Abstract
The objective of this review is to identify and review publications describing the impact of reduced somatosensation on balance. Based on knowledge of the association between specific somatosensory loss and deterioration of balance, conclusions can be made about role of somatosensation in standing balance.
A systematic literature review is presented in which publications from the years 1993 through 2007 were searched in Medline and Embase. Medical Subject Headings (MESH) terms and free text words (related to balance, somatosensory loss, and lower limb) were used to perform the searches. Fifteen articles were selected for detailed review based on predetermined inclusion criteria, and three of the included articles described the effect of experimentally reduced somatosensation on balance in healthy subjects. Ten of the articles described balance in diabetic neuropathy (DN). The last two included articles described balance in Charcot-Marie-Tooth (CMT) disease type 1A (CMT1A) or type 2 (CMT2).
The literature indicates that the tactile sensation is reduced in DN, CMT1A, and CMT2 and when the plantar surface of the feet was hypothermically anesthetized. Joint motion sensation seems to be impaired in patients with DN, and passive joint position sensation appears to be reduced in healthy subjects with anesthesia of ankle and foot from prolonged ischemia. This reduced somatosensation seems to have a negative effect on balance in patients with DN and CMT2; however, this appeared not to be the case in patients with CMT1A and in healthy subjects.
Keywords: balance, healthy, peripheral nervous system disorder, somatosensation
Introduction
To stay in an upright position, it is essential that the central nervous system receives and integrates the position of different body segments and their relation with each other and the surroundings. The maintenance of balance requires the central integration of afferent information1,2 and is highly dependent on vestibular, visual, and somatosensory information.3–5 Healthy persons predominately rely on their somatosensory system when they are in a lightened environment with a solid base of support.4,6 This somatosensory system includes both the tactile and proprioceptive system.5,7
The tactile system is associated with sensations of touch and pressure and more complex sensations like vibration.8 The receptors involved in providing these tactile sensations to the central nervous system are Merkel's cells, Pacinian corpuscles, Meissner's corpuscles, and Ruffini endings.8 As these cutaneous mechanoreceptors can be found in the feet, being the boundary between the body and the ground, they might play an important role in controlling upright stance.9 A change in upright position is often related to a change in pressure under the feet. Different studies tried to confirm this assumption using different experimental designs to influence the tactile afferent information.5,9–12 In these experiments, postural stability decreased by reducing sensibility by cooling the plantar cutaneous mechanoreceptors,5 anesthetizing the receptors,10 or changing the characteristics of the supporting surface on which the subject is standing.12 Also, when vibration is applied to the skin covering the main foot-supporting areas of a standing subject, involuntary whole-body tilt was induced as a reaction to this vibration.9,11 The contribution of plantar cutaneous afferents to balance control is largely evidenced by these protocols. However, the extent of this contribution remains unclear.5,8,9
The proprioceptive system contributes to joint position sense, joint motion sense, and kinesthesia. This includes the sensations of muscle length and tension, joint angles, and changes in these angles.8 The receptors providing the central nervous system with this information are muscle spindles, joint afferents, and Golgi tendon organs.8,13 The proprioceptive receptors in the lower legs or feet are sensitive to ankle rotation and can give information of balance since most postural sway occurs at the ankles.14 The lower leg proprioceptive feedback is considered critical for human automatic balance correcting responses.9,13,15,16 However, this assumption is not supported by a study that has shown that balance-correcting responses can be triggered in subjects whose lower leg proprioceptive feedback has been blocked.17 This effect is created by “nulling” the ankle rotation during translational movements of a support surface.17 This observation suggests that lower leg proprioception is not required for triggering many balance corrections.18 Therefore, the role and importance of lower leg proprioception in balance-correcting responses remains unclear.
When both tactile and proprioceptive information is not conducted to the central nervous system as it is supposed to be, like in neuropathy or after anesthesia, a decline in control of balance may occur, associated with an increased risk of falling.13,15,19 Patients with peripheral nervous system disorders (PNSDs) (e.g., diabetic neuropathy [DN], hereditary motor and sensory neuro-pathies, and nerve compression syndromes) experience several somatosensory deficits such as loss of position, vibration, and tactile sensation.13,16 In this review, older people, which is a large group of people with somatosensory loss, are not included. This group often has many other problems (e.g., motor problems) that can affect balance as well. Therefore, the balance problems cannot be addressed to the loss of somatosensation alone.
Quantifying the somatosensory loss in feet or ankles cannot fully predict dysfunction of the balance system, because function also depends on strategies that individuals use to accomplish stability.4 This, and the contradicting results of different studies on proprioceptive influence of controlling balance,18 leads to the conclusion that the exact relation between PNSDs and controlling upright position remains unclear. Therefore, the purpose of this systematic review is to investigate the impact of reduced somatosensation of the lower leg on standing balance and the relative role of both ankle proprioception and tactile sensation from the plantar side of the feet.
Methods
Search Strategy
Medline and Embase databases were searched for publications from 1993 until the end of 2007 to identify the those concerning the effect of reduced somatosensation on balance. Keywords (MESH terms and free text words) used to perform the searches were “balance” and “posture,” “lower extremity,” “PNSD,” “peripheral nervous system,” “somatosensation,” and “sensory deprivation.” Only Medline and Embase were used, because these two databases contain the most sufficient, high-quality articles, and these databases could be structurally and systematically used. In order to include all important articles, the references of all primarily selected articles were checked. The Medline search is presented in Appendix A as an example.
Selection
Titles and abstracts of the articles identified by these searches were read by two reviewers (Kars and Hijmans) for initial selection. An article was initially selected if it met all the following selection criteria: (1) the study population consisted of patients suffering from PNSD, a subgroup of patients with PNSD was presented separately, or the study population consisted of healthy subjects with experimentally reduced somatosensation; (2) the reduced somatosensation was located in the lower leg; (3) one of the main outcomes of the study was a kinetic or kinematic standing balance measurement; (4) the study used a standardized norm for balance or a healthy control group to which the study population or single case was compared, or in case of healthy subjects, the study had a baseline measurement; (5) the absolute values of the balance measurements were given; and (6) the article was a full report published in English, Dutch, or German.
An article was excluded for initial selection if (1) the study population consisted of patients suffering from central nervous system disorder; (2) patients used orthopedic aids during all measurements; (3) it was a review article; (4) a vibratory stimulation was used to affect somatosensation and induce postural illusions; and (5) perturbations were given during measurements of the standing balance.
Reference lists of the initially selected studies were checked to identify additional published research from 1993 until the end of 2007. These were added to the initially selected papers.
Subsequently, each initially selected paper was scored by the reviewers independently, according to a standardized set of predefined inclusion criteria (Table 1). The criteria were adapted from Downs and Black,20 Dijkstra and colleagues,21 and Hijmans and associates.7 When criteria 1a, 2, 4, 5, and 6, criteria 1b, 3, 5, and 6, or criteria 1c, 3, 5, and 6 were met, the study was included for detailed review. A consensus meeting was held between the two reviewers to discuss discrepancies in assessment. When no agreement could be reached the assessment of a third referee (Zijlstra) would be binding.
Table 1.
Standardized Set of Predefined Criteria
| Criteria | |
|---|---|
| 1. Study populationa | |
| 1a. Healthy people with experimentally reduced somatosensation | Yes / No |
| 1b. People with PNSD | Yes / No |
| 1c. Case study of a patient with PNSD | Yes / No |
| 2. Study design healthy subjects | |
| 2a. Prospective study design | Yes / No |
| 2b. Observational study with baseline measurement (T0), an intervention, and a measurement after intervention (T1) | Yes / No |
| 2c. Results of T0 and T1 published | Yes / No |
| 3. Study design subjects with PNSD | |
| 3a. Comparison with control group or standardized norm | Yes / No |
| 4. Intervention | |
| 4a. Description of intervention | Yes / No |
| 4b. The intervention involves experimentally reduced somatosensation | Yes / No |
| 5. Outcome measures | |
| 5a. Values of somatosensation measures published | Yes / No |
| 5b. Balance measurement is main or one of the three main outcomes | Yes / No |
| 5c. Balance measurement is a standing balance measurement | Yes / No |
| 5d. Values of balance measures published | Yes / No |
| 6. Statistical tests | |
| 6a. Descriptive statistics / case definition published | Yes / No |
If 1a was met, criteria 2, 4, 5, and 6 had to be met. If 1b or 1c were met, criteria 3, 5, and 6 had to be met.
Results
Initially, the search resulted in 594 articles (Medline 351 hits and Embase 243 hits). Due to the use of different databases, duplicate articles were found. In total, 489 articles were identified. A flow chart of the article selection is presented in Figure 1. Based on title and abstract, 453 articles were excluded. Three articles19,22,23 were added after examining the references of the 36 selected articles, resulting in 39 articles1,2,5,10,15,16,18,19,22–52 to be assessed for detailed review.
Figure 1.
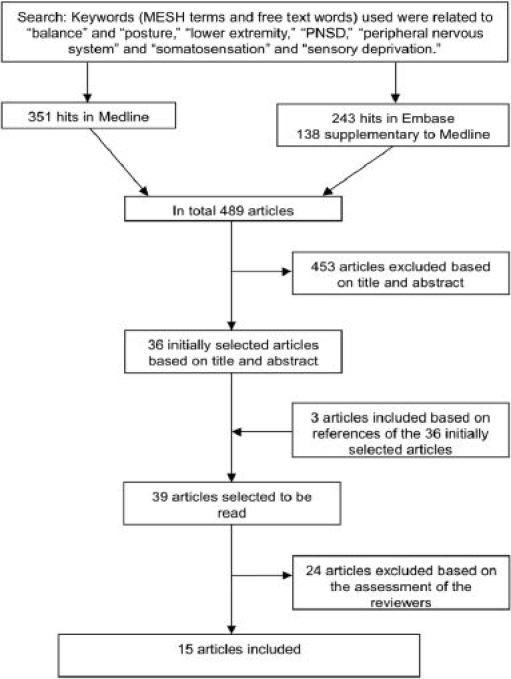
Flow chart of the article selection.
Before the consensus meeting, a disagreement originated from misreading sentences or misunderstanding the terminology used in the articles, between the two reviewers, about 14 articles. However, after the consensus meeting, all disagreements were resolved. Based on the assessment of the reviewers, 15 articles were included for detailed review.5,19,23–26,32,34–36,41,42,46,47,50 Thirteen of the 39 initially selected articles were excluded because no measures of somatosensation were presented.1,22,27–30,38,40,43–45,48,51Six of the selected articles10,18,31,33,49,52 were excluded because the balance measurements did not refer to standing balance. Two articles2,16 were excluded because there was no comparison with a control group or a standardized norm. Another two articles15,37 were excluded because their results were not published as absolute values. Finally, one article39 was excluded based on a lack of publishing the results of the baseline measurement and the measurement after intervention. In total, 24 articles were excluded from detailed review.1,2,10,15,16,18,22,27–31,33,37–40,43–45,48,49,51,52
Ten of the articles19,23–26,34,36,46,47,50 described the effect of DN on balance. Two included articles41,42 described the effect of Charcot-Marie-Tooth disease (CMT) type 1A (CMT1A) or type 2 (CMT2) on balance. The last three of the 15 included articles5,32,35 described the effect of experimentally reduced somatosensation in healthy subjects. An outline of the measurements of somatosensation of the included articles is presented in Table 2. In Table 3, the results of the measurements of balance are presented. Because the studies used different outcome measures and different units, differences between study and control groups, norm, and baseline measurement (T0) on vibration perception threshold (VPT) (Table 2) and the different balance measurements (Table 3) are given in percentages.
Table 2.
Outline of the Somatosensation Measurements of the Included Papers (n = 15) and the Difference of VPT between Study and Control Groups
| First author (year) | Type of study | Subjects | Measurements of somatosensation method (location) | Results | Differences in VPT scores | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group | N | Age in years (mean ± SD) | Control | Study | ||||||||
| Group | Mean ± SD | Group | Mean ± SD | Group versus group | % | |||||||
| PNSD patients | ||||||||||||
| Bergin (1995)34 | CS | (C) | 32 | (42 ± 18) | VPT: neurothesiometer, 100 Hz (V) (medial malleoli) | (C) | 12.56a | (DN) | 29.65a | (C)-(DN) | 263 | |
| (DN) | 25 | (55 ± 14) | VPT: audiometer, 250 Hz (dB)(medial malleoli) | (C) | 36.00a | (DN) | 55.30a | |||||
| VPT: tuning fork, 64 Hz (arbitrary units)(medial malleoli) | (C) | 7.90a | (DN) | 4.65a | ||||||||
| VPT: neurothesiometer, 100 Hz (V)(tibial tuberosities) | (C) | 14.10a | (DN) | 27.15a | (C)-(DN) | 193 | ||||||
| VPT: audiometer, 250 Hz (dB)(tibial tuberosities) | (C) | 35.05a | (DN) | 52.85a | ||||||||
| VPT: tuning fork, 64 Hz (arbitrary units)(tibial tuberosities) | (C) | 7.85a | (DN) | 5.05a | ||||||||
| Boucher (1995)25 | CSR | (C) | 7 | (60.6 ± 5.6) | Valk scoreb | (C) | 0 | (DN) | 12.0 ± 7.4 | |||
| (DN) | 12 | (62.5 ± 7.4) | ||||||||||
| Corriveau (2000)26 | CS | (C) | 15 | (69.3 ± 5.1) | Valk scoreb | (C) | 0.4 ± 0.8 | (DN) | 15 ± 8.2 | |||
| (DN) | 15 | (68.6 ± 5.5) | VPT: vibrometer, 120 Hz (μm)(halluces) | (C) | 1 ± 0.53 | (DN) | 26.8 ± 35 | (C)-(DN) | 2680 | |||
| TPST: SW monofilaments (% > 3.84)(halluces) | (C) | 20.0 | (DN) | 87.7 | ||||||||
| Katoulis (1996)34 | CS | (C) | 20 | (50.6 ± 8.6) | NDSc | (C) | 0 | (DN-NU) | 7.5 ± 6.8 | (C)-(DN-NU) | 271 | |
| (DC) | 20 | (47.6 ± 10.7) | (DC) | 0 | (DN-U) | 8 ± 7.9 | (C)-(DN-U) | 311 | ||||
| (DC)-(DN-NU) | 265 | |||||||||||
| (DN-NU) | 20 | (52.9 ± 8.8) | VPT: biothesiometer, max 50 V (V)(halluces) | (C) | 11.8 ± 3.6 | (DN-NU) | 32 ± 5.6 | (DC)-(DN-U) | 303 | |||
| (DN-U) | 20 | (54.1 ± 7.1) | (DC) | 12.1 ± 4.6 | (DN-U) | 36.7 ± 8.3 | ||||||
| Lafond (2004)36 | CS | (C) | 20 | (72.3 ± 5.8) | Valk scoreb | (C) | 0.3 ± 0.7 | (DN) | 12.6 ± 7.0 | |||
| (DN) | 11 | (69.1 ± 5.1) | VPT (μm) | (C) | 1.0 ± 0.6 | (DN) | 20.3 ± 30.2 | (C)-(DN) | 2032 | |||
| TPST: SW monofilaments (% > 3.84) | (C) | 15 | (DN) | 82 | ||||||||
| Nardone (2000)41 | CS | (C) | 46 | (44.1 ± 9.7) | Neurological disability scored | (CMT1A) | 27.3 | |||||
| (CMT1A) | 15 | (40.3 ± 16.6) | ||||||||||
| Nardone (2006)42 | CS | (C) | 20 | 29-77 | Neurological disability scored | (DN) | 24.1 ± 14.8 | |||||
| (DN) | 14 | 43-77 | (CMT1A) | 31.4 ± 14.9 | ||||||||
| (CMT1A) | 5 | 32-63 | (CMT2) | 27.4 ± 18.6 | ||||||||
| (CMT2) | 8 | 18-61 | ||||||||||
| Richerson (2007)46 | CS | (C) | 11 | (72.92 ± 5.21) | VPT: Medoc vibrometer (V)(halluces and third metatarsal dominant foot)(before Tai Chi training) | (C) | 11.4 ± 8.3 | (DNmod) | 39.4 ± 17.9 | (C)-(DNmod) | 346 | |
| (DNmod) | 11 | (72.57 ± 5.38) | (DNsev) | 114.3 ± 17.5 | (C)-(DNsev) | 1003 | ||||||
| (DNsev) | (74.50 ± 7.72) | |||||||||||
| Rogers (2001)47e | CS | (YC) | 8 | (26.9) | VPT: vibrator, 200 Hz (μm)(tibial tuberosities) | (YC) | 5.45 | (DN) | 33.63 | (YC)-(DN) | 617 | |
| (E-NF) | 15 | (74.7 ± 4.5) | (E-NF) | 33.63 | (E-F) | 43.63 | (E-NF)-(DN) | 100 | ||||
| (DN) | 14 | (60.0) | TPST: SW monofilaments (mN)(lateral malleoli) | (YC) | 0.92 | (DN) | 2.00 | (E-F)-(DN) | 77 | |||
| (E-F) | 10 | (74.2 ± 2.9) | (E-NF) | 1.39 | (E-F) | 1.43 | ||||||
| TPST: SW monofilaments (mN)(fibula head) | (YC) | 0.50 | (DN) | 1.25 | ||||||||
| (E-NF) | 1.32 | (E-F) | 1.36 | |||||||||
| Simoneau (1994)19 | CSR | (C) | 17 | (54.7 ± 8.5) | VPT: vibrometer, 60 Hz and max 50 V (V)(halluces, plantar surface) | (C) | 11.8 ± 4.7 | (DN) | 47.4 ± 3.3 | (C)-(DN) | 402 | |
| (DC) | 17 | (54.2 ± 8.1) | (DC) | 13.9 ± 6.4 | (DC)-(DN) | 341 | ||||||
| (DN) | 17 | (55.0 ± 7.9) | TPST: SW monafilaments (SW ratingf)(halluces, plantar surface) | (C) | 2.9 ± 0.5 | (DN) | 4.6 ± 1.4 | |||||
| (DC) | 3.3 ± 0.5 | |||||||||||
| JMPT: Two individually movable foot plates (degrees)(ankle) | (C) | 1.8 ± 1.4 | (DN) | 3.8 ± 3.6 | ||||||||
| (DC) | 1.5 ± 1.0 | |||||||||||
| Uccioli (1995)23 | CS | (C) | 21 | (31 ± 0.9) | VPT: biothesiometer (V)(lateral malleoli) | (C) | 10.3 ± 0.6 | (DN) | 23.5 ± 3.6 | (C)-(DN) | 228 | |
| (DC) | 23 | (31 ± 1.1) | (DC) | 9.7 ± 0.4 | (DC)-(DN) | 242 | ||||||
| (DN) | 10 | (35 ± 1.9) | VPT: biothesiometer (V)(hallucis, dorsal surface) | (C) | 9.6 ± 0.3 | (DN) | 29.5 ± 5.0 | (C)-(DN) | 307 | |||
| (DC) | 7.4 ± 0.3 | (DC)-(DN) | 399 | |||||||||
| Uccioli (1997)50 | CS | (C) | 31 | (31.9 ± 0.9) | VPT: biothesiometer (V)(lateral malleoli) | (C) | 9.18 ± 0.12 | (DN) | 28.15 ± 5.41 | (C)-(DN) | 307 | |
| (DC) | 18 | (31.3 ± 1.8) | (DC) | 10.33 ± 0.61 | (DC)-(DN) | 273 | ||||||
| (DN) | 7 | (35.1 ± 3.1) | VPT: biothesiometer (V)(hallucis, dorsal surface) | (C) | 9.01 ± 0.11 | (DN) | 34.82 ± 6.68 | (C)-(DN) | 387 | |||
| (DC) | 7.22 ± 0.45 | (DC)-(DN) | 482 | |||||||||
| Healthy subjects | ||||||||||||
| Hertel (1996)32e | (H) | 16 | (22.6 ± 1.9) | JMPT: isokinetic dynamometer (degrees) with 10° eversion | (H) | 2.1 | (H-H) | 2.4 | ||||
| JMPT: isokinetic dynamometer (degrees) with 20° inversion | (H) | 4.2 | (H-H) | 3.9 | ||||||||
| JMPT: isokinetic dynamometer (degrees) with 30° inversion | (H) | 4.0 | (H-H) | 3.6 | ||||||||
| Konradsen (1993)35 | CS | (HM) | 7 | 27 till 38 | JMPT: active joint positioning (degrees)g | (HM) | 1.7 | (HM-H) | 1.8 | |||
| JMPT: passive joint positioning (degrees)h | (HM) | 1.8 | (HM-H) | 5.4 | ||||||||
| McKeon (2007)5 | CSR | (HM) | 16 | (26.4 ± 6.5) | TpD: anesthesiometer (mm) | (H) | 12.4 ± 2.7 | (H-H) | 16.9 ± 6.3 | |||
| (HW) | 16 | (21.4 ± 2.6) | Pressure algometry: algometer (kg) | (H) | 1.5 ± 0.5 | (H-H) | 2.1 ± 0.7 | |||||
CS, cross-sectional study; CSR, cross-sectional study with randomization of trials; C, control subjects; DN, diabetic neuropathy patients; DC, diabetes control subjects; DN-NU, diabetic neuropathy patients without history of ulceration; DN-U, diabetic neuropathy patients with history of ulceration; DNmod, moderate diabetic neuropathy; DNsev, severe diabetic neuropathy; YC, young control subjects; E-NF, elderly nonfallers; E-F, elderly fallers; H, healthy subjects, men and women; H-H, healthy subjects hypoesthesia; HM, healthy men; HM-H, healthy men hypoesthesia; HW, healthy women; TPST, touch-pressure sensation threshold; SW, Semmes Weinstein; JMPT, joint movement perception threshold; TpD, two-point discrimination; SD, standard deviation.
The measures of left and right were averaged.
The scoring system has four levels of neuropathy: normal, mild, moderate, and severe. It consists of clinical testing of sensory modalities (light touch, vibration, and pain), anatomic level below which light touch sensation is impaired, muscle strength, and ankle jerk. A total score of 0 is graded as no polyneuropathy, 1–9 as mild polyneuropathy, 10–18 as moderate polyneuropathy, and 19–33 as severe polyneuropathy.
The NDS is the product of scoring ankle reflexes plus vibration, pin prick, and temperature (cold tuning fork) sensation at the great toe. The maximum NDS is 10, and scores of 3–5, 6–8, and 9–10 were defined as evidence of mild, moderate, and severe signs, respectively.
The neurological disability score exists of lower limb muscle strength (distal and proximal muscle groups), touch pressure, vibration, joint position, pricking pain, and quadriceps and Achilles tendon reflexes.
The values described in the table are measured of the graphs in the original article.
Rating of measurements with Semmes Weinstein monofilaments ranging form 1.65 to 6.65. The higher the number, the more reduced somatosensation.
The subject inverts the ankle from a neutral position at a speed of approximately 15 /s. The foot was then held by the investigator at one of five positions of inversion (5°, 10°, 15°, 20°, or 25°) for 5 s. The subject moved his foot back to neutral and then attempted to replicate the test position actively. A mean error of active positioning was calculated.
The ankle was passively moved by the investigator to one of five positions of inversion (5°, 10°, 15°, 20°, or 25°). The inversion position was reached in 1 s and was held for 5 s. The ankle was then returned to neutral and then gradually inverted at a speed of 2°/s. The subject was asked to say when he thought that his foot had regained the initial position. The error in reproduction of the initial position was recorded, and the mean error was calculated.
Table 3.
Outline of the Balance Measurements of the Included Papers (n = 15) and the Differences in Balance Measures with Eyes Closed
| First author | Balance measurements/intervention | Results eyes open | Results eyes closed | Differences in balance measures with eyes closed | Author conclusions | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | Study | Control | Study | |||||||||||||
| Group | Mean ± SD | Group | Mean ± SD | Group | Mean ± SD | Group | Mean ± SD | Group versus group | % | |||||||
| PNSD patients | ||||||||||||||||
| Bergin24 | Displacement of CoP with eyes open and closed, standing on a normal floor and foam for 60 s, with the Romberg coefficienta calculated | A/P CoP displacement normal floor (mm/min) | In all conditions, patients had significantly larger CoP displacements than control subjects, and their VPT values were well correlated with this CoP displacement | |||||||||||||
| (C) | 145.2 ± 43.4 | (DN) | 497.2 ± 678.7 | (C) | 280.4 ± 156.5 | (DN) | 1150.3 ± 1179.5 | (DN)-(C) | 410 | |||||||
| Romberg coefficienta normal floor | ||||||||||||||||
| (DN) | 2.7 ± 1.0 | (C) | 1.9 ± 0.6 | |||||||||||||
| A/P CoP displacement foam floor (mm/min) | ||||||||||||||||
| (C) | 178.1 ± 66.7 | (DN) | 468.7 ± 576.5 | (C) | 356.8 ± 128.9 | (DN) | 1118.7 ± 790.7 | (DN)-(C) | 314 | |||||||
| Romberg coefficienta foam floor | ||||||||||||||||
| (DN) | 3.0 ± 1.2 | (C) | 2.1 ± 0.6 | |||||||||||||
| Boucher25 | Displacement of CoP during stance with feet together, eyes open and closed, and during a recovery interval (initial eyes were closed, during the trial the eyes were opened for 10 s) | A/P CoP displacement (mm) during 5 s (averaged by original authors) | The postural instability increased linearly with the severity of the neuropathy | |||||||||||||
| (DN) | 21.0 | (C) | 12.4 | |||||||||||||
| (DNmild) | 15.5 | |||||||||||||||
| M/L CoP displacement (mm) during 5 s (averaged by original authors) | ||||||||||||||||
| (DN) | 20.1 | (C) | 13.5 | |||||||||||||
| (DNmild) | 17.0 | |||||||||||||||
| Scalar range of CoP displacement (mm) during 5 s (averaged by original authors) | ||||||||||||||||
| (DN) | 18.8 | (C) | 12.0 | |||||||||||||
| (DNmild) | 15.1 | |||||||||||||||
| Velocity of CoP (mm/s) (averaged by original authors) | ||||||||||||||||
| (DN) | 22.0 | (C) | 14.8 | |||||||||||||
| (DNmild) | 18.2 | |||||||||||||||
| Corriveau26 | Displacement of CoP-CoM when standing for 120 s on two adjacent force plates with shoes and eyes open and closed; three optotrack sensors recorded marker displacement (14 segment mode) | RMS of A/P CoP-CoM displacement (cm) | In all conditions, patients had significantly larger CoP-CoM displacements than control subjects, this could underscore their reliance on vision to compensate for their somatosensory impairment | |||||||||||||
| (C) | 0.09 ± 0.02 | (DN) | 0.13 ± 0.05 | (C) | 0.13 ± 0.04 | (DN) | 0.20 ± 0.09 | (DN)-(C) | 154 | |||||||
| RMS of M/L CoP-CoM displacement (cm) | ||||||||||||||||
| (C) | 0.07 ± 0.01 | (DN) | 0.11 ± 0.04 | (C) | 0.08 ± 0.02 | (DN) | 0.14 ± 0.06 | (DN)-(C) | 175 | |||||||
| Katoulis34 | Displacement of CoP during a Romberg test (calculating the SD of the movement of CoP while the subject is standing) for 30 s | A/P CoP displacement (geometric mean in mm with 95% confidence interval) | A significant adverse effect of patients with DN and with foot ulceration on postural control was shown, while DM itself, without DN, had no effect on postural control | |||||||||||||
| (C) | 4.2 (3.6–4.9) | (DN-NU) | 5.3 (4.6–6.2) | (C) | 5.6 (4.8–6.6) | (DN-NU) | 6.3 (5.5–7.2) | (DN-NU)-(C) | 113 | |||||||
| (DC) | 4.2 (3.7–4.7) | (DN-U) | 6.8 (6.0–7.7) | (DC) | 5.3 (4.5–6.2) | (DN-U) | 9.1 (7.1–11.5) | (DN-NU)-(DC) | 119 | |||||||
| (DN-U)-(C) | 163 | |||||||||||||||
| (DN-U)-(DC) | 172 | |||||||||||||||
| M/L CoP displacement (geometric mean in mm with 95% confidence interval) | ||||||||||||||||
| (C) | 3.1 (2.6–3.6) | (DN-NU) | 3.5 (2.9–4.2) | (C) | 3.4 (2.7–4.2) | (DN-NU) | 4.6 (3.7–5.7) | (DN-NU)-(C) | 135 | |||||||
| (DC) | 3.2 (2.8–3.7) | (DN-U) | 5.3 (4.4–6.3) | (DC) | 3.7 (3.1–4.4) | (DN-U) | 6.6 (5.0–8.8) | (DN-NU)-(DC) | 124 | |||||||
| (DN-U)-(C) | 194 | |||||||||||||||
| (DN-U)-(DC) | 178 | |||||||||||||||
| Lafond36 | Displacement of the CoPnet during standing balance for 120 s, with eyes open or closed on two adjacent force plates | RMS of A/P CoPnet (mm) | The displacement of CoPnet is significantly larger of patients compared to the control, which means that left and right evertor/ invertor motor activities are not as well matched in patients as seen in control subjects | |||||||||||||
| (C) | 3.58 ± 1.02 | (DN) | 4.91 ± 1.56 | (C) | 3.92 ± 1.02 | (DN) | 5.53 ± 1.56 | (DN)-(C) | 141 | |||||||
| RMS of M/L CoPnet (mm) | ||||||||||||||||
| (C) | 1.97 ± 0.53 | (DN) | 2.77 ± 0.97 | (C) | 2.39 ± 0.81 | (DN) | 4.13 ± 3.69 | (DN)-(C) | 173 | |||||||
| Nardone41 | Area of the line joining the average CoF to the instantaneous CoP during stance for 51 s, with eyes open and closed | Surface area with feet 10 cm apart (mm2) | Most CMT1A patients are able to stand upright normally because of the negligible anatomical or functional changes of their spindle group Aβ fibers | |||||||||||||
| (C) | 321.1 ± 256.1 | (CMT-M) | 391.7 ± 230.9 | (C) | 590.2 ± 501.3 | (CMT-M) | 936.2 ± 621.4 | (CMT-M)-(C) | 159 | |||||||
| (CMT-S) | 677.9 ± 300.3 | (CMT-S) | 2185.4 ± 1005.6 | (CMT-S)-(C) | 370 | |||||||||||
| Surface area with feet together (mm2) | ||||||||||||||||
| (C) | 641.5 ± 266.2 | (CMT-M) | 838.7 ± 321.0 | (C) | 1937.0 ± 1050.2 | (CMT-M) | 2722.0 ± 1378.8 | (CMT-M)-(C) | 141 | |||||||
| (CMT-S) | 1181.8 ±441.2 | (CMT-S) | 6883.1 ± 5303.2 | (CMT-S)-(C) | 355 | |||||||||||
| Nardone42b | Area of the line joining the average CoF to the instantaneous CoP, and the mean SD of A-P area of the CoP during stance for 51 s, eyes open and closed | Body sway area (log10 mm2) | While patients with DN and CMT2 are unstable during quiet stance, those with CMT1A are not this due to their relative sparing of group Aβ fibers | |||||||||||||
| (C) | 2.38 | (DN) | 2.80 | (C) | 2.63 | (DN) | 3.18 | (DN)-(C) | 121 | |||||||
| (CMT1A) | 2.45 | (CMT1A) | 2.82 | (CMT1A)-(C) | 107 | |||||||||||
| (DMT2) | 2.75 | (DMT2) | 3.18 | (DMT2)-(C) | 121 | |||||||||||
| Mean SD of A-P sway of the CoP (log10 mm2) | ||||||||||||||||
| (C) | 0.45 | (DN) | 0.65 | (C) | 0.63 | (DN) | 0.85 | (DN)-(C) | 135 | |||||||
| (CMT1A) | 0.42 | (CMT1A) | 0.64 | (CMT1A)-(C) | 102 | |||||||||||
| (DMT2) | 0.63 | (DMT2) | 0.86 | (DMT2)-(C) | 137 | |||||||||||
| Richerson46 | Range of RMS of sway during stance for 30 s on a force platform, with eyes open and eyes closed (only measures before Tai Chi training are reported) | Range of RMS of sway (mm) | No author conclusions associated with reduced somatosensation in relation with decreased balance control | |||||||||||||
| (C) | 2.9 ± 1.9 | (DNmod) | 1.9 ± 0.5 | (C) | 2.9 ± 1.6 | (DNmod) | 2.7 ± 1.3 | (DNmod)-(C) | 93 | |||||||
| (DNsev) | 3.5 ± 1.6 | (DNsev) | 2.4 ± 1.4 | (DNsev)-(C) | 83 | |||||||||||
| Rogers47b | RMS values of ankle rotation were calculated during stance for 40 s, with eyes open and closed with use of an optical displacement device that was targeted at the right tibial tuberosity | RMS of ankle rotation on a normal floor (degrees) | No authors conclusions associated with reduced somatosensation in relation with decreased balance control | |||||||||||||
| (YC) | 0.15 | (DN) | 0.22 | (YC) | 0.22 | (DN) | 0.29 | (DN)-(YC) | 132 | |||||||
| (E-NF) | 0.16 | (E-F) | 0.23 | (E-NF) | 0.23 | (E-F) | 0.49 | (DN)-(E-NF) | 126 | |||||||
| (DN)-(E-F) | 59 | |||||||||||||||
| RMS of ankle rotation on a foam floor (degrees) | ||||||||||||||||
| (YC) | 0.23 | (DN) | 0.39 | (YC) | 0.35 | (DN) | 0.47 | (DN)-(YC) | 134 | |||||||
| (E-NF) | 0.33 | (E-F) | 0.49 | (E-NF) | 0.51 | (E-F) | 0.77 | (DN)-(E-NF) | 92 | |||||||
| (DN)-(E-F) | 61 | |||||||||||||||
| Simoneau19b | Total of CoP displacement during stance for 30 s, with eyes open and closed, head straight and head back | Total CoP excursion with the head straight (cm) | The values of VPT, TPST, and JMPT were significantly higher for the patients compared with control subjects, and they were all equally associated with instability | |||||||||||||
| (C) | 20.0 | (DN) | 33.3 | (C) | 28.3 | (DN) | 50.0 | (DN)-(C) | 177 | |||||||
| (DC) | 22.2 | (DC) | 32.2 | (DN)-(DC) | 155 | |||||||||||
| Total CoP excursion with the head back (cm) | ||||||||||||||||
| (C) | 21.1 | (DN) | 34.4 | (C) | 32.2 | (DN) | 70.0 | (DN)-(C) | 217 | |||||||
| (DC) | 22.2 | (DC) | 35.0 | (DN)-(DC) | 200 | |||||||||||
| Uccioli23b | Total displacement and velocity of CoP during stance for 90 s, with eyes open and eyes closed, and with the Romberg coefficienta calculated | Trace length of CoP (cm) | There is strong evidence that DN patients demonstrate a deficit in their ability to maintain posture even when adequate function of the other sensory organs is present | |||||||||||||
| (C) | 377.78 | (DN) | 555.56 | (C) | 566.67 | (DN) | 1033.33 | (DN)-(C) | 182 | |||||||
| (DC) | 333.33 | (DC) | 477.78 | (DN)-(DC) | 216 | |||||||||||
| Trace surface of CoP (cm2) | ||||||||||||||||
| (C) | 200.00 | (DN) | 486.66 | (C) | 341.67 | (DN) | 766.67 | (DN)-(C) | 224 | |||||||
| (DC) | 200.00 | (DC) | 275.00 | (DN)-(DC) | 279 | |||||||||||
| Mean velocity of CoP (mm/s) | ||||||||||||||||
| (C) | 4.58 | (DN) | 11.67 | (C) | 6.04 | (DN) | 19.79 | (DN)-(C) | 328 | |||||||
| (DC) | 7.50 | (DC) | 10.63 | (DN)-(DC) | 186 | |||||||||||
| Romberg coefficienta | ||||||||||||||||
| (DN) | 2.12 | (C) | 1.91 | |||||||||||||
| (DC) | 1.59 | |||||||||||||||
| Uccioli50 | Total displacement and velocity of the CoP during stance for 90 s, with open and closed eyes | Trace length of CoP (cm) | Patients with DN show decreased somatosensation and postural instability may be fully explained by the presence of DN | |||||||||||||
| (C) | 376.30 ± 17.64 | (DN) | 537.27 ± 133.17 | (C) | 555.40 ± 36.21 | (DN) | 1028.51 ± 244.81 | (DN)-(C) | 185 | |||||||
| (DC) | 335.88 ± 34.81 | (DC) | 481.09 ± 82.52 | (DN)-(DC) | 214 | |||||||||||
| Trace surface of CoP (cm2) | ||||||||||||||||
| (C) | 204.62 ± 27.38 | (DN) | 464.36 ± 113.51 | (C) | 313.19 ± 33.26 | (DN) | 737.30 ± 171.09 | (DN)-(C) | 235 | |||||||
| (DC) | 188.92 ± 26.93 | (DC) | 264.27 ± 45.78 | (DN)-(DC) | 279 | |||||||||||
| Mean velocity of CoP (mm/s) | ||||||||||||||||
| (C) | 7.88 ± 0.19 | (DN) | 14.10 ± 2.57 | (C) | 10.01 ± 0.25 | (DN) | 25.19 ± 3.15 | (DN)-(C) | 252 | |||||||
| (DC) | 8.03 ± 0.85 | (DC) | 10.84 ± 1.34 | (DN)-(DC) | 232 | |||||||||||
| Healthy subjects | ||||||||||||||||
| Hertel32b | Total postural sway (distance traveled away from the mean center of balance) and center of balance during unilateral stance for 10 s, with eyes closed/lateral aspect of the ankle anesthetized | Postural sway (cm) | Lateral ankle joint anesthesia does not appear to alter postural sway or JMPT but does affect the center of balance | |||||||||||||
| (H) | 1.64 | (H-H) | 1.73 | (H-H)-(H) | 105 | |||||||||||
| Center of balance for the x axis (cm) | ||||||||||||||||
| (H) | 0.32 | (H-H) | 0.86 | (H-H)-(H) | 269 | |||||||||||
| Konradsen35 | Displacement of the CoP during single-leg stance for 60 s, with eyes open, a tourniquet inflated to a minimum pressure of 350 mmHg just above the ankle and anesthesia injected into two distal veins of the foot | Mean CoP displacement (mm) | Postural stability was maintained equally well with or without anesthesia of the normal ankle and foot | |||||||||||||
| (HM) | 5.4 | (HM-H) | 5.5 | |||||||||||||
| McKeon5 | Area and velocity of the CoP during double-limb stance for 10 s, with eyes open and closed/10 min of ice immersion of the plantar aspect of the feet | 95% confidence ellipse of the area of CoP (cm2) | The area of CoP excursions may have been reduced to restrict exploratory postural behavior given the decreased sensation from plantar receptors | |||||||||||||
| (H) | 0.81 ± 0.46 | (H-H) | 0.75 ±0.43 | (H) | 1.01 ± 0.78 | (H-H) | 0.74 ± 0.38 | (H-H)-(H) | 73 | |||||||
| Average velocity (cm/s) | ||||||||||||||||
| (H) | 1.12 ± 0.25 | (H-H) | 1.12 ± 0.22 | (H) | 1.28 ±0.31 | (H-H) | 1.21 ± 0.32 | (H-H)-(H) | 95 | |||||||
C, control subjects; DN, diabetic neuropathy patients; DC, diabetes control subjects; DN-NU, diabetic neuropathy patients without history of ulceration; DN-U, diabetic neuropathy patients with history of ulceration; DNmild, mild diabetic neuropathy; DNmod, moderate diabetic neuropathy; DNsev, severe diabetic neuropathy; YC, young control subjects; NF, nonfallers; F, fallers; E-F, elderly fallers; H, healthy subjects, men and women; H-H, healthy subjects hypoesthesia; HM, healthy men; HM-H, healthy men hypoesthesia; HW, healthy women; TPST, touch-pressure sensation threshold; JMPT, joint movement perception threshold; SD, standard deviation; A/P, anterior/posterior, M/L, medial/lateral; RMS, root mean square; CoP, center of pressure; CoM, center of motion; CoP-CoM, the scalar distance at a given time between CoP and CoM; CoF, center of foot pressure.
Sway with eyes open divided by sway with eyes closed.
The values described in the table are measured of the graphs in the original article.
Discussion
The aim of this systematic review is to evaluate the literature concerning the impact of reduced somatosensation of the lower leg on standing balance and its relation with the underlying morphology of the somatosensory impairment. Fifteen articles met the inclusion criteria for detailed review. Three articles described the impact of experimentally reduced somatosensation on balance. The other twelve articles were about the impact of PNSD on balance. Ten of the articles were about the impact of DN on balance, and two of the articles were about the impact of CMT on balance. Based on the morphology of the somatosensory impairment, conclusions can be made about the impact on standing balance of the loss of the specific somatosensory components. However, these conclusions should be regarded with caution, because no randomized, controlled trials were found, the samples sizes were small, and the included studies cannot be merged because of the differences in outcome measures and methodology.
Tactile Sensation
Many different ways to measure the somatosensation were used. Of the 15 included articles, 919,23,24,26,34,36,46,47,50 used the VPT as a measure of somatosensation. Other measurements used were touch pressure sensation threshold,19,26,36,47 two-point discrimination, pressure algometry,5 joint motion perception threshold,19 and active and passive joint position sense.19,32,35 Also, more extensive somatosensation measurements were taken, like the Valk score25,26,36 and the neurological disability score (NDS).34,41,42 This spectrum of somatosensation measurements complicates the presentation of an overall conclusion. However, by analyzing the results of the used outcome measures separately, conclusions can be drawn.
In six articles, VPT, measured with different techniques, was significantly increased in patients with DN compared to controls.19,23,24,26,36,50 The VPT was between 139% and 2680% larger, compared to the healthy. The other three studies using VPT also showed increased values of somatosensation in patients compared to control subjects; however, this was not significant34 or not statistically tested.46,47 The increased VPT of patients with DN demonstrate that, in DN somatosensation, or more precisely, the vibrotactile, sensation is deteriorated. Another outcome measure of the tactile sensation is the touch pressure sensation threshold measured using Semmes Weinstein monofilaments. Corriveau and coworkers26 and Lafond and colleagues36 demonstrated a significantly increased touch pressure sensation threshold in patients with DN.26,36 Rogers and associates47 found differences of the touch sensation at the lateral malleoli, but not on the more proximally located fibula head. The increased touch pressure sensation threshold supports the finding of increased VPT.
Somatosensation of patients with CMT1A and CMT2 was measured using the NDS.41,42 The various items of the NDS were significantly different between CMT1A and control subjects; however, NDS scores were presented only for the patients and not for the control group.41 In the second included article of Nardone and coworkers,42 patients with CMT1A appeared to have the most severe neuropathy followed by CMT2 and DN, with DN causing the least impairment. The increased NDS demonstrates that the somatosensation of patients with CMT1A and CMT2 is decreased. It should be mentioned that NDS not only measures tactile sensation, but also proprioceptive sensation.
Vibration perception threshold, touch pressure sensation threshold, and NDS were used for measuring tactile sensation of the different patient groups. Two-point discrimination and pressure algometry were used to measure tactile sensation in healthy subjects.5 Tactile sensation in healthy subjects was impaired when the feet were hypothermically anesthetized. This was only measured in one article,5 the other two studies32,35 in healthy subjects only measured proprioceptive sensation. This means that only a preliminary conclusion can be drawn about decreased tactile sensation caused by hypothermically anesthetising the plantar surface of the feet of healthy subjects.
Proprioception
Proprioceptive sensation was tested in three different studies.19,32,35 One study was in patients with DN,19 and the other two studies were about experimentally reduced somatosensation in healthy subjects.32,35 Patients with DN had significantly increased passive joint motion perception threshold compared to the controls.19 In healthy subjects who received an anesthetic injection at the lateral aspect of the ankle, passive joint position sense was not affected.32In contrast, when a tourniquet was applied just above the ankle, inducing a local anesthesia from prolonged ischemia, passive joint position sense was reduced.35 However, active joint position sense was not affected.35
The included articles showed that DN, CMT1A, CMT2, and cooling the plantar surface of the foot of healthy subjects negatively affected tactile sensation.19,23–26,34,36,41,42,46,47,50Proprioceptive sensation is also deteriorated in DN and by local ischemic anesthesia of the ankle and foot in healthy subjects.19,35
Balance
Nine of the 10 studies demonstrated that patients with DN have a poor postural control during quiet stance with eyes open and with eyes closed compared to healthy individuals.19,23–26,34,36,47,50 The 10th study is not further discussed because of contradiction between their results and discussion.46 Patients with DN showed an increased area of center of pressure (CoP),19,23,25,50 velocity of CoP,23,25,50 CoP trace length,23–25,34,50 ankle rotation,47 root mean square values of the CoP–CoM variable [the scalar distance at a given time between CoP and center of motion (CoM)],26 and values of CoPnet, which is the weighted sum of the time-varying position of the CoP from two force plates.36 When the eyes were closed, the percentages of differences between DN and healthy controls even ran up to 410%.
The two studies about the patients with CMT demonstrated no increase in sway area in patients with CMT1A compared to control subjects, when the eyes were both opened and closed.41,42 However, when the CMT1A group was subdivided based on severity of the disease, sway area of the less severely affected patients was not different from the controls, while sway area of the more severely affected patients was significantly increased.41 The second article about CMT demonstrated significantly increased sway area of CMT2 compared to CMT1A.42 Patients with CMT2 had a sway area similar to the sway area of patients with DN, whereas the patients with CMT1A had a sway area comparable with those of healthy control.42 It should be noticed that, in both articles, the sway area was measured during single-limb stance.41,42 The single-limb stance is more challenging to the postural control system than double-limb stance measured in the other included articles. This means that the increased body sway area of patients with CMT1A (107%) and CMT2 (121%) found during single-limb stance is not comparable with the increased body sway area found using double-limb stance in patients with DN.
The differences in CoP-related outcomes were not distinct between control measurements and experimental reduced somatosensation measurements of healthy subjects.5,32,35 In two of the studies,32,35 postural stability was maintained equally well with or without anesthesia of lateral aspect of the ankle or local ischemic anesthesia of the ankle and foot. In the third study,5 the plantar surface of the feet was cooled with ice for 10 min. The effect of this intervention was a decreased CoP area compared to the control measurements, which is usually associated with improved balance.5 The contradicting results found after experimentally reduced somatosensation imply that no statement can be made about the effect of this experimentally reduced somatosensation on balance.
The increase in body sway of patients with DN or CMT2 during the eyes-closed condition compared to the healthy control groups under the same condition could emphasize their reliance on vision to compensate for their somatosensory impairment. It is striking, however, that, even during eyes-open conditions, the patients with DN or CMT2 showed increased body sway, demonstrating a poorer balance performance than the healthy control.19,23–26,34,36,41,42,47,50 The finding that the patients with DN and CMT2 differed from control subjects in all sway testing conditions indicates that vision cannot fully compensate for the reduced somatosensation.24–26,42
Somatosensation and Balance
An important relationship between the severity of the neuropathy and postural stability is found. Somatosensation in patients with DN correlated well with body sway.9,24–26,47 Postural instability increased linearly with the severity of the neuropathy.25 However, no significant relationship was found between postural stability and the NDS of CMT.41,42 This could be the consequence of the differences between peripheral nerve fibers that are affected by DN, CMT1A and CMT2.
A morphologic study53 has demonstrated that CMT1A features a loss of large sensory nerve fibers, whereas smaller caliber fibers are less affected. The large sensory nerve fibers, or the Aα fibers, are mostly responsible for the innervation of primary muscle spindles and the Golgi tendon organs,54 which are part of the proprioceptive system.8 Smaller fibers, the group of Aβ fibers, are responsible for the innervation of the cutaneous receptors54 (e.g., Merkel's cells, Pacinian corpuscles, Meissner's corpuscles, and Ruffini endings in the skin).8,54 However, muscle spindles, Ruffini's joint receptors, and Pacinian joint receptors are also innervated by Aβ,54 which means that the Aβ fibers are both responsible for tactile and proprioceptive sensation.
Nardone and colleagues41,42 confirmed the findings of Dyck and associates,53 stating that CMT1A is featured by a complete functional loss of Aα fibers. Patients with CMT1A still showed good postural stability, which may be due to the fact that the smaller Aβ were relatively spared by the disease.41,42 The vibrotactile sensitivity, however, innervated by Aβ fibers, was impaired in CMT1A.41 It seems plausible, therefore, that the Meissner's and Pacinian corpuscles (fast-adapting receptors), both responsible for the vibrotactile sensation,54 are less involved in the control of standing balance than other cutaneous receptors. This is a reasonable argument; given the velocity sensitivity of the receptors, one would predict a larger role of the slow-adapting receptors (e.g., Merkel's cells and Ruffini endings), where very slow movements are concerned, as during maintenance of quiet stance.41,54 This is in line with a study of Perry and coworkers,55 suggesting that, based on the slow-adapting properties of Merkel's cells and Ruffini endings, they play a key role in quiet stance.
When both Aα and Aβ fibers were affected, as seen in CMT242 and DN,19,23–26,34,36,47,50 patients were unstable. This postural imbalance could be ascribed to the decreased function of Aβ fibers.42 When there is only a loss of Aα fibers (CMT1A) the standing balance is maintained.41,42 The Aβ fibers seem to conduct more important information for stance control than Aα fibers.
Reducing proprioception due to experimentally impairing specific proprioceptive receptors may suggest which of the receptors, innervated by Aβ fibers, are responsible for maintaining balance. When the anterior talofibular ligament of the ankle is anesthetized, proprioceptive sensation of the ankle is thought to be decreased.32 However, this specific anesthesia did not affect joint position sense in the study of Hertel and colleagues,32 suggesting that the joint position sense was maintained by the other tissues in the ankle (e.g., muscles and tendons). This suggestion of maintained joint position sense might explain the preserved standing balance found in the same study. The intervention of anesthetizing the anterior talofibular ligament was not sufficient to disturb the proprioceptive system and the standing balance.32
The two other studies about experimentally reducing proprioceptive and tactile sensation35 or the tactile sensation alone5 could not provide any insight in the discussion of which type of receptors are responsible for the control of standing balance. Konradsen et al.35 tested the effect of reducing both the proprioceptive and the tactile sensation. Therefore, no clear differentiation between these two parts of somatosensation can be made. McKeon and Hertel5 found a decrease in CoP excursion after cooling the plantar receptors. The authors explained this unexpected finding as a defending mechanism to maintain postural control by limiting the CoP excursions toward the boundaries of the base of support.5 However, the question arises if this is a plausible explanation based on the fact that in 11 out of the 15 articles included in this review found an increase in CoP-related outcomes when the tactile sensation was deteriorated by a disease.19,23–26,34,36,41,42,47,50
This review has demonstrated that the tactile sensation is deteriorated in DN, CMT1A, and CMT2 and when the plantar surfaces of the feet were cooled with ice. Proprioception was less thoroughly investigated compared to the tactile sensation. No conclusion could be drawn about the impact of CMT1A or CMT2 on proprioception. Joint motion perception threshold in patients with DN and measurements of joint position sense in healthy people with local ischemic anesthesia of the ankle and foot was decreased. Joint motion perception threshold and joint position sense are both part of the proprioceptive sensation. Reduced somatosensation had an impairing effect on balance in patients with DN and CMT2, however, not in patients with CMT1A. This may be due to the nerve fibers affected by the diseases. The Aβ fibers conduct more important sensory information for controlling standing balance than Aα fibers. Which receptors are the most involved is not clear. To test the receptors individually, it is important in future research to investigate the involvement step by step. This can be done by reducing the tactile or proprioceptive sensation individually by experimentally reducing the sensation. However, this review showed that experimentally reducing tactile or proprioceptive sensation does not mimic somatosensory loss by DN or CTM. The results from the studies that experimentally reduce somatosensation show different effects compared to patients with DN or CTM. The distinct deviation between tactile and proprioceptive sensation is clearly not present in DN and CMT. Moreover, long-term compensation for the somatosensory loss can explain the differences between patients and healthy people with experimentally reduced somatosensation.
Abbreviations
- CMT
Charcot-Marie-Tooth disease
- CMT1A
Charcot-Marie-Tooth disease type 1A
- CMT2
Charcot-Marie-Tooth disease type 2
- CoM
center of motion
- CoP
center of pressure
- DN
diabetic neuropathy
- MESH
Medical Subject Headings
- NDS
neurological disability score
- PNSD
peripheral nervous system disorders
- VPT
vibration perception threshold
Appendix A
-
Explode “Posture”/ all subheadings
The thesaurus term is exploded with
Head-down tilt
Prone position
Supine position
“Musculoskeletal-Equilibrium”/ all subheadings
Balance*
Posture*
1 or 2 or 3 or 4
-
Explode “Lower-Extremity”/ all subheadings
The thesaurus term is exploded with
Buttocks
Foot
Hip
Knee
Leg
Thigh
-
Explode “Foot”/ all subheadings
The thesaurus term is exploded with
Ankle
Forefoot, human
Heel
Foot*
Feet
Lower extremity*
Lower limb*
Leg*
8 or 9 or 10 or 11 or 12
(13 in ti) or (13 in mime) or (13 in mjme) or (13 in ab)
6 or 7 or 14
-
Explode “Peripheral-Nervous-System-Diseases”/ all subheadings
The thesaurus term is exploded with
Acrodynia
Amyloid neuropathies
Brachial plexus neuropathies
Complex regional pain syndromes
Diabetic neuropathies
Guillain-Barre syndrome
Hand-arm vibration syndrome
Isaacs syndrome
Mononeuropathies
Nerve compression syndromes
Neuralgia
Neuritis
Neurofibromatosis
Pain insensitivity, congenital
Peripheral nervous system neoplasms
Polyneuropathies
Radiculopathy
Tarlov cysts
Neuropathy
16 or 17
5 and 15 and 18
-
Explode “Peripheral-Nervous-System”/ all subheadings
The thesaurus term is exploded with
Autonomic nervous system
Ganglia, sensory
Nerve endings
Peripheral nerves
-
Explode “Mechanoreceptors”/ all subheadings
The thesaurus term is exploded with
Golgi-Mazzoni corpuscles
Merkel cells
Muscle spindles
Neuroepithelial cells
Pacinian corpuscles
Pressorreceptors
Pulmonary stretch receptors
-
Explode “Proprioception”/ all subheadings
The thesaurus term is exploded with
Kinesthesis
Musculoskeletal equilibrium
-
Explode “Afferent-Pathways”/ all subheadings
The thesaurus term is exploded with
Auditory pathways
Olfactory pathways
Spinocerebellar tracts
Spinothalamic tracts
Visceral afferents
Visual pathways
Somatosen*
20 or 21 or 22 or 23 or 24
-
Explode “Anesthesia”/ all subheadings
The thesaurus term is exploded with
Anesthesia, conduction
Anesthesia, dental
Anesthesia, general
Anesthesia, intratracheal
Anesthesia, intravenous
Anesthesia, obstetrical
Cryoanesthesia
Electroacupuncture
Hypnosis, anesthetic
“Sensory-Deprivation”/ all subheadings
“Vibration”/ all subheadings
Cool*
26 or 27 or 28 or 29
5 and 15 and 25 and 30
19 or 31
(English in la) or (Dutch in la) or (German in la)
32 and 33
References
- 1.Ducic I, Short KW, Dellon AL. Relationship between loss of pedal sensibility, balance, and falls in patients with peripheral neuropathy. Ann Plast Surg. 2004;52(6):535–540. doi: 10.1097/01.sap.0000122654.65588.f0. [DOI] [PubMed] [Google Scholar]
- 2.Ducic I, Taylor NS, Dellon AL. Relationship between peripheral nerve decompression and gain of pedal sensibility and balance in patients with peripheral neuropathy. Ann Plast Surg. 2006;56(2):145–150. doi: 10.1097/01.sap.0000194246.18332.23. [DOI] [PubMed] [Google Scholar]
- 3.Jeka J, Oie K, Schöner G, Dijkstra T, Henson E. Position and velocity coupling of postural sway to somatosensory drive. J Neurophysiol. 1998;79(4):1661–1674. doi: 10.1152/jn.1998.79.4.1661. [DOI] [PubMed] [Google Scholar]
- 4.Horak FB. Postural orientation and equilibrium: what do we need to know about neural control of balance to prevent falls? Age Ageing. 2006;35(Suppl 2):ii7–ii11. doi: 10.1093/ageing/afl077. [DOI] [PubMed] [Google Scholar]
- 5.McKeon PO, Hertel J. Diminished plantar cutaneous sensation and postural control. Percept Mot Skills. 2007;104(1):56–66. doi: 10.2466/pms.104.1.56-66. [DOI] [PubMed] [Google Scholar]
- 6.Peterka RJ. Sensorimotor integration in human postural control. J Neurophysiol. 2002;88(3):1097–1118. doi: 10.1152/jn.2002.88.3.1097. [DOI] [PubMed] [Google Scholar]
- 7.Hijmans JM, Geertzen JH, Dijkstra PU, Postema K. A systematic review of the effects of shoes and other ankle or foot appliances on balance in older people and people with peripheral nervous system disorders. Gait Posture. 2007;25(2):316–323. doi: 10.1016/j.gaitpost.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 8.Bray JJ, Cragg PA, Macknight ADC, Mills RG. Waite PME. Human physiology. 4th ed. Oxford: Blackwell Science; 1999. Sensory system; pp. 128–143. [Google Scholar]
- 9.Kavounoudias A, Roll R, Roll JP. The plantar sole is a ‘dynamometric map’ for human balance control. Neuroreport. 1998;9(14):3247–3252. doi: 10.1097/00001756-199810050-00021. [DOI] [PubMed] [Google Scholar]
- 10.Meyer PF, Oddsson LI, De Luca CJ. The role of plantar cutaneous sensation in unperturbed stance. Exp Brain Res. 2004;156(4):505–512. doi: 10.1007/s00221-003-1804-y. [DOI] [PubMed] [Google Scholar]
- 11.Kavounoudias A, Roll R, Roll JP. Foot sole and ankle muscle inputs contribute jointly to human erect posture regulation. J Physiol. 2001;532(Pt 3):869–878. doi: 10.1111/j.1469-7793.2001.0869e.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu G, Chiang JH. The significance of somatosensory stimulations to the human foot in the control of postural reflexes. Exp Brain Res. 1997;114(1):163–169. doi: 10.1007/pl00005616. [DOI] [PubMed] [Google Scholar]
- 13.Van Deursen RW, Simoneau GG. Foot and ankle sensory neuropathy, proprioception, and postural stability. J Orthop Sports Phys Ther. 1999;29(12):718–726. doi: 10.2519/jospt.1999.29.12.718. [DOI] [PubMed] [Google Scholar]
- 14.Fitzpatrick R, McCloskey DI. Proprioceptive, visual and vestibular thresholds for the perception of sway during standing in humans. J Physiol. 1994;478(Pt 1):173–186. doi: 10.1113/jphysiol.1994.sp020240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Simmons RW, Richardson C, Pozos R. Postural stability of diabetic patients with and without cutaneous sensory deficit in the foot. Diabetes Res Clin Pract. 1997;36(3):153–160. doi: 10.1016/s0168-8227(97)00044-2. [DOI] [PubMed] [Google Scholar]
- 16.Rao N, Aruin AS. Automatic postural responses in individuals with peripheral neuropathy and ankle-foot orthoses. Diabetes Res Clin Pract. 2006;74(1):48–56. doi: 10.1016/j.diabres.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 17.Bloem BR, Allum JH, Carpenter MG, Honegger F. Is lower leg proprioception essential for triggering human automatic postural responses? Exp Brain Res. 2000;130(3):375–391. doi: 10.1007/s002219900259. [DOI] [PubMed] [Google Scholar]
- 18.Bloem BR, Allum JH, Carpenter MG, Verschuuren JJ, Honegger F. Triggering of balance corrections and compensatory strategies in a patient with total leg proprioceptive loss. Exp Brain Res. 2002;142(1):91–107. doi: 10.1007/s00221-001-0926-3. [DOI] [PubMed] [Google Scholar]
- 19.Simoneau GG, Ulbrecht JS, Derr JA, Becker MB, Cavanagh PR. Postural instability in patients with diabetic sensory neuropathy. Diabetes Care. 1994;17(12):1411–1421. doi: 10.2337/diacare.17.12.1411. [DOI] [PubMed] [Google Scholar]
- 20.Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. 1998;52(6):377–384. doi: 10.1136/jech.52.6.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dijkstra PU, Kalk WW, Roodenburg JL. Trismus in head and neck oncology: a systematic review. Oral Oncol. 2004;40(9):879–889. doi: 10.1016/j.oraloncology.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 22.Giacomini PG, Bruno E, Monticone G, Di Girolamo S, Magrini A, Parisi L, Menzinger G, Uccioli L. Postural rearrangement in IDDM patients with peripheral neuropathy. Diabetes Care. 1996;19(4):372–374. doi: 10.2337/diacare.19.4.372. [DOI] [PubMed] [Google Scholar]
- 23.Uccioli L, Giacomini PG, Monticone G, Magrini A, Durola L, Bruno E, Parisi L, Di Girolamo S, Menzinger G. Body sway in diabetic neuropathy. Diabetes Care. 1995;18(3):339–344. doi: 10.2337/diacare.18.3.339. [DOI] [PubMed] [Google Scholar]
- 24.Bergin PS, Bronstein AM, Murray NM, Sancovic S, Zeppenfeld DK. Body sway and vibration perception thresholds in normal aging and in patients with polyneuropathy. J Neurol Neurosurg Psychiatry. 1995;58(3):335–340. doi: 10.1136/jnnp.58.3.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boucher P, Teasdale N, Courtemanche R, Bard C, Fleury M. Postural stability in diabetic polyneuropathy. Diabetes Care. 1995;18(5):638–645. doi: 10.2337/diacare.18.5.638. [DOI] [PubMed] [Google Scholar]
- 26.Corriveau H, Prince F, Hébert R, Raîche M, Tessier D, Maheux P, Ardilouze JL. Evaluation of postural stability in elderly with diabetic neuropathy. Diabetes Care. 2000;23(8):1187–1191. doi: 10.2337/diacare.23.8.1187. [DOI] [PubMed] [Google Scholar]
- 27.Dewhurst S, Riches PE, De Vito G. Moderate alterations in lower limbs muscle temperature do not affect postural stability during quiet standing in both young and older women. J Electromyogr Kinesiol. 2007;17(3):292–298. doi: 10.1016/j.jelekin.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 28.Dickstein R, Shupert CL, Horak FB. Fingertip touch improves postural stability in patients with peripheral neuropathy. Gait Posture. 2001;14(3):238–247. doi: 10.1016/s0966-6362(01)00161-8. [DOI] [PubMed] [Google Scholar]
- 29.Dickstein R, Peterka RJ, Horak FB. Effects of light fingertip touch on postural responses in subjects with diabetic neuropathy. J Neurol Neurosurg Psychiatry. 2003;74(5):620–626. doi: 10.1136/jnnp.74.5.620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fiolkowski P, Brunt D, Bishop M, Woo R. Does postural instability affect the initiation of human gait? Neurosci Lett. 2002;323(3):167–170. doi: 10.1016/s0304-3940(02)00158-1. [DOI] [PubMed] [Google Scholar]
- 31.Fujiwara K, Asai H, Miyaguchi A, Toyama H, Kunita K, Inoue K. Perceived standing position after reduction of foot-pressure sensation by cooling the sole. Percept Mot Skills. 2003;96(2):381–399. doi: 10.2466/pms.2003.96.2.381. [DOI] [PubMed] [Google Scholar]
- 32.Hertel JN, Guskiewicz KM, Kahler DM, Perrin DH. Effect of lateral ankle joint anesthesia on center of balance, postural sway, and joint position sense. J Sport Rehabil. 1996;5(2):111–119. [Google Scholar]
- 33.Inglis JT, Horak FB, Shupert CL, Jones-Rycewicz C. The importance of somatosensory information in triggering and scaling automatic postural responses in humans. Exp Brain Res. 1994;101(1):159–164. doi: 10.1007/BF00243226. [DOI] [PubMed] [Google Scholar]
- 34.Katoulis EC, Ebdon-Parry M, Hollis S, Harrison AJ, Vileikyte L, Kulkarni J, Boulton AJ. Postural instability in diabetic neuropathic patients at risk of foot ulceration. Diabet Med. 1997;14(4):296–300. doi: 10.1002/(SICI)1096-9136(199704)14:4<296::AID-DIA344>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 35.Konradsen L, Ravn JB, Sørensen AI. Proprioception at the ankle: the effect of anaesthetic blockade of ligament receptors. J Bone Joint Surg Br. 1993;75(3):433–436. doi: 10.1302/0301-620X.75B3.8496215. [DOI] [PubMed] [Google Scholar]
- 36.Lafond D, Corriveau H, Prince F. Postural control mechanisms during quiet standing in patients with diabetic sensory neuropathy. Diabetes Care. 2004;27(1):173–178. doi: 10.2337/diacare.27.1.173. [DOI] [PubMed] [Google Scholar]
- 37.Lin SI, Lin RM. Sensorimotor and balance function in older adults with lumbar nerve root compression. Clin Orthop Relat Res. 2002;(394):146–153. doi: 10.1097/00003086-200201000-00017. [DOI] [PubMed] [Google Scholar]
- 38.Menz HB, Lord SR, Fitzpatrick RC. A tactile stimulus applied to the leg improves postural stability in young, old and neuropathic subjects. Neurosci Lett. 2006;406(1-2):23–26. doi: 10.1016/j.neulet.2006.07.014. [DOI] [PubMed] [Google Scholar]
- 39.Meyer PF, Oddsson LI, De Luca CJ. Reduced plantar sensitivity alters postural responses to lateral perturbations of balance. Exp Brain Res. 2004;157(4):526–536. doi: 10.1007/s00221-004-1868-3. [DOI] [PubMed] [Google Scholar]
- 40.Muraskin SI, Conrad B, Zheng N, Morey TE, Enneking FK. Falls associated with lower-extremity-nerve blocks: a pilot investigation of mechanisms. Reg Anesth Pain Med. 2007;32(1):67–72. doi: 10.1016/j.rapm.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 41.Nardone A, Tarantola J, Miscio G, Pisano F, Schenone A, Schieppati M. Loss of large-diameter spindle afferent fibres is not detrimental to the control of body sway during upright stance: evidence from neuropathy. Exp Brain Res. 2000;135(2):155–162. doi: 10.1007/s002210000513. [DOI] [PubMed] [Google Scholar]
- 42.Nardone A, Grasso M, Schieppati M. Balance control in peripheral neuropathy: are patients equally unstable under static and dynamic conditions? Gait Posture. 2006;23(3):364–373. doi: 10.1016/j.gaitpost.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 43.Nardone A, Galante M, Pareyson D, Schieppati M. Balance control in Sensory Neuron Disease. Clin Neurophysiol. 2007;118(3):538–550. doi: 10.1016/j.clinph.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 44.Priplata AA, Patritti BL, Niemi JB, Hughes R, Gravelle DC, Lipsitz LA, Veves A, Stein J, Bonato P, Collins JJ. Noise-enhanced balance control in patients with diabetes and patients with stroke. Ann Neurol. 2006;59(1):4–12. doi: 10.1002/ana.20670. [DOI] [PubMed] [Google Scholar]
- 45.Richardson JK, Ashton-Miller JA, Lee SG, Jacobs K. Moderate peripheral neuropathy impairs weight transfer and unipedal balance in the elderly. Arch Phys Med Rehabil. 1996;77(11):1152–1156. doi: 10.1016/s0003-9993(96)90139-2. [DOI] [PubMed] [Google Scholar]
- 46.Richerson S, Rosendale K. Does Tai Chi improve plantar sensory ability? A pilot study. Diabetes Technol Ther. 2007;9(3):276–286. doi: 10.1089/dia.2006.0033. [DOI] [PubMed] [Google Scholar]
- 47.Rogers MW, Wardman DL, Lord SR, Fitzpatrick RC. Passive tactile sensory input improves stability during standing. Exp Brain Res. 2001;136(4):514–522. doi: 10.1007/s002210000615. [DOI] [PubMed] [Google Scholar]
- 48.Schieppati M, Tacchini E, Nardone A, Tarantola J, Corna S. Subjective perception of body sway. J Neurol Neurosurg Psychiatry. 1999;66(3):313–322. doi: 10.1136/jnnp.66.3.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stål F, Fransson PA, Magnusson M, Karlberg M. Effects of hypothermic anesthesia of the feet on vibration-induced body sway and adaptation. J Vestib Res. 2003;13(1):39–52. [PubMed] [Google Scholar]
- 50.Uccioli L, Giacomini PG, Pasqualetti P, Di Girolamo S, Ferrigno P, Monticone G, Bruno E, Boccasena P, Magrini A, Parisi L, Menzinger G, Rossini PM. Contribution of central neuropathy to postural instability in IDDM patients with peripheral neuropathy. Diabetes Care. 1997;20(6):929–934. doi: 10.2337/diacare.20.6.929. [DOI] [PubMed] [Google Scholar]
- 51.Van Geffen JA, Dijkstra PU, Hof AL, Halbertsma JP, Postema K. Effect of flat insoles with different Shore A values on posture stability in diabetic neuropathy. Prosthet Orthot Int. 2007;31(3):228–235. doi: 10.1080/03093640600994557. [DOI] [PubMed] [Google Scholar]
- 52.Wu G. Real-time feedback of body center of gravity for postural training of elderly patients with peripheral neuropathy. IEEE Trans Rehabil Eng. 1997;5(4):399–402. doi: 10.1109/86.650298. [DOI] [PubMed] [Google Scholar]
- 53.Dyck PJ, Chance P, Lebo R, Carney JA. Hereditary motor and sensory neuropathies. In: Dyck PJ, Thomas PK, Griffin JW, editors. Peripheral neuropathy. Philadelphia: Saunders; 1993. pp. 1094–1136. [Google Scholar]
- 54.Shaffer SW, Harrison AL. Aging of the somatosensory system: a translational perspective. Phys Ther. 2007;87(2):193–207. doi: 10.2522/ptj.20060083. [DOI] [PubMed] [Google Scholar]
- 55.Perry SD, McIlroy WE, Maki BE. The role of plantar cutaneous mechanoreceptors in the control of compensatory stepping reactions evoked by unpredictable, multi-directional perturbation. Brain Res. 2000;877(2):401–406. doi: 10.1016/s0006-8993(00)02712-8. [DOI] [PubMed] [Google Scholar]


