AdEF1a-hKGF transduced; L, AdLTR2EF1α-hKGF transduced. The hKGF is seen as an ~ 22-kDa protein. The data shown in A to C are mean values ± SE (n = 4 mice per treatment). Note that for several time points (A and B) the error bars are too small to be seen.
Oral mucositis-ulceration at the base of tongue
In this study, ulcers that formed at the base of the tongue after irradiation were used as an indicator of mucositis. The absence of toluidine blue staining indicated that no ulcer occurred, whereas a dark blue color indicated that an ulcer was present.
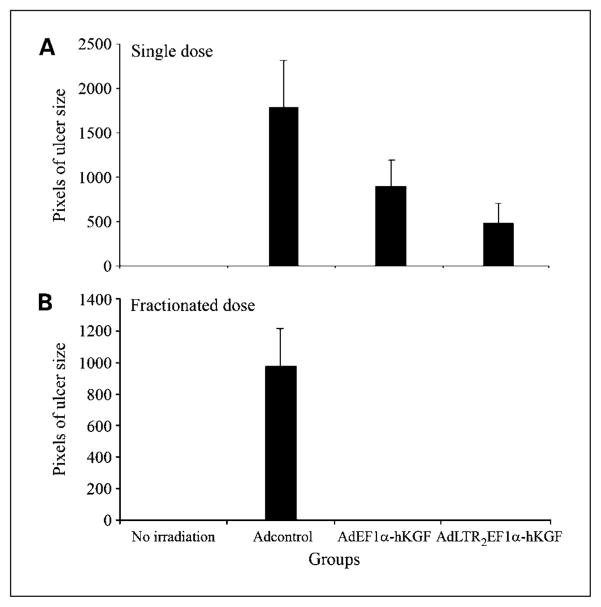
The single-dose irradiation experiment was repeated thrice. Results (Fig. 1) are from 7 days posttransduction, i.e., 6 days postirradiation. For nonirradiated controls (Fig. 1A), no staining on the surface of the tongue is seen. For irradiated mice receiving no vector, all tongues exhibited ulcerations (not shown). Figures 1B – D were from vector-transduced groups. The number and size of ulcers seen clearly show that the tongues from the AdEF1a-hKGF – and AdLTR2EF1α-hKGF – transduced mice were protected compared with those of control vector-transduced mice. To quantify the extent of ulceration, the areas of ulceration shown in Fig. 1A – D were measured. Ulcer size in the vector control group was the largest, and these ulcers were significantly greater than those found in both hKGF vector groups (P < 0.05; Fig. 4A). Table 1 includes a summary of all three single irradiation dose experiments. In the Adcontrol group, 85% of tongues showed ulceration, 50% in the AdEF1α-hKGF group, and 38% in the AdLTR2EF1α-hKGF group. The ulcers in the Adcontrol group also were more severe and larger than those of the hKGF vector groups (see legend for details). In addition, Table 1 shows the hKGF expression levels found in serum, saliva, and gland extracts, as well as vector copy numbers in transduced glands, at the day 7 time point.
Fig. 4.
Measurement of ulcer size on mouse tongues using imageJ software. A, single-dose irradiation experiments. B, fractionated irradiation dose experiments. The units are in pixels, i.e., the more pixels are, the larger the size of the ulcers observed. Columns, mean values; bars, SE. Data shown are from all experiments combined for each irradiation scheme.
Figure 2 shows that the fractionated irradiation scheme used yielded generally similar results to the single-dose experiments. The fractionated irradiation experiment was repeated twice. Tongues from mice given either AdEF1a-hKGF or AdLTR2EF1α-hKGF had no ulcers; i.e., tongues were fully protected from mucositis. In contrast, in the experiment shown, mice given Adcontrol exhibited severe ulcers on three of four tongues (Fig. 2B) whereas the other had a mild ulcer. When the ulcers were measured, the results were consistent with the direct visual impression (Fig. 4B). Table 1 also provides a summary of the two fractionated dose experiments. In addition, Table 1 shows the hKGF expression levels found in serum, saliva, and gland extracts, as well as vector copy numbers in transduced glands, at the day 10 time point.
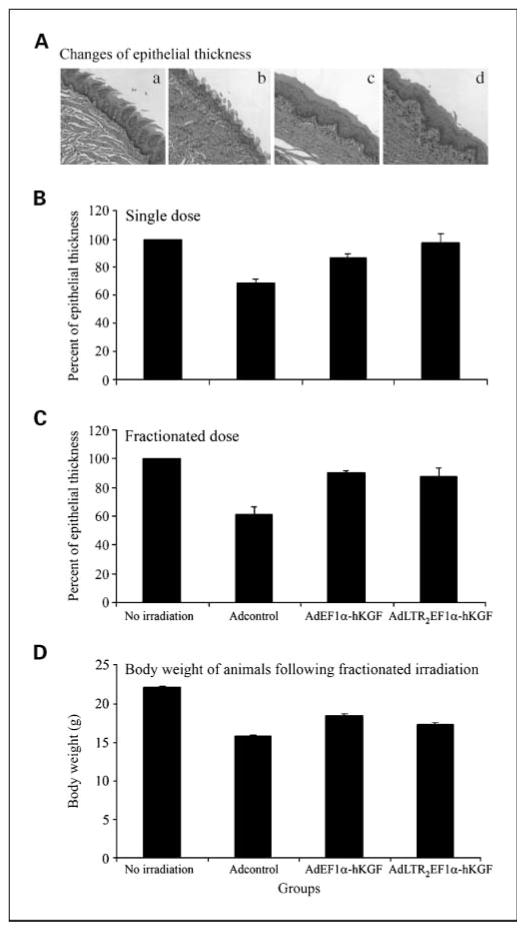
To further evaluate the extent of ulceration, tongue sections were stained with H&E and examined histologically, and the thickness of remaining epithelium was measured digitally. Figure 5A shows representative sections from each experimental group. The Adcontrol-treated group displayed a loss of epithelial integrity, surface ulceration, and the presence of a pseudomembrane, along with infiltration of inflammatory cells in the adjacent connective tissue. Both hKGF vector-treated groups essentially had a similar epithelial thickness to the no irradiation group but showed a loss in filiform papillae. Quantitative analysis of epithelial thickness from the single-dose and fractionated irradiation experiments is shown in Fig. 5B and C. The rank order of epithelial cell thickness in both studies was no irradiation group > two hKGF vector groups > Adcontrol group. In both irradiation schemes, the differences between the no irradiation group and Adcontrol group were statistically significant (P < 0.05). For the single-dose experiment, the differences seen between the AdLTR2EF1α-hKGF and Adcontrol groups were also significant (P < 0.05). These results are generally consistent with those shown in Figs. 1 and 2.
Fig. 5.
Determination of epithelial thickness on mouse tongues and body weight after irradiation. A, representative histologic sections from each study group: a, no irradiation control with normal stratified squamous epithelium and integrity of papillae; b, Adcontrol plus irradiation showing loss of epithelial integrity resulting in ulceration covered by a pseudo membrane, along with the presence of inflammatory cells in the adjacent connective tissue; c, AdEF1a-hKGF plus irradiation showing preserved stratified squamous epithelium with an absence of papillae; d, AdLTR2EF1α-hKGF plus irradiation showing preserved stratified squamous epithelium with an absence of papillae. B, epithelial thickness in single-dose irradiation experiments. Data shown here are from all three experiments. C, epithelial thickness in fractionated irradiation experiments. Data shown here were from both experiments done. D, body weight of animals after fractionated irradiation. In Band C, the epithelial thickness of the no irradiation group was set as100%. All other values were calculated relative to this no irradiation control group. Columns, mean values; bars, SE. For the single irradiation dose studies (B), a Kruskal-Wallis one way ANOVA on ranks was significant (H = 12.85, P =0.005). Multiple pairwise comparisons (Tukey test) of the groups indicated that the no irradiation group and the AdLTR2EF1α-hKGF groups were significantly different from the Adcontrol plus irradiation group (P < 0.05). For the fractionated irradiation dose studies(C), the Kruskal-Wallis one-way ANOVA was significant (H = 9.6, P = 0.022). The subsequent multiple pairwise comparison showed that the only significant difference was between the no irradiation and Adcontrol plus irradiation groups (P < 0.05). For the body weight determinations (D), the data shown are mean values ± SE. A one-way ANOVA of the data was significant: F = 33.8 and P < 0.001. A multiple pairwise comparison (Tukey test) showed that the no irradiation group was significantly different from all other groups (P < 0.001). Additionally, both hKGF vector groups were significantly different from the Adcontrol group (P ≤ 0.006), whereas the hKGF vector groups were not significantly different from each other.
Finally, the presence of severe damage to the oral mucosa can affect food consumption and cause loss of body weight. As shown in Fig. 5D, body weight in the no irradiation group was significantly greater than all other study groups. Additionally, body weight in the Adcontrol group was significantly less (P < 0.05) than that in both hKGF vector groups, which did not differ from each other.
Discussion
Oral mucositis is a serious side effect in patients with head and neck cancers who receive radiotherapy with and without chemotherapy. Current clinical strategies cannot efficiently or fully prevent the occurrence of oral mucositis, and it remains a significant clinical problem (1 – 3). Herein, two radiation-induced oral mucositis models were used in mice to test a gene transfer strategy to prevent this condition: a 22.5-Gy single dose and a 5-day × 8 Gy fractionated scheme. Both models yielded severe and frequent oral mucositis. Importantly, our results show that hKGF gene transfer to salivary glands, presumably via the secreted hKGF found in saliva, can effectively protect oral mucosa and prevent mucositis/ulceration after both single and fractionated schemes.
Radiation causes DNA damage in basal epithelial cells and generates reactive oxygen species. Further damage occurs to cells and blood vessels in the submucosa (34). Reactive oxygen species lead to apoptosis and to the up-regulation of inflammatory cytokines in the mucosa (35, 36). The latter can produce further tissue damage, amplifying signaling cascades and the entire injury process (35, 36). KGF has multiple functions in epithelial cells to help maintain their integrity (17). Our results suggest that the hKGF secreted from submandibular glands can lead to the prevention of radiation damage in epithelial cells on murine tongue, i.e., the absence of ulcerations. This occurred when Ad5 vectors encoding transgenic hKGF were delivered into glands 1 day before mice were irradiated. In contrast, hKGF gene transfer could not effectively protect epithelial cells or prevent lingual ulcerations when the Ad5 vectors were delivered 1 day after mice were irradiated (data not shown). Because irradiation damage begins very quickly, these results suggest that the transgenic hKGF acts soon after irradiation. Whereas many studies have shown that recombinant hKGF is useful to protect damaged epithelial cells (17, 19 – 23), our results are the first to show that hKGF gene transfer can prevent oral mucositis. Vector (Ad5, nonviral) – mediated KGF gene transfer strategies to protect hyperoxia-induced lung injury and facilitate cutaneous wound healing have also been shown to be effective (24, 37, 38).
Previously, we showed that salivary glands are a promising target site for applications of gene therapeutics (25). Classically, salivary glands are considered an exocrine gland; however, physiologically, they can secrete proteins into the bloodstream (26). Herein, we show that transgenic hKGF is found in both saliva and serum (Fig. 3; Table 1). Indeed, the hKGF levels in serum were much higher than those of saliva. This observation suggests that, in part, the effects of hKGF on oral epithelial cells in our study may also come from serum as well as saliva. In a recently reported phase II clinical trial, recombinant hKGF was given by an i.v. bolus injection (39).
The systemic delivery of hKGF could be a concern because of the effects it might have on a malignant tumor (13, 40, 41). However, in the above clinical trial, i.v. delivery did not alter tumor response or patient survival (ref. 39; 44.8 months median follow-up). Additionally, using murine xenograft models for head and neck and colorectal carcinoma, recombinant hKGF given s.c. had no effect on both tumor growth and antitumor chemotherapeutic activity (42). We suggest that, for vector targeted hKGF delivery to be most useful clinically, its secretion into saliva should be optimized. Interestingly, salivary glands of mice can sort transgenic secretory proteins, e.g., erythropoietin, quite differently than salivary glands of larger animals (miniature pig and rhesus macaque; refs. 43, 44). In both large species, transgenic erythropoietin sorts more frequently into saliva than the bloodstream, whereas in mice almost all erythropoietin is found in serum. Thus, it is important that future studies with the Ad5-encoding hKGF vectors use a large animal mucositis model. Additionally, after stimulation of salivary flow by pilocarpine in the present studies, there were still high levels of hKGF remaining in murine submandibular glands. This theoretically suggests that decreased doses of vector can be used in the future if efficient exocrine secretion can be attained.
We also compared two types of Ad5 vectors, AdEF1a-hKGF (a conventional first generation Ad5 vector) and AdLTR2EF1α-hKGF (a hybrid adenoretroviral vector; ref. 27). Previously, many published studies showed that transgene expression from conventional Ad5 vectors is transient, lasting 1 to 2 weeks in salivary glands. However, we showed using a hybrid vector that extended transgene expression occurs for at least 2 to 3 months (27). The LTR element from Moloney murine leukemia virus used to construct the AdLTR2EF1α-hKGF vector also serves as an enhancer to increase EF1α promoter activity (27). Indeed, results presented in Fig. 3 and Table 1 indicate that the AdLTR2EF1α-hKGF – transduced gland cells produce more transgenic hKGF than found with the conventional vector. Overall, our results show that the AdLTR2EF1α-hKGF group received a little more protection than the AdEF1α-hKGF group (Figs. 1, 2 and 4; Table 1). Both vectors lead to high levels of transgenic hKGF from transduced glands by days 7 or 10 and are effective in the murine models used. However, because fractionated irradiation regimens used clinically to treat the head and neck cancers occur over 1 to 3 months, much longer than our fractionated dose model (5 days, 8 Gy/d), it is likely that the hybrid vector tested herein may be more advantageous for clinical applications.
Conclusions
The present study shows, using both single dose and fractionated dose mouse irradiation models, that transgenic hKGF secreted from vector-transduced submandibular glands effectively protects oral mucosal epithelial cells, preventing oral mucositis/ulceration and/or accelerating regeneration of the surface epithelium. In aggregate, the data suggest that salivary gland hKGF gene transfer may be beneficial to prevent radiation-induced oral mucositis in patients being treated for head and neck cancers.
Acknowledgments
Grant support: The Divisions of Intramural Research of the National Institute of Dental and Craniofacial Research and the National Cancer Institute provided support.
Footnotes
Note: Supplementary data for this article are available at Clinical Cancer Research Online (http://clincancerres.aacrjournals.org/).
Translational Relevance
Oral mucositis, a common side effect of radiotherapy for head and neck cancers, can lead to significant patient discomfort and morbidity and can limit therapy itself. Using two different adenoviral vectors in a novel gene transfer approach, the cDNA for keratinocyte growth factor was transferred into murine salivary glands 1 day before either a single or fractionated radiation dose scheme. Both lead to significant oral mucositis. The mucositis after the single dose was dramatically reduced, whereas that after fractionated radiation was completely prevented. Gene transfer to salivary glands, via cannulation and retrograde delivery into the main excretory duct opening in the mouth, is not difficult and mimics a common procedure used for contrast X-rays. The results suggest a real potential for applying this approach to head and neck cancer patients before radiotherapy and preventing oral mucositis.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Shih A, Miaskowski C, Dodd MJ, Stotts NA, Mac-Phail L. A research review of the current treatments for radiation-induced oral mucositis in patients with head and neck cancer. Oncol Nurs Forum. 2002;29:1063–80. doi: 10.1188/02.ONF.1063-1080. [DOI] [PubMed] [Google Scholar]
- 2.Scully C, Epstein J, Sonis S. Oral mucositis: a challenging complication of radiotherapy, chemotherapy, and radiochemotherapy: Part 1. Pathogenesis and prophylaxis of mucositis. Head Neck. 2003;25:1057–70. doi: 10.1002/hed.10318. [DOI] [PubMed] [Google Scholar]
- 3.Elting LS, Keefe DM, Sonis ST, et al. Burden of Illness Head and Neck Writing Committee. Patient-reported measurements of oral mucositis in head and neck cancer patients treated with radiotherapy with or without chemotherapy: demonstration of increased frequency, severity, resistance to palliation, and impact on quality of life. Cancer. 2008;113:2704–13. doi: 10.1002/cncr.23898. [DOI] [PubMed] [Google Scholar]
- 4.Jemal A, Siegel R, Ward E, et al. Cancer statistics. CA Cancer J Clin. 2006;56:106–30. doi: 10.3322/canjclin.56.2.106. [DOI] [PubMed] [Google Scholar]
- 5.Grdina DJ, Murley JS, Kataoka Y. Radioprotectants: current status and new directions. Oncology. 2002;63:2–10. doi: 10.1159/000067146. [DOI] [PubMed] [Google Scholar]
- 6.Rosenthal DI, Trotti A. Strategies for managing radiation-induced mucositis in head and neck cancer. Semin Radiat Oncol. 2009;19:29–34. doi: 10.1016/j.semradonc.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 7.Putwatana P, Sanmanowong P, Oonprasertpong L, Junda T, Pitiporn S, Narkwong L. Relief of radiation-induced oral mucositis in head and neck cancer. Cancer Nurs. 2009;32:82–7. doi: 10.1097/01.NCC.0000343362.68129.ed. [DOI] [PubMed] [Google Scholar]
- 8.Genot MT, Klastersky J. Low-level laser for prevention and therapy of oral mucositis induced by chemotherapy or radiotherapy. Curr Opin Oncol. 2005;17:236–40. doi: 10.1097/01.cco.0000156196.22249.76. [DOI] [PubMed] [Google Scholar]
- 9.Worthington HV, Clarkson JE. Prevention of oral mucositis and oral candidiasis for patients with cancer treated with chemotherapy: cochrane systematic review. J Dent Educ. 2002;66:903–11. [PubMed] [Google Scholar]
- 10.Rubenstein EB, Peterson DE, Schubert M, et al. Mucositis Study Section of the Multinational Association for Supportive Care in Cancer; International Society for Oral Oncology. Clinical practice guidelines for the prevention and treatment of cancer therapy-induced oral and gastrointestinal mucositis. Cancer. 2004;100:2026–46. doi: 10.1002/cncr.20163. [DOI] [PubMed] [Google Scholar]
- 11.Plevová P. Prevention and treatment of chemotherapy- and radiotherapy-induced oral mucositis: a review. Oral Oncol. 1999;35:453–70. doi: 10.1016/s1368-8375(99)00033-0. [DOI] [PubMed] [Google Scholar]
- 12.Ornitz DM, Itoh N. Fibroblast growth factors. Genome Biol. 2001;2:reviews3005.1–3005.12. doi: 10.1186/gb-2001-2-3-reviews3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Finch PW, Rubin JS. Keratinocyte growth factor expression and activity in cancer: implications for use in patients with solid tumors. J Natl Cancer Inst. 2006;98:812–24. doi: 10.1093/jnci/djj228. [DOI] [PubMed] [Google Scholar]
- 14.Finch PW, Rubin JS, Miki T, Ron D, Aaronson SA. Human KGF is FGF-related with properties of a paracrine effector of epithelial cell growth. Science. 1989;245:752–5. doi: 10.1126/science.2475908. [DOI] [PubMed] [Google Scholar]
- 15.Rubin JS, Osada H, Finch PW, Taylor WG, Rudikoff S, Aaronson SA. Purification and characterization of a newly identified growth factor specific for epithelial cells. Proc Natl Acad Sci U S A. 1989;86:802–6. doi: 10.1073/pnas.86.3.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miki T, Fleming TP, Bottaro DP, Rubin JS, Ron D, Aaronson SA. Expression cDNA cloning of the KGF receptor by creation of a transforming autocrine loop. Science. 1991;251:72–5. doi: 10.1126/science.1846048. [DOI] [PubMed] [Google Scholar]
- 17.Finch PW, Rubin JS. Keratinocyte growth factor/fibroblast growth factor 7, a homeostatic factor with therapeutic potential for epithelial protection and repair. Adv Cancer Res. 2004;91:69–136. doi: 10.1016/S0065-230X(04)91003-2. [DOI] [PubMed] [Google Scholar]
- 18.Dörr W, Spekl K, Farrell CL. The effect of keratinocyte growth factor on healing of manifest radiation ulcers in mouse tongue epithelium. Cell Prolif. 2002;35 Suppl 1:86–92. doi: 10.1046/j.1365-2184.35.s1.9.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen L, Brizel DM, Rabbani ZN, et al. The protective effect of recombinant human keratinocyte growth factor on radiation-induced pulmonary toxicity in rats. Int J Radiat Oncol Biol Phys. 2004;60:1520–9. doi: 10.1016/j.ijrobp.2004.07.729. [DOI] [PubMed] [Google Scholar]
- 20.Farrell CL, Bready JV, Rex KL, et al. Keratinocyte growth factor protects mice from chemotherapy and radiation-induced gastrointestinal injury and mortality. Cancer Res. 1998;58:933–9. [PubMed] [Google Scholar]
- 21.Farrell CL, Rex KL, Kaufman SA, et al. Effects of keratinocyte growth factor in the squamous epithelium of the upper aerodigestive tract of normal and irradiated mice. Int J Radiat Biol. 1999;75:609–20. doi: 10.1080/095530099140258. [DOI] [PubMed] [Google Scholar]
- 22.Yi ES, Williams ST, Lee H, et al. Keratinocyte growth factor ameliorates radiation-and bleomycin-induced lung injury and mortality. Am J Pathol. 1996;149:1963–70. [PMC free article] [PubMed] [Google Scholar]
- 23.Gibson RJ, Bowen JM, Keefe DM. Palifermin reduces diarrhea and increases survival following irinotecan treatment in tumor-bearing DA rats. Int J Cancer. 2005;116:464–70. doi: 10.1002/ijc.21082. [DOI] [PubMed] [Google Scholar]
- 24.Baba Y, Yazawa T, Kanegae Y, et al. Keratinocyte growth factor gene transduction ameliorates acute lung injury and mortality in mice. Hum Gene Ther. 2007;18:130–41. doi: 10.1089/hum.2006.137. [DOI] [PubMed] [Google Scholar]
- 25.Baum BJ, Wellner RB, Zheng C. Gene transfer to salivary glands. Int Rev Cytol. 2002;213:93–146. doi: 10.1016/s0074-7696(02)13013-0. [DOI] [PubMed] [Google Scholar]
- 26.Kagami H, O’Connell BC, Baum BJ. Evidence for the systemic delivery of a transgene product from salivary glands. Hum Gene Ther. 1996;7:2177–84. doi: 10.1089/hum.1996.7.17-2177. [DOI] [PubMed] [Google Scholar]
- 27.Zheng C, Vitolo JM, Zhang W, Mineshiba F, Chiorini JA, Baum BJ. Extended transgene expression from a nonintegrating adenoviral vector containing retroviral elements. Mol Ther. 2008;16:1089–97. doi: 10.1038/mt.2008.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zheng C, Baum BJ. Evaluation of viral and mammalian promoters for use in gene delivery to salivary glands. Mol Ther. 2005;12:528–36. doi: 10.1016/j.ymthe.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 29.Graham FL, Smiley J, Russel WC, Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol. 1977;36:59 – 74. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- 30.Amalfitano A, Chamberlain JS. Isolation and characterization of packaging cell lines that coexpress the adenovirus E1, DNA polymerase, and preterminal protein: implication for gene therapy. Gene Ther. 1996;4:258–63. doi: 10.1038/sj.gt.3300378. [DOI] [PubMed] [Google Scholar]
- 31.Brown AM, Rusnock EJ, Sciubba JJ, Baum BJ. Establishment and characterization of an epithelial cell line from the rat submandibular gland. J Oral Pathol Med. 1989;18:206–13. doi: 10.1111/j.1600-0714.1989.tb00764.x. [DOI] [PubMed] [Google Scholar]
- 32.Adesanya MR, Redman RS, Baum BJ, O’Connell BC. Immediate inflammatory responses to adenovirus-mediated gene transfer in rat salivary glands. Hum GeneTher. 1996;7:1085–93. doi: 10.1089/hum.1996.7.9-1085. [DOI] [PubMed] [Google Scholar]
- 33.Muanza TM, Cotrim AP, McAuliffe M, et al. Evaluation of radiation-induced oral mucositis by optical coherence tomography. Clin Cancer Res. 2005;11:5121 – 7. doi: 10.1158/1078-0432.CCR-05-0403. [DOI] [PubMed] [Google Scholar]
- 34.Shea TC, Bruner R, Wiley JM, et al. An expanded Phase I/II trial of cyclophosphamide, etoposide, and carboplatin plus total-body irradiation with autologous marrow or stem cell support for patients with hematologic malignancies. Biol Blood Marrow Transplant. 2003;9:443–52. doi: 10.1016/s1083-8791(03)00204-0. [DOI] [PubMed] [Google Scholar]
- 35.Sonis ST. The pathobiology of mucositis. Nat Rev Cancer. 2004;4:277–84. doi: 10.1038/nrc1318. [DOI] [PubMed] [Google Scholar]
- 36.Sonis ST, Elting LS, Keefe D, et al. Perspectives on cancer therapy induced mucosal injury: pathogenesis, measurement, epidemiology, and consequences for patients. Cancer. 2004;100:1995–2025. doi: 10.1002/cncr.20162. [DOI] [PubMed] [Google Scholar]
- 37.Escámez MJ, Carretero M, García M, et al. Assessment of optimal virus-mediated growth factor gene delivery for human cutaneous wound healing enhancement. J Invest Dermatol. 2008;128:1565–75. doi: 10.1038/sj.jid.5701217. [DOI] [PubMed] [Google Scholar]
- 38.Pereira CT, Herndon DN, Rocker R, Jeschke MG. Liposomal gene transfer of keratinocyte growth factor improves wound healing by altering growth factor and collagen expression. J Surg Res. 2007;139:222 – 8. doi: 10.1016/j.jss.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 39.Brizel DM, Murphy BA, Rosenthal DI, et al. Phase II study of Palifermin and concurrent chemoradiation in head and neck squamous cell carcinoma. J Clin Oncol. 2008;26:2489–96. doi: 10.1200/JCO.2007.13.7349. [DOI] [PubMed] [Google Scholar]
- 40.Housley RM, Morris CF, Boyle W, et al. Keratinocyte growth factor induces proliferation of hepatocytes and epithelial cells throughout the rat gastrointestinal tract. J Clin Invest. 1994;94:1764–77. doi: 10.1172/JCI117524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tsuboi R, Sato C, Kurita Y, Ron D, Rubin JS, Ogawa H. Keratinocyte growth factor (FGF-7) stimulates migration and plasminogen activator activity of normal human keratinocytes. J Invest Dermatol. 1993;101:49–53. doi: 10.1111/1523-1747.ep12358892. [DOI] [PubMed] [Google Scholar]
- 42.Brake R, Starnes C, Lu J, et al. Effects of Palifermin on antitumor activity of chemotherapeutic and biological agents in human head and neck and colorectal carcinoma xenograft models. Mol Cancer Res. 2008;6:1337–46. doi: 10.1158/1541-7786.MCR-07-2131. [DOI] [PubMed] [Google Scholar]
- 43.Yan X, Voutetakis A, Zheng C, et al. Sorting of transgenic secretory proteins in miniature pig parotid glands following adenoviral-mediated gene transfer. J Gene Med. 2007;9:779–87. doi: 10.1002/jgm.1081. [DOI] [PubMed] [Google Scholar]
- 44.Voutetakis A, Zheng C, Metzger M, et al. Sorting of transgenic secretory proteins in rhesus macaque parotid glands following adenoviral mediated gene transfer. Hum Gene Ther. 2008;19:1401–6. doi: 10.1089/hum.2008.034. [DOI] [PMC free article] [PubMed] [Google Scholar]