Abstract
Type 2 diabetes mellitus is one of the major public health threats in the United States today, reaching epidemic rates. Epidemiological evidence suggests a strong link between obesity and the risk of developing diabetes. Increasing evidence demonstrates that lifestyle interventions can significantly delay or possibly prevent the onset of type 2 diabetes in persons with increased risk. Despite these findings, there remain important barriers to the translation of this research to the public health. These include identifying persons with an increased risk for developing the disease and the lack of easily accessible, cost-effective intervention programs. At least one study, however, has effectively implemented an evidenced-based intervention in community settings, suggesting that it may be possible to develop a model for the national scalability of primary prevention in the United States.
Keywords: community programs, lifestyle interventions, primary prevention, review, type 2 diabetes
Type 2 diabetes mellitus is one of the major public health threats in the United States today. There are currently 24 million Americans estimated to have diabetes.1–5Moreover, there are over 65 million Americans with evidence of “prediabetes,” characterized by evidence of a metabolic defect defined by the presence of either impaired glucose tolerance (IGT) or impaired fasting glucose.1,3,5,6Persons with prediabetes have a significantly increased risk for developing type 2 diabetes—between 5 and 15% per year depending on the presence of other risk factors.4,5 These statistics illuminate a frightening possibly; the number of persons with diabetes is expected to nearly double by 2030 if current trends continue unabated.7–10
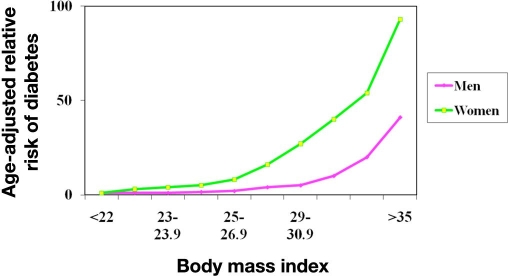
Epidemiological evidence suggests a strong link between obesity and the risk of developing diabetes. Figure 1 shows data combined from two studies, the Nurses Health Study11 and the U.S. Health Professionals Study.12,13 Both are longitudinal studies that look at the effect of lifestyle on chronic illness. In the male health professional study, men with a body mass index (BMI) greater than 35 had an age-adjusted risk of 42.1 times greater than a man with a BMI of less than 23 for developing diabetes. A similar trend was observed in female nurses, with the risk increasing to a staggering 93.2 of developing diabetes with a BMI of greater than 35 kg/m2. Moreover, the longer a person remains obese, the higher their risk of developing type 2 diabetes. In one study, people who have been at a BMI of greater than 30 for more than 10 years have over twice the risk of type 2 diabetes compared with those who have been obese for less than 5 years.12
Figure 1.
Obesity and type 2 diabetes.
Fortunately, three major clinical trials have demonstrated convincingly that personalized lifestyle interventions can significantly delay or possibly prevent the onset of type 2 diabetes in persons with prediabetes. The first was the Da Qing study, which screened over 110,000 men and women for IGT in 33 health centers in China.14 The investigators identified 577 persons (mean age 45 and mean BMI 25.8 kg/m2) with IGT and randomized them by clinic to receive either standard care (i.e., the control condition) or one of three interventions: diet only, exercise only, or a combined diet and exercise intervention. These interventions were selected to address insulin resistance via weight loss and physical activity. Each of the interventions was personalized to accommodate subjects' lifestyle and situation. In the diet-only condition, subjects were encouraged to eat a diet that provided approximately 55–65% of total daily calories from carbohydrate, increased consumption of vegetables while reducing simple sugars and alcohol, and were within a calorie goal of 25–30 kcal per kilogram of body weight. Subjects consulted individually with physicians and in small groups to achieve these goals. The exercise intervention was also tailored to subject's level of fitness. They were encouraged to increase the amount of their leisure physical exercise by one “unit” per day. Units were defined as either 30 minutes of mild, 20 minutes of moderate, 10 minutes of strenuous, or 5 minutes of very strenuous exercise. As was the case in diet intervention, the exercise prescription was personalized to account for differences in subject's level of fitness and capacity. The diet plus exercise intervention simply combined the two personalized approaches.
After 6 years of observation, the Da Qing study showed a 31% reduction in risk of developing diabetes for the diet intervention, a 46% reduction for the exercise intervention, and a 41% reduction for the combined diet and exercise intervention. This was the first study that clearly demonstrated that implementing a personalized lifestyle intervention was both feasible and effective for the prevention of type 2 diabetes.
The next major clinical trial of lifestyle as a prevention modality for type 2 diabetes was the Finish Diabetes Prevention (FDP) study.15 The FDP recruited and randomized 522 adults age 40–65 with IGT to receive either a control or a lifestyle intervention. Subjects assigned to the control condition received written and oral general information about a weight loss diet and benefits of exercise provided at baseline and annually. They also completed a 3-day food diary annually, but this was used for data collection purposes only. Subjects assigned to the intervention condition were provided detailed, individualized instructions on achieving study goals: weight loss of at least 5% of their body weight at entry into the study, a reduction in total daily calories derived from fat to 30%, an increase in fiber intake to ≥15 g/1000 kcal, and moderate exercise for at least 30 minutes per day. This was accomplished by meeting with a registered dietician seven times in the first year and quarterly thereafter. In addition, supervised exercise programs were offered. At the end of 4 years, subjects in the intervention condition showed a 58% reduction in risk of developing diabetes compared to controls.
The largest and most rigorous prevention trial to date is the Diabetes Prevention Program (DPP).16 The DPP recruited 3234 subjects age 25–75 with IGT. In addition, unlike the studies reviewed earlier, which studied subjects with homogeneous characteristics, almost half of the DPP subjects were from minority groups. Subjects were randomized into one of three conditions: an intensive lifestyle intervention, a medication intervention, or a medication placebo control condition. Subjects in the lifestyle intervention were encouraged to lose at least 7% of their initial body weight and to maintain this weight loss throughout the trial. The primary method advocated for weight loss was to reduce fat intake to 25% of total daily calories and restrict daily caloric consumption. In addition, each participant was encouraged to achieve and maintain at least 150 minutes per week of physical activity, with an intensity similar to brisk walking. Subjects in the medication arm were given metformin and were recommended to maintain a healthy lifestyle but without specific advice as to how to accomplish this. Control subjects were given placebo metformin and were also provided the same lifestyle recommendations.
At the end of 3 years, trial data warranted an early termination of the study. Subjects in the lifestyle condition had a 58% reduction in risk of developing diabetes, and subjects in the medication arm had a 31% reduction when compared to control subjects. In addition, the interventions were effective regardless of race or age.
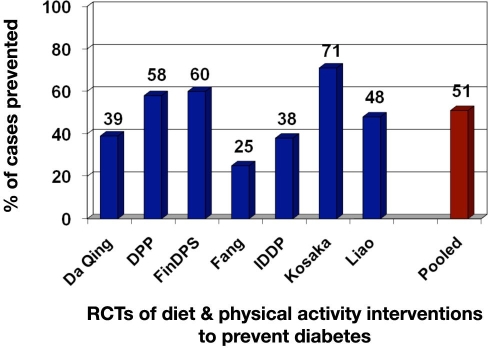
Since the DPP, at least four trials have been conducted that reinforce the ability of lifestyle modification to significantly reduce the risk of developing diabetes in persons with prediabetes.17 Collectively, if results of the primary prevention trials that assessed lifestyle interventions are pooled, they average a 51% reduction in risk (Figure 2). Moreover, all of these studies have demonstrated that to be effective, the intervention programs must be personalized to meet the unique situational and cultural aspects of its recipients.
Figure 2.
Reduction in type 2 diabetes—lifestyle intervention trials. FinDPS, Finnish Diabetes Prevention Study; IDDP, Indian Diabetes Prevention Program; RCTs, randomized controlled trials. Adapted from Gillies and colleagues.17
So why don't we have a diabetes prevention program on every street corner? Simply put, in the United States today, there remain important barriers to the translation of the DPP to public health. First, to develop an evidenced-based program, it is important to identify persons with prediabetes. While there are a variety of pen and paper methods to assess risk, the gold standard is still a blood test identifying prediabetes, either by fasting glucose values or, more appropriately, an oral glucose tolerance test to define impaired glucose tolerance. Currently, performing such tests is not routine in primary care settings. Indeed, only recently were primary care physicians able to be reimbursed for screening for prediabetes.
The second and perhaps more problematic barrier is the lack of established lifestyle intervention programs. Lifestyle interventions are resource and time intensive, exceeding both the resources and the training of most primary care providers. In addition, implementing programs such as the one used in the DPP is costly. Thus, it begs the question, why screen for prediabetes if there is no reasonable, cost-effective way to treat those who screen positive?
Clearly to overcome these barriers and provide “real-world” implementation, it will be necessary to simplify testing to identify high-risk patients and provide lower cost, more easily accessible evidence-based interventions. This will require that new partnerships be developed between health care systems, where diabetes risk can be assessed accurately and community agencies where the necessary resources to mount more accessible, long-term lifestyle interventions are available.
One example of this partnership approach has been pilot tested by the Diabetes Translational Research Center (DTRC) at the Indiana University School of Medicine. The DTRC tested the feasibility and effectiveness of training Young Men's Christian Association (YMCA) employees to deliver a group-based version of the DPP lifestyle intervention in YMCA branch facilities.18,19 The YMCA was selected for its ability to address the barriers to translation described earlier. The intervention model used in the DPP was adapted for delivery in groups using lower cost “lay leaders” employed by the YMCA. In addition, programs can be implemented at reduced expense because the YMCA operates on a cost recovery basis rather than for profit. In addition, the YMCA has the potential for considerable reach; there are over 2600 YMCAs in the United States with over 46 million persons living within 3 miles of a YMCA facility.
The study was a control trial in which YMCA facilities were randomized to receive either the group-based adaptation of the DPP or counseling regarding how persons identified as being at high risk could individually reduce their risk using materials developed by the National Diabetes Education Program (NDEP).19,20Risk assessment was done by mailing brochures to households within 5 km of the participating YMCAs, inviting them to attend a diabetes risk screening event held at the YMCA facility. This screening event was staffed by members of the Indiana University School of Medicine. All participants were tested for BMI and blood pressure; random or casual capillary glucose, cholesterol, and hemoglobin A1c levels were assessed using point-of-care technology. In combination with their responses to a family history of diabetes, levels of physical activity, and age, a risk score was assigned. Those deemed being a high risk were either enrolled in the group adaptation of the DPP curriculum delivered by trained YMCA staff or received brief counseling regarding the meaning of their risk assessment data by medical staff conducting the screening event. This counseling was supported by NDEP materials.
The cost for delivering the 16 session DPP curriculum using the YMCA was a fraction of that required when the original DPP intervention was implemented; $205 dollars per participant vs $1476 required in the original study. Is it possible for the YMCA to achieve a 5–7% weight loss for a fraction of the cost of the DPP? Tables 1 and 2 show outcome data for this pilot. Subjects in the group intervention achieved a 6% weight loss that was maintained at 6 and 14 months postintervention contact. Moreover, data show a significant reduction in total cholesterol over the same time period.
Table 1.
Results of DTRC–YMCA Study after 4–6 Months Postintervention
| Brief advice (N = 38) | DPP (N = 39) | p value | |
|---|---|---|---|
| Weight (% reduction) | −2.0 | −6.0 | <0.001 |
| Change systolic blood pressure (mmHg) | −2.3 | −1.9 | 0.88 |
| Change hemoglobin A1c (%) | −0.1 | −0.1 | 0.96 |
| Change total cholesterol (mg/dl) | +6.0 | −21.6 | <0.001 |
| Change high-density lipoprotein (mg/dl) | +2.1 | +1.1 | 0.68 |
Table 2.
Results of DTRC–YMCA Study after 12–14 Months Postintervention
| Brief advice (N = 33) | DPP (N = 29) | p value | |
|---|---|---|---|
| Weight (% reduction) | −1.8 | −6.0 | 0.008 |
| Change systolic blood pressure (mmHg) | −2.7 | −1.6 | 0.78 |
| Change hemoglobin A1c (%) | +0.03 | −0.1 | 0.28 |
| Change total cholesterol (mg/dl) | +11.8 | −13.5 | 0.002 |
| Change high-density lipoprotein (mg/dl) | −1.4 | +1.9 | 0.10 |
Convincing evidence demonstrates that implementing personalized programs to help persons adopt risk-reducing lifestyle behaviors can help reduce the epidemic of type 2 diabetes. Data from the YMCA study suggest that it may be possible to develop a model for the national scalability of primary prevention in the United States. Such a model should combine a community agency such as the YMCA in collaboration with medical centers to address the current barriers to translation of the DPP in the United States so that evidence-based, cost-effective primary prevention programs can be implemented.
Abbreviations
- BMI
body mass index
- DPP
Diabetes Prevention Program
- DTRC
Diabetes Translational Research Center
- FDP
Finish Diabetes Prevention
- IGT
impaired glucose tolerance
- NDEP
National Diabetes Education Program
- YMCA
Young Men's Christian Association
References
- 1.National Diabetes Fact Sheet: general information and national estimates on diabetes in the United States, 2003. Department of Health and Human Services, Centers for Disease Control and Prevention [cited 2004 May 5] Available from: http://www.cdc.gov/diabetes/pubs/pdf/ndfs_2003.pdf.
- 2.Diabetes: disabling, deadly, and on the rise. Centers for Disease Control and Prevention, Department of Health and Human Services [cited 2004 Apr 15] Available from: http://www.cdc.gov/nccdphp/aag/pdf/aag_ddt2004.pdf.
- 3.Harris MI, Flegal KM, Cowie CC, Eberhardt MS, Goldstein DE, Little RR, Wiedmeyer HM, Byrd-Holt DD. Prevalence of diabetes, impaired fasting glucose, and impaired glucose tolerance in U.S. adults. The Third National Health and Nutrition Examination Survey, 1988-1994. Diabetes Care. 1998;21(4):518–524. doi: 10.2337/diacare.21.4.518. [DOI] [PubMed] [Google Scholar]
- 4.Mokdad AH, Bowman BA, Ford ES, Vinicor F, Marks JS, Koplan JP. The continuing epidemics of obesity and diabetes in the United States. JAMA. 2001;286(10):1195–1200. doi: 10.1001/jama.286.10.1195. [DOI] [PubMed] [Google Scholar]
- 5.National Diabetes Fact Sheet. American Diabetes Association [cited 2004 May 2] Available from: http://www.diabetes.org/diabetes-statistics/national-diabetes-fact-sheet.jsp.
- 6.HHS. U.S. Department of Health and Human Services; ADA warn Americans of “pre-diabetes,” encourage people to take healthy steps to reduce risks [cited 2004 May 5] Available from: http://www.hhs.gov/news/press/2002pres/20020327.html. [Google Scholar]
- 7.King H, Aubert RE, Herman WH. Global burden of diabetes, 1995-2025: prevalence, numerical estimates, and projections. Diabetes Care. 1998;21(9):1414–1431. doi: 10.2337/diacare.21.9.1414. [DOI] [PubMed] [Google Scholar]
- 8.Boyle JP, Honeycutt AA, Narayan KM, Hoerger TJ, Geiss LS, Chen H, Thompson TJ. Projection of diabetes burden through 2050: impact of changing demography and disease prevalence in the U.S. Diabetes Care. 2001;24(11):1936–1940. doi: 10.2337/diacare.24.11.1936. [DOI] [PubMed] [Google Scholar]
- 9.Engelgau MM, Geiss LS, Saaddine JB, Boyle JP, Benjamin SM, Gregg EW, Tierney EF, Rios-Burrows N, Mokdad AH, Ford ES, Imperatore G, Narayan KM. The evolving diabetes burden in the United States. Ann Intern Med. 2004;140(11):945–950. doi: 10.7326/0003-4819-140-11-200406010-00035. [DOI] [PubMed] [Google Scholar]
- 10.Honeycutt AA, Boyle JP, Broglio KR, Thompson TJ, Hoerger TJ, Geiss LS, Narayan KM. A dynamic Markov model for forecasting diabetes prevalence in the United States through 2050. Health Care Manag Sci. 2003;6(3):155–164. doi: 10.1023/a:1024467522972. [DOI] [PubMed] [Google Scholar]
- 11.Carey VJ, Walters EE, Colditz GA, Solomon CG, Willet WC, Rosner BA, Speizer FE, Manson JE. Body fat distribution and risk of non-insulin-dependent diabetes mellitus in women: the Nurses' Health Study. Am J Epidemiol. 1997;145(7):614–619. doi: 10.1093/oxfordjournals.aje.a009158. [DOI] [PubMed] [Google Scholar]
- 12.Chan JM, Rimm EB, Colditz GA, STampfer MJ, Willett WC. Obesity, fat distribution, and weight gain as risk factors for clinical diabetes in men. Diabetes Care. 1994;17(9):961–969. doi: 10.2337/diacare.17.9.961. [DOI] [PubMed] [Google Scholar]
- 13.Mokdad AH, Ford ES, Bowman BA, Dietz WH, Vinicor F, Bales VS, Marks JS. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA. 2003;289(1):76–79. doi: 10.1001/jama.289.1.76. [DOI] [PubMed] [Google Scholar]
- 14.Pan XR, Li GW, Hu YH, Wang JX, Yang WY, An ZX, Hu ZX, Lin J, Xiao JZ, Cao HB, Liu PA, Jiang XG, Jiang YY, Wang JP, Zheng H, Zhang H, Bennett PH, Howard BV. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and Diabetes Study. Diabetes Care. 1997;20(4):537–544. doi: 10.2337/diacare.20.4.537. [DOI] [PubMed] [Google Scholar]
- 15.Tuomilehto J, Lindström J, Eriksson JG, Valle TT, Hämäläinen H, Ilanne-Parikka P, Keinänen-Kiukaanniemi S, Laakso M, Louheranta A, Rastas M, Salminen V, Uusitupa M. Finnish Diabetes Prevention Study Group. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344:1343–1350. doi: 10.1056/NEJM200105033441801. [DOI] [PubMed] [Google Scholar]
- 16.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM. Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gilles CL, Abrams KR, Lambert PC, Cooper NJ, Sutton AJ, Hsu RT, Khunti K. Pharmacological and lifestyle interventions to prevent or delay type 2 diabetes in people with impaired glucose tolerance: systematic review and meta-analysis. BMJ. 2007;334(7588):299. doi: 10.1136/bmj.39063.689375.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ackermann RT, Marrero DG. Adapting the Diabetes Prevention Program lifestyle intervention for delivery in the community: the YMCA model. Diabetes Educ. 2007;33(1):1–6. doi: 10.1177/0145721706297743. [DOI] [PubMed] [Google Scholar]
- 19.Ackermann RT, Finch EA, Brizendine E, Zhou H, Marrero DG. Translating the Diabetes Prevention Study into the community. The DEPLOY Pilot Study. Am J Prev Med. 2008;35(4):357–363. doi: 10.1016/j.amepre.2008.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.The National Diabetes education program. Small steps. Big rewards: your gameplan to prevent type 2 diabetes package. Prevent type 2 diabetes. Available from: www.ndep.nih.gov.