Summary
Low steady state haemoglobin oxygen saturation in patients with sickle cell anaemia has been associated with the degree of anaemia and haemolysis. How much pulmonary dysfunction contributes to low saturation is not clear. In a prospective study of children and adolescents with sickle cell disease aged 3–20 years at steady state and matched controls, 52% of 391 patients versus 24% of 63 controls had steady state oxygen saturation <99% (P < 0·0001), 9% of patients versus no controls had saturation <95% (P = 0·008) and 8% of patients versus no controls had exercise-induced reduction in saturation ≥3%. Decreasing haemoglobin concentration (P ≤ 0·001) and increasing haemolysis (P ≤ 0·003) but not pulmonary function tests were independent predictors of both lower steady-state saturation and exercise-induced reduction in saturation. Neither history of stroke nor history of acute chest syndrome was significantly associated with lower steady-state oxygen saturation or exercise-induced reduction in saturation. Tricuspid regurgitation velocity was higher in patients with lower steady state haemoglobin oxygen saturation (P = 0·003) and with greater decline in oxygen saturation during the six-minute walk (P = 0·022). In conclusion, lower haemoglobin oxygen saturation is independently associated with increasing degrees of anaemia and haemolysis but not pulmonary function abnormalities among children and adolescents with sickle cell disease.
Keywords: sickle cell disease, paediatric, oxygen saturation, six-minute walk, pulmonary hypertension
A lowering of the haemoglobin oxygen saturation as determined by pulse oximetry is common in patients with sickle cell disease at steady state despite the lack of overt evidence of pulmonary dysfunction or intrinsic lung disease (Rackoff et al, 1993; Homi et al, 1997; Setty et al, 2003; Quinn & Ahmad, 2005). Decreased affinity of haemoglobin S for oxygen (Seakins et al, 1973; Ueda et al, 1979) and increased erythrocyte 2,3-bisphosphoglycerate (Milner, 1974) may partially, but probably not fully, explain decreased oxygen saturation in sickle cell disease given the wide range of saturations in patients. Several groups have reported the association of low with elevated serum lactate dehydrogenase, severity of anaemia and/or reticulocytosis, suggesting a possible aetiological relationship of haemolysis with hypoxemia (Rackoff et al, 1993; Homi et al, 1997; Setty et al, 2003; Quinn & Ahmad, 2005; Kato et al, 2006). It has been proposed that this association might involve haemolysis-associated ventilation-perfusion mismatch (Kato et al, 2007a).
A developing paradigm of sickle cell disease manifestations proposes two overlapping but distinctively characteristic subphenotypes (Kato et al, 2007a). In the vasculopathy subphenotype, complications such as stroke, pulmonary hypertension, priapism and leg ulcers predominate and are linked to the degree of haemolysis and associated vascular dysfunction. In the vaso-occlusive subphenotype, markers of increased blood viscosity predict risk for infarctive complications, such as frequent pain crises, acute chest syndrome and avascular necrosis of bone. Steady state haemoglobin oxygen desaturation is a risk factor for overt stroke (Quinn & Sargent, 2008) and for pulmonary hypertension (Pashankar et al, 2008; Liem et al, 2009; Minniti et al, 2009) in sickle cell disease, two of the prominent manifestations of the haemolytic subphenotype. Nocturnal oxygen desaturation is also a risk factor for stroke, silent infarct or seizure (Kirkham et al, 2001). On the other hand, reported associations of lower haemoglobin oxygen saturation with increased episodes of pain (Hargrave et al, 2003) and acute chest syndrome (Rackoff et al, 1993), manifestations of the vaso-occlusive subphenotype, have not been confirmed in other studies (Quinn & Ahmad, 2005; Uong et al, 2006).
We recently reported the initial results of an ongoing prospective, multicenter study of the prevalence and risk factors for developing pulmonary hypertension in children with sickle cell disease (PUSH Study) (Minniti et al, 2009). Both haemolysis and oxygen desaturation were independently associated with elevated tricuspid regurgitation velocity in this study. Pulmonary function testing and a six-minute walk were performed as a part of this study and pulse oximetry measurements were made before and after the walk. We hypothesized that lower oxygen saturations at steady state and oxygen desaturation during the six-minute walk may reflect pulmonary ventilation/perfusion mismatch related to the vascular effects of accelerated haemolysis. We investigated the relationship of oxygen saturation with medical history, echocardiographic measurements, pulmonary function testing and laboratory test results in this cohort.
Methods
Enrollment of participants
A total of 391 patients with sickle cell disease and 63 controls were recruited at three tertiary medical centers: Children’s National Medical Center and Howard University Hospital in Washington DC, and University of Michigan, in Ann Arbor, Michigan. Of these, 310 patients and 54 controls were included in the initial report (Minniti et al, 2009). The patients were of 3–20 years of age and had a diagnosis of sickle cell disease confirmed with haemoglobin electrophoresis or high performance liquid chromatography. All phenotypes were included, such as haemoglobins SS, SC, Sb-thalassemia, SD, SO-Arab and others. The controls were matched by age, sex and ethnicity to every sixth patient with sickle cell disease. Children with sickle cell trait (n = 17) and haemoglobin C trait (n = 5) also qualified as controls. Patients were invited to participate on a consecutive basis, as they presented for routine outpatient care; no attempt was made to select them by known or perceived risk factors. To ensure that patients were enrolled at steady state, at least 3 weeks had to have elapsed since hospitalization, emergency department or clinic visit for acute chest syndrome, pain crisis, infection or other sickle cell disease-related complication. Written informed consent was obtained for all participants. Parents or legal guardians provided consent for children ages 3–17 years. Participants from ages 18 to 20 years provided consent for themselves. Participants from 7 to 17 years provided additional written informed assent. Patients receiving regular blood transfusions or hydroxycarbamide therapy were not excluded.
Clinical evaluation
Patients underwent medical history, physical examination and measurement of oxygen saturation by pulse oximetry. Venous blood was drawn for complete blood count, reticulocyte count and serum chemistries.
Six-minute walk test
An unencouraged six-minute walk test was conducted in a predetermined course, at least six feet wide, as per the guidelines of the American Thoracic Society (ATS) (ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories 2002). This test measures the distance that an individual can quickly walk on a flat, hard surface in a period of 6 min. It is self-paced and rest is allowed during the exercise. The distance walked was recorded in metres for all subjects. Transcutaneous pulse oximetry haemoglobin oxygen saturation measurements were obtained before, during, and immediately after the six-minute walk test. Only children older than 5 years of age were given this test, due to the difficulty in obtaining reliable information from younger participants.
Laboratory studies
Complete blood count and reticulocyte count were performed by Beckman Coulter LH 750 Analyzer (Fullerton, CA, USA) at Howard University and the University of Michigan, and by Sysmex 2100QC (Sysmex America, Inc., Mundelein, IL, USA) at the Children’s National Medical Center. Serum chemistries were performed by Beckman Coulter Unicel DXC800 at Howard University, by RXL 2 Max, Model 973626, (Dade-Behring, Inc., Dover, DE, USA) at the Children’s National Medical Center and by Siemens Advia 2400 (Deerfield, IL, USA) at the University of Michigan. Pulse oximetry was measured by Criticare Model 506 Series (Waukesha, WI, USA) at Howard University, a Welch Allyn instrument (Beaverton, OR, USA) or a SureSigns VS3 No. 3000 (Philips Medical System, Andover, MA, USA) at the Children’s National Medical Center and by a Masimo Rad 8 Signal Extraction Pulse Oximeter at the University of Michigan. Reticulocyte count and serum concentrations of lactate dehydrogenase, aspartate aminotransferase and total bilirubin were prospectively considered to be markers of haemolysis. Reference ranges for Howard University, Children’s National Medical Center and University of Michigan, respectively, were 25–75, 42–95 and 26–104 × 109/l for reticulocyte count; 0–250, 141–231 and 150–300 u/l for lactate dehydrogenase; 0–50, 10–36 and 5–60 u/l for aspartate aminotransferase; 3·4–20·5, <13·7 and 1·7–17·1 µmol/l for total bilirubin.
Echocardiography
Transthoracic echocardiography was performed using the Philips Sonos 5500/7500 or iE33 (Philips Medical Systems, Best, Holland), Acuson Sequoa (Siemans Medical Systems, Mountain View, CA, USA), or General Electric VIVID 7 or VIVID I (General Electric, Milwaukee, WI, USA). All images were recorded digitally and subsequently reviewed on an offline digital work station. Cardiac images were obtained, measurements were performed, and the studies were interpreted according to the guidelines of the American Society of Echocardiography (Lai et al, 2006). Tricuspid regurgitation was assessed in the parasternal long and short-axis, and apical four-chamber views. Continuous-wave Doppler sampling was used to determine the peak tricuspid regurgitation velocity, which reflects systolic pulmonary artery pressure (Berger et al, 1985). Left ventricular diastolic function was assessed by measuring the tissue Doppler E wave at the basilar segments of the left ventricular free wall and the interventricular septum. The mitral inflow E wave to tissue Doppler E wave (E/Etdi) ratio reflects left atrial and left ventricular filling pressures (Nagueh et al, 1997). Based on the systemic blood pressure-and age-adjusted mean + 2 SDs in the controls of this study, a peak tricuspid regurgitation velocity of ≥2·60 m/s and an E/Etdi ≥ 8·9 was considered to be elevated.
Pulmonary function testing
Participants underwent spirometry and plethysmography, according to the ATS standard protocols (Macintyre et al, 2005; Miller et al, 2005; Wanger et al, 2005). Pulmonary function test results were expressed as a percentage of the predicted value based on ethnic appropriate reference standards for spirometry (Hankinson et al, 1999). For plethysmography, results were expressed as a percentage of the predicted race-adjusted reference standards (Pellegrino et al, 2005). The pulmonary function testing systems used were VMAX 62J (Sensomedics, Vienna, Austria) at the University of Michigan and Howard University and Elite DX (Medical Graphics, St. Paul, MN, USA) at the Children’s National Medical Center. The following parameters were included in the analysis: forced vital capacity (FVC), forced expiratory volume in 1 s (FEV1), FEV1/FVC, total lung capacity (TLC).
Statistical analysis
Analysis of variance was used to compare continuous variables among groups of patients according to different baseline haemoglobin oxygen saturations. The post hoc Bonferroni test was used for pair-wise comparisons. The Student’s t-test or the Kolmogorov–Smirnov nonparametric test (whichever appropriate) was used to compare continuous variables between cases and controls, or two groups of patients according to six-minute walk desaturation. For categorical variables we applied the chi-square test. A stepwise linear regression model was developed to identify independent predictors of steady-state haemoglobin oxygen saturation. A stepwise logistic regression model was developed to determine independent predictors of a decline in oxygen saturation of three points or more during the six-minute walk. P values <0·05 were considered significant. Analyses were performed with stata 10 (StataCorp, College Station, TX, USA).
Results
Steady-state haemoglobin oxygen saturation
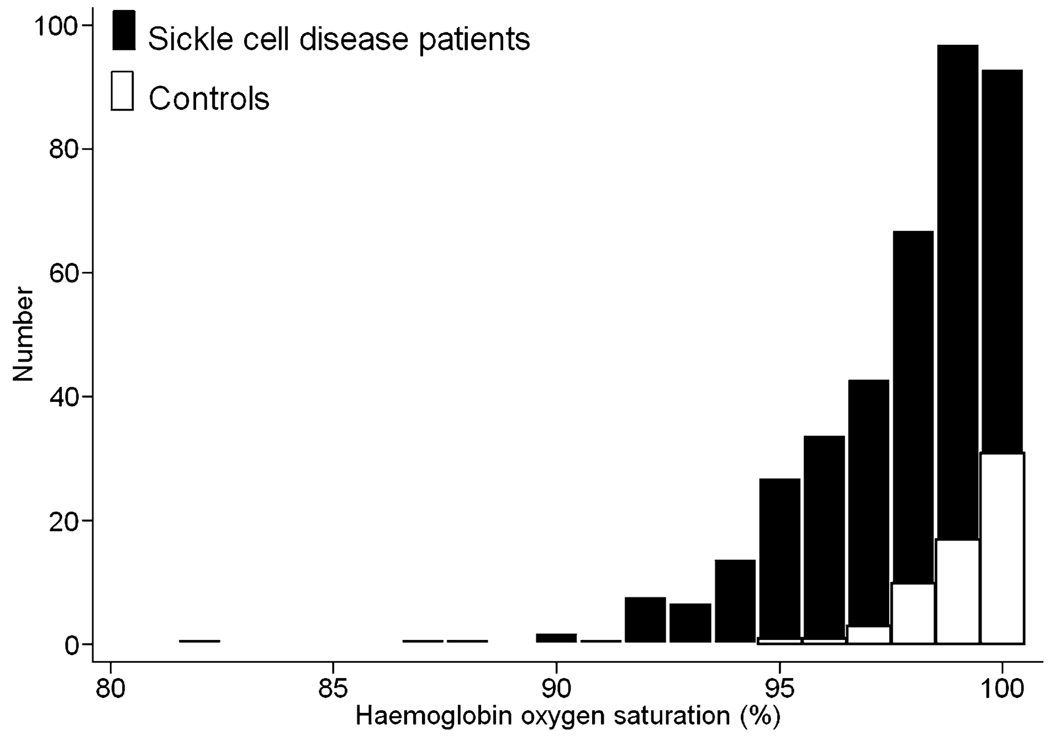
The distribution of steady state haemoglobin oxygen saturation in 391 children with sickle cell disease and 63 controls is depicted in Fig 1. Two hundred and two (52%) of the patients with sickle cell disease compared to 15 (24%) of the controls had haemoglobin oxygen saturation <99% (P < 0·0001) while 9% of patients versus no controls had saturation <95% (P = 0·008). Table I presents clinical findings stratified according to those that might be predictors of steady state oxygen saturation level and those that could be viewed as outcomes associated with the degree of saturation. In bivariate analysis of potential predictors of oxygen saturation, the following showed significant correlations with lower saturation: male sex, severe sickling phenotype, lower values for weight-for-age z-score, haemoglobin concentration and TLC, and higher values for four markers of haemolysis and a haemolytic component derived from these markers. In bivariate analysis of potential outcomes, lower oxygen saturation was associated with higher values for tricuspid regurgitation velocity, which reflects systolic pulmonary artery pressure, and with more frequently having an exercise-induced decline in haemoglobin oxygen saturation. Lower oxygen saturation was not associated with increasing mitral valve E/Etdi, which reflects left ventricular diastolic dysfunction, nor with shorter distance walked in the six-minute walk or histories of acute chest syndrome, frequent pain episodes or stroke.
Fig 1.
Steady-state haemoglobin oxygen saturation in patients with sickle cell disease and controls. The steady state distribution of haemoglobin oxygen saturation in 391 children with sickle cell disease and 63 controls is depicted. Two hundred and two (52%) of the patients with sickle cell disease compared to 15 (24%) of the controls had haemoglobin oxygen saturation <99% while 9% of patients versus no controls had saturation <95% (P = 0·008).
Table I.
Clinical data by haemoglobin oxygen saturation group. Results in median and interquartile range unless otherwise indicated
| N | O2 sat. > 98% | N | O2 sat. 95–98% | N | O2 sat. < 95% | P | |
|---|---|---|---|---|---|---|---|
| Potential predictors of O2 saturation | |||||||
| Age (years) | 189 | 12 (7–16) | 168 | 13 (8–16) | 34 | 12 (8–16) | 0·7† |
| Female sex* | 189 | 104 (55%) | 168 | 69 (41%) | 34 | 17 (50%) | 0·030‡ |
| Severe sickling phenotype | 185 | 119 (64%) | 167 | 137 (82%) | 34 | 34 (100%) | <0·0001‡ |
| (Hb SS, Sβ0 thal or SDLA)* | |||||||
| Regular transfusion programme* | 183 | 25 (14%) | 162 | 24 (15%) | 32 | 4 (13%) | 0·9‡ |
| Weight for age Z score | 175 | 0·2 (−0·5–1·0) | 161 | 0 (−0·8–0·8) | 34 | −0·5 (−1·1–0·1) | 0·002† |
| Hydroxycarbamide therapy* | 185 | 68 (37%) | 163 | 70 (43%) | 32 | 6 (19%) | 0·033‡ |
| History of asthma* | 186 | 43 (23%) | 163 | 45 (28%) | 31 | 9 (29%) | 0·6‡ |
| History of obstructive sleep apnea* | 186 | 19 (10%) | 163 | 15 (9%) | 32 | 6 (19%) | 0·3‡ |
| Haemoglobin (g/l) | 184 | 98 (88–111) | 159 | 88 (79–99) | 33 | 78 (69–85) | <0·0001† |
| Lactate dehydrogenase (u/l) | 168 | 334 (250–461) | 150 | 411 (300–549) | 32 | 552 (458–694) | <0·0001† |
| Reticulocytes (× 109/l) | 177 | 173 (127–255) | 159 | 244 (168–351) | 33 | 298 (248–435) | <0·0001† |
| Aspartate amino-transferase (u/l) | 183 | 35 (27–50) | 161 | 42 (31–53) | 34 | 53 (46–66) | <0·0001† |
| Total bilirubin (µmol/l) | 184 | 29 (19–46) | 160 | 43 (29–68) | 34 | 56 (39–82) | <0·0001† |
| Haemolytic component | 160 | −0·6 (−1·6–0·8) | 145 | 0·4 (−0·7–1·3) | 31 | 1·3 (0·5–2·4) | <0·0001† |
| FEV1/FVC | 64 | 84 (81–89) | 55 | 86 (80–89) | 18 | 84 (79–87) | 0·5† |
| TLC | 47 | 104 (94–114) | 36 | 103 (89–111) | 14 | 88 (84–92) | 0·006† |
| DLCO | 47 | 94 (85–105) | 38 | 102 (88–113) | 13 | 93 (91–98) | 0·1† |
| Potential outcomes associated with level of O2 saturation | |||||||
| TRV (m/s) | 167 | 2·3 (2·1–2·4) | 149 | 2·4 (2·2–2·5) | 30 | 2·4 (2·3–2·5) | 0·003† |
| TRV ≥ 2·6* | 167 | 10 (6%) | 149 | 23 (15%) | 30 | 5 (17%) | 0·016‡ |
| Mitral valve E/Etdi | 173 | 6·3 (5·5–7·5) | 157 | 6·4 (5·6–7·3) | 33 | 6·6 (5·7–8·2) | 0·3† |
| Mitral valve E/Etdi ≥ 8·9* | 173 | 16 (9%) | 157 | 9 (6%) | 33 | 4 (12%) | 0·3‡ |
| Six-minute walk distance (m) | 146 | 451 (402–508) | 141 | 433 (388–501) | 28 | 478 (398–520) | 0·4§ |
| Decline in oxygen saturation | 146 | 7 (5%) | 140 | 13 (9%) | 27 | 7 (26%) | 0·001 |
| of ≥3% during walk | |||||||
| History of acute chest syndrome* | 186 | 51 (27%) | 163 | 53 (32%) | 32 | 8 (25%) | 0·5‡ |
| History of ≥2 severe pain | 189 | 55 (29%) | 168 | 55 (33%) | 34 | 7 (21%) | 0·3‡ |
| episodes past year* | |||||||
| History >20 units of | 185 | 25 (14%) | 160 | 19 (12%) | 32 | 6 (19%) | 0·6‡ |
| blood transfused* | |||||||
| History of stroke* | 185 | 18 (10%) | 163 | 17 (10%) | 31 | 6 (19%) | 0·3‡ |
| History of priapism* | 81 | 18 (22%) | 92 | 18 (20%) | 15 | 2 (13%) | 0·7‡ |
FEV, forced expiratory volume in 1 s; FVC, forced vital capacity; TLC, total lung capacity; DLCO, Diffusion capacity for carbon monoxide; TRV, tricuspid regurgitation velocity; E/Etdi, E wave to tissue Doppler E wave.
Number (%).
From anova.
From Chi-square test.
Adjusted for height.
Independent predictors of low baseline oxygen saturation by multiple linear regression analysis
Both decreasing haemoglobin concentration (P = 0·001) and increasing degree of haemolysis (P < 0·0001) were independent predictors of lower steady state oxygen saturation among the patients overall and age was also a predictor (P = 0·043) (Table II). TLC did not have an independent association with lower oxygen saturation. According to this model and with other variables held constant, an increase in the haemolytic index of 2 standard deviations (SD) was associated with a decrease in the oxygen saturation of 1·4% (95% confidence interval of 0·9–1·9%). Likewise, a reduction in the haemoglobin concentration of 40 g/l is associated with a decrease in the oxygen saturation of 1·0% (0·4–1·6%). FEV1, FVC, FEV1/FVC ratio and TLC did not have independent associations with oxygen saturation.
Table II.
Independent predictors of steady state haemoglobin oxygen saturation among 391 sickle cell disease patients by multiple linear regression analysis*.
| 95% Confidence | |||
|---|---|---|---|
| Beta | interval | P value | |
| Haemolytic component | −0·71 | −0·97 to −0·45 | <0·0001 |
| (1 SD increase) | |||
| Haemoglobin | −0·25 | −0·11 to −0·40 | 0·001 |
| (10 g/l decrease) | |||
| Age (1 year increase) | −0·04 | −0·08 to −0·001 | 0·043 |
FEV, forced expiratory volume in 1 s; FVC, forced vital capacity; TLC, total lung capacity; DLCO, diffusion capacity for carbon monoxide; SD, standard deviation.
r-square = 0·25 for model. The following variables were entered into the model initially: sex, age, hydroxycarbamide therapy, history of asthma, history of sleep apnea, weight for age z-score, haemoglobin, haemolytic component, TLC, FEV1/FVC and DLCO.
Decline in haemoglobin oxygen desaturation during the six-minute walk test (Table III)
Table III.
Clinical and laboratory results by six-minute walk-induced decline in oxygen saturation groups among participants with sickle cell disease (results in median and interquartile range unless otherwise indicated)
| N | O2 saturation reduction < 3 | N | O2 saturation reduction ≥ 3 | P value | |
|---|---|---|---|---|---|
| Potential predictors of exercise-induced decline in oxygen saturation | |||||
| Age (year) | 296 | 13 (9–17) | 27 | 11 (9–14) | 0·030† |
| Female sex* | 296 | 145 (49%) | 27 | 9 (33%) | 0·12‡ |
| Severe sickling phenotype | 292 | 214 (73%) | 27 | 25 (93%) | 0·027‡ |
| (Hb SS, Sβ0 thal or SDLA)* | |||||
| Weight for age Z score | 271 | 0·1 (−0·7–0·8) | 26 | −0·4 (−1·1–0·2) | 0·003† |
| Regular transfusion programme* | 287 | 38 (13%) | 27 | 6 (22%) | 0·2‡ |
| Hydroxycarbamide therapy* | 291 | 123 (42%) | 27 | 8 (27%) | 0·2‡ |
| History of asthma* | 291 | 72 (25%) | 27 | 9 (33%) | 0·3‡ |
| History of obstructive sleep apnea* | 292 | 28 (10%) | 27 | 5 (19%) | 0·15‡ |
| History of >20 units of blood transfused* | 289 | 42 (15%) | 27 | 1 (4%) | 0·12‡ |
| Baseline oxygen saturation (%) | 296 | 99 (97–100) | 27 | 97 (96–99) | 0·010‡ |
| Haemoglobin (g/l) | 285 | 94 (83–108) | 26 | 75 (69–83) | <0·0001† |
| Haemolytic component | 261 | −0·1 (−1·2–0·9) | 24 | 1·1 (0·3–2·4) | <0·0001† |
| FEV1/FVC | 112 | 84 (80–89) | 17 | 85 (79–87) | 0·4† |
| TLC | 80 | 103 (91–112) | 12 | 92 (81–107) | 0·2† |
| DLCO | 79 | 97 (86–109) | 14 | 103 (92–113) | 0·4† |
| Potential outcomes associated with exercise-induced decline in oxygen saturation | |||||
| TRV (m/s) | 257 | 2·3 (2·1–2·5) | 24 | 2·4 (2·2–2·6) | 0·022† |
| TRV ≥ 2·6* | 257 | 26 (10·1%) | 24 | 7 (29·2%) | 0·006‡ |
| Mitral valve E/Etdi | 267 | 6·3 (5·5–7·4) | 25 | 6·9 (6·0–7·4) | 0·1† |
| Mitral valve E/Etdi ≥8·9* | 267 | 21 (7·9%) | 25 | 3 (12·0%) | 0·5‡ |
| Six-minute walk distance (m) | 296 | 443 (402–504) | 27 | 431 (357–515) | 0·8§ |
| History of acute chest syndrome * | 292 | 85 (29%) | 27 | 9 (33%) | 0·6‡ |
| History of stroke* | 290 | 30 (10%) | 27 | 2 (7%) | 0·6‡ |
| History of priapism* | 145 | 26 (18%) | 18 | 6 (33%) | 0·12‡ |
FEV, forced expiratory volume in 1 s; FVC, forced vital capacity; TLC, total lung capacity; DLCO, diffusion capacity for carbon monoxide; TRV, tricuspid regurgitation velocity; E/Etdi, E wave to tissue Doppler E wave.
Number (%).
From Student’s t-test.
Chi-square test.
Adjusted for height.
Oxygen saturation was measured before and after the six-minute walk test. A cut-off point of 3% or greater decline in saturation during the walk was used to categorize the patients into two groups. Among 323 patients with this available data, 27 (8%) had a reduction in oxygen saturation of three points or more during the six-minute walk. None of the controls had such a decline during the six-minute walk. In bivariate analyses, younger age, severe sickling phenotype, lower values for weight-for-age z-score, haemoglobin concentration and baseline oxygen saturation and higher values for the haemolytic component were predictors of exercise-induced decline in oxygen saturation of three points or more. Histories of asthma and obstructive sleep apnea did not predict desaturation during exercise. Of potential outcomes, exercise-induced decline in oxygen saturation had a significant association with higher tricuspid regurgitation velocity but not with history of acute chest syndrome or frequent severe pain episodes. Interestingly, exercise-induced decline in oxygen saturation appeared to be a better predictor of elevated tricuspid regurgitation velocity than steady-state saturation: 29% of patients with a decline in saturation of three points or more had elevated tricuspid regurgitation velocity compared to 17% of patients with steady state saturation <95%.
Independent predictors of exercise-induced decrease in oxygen saturation by logistic regression analysis
By multivariate analysis, lower haemoglobin concentration (P = 0·0003) and greater degree of haemolysis (P = 0·003) were each independently associated with increased odds of exercise-induced decrease in oxygen saturation (Table IV). Age, height, sex, history of acute chest syndrome, baseline oxygen saturation and histories of asthma or sleep apnea were not significantly related to oxygen desaturation during the six-minute walk test. Pulmonary function tests were not independent predictors of exercise-induced decline in haemoglobin oxygen saturation.
Table IV.
Independent predictors of decline in haemoglobin O2 saturation of three percentage points or more during the six-minute walk among 323 patients by logistic regression*.
| Odds ratio | 95% CI | P value | |
|---|---|---|---|
| Haemolytic index | 2·5 | 1·4–4·7 | 0·003 |
| (increase by 1 SD) | |||
| Haemoglobin | 3·3 | 1·8–6·3 | 0·0003 |
| (decrease by 10 g/l) |
FEV, forced expiratory volume in 1 s; FVC, forced vital capacity; TLC, total lung capacity; DLCO, diffusion capacity for carbon monoxide; SD, standard deviation.
Variables entered into model: baseline O2 saturation, age, height, sex, history of acute chest syndrome, history of asthma, haemoglobin, haemolytic index, TLC, FEV1/FVC, DLCO. Seven cases with residuals out of range were excluded. Area under the curve for the Receiver-operated characteristic = 0·94. P for goodness of fit = 0·99.
Discussion
Both the degree of anaemia and the degree of haemolysis, as indicated by a haemolytic component derived from four markers, were found to be independent predictors of lower steady state oxygen saturation and of oxygen-induced decline in saturation in children and adolescents with sickle cell disease. Increasing age was an additional independent predictor of lower steady state oxygen saturation. Lower values for FEV1, FVC, FEV1/FVC ratio and TLC were not independent predictors of lower steady state oxygen saturation or exercise-induced reduction in saturation. Neither lower steady state oxygen saturation nor desaturation during exercise were predictors of stroke, acute chest syndrome or severe pain history.
Lower oxygen saturation in the setting of sickle cell disease at steady state might be due to several factors including intrinsic lung disease leading to reduced gas exchange and alterations in the pulmonary vasculature or airways leading to ventilation-perfusion mismatching. Further, altered affinity of haemoglobin for oxygen and increased levels of methaemoglobin or carboxyhaemoglobin may be other contributing factors (Hurford & Kratz, 2004). Several factors could contribute to decreased oxygen affinity, including the intrinsic reduced affinity of haemoglobin S (Seakins et al, 1973; Ueda et al, 1979), an increase in red blood cell 2,3-biphosphoglycerate related to the degree of anaemia (Milner, 1974) and/or to haemoglobin S polymer formation in the red cells that precedes overt sickling and haemolysis (Sunshine et al, 1982). We did not have strong evidence of intrinsic lung disease in the children with sickle cell disease in this study, and the lack of an independent association of lower oxygen saturation with pulmonary function test abnormalities or histories of asthma, acute chest syndrome, and sleep apnea is also against intrinsic lung disease as a major contributing factor. On the other hand, the independent association of lower haemoglobin with lower oxygen saturation might plausibly be related to increased levels of red cell 2, 3-biphosphoglycerate and the independent association with haemolysis to increased haemoglobin S polymer formation. In addition, our finding of an association of greater haemolysis with lower oxygen saturation in all of the statistical analyses that we performed was consistent with the possibility that haemolysis induces changes in the pulmonary vasculature and airways that limit the acquisition of oxygen by haemoglobin in sickle cell disease, thus supporting ventilation-perfusion mismatching as a consequence of haemolysis-induced pulmonary vasculopathy (Kato et al, 2007a).
This study is limited by the fact that arterial blood gases were not performed to confirm pO2 and oxygen saturation, and some evidence suggests that pulse oximetry is not reliable in sickle cell disease (Blaisdell et al, 2000). However, a number of other studies have indicated that pulse oximetry is reliable for diagnosing hypoxia during steady state conditions and with acute complications in sickle cell disease patients (Rackoff et al, 1993; Kress et al, 1999; Ortiz et al, 1999). Another limitation of our study is that it is cross-sectional rather than longitudinal. Nevertheless, the findings presented here confirm, in one prospective study, the observations of a number of other investigators. In particular, other investigations have also shown relationships of lower oxygen saturation in sickle cell disease patients with severe anaemia, more severe sickling phenotype (haemoglobins SS and Sβ0 thalassemia) (Rackoff et al, 1993; Homi et al, 1997) and/or with haemolysis (Setty et al, 2003; Quinn & Ahmad, 2005; Kato et al, 2006; Pashankar et al, 2008; Quinn & Sargent, 2008; Liem et al, 2009). For example, the Dallas Newborn Cohort Study of 585 patients showed independent associations of both the haemolytic marker, reticulocyte count, and low haemoglobin concentration with lower oxygen saturation, with the haemolytic marker explaining more of the oxygen saturation than the haemoglobin concentration (Quinn & Ahmad, 2005); our study was able to duplicate these observations. Our findings point to a potential interrelationship between haemolysis and hypoxia in predicting certain complications of sickle cell disease. An increase in the haemolytic index of 2 SD was associated with a 1·4% decrease in the oxygen saturation within our cohort. Increased haemolysis may contribute to the development of hypoxia by inducing changes in the pulmonary vessels and airways that lead to ventilation-perfusion mismatching. In turn, hypoxia may contribute to the development of haemolytic complications, such as pulmonary hypertension.
Patients in the present study with the lower steady state oxygen saturation or ≥3% reduction in oxygen saturation with exercise had similar statistically significant clinical and laboratory correlates (i.e. markers of haemolysis, lower haemoglobin concentration and higher tricuspid regurgitation velocity). Surrogate markers for diagnosing pulmonary hypertension and predicting its severity in sickle cell disease adult patients are being developed (Machado et al, 2006). However, there are no models for predicting the early development of pulmonary hypertension in children (Kato et al, 2007b). Inferring from the results of our study, lower steady state oxygen saturation and an acute decline of 3% in oxygen saturation from baseline during the six-minute walk test may serve as early markers for future development of pulmonary hypertension in children. Therefore, future studies are warranted to longitudinally follow children and adolescents with sickle cell disease.
In conclusion, our findings provide evidence that low haemoglobin oxygen saturation should be considered as a potential feature of the haemolytic vasculopathy sub-pheno-type of sickle cell disease. Haemolysis-related pulmonary vascular dysfunction may underlie both oxygen desaturation through ventilation/perfusion mismatch and shunting as well as pulmonary hypertension.
Acknowledgements
Supported in part by grant nos. 2 R25 HL003679-08 and 1 R01 HL079912-02 from NHLBI, by Howard University GCRC grant no 2MOI RR10284-10 from NCRR, NIH, Bethesda, MD, and by the intramural research program of the National Institutes of Health.
References
- ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the sixminute walk test. American Journal of Respiratory and Critical Care Medicine. 2002;166:111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- Berger M, Haimowitz A, Van Tosh A, Berdoff RL, Goldberg E. Quantitative assessment of pulmonary hypertension in patients with tricuspid regurgitation using continuous wave Doppler ultrasound. Journal of the American College of Cardiology. 1985;6:359–365. doi: 10.1016/s0735-1097(85)80172-8. [DOI] [PubMed] [Google Scholar]
- Blaisdell CJ, Goodman S, Clark K, Casella JF, Loughlin GM. Pulse oximetry is a poor predictor of hypoxemia in stable children with sickle cell disease. Archives of Pediatrics and Adolescent Medicine. 2000;154:900–903. doi: 10.1001/archpedi.154.9.900. [DOI] [PubMed] [Google Scholar]
- Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. American Journal of Respiratory and Critical Care Medicine. 1999;159:179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- Hargrave DR, Wade A, Evans JP, Hewes DK, Kirkham FJ. Nocturnal oxygen saturation and painful sickle cell crises in children. Blood. 2003;101:846–848. doi: 10.1182/blood-2002-05-1392. [DOI] [PubMed] [Google Scholar]
- Homi J, Levee L, Higgs D, Thomas P, Serjeant G. Pulse oximetry in a cohort study of sickle cell disease. Clinical and Laboratory Haematology. 1997;19:17–22. doi: 10.1046/j.1365-2257.1997.00215.x. [DOI] [PubMed] [Google Scholar]
- Hurford WE, Kratz A. Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises. Case23-2004. A 50-year-old woman with low oxygen saturation. New England Journal of Medicine. 2004;351:380–387. doi: 10.1056/NEJMcpc049013. [DOI] [PubMed] [Google Scholar]
- Kato GJ, McGowan V, Machado RF, Little JA, Taylor JG, Morris CR, Nichols JS, Wang X, Poljakovic M, Morris SM, Jr, Gladwin MT. Lactate dehydrogenase as a biomarker of hemolysis-associated nitric oxide resistance, priapism, leg ulceration, pulmonary hypertension, and death in patients with sickle cell disease. Blood. 2006;107:2279–2285. doi: 10.1182/blood-2005-06-2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato GJ, Gladwin MT, Steinberg MH. Deconstructing sickle cell disease: reappraisal of the role of hemolysis in the development of clinical subphenotypes. Blood Reviews. 2007a;21:37–47. doi: 10.1016/j.blre.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato GJ, Onyekwere OC, Gladwin MT. Pulmonary hypertension in sickle cell disease: relevance to children. Pediatric Hematology and Oncology. 2007b;24:159–170. doi: 10.1080/08880010601185892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkham FJ, Hewes DK, Prengler M, Wade A, Lane R, Evans JP. Nocturnal hypoxaemia and central-nervous-system events in sickle-cell disease. Lancet. 2001;357:1656–1659. doi: 10.1016/s0140-6736(00)04821-2. [DOI] [PubMed] [Google Scholar]
- Kress JP, Pohlman AS, Hall JB. Determination of hemoglobin saturation in patients with acute sickle chest syndrome: a comparison of arterial blood gases and pulse oximetry. Chest. 1999;115:1316–1320. doi: 10.1378/chest.115.5.1316. [DOI] [PubMed] [Google Scholar]
- Lai WW, Geva T, Shirali GS, Frommelt PC, Humes RA, Brook MM, Pignatelli RH, Rychik J. Guidelines and standards for performance of a pediatric echocardiogram: a report from the Task Force of the Pediatric Council of the American Society of Echocardiography. Journal of the American Society of Echocardiography. 2006;19:1413–1430. doi: 10.1016/j.echo.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Liem RI, Nevin MA, Prestridge A, Young LT, Thompson AA. Tricuspid regurgitant jet velocity elevation and its relationship to lung function in pediatric sickle cell disease. Pediatric Pulmonology. 2009;44:281–289. doi: 10.1002/ppul.20996. [DOI] [PubMed] [Google Scholar]
- Machado RF, Anthi A, Steinberg MH, Bonds D, Sachdev V, Kato GJ, Taveira-DaSilva AM, Ballas SK, Blackwelder W, Xu X, Hunter L, Barton B, Waclawiw M, Castro O, Gladwin MT. N-terminal pro-brain natriuretic peptide levels and risk of death in sickle cell disease. Journal of the American Medical Association. 2006;296:310–318. doi: 10.1001/jama.296.3.310. [DOI] [PubMed] [Google Scholar]
- Macintyre N, Crapo RO, Viegi G, Johnson DC, van der Grinten CP, Brusasco V, Burgos F, Casaburi R, Coates A, Enright P, Gustafsson P, Hankinson J, Jensen R, McKay R, Miller MR, Navajas D, Pedersen OF, Pellegrino R, Wanger J. Standardisation of the single-breath determination of carbon monoxide uptake in the lung. European Respiratory Journal. 2005;26:720–735. doi: 10.1183/09031936.05.00034905. [DOI] [PubMed] [Google Scholar]
- Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Crapo R, Enright P, van der Grinten CP, Gustafsson P, Jensen R, Johnson DC, MacIntyre N, McKay R, Navajas D, Pedersen OF, Pellegrino R, Viegi G, Wanger J. Standardisation of spirometry. European Respiratory Journal. 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- Milner PF. Oxygen transport in sickle cell anemia. Archives of Internal Medicine. 1974;133:565–572. [PubMed] [Google Scholar]
- Minniti CP, Sable C, Campbell A, Rana S, Ensing G, Dham N, Onyekwere O, Nouraie M, Kato GJ, Gladwin MT, Castro OL, Gordeuk VR. Elevated tricuspid regurgitant jet velocity in children and adolescents with sickle cell disease: association with hemolysis and hemoglobin oxygen desaturation. Haematologica. 2009;94:340–347. doi: 10.3324/haematol.13812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagueh SF, Middleton KJ, Kopelen HA, Zoghbi WA, Quinones MA. Doppler tissue imaging: a noninvasive technique for evaluation of left ventricular relaxation and estimation of filling pressures. Journal of the American College of Cardiology. 1997;30:1527–1533. doi: 10.1016/s0735-1097(97)00344-6. [DOI] [PubMed] [Google Scholar]
- Ortiz FO, Aldrich TK, Nagel RL, Benjamin LJ. Accuracy of pulse oximetry in sickle cell disease. American Journal of Respiratory and Critical Care Medicine. 1999;159:447–451. doi: 10.1164/ajrccm.159.2.9806108. [DOI] [PubMed] [Google Scholar]
- Pashankar FD, Carbonella J, Bazzy-Asaad A, Friedman A. Longitudinal follow up of elevated pulmonary artery pressures in children with sickle cell disease. British Journal of Haematology. 2008;144:736–741. doi: 10.1111/j.1365-2141.2008.07501.x. [DOI] [PubMed] [Google Scholar]
- Pellegrino R, Viegi G, Brusasco V, Crapo RO, Burgos F, Casaburi R, Coates A, van der Grinten CP, Gustafsson P, Hankinson J, Jensen R, Johnson DC, MacIntyre N, McKay R, Miller MR, Navajas D, Pedersen OF, Wanger J. Interpretative strategies for lung function tests. European Respiratory Journal. 2005;26:948–968. doi: 10.1183/09031936.05.00035205. [DOI] [PubMed] [Google Scholar]
- Quinn CT, Ahmad N. Clinical correlates of steady-state oxyhaemoglobin desaturation in children who have sickle cell disease. British Journal of Haematology. 2005;131:129–134. doi: 10.1111/j.1365-2141.2005.05738.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn CT, Sargent JW. Daytime steady-state haemoglobin desaturation is a risk factor for overt stroke in children with sickle cell anaemia. British Journal of Haematology. 2008;140:336–339. doi: 10.1111/j.1365-2141.2007.06927.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rackoff WR, Kunkel N, Silber JH, Asakura T, Ohene-Frempong K. Pulse oximetry and factors associated with hemoglobin oxygen desaturation in children with sickle cell disease. Blood. 1993;81:3422–3427. [PubMed] [Google Scholar]
- Seakins M, Gibbs WN, Milner PF, Bertles JF. Erythrocyte Hb-S concentration. An important factor in the low oxygen affinity of blood in sickle cell anemia. Journal of Clinical Investigation. 1973;52:422–432. doi: 10.1172/JCI107199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setty BN, Stuart MJ, Dampier C, Brodecki D, Allen JL. Hypoxaemia in sickle cell disease: biomarker modulation and relevance to pathophysiology. Lancet. 2003;362:1450–1455. doi: 10.1016/S0140-6736(03)14689-2. [DOI] [PubMed] [Google Scholar]
- Sunshine HR, Hofrichter J, Ferrone FA, Eaton WA. Oxygen binding by sickle cell hemoglobin polymers. Journal of Molecular Biology. 1982;158:251–273. doi: 10.1016/0022-2836(82)90432-6. [DOI] [PubMed] [Google Scholar]
- Ueda Y, Nagel RL, Bookchin RM. An increased Bohr effect in sickle cell anemia. Blood. 1979;53:472–480. [PubMed] [Google Scholar]
- Uong EC, Boyd JH, DeBaun MR. Daytime pulse oximeter measurements do not predict incidence of pain and acute chest syndrome episodes in sickle cell anemia. Journal of Pediatrics. 2006;149:707–709. doi: 10.1016/j.jpeds.2006.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanger J, Clausen JL, Coates A, Pedersen OF, Brusasco V, Burgos F, Casaburi R, Crapo R, Enright P, van der Grinten CP, Gustafsson P, Hankinson J, Jensen R, Johnson D, Macintyre N, McKay R, Miller MR, Navajas D, Pellegrino R, Viegi G. Standardisation of the measurement of lung volumes. European Respiratory Journal. 2005;26:511–522. doi: 10.1183/09031936.05.00035005. [DOI] [PubMed] [Google Scholar]