Abstract
Introduction
There are several reports from locations in the northern hemisphere of seasonal variation in hemoglobin A1c (HbA1c) levels with higher values noted in the cooler months. The variation has been attributed to holiday seasons, temperature differences, and changes in diet. This article describes the seasonal variation in both hemispheres and in a country on the equator with minimal temperature variation.
Methods
The mean and median HbA1c by month was calculated for a maximum of 2 years for HbA1c data from the different locations: Edmonton and Calgary, Canada; Singapore; Melbourne, Australia; and Marshfield, Wisconsin. The mean monthly temperature for each location was found from available meteorological information.
Results
In both northern and southern hemispheres, the HbA1c was higher in cooler months and lower in the warmer months. In Singapore, where there is minimal temperature variation, there is also minimal variation in HbA1c values over the year. The difference in HbA1c over a year appears to be related to the difference in temperature.
Conclusion
Hemoglobin A1c is higher in cooler months and lower in the warmer months in both hemispheres. In a country with minimal monthly temperature variation, there is only minimal variation in HbA1c values through the year. In all locations, the mean and median HbA1c declined over the study period, possibly due to better glycemic control of patients with diabetes or an increase in use of HbA1c as a screening test for diabetes or a combination of both.
Keywords: hemoglobin A1c, seasonal, variation
Introduction
Hemoglobin A1c (HbA1c) is the primary test for the assessment of glycemic control for patients with both type 1 and type 2 diabetes. Both the American and the Canadian Diabetes Association have advocated a treatment target goal for HbA1c of 7% or lower (as close to the nondiabetes range) in order to minimize micro-vascular and macrovascular complications.1 If it can be safely achieved, a HbA1c target value of ≤6.0% should be considered.
Analytical imprecision performance goals for HbA1c testing have been set by the National Academy of Clinical Biochemistry at ideally less than 3%.2 Optimal testing frequency1 has not been determined, although various expert committees recommend a testing frequency of every 6 months in patients who are meeting treatment goals and more frequently in patients not meeting glycemic goals. Biological variation for HbA1c has been determined to be in the region of 1.7% to 3%.2–4
However, there are several reports of a seasonal variation in HbA1c values dating back to 19855 when Mortensen found lower HbA1c values in June and July compared to other months. Ishii and colleagues6 reported a difference of 0.5% between winter and spring/autumn in type 2 diabetes patients in Japan. This change was attributed to increased caloric intake accompanied by decreased physical activity in winter. Also in Japan, Sohmiya and associates7 found that, in male patients with type 2 diabetes treated with insulin, the HbA1c value was higher in February compared to the nadir in July. They attributed this change to increased insulin resistance. In Taiwan, Chen and coworkers8 attributed changes in HbA1c to the winter holiday season. In Sweden, Aspenlund9 showed lower HbA1c values in July with higher values in autumn and winter. He attributed this variation to possible differences in exercise and diet regimens in summer/winter as well as increased insulin sensitivity in the summer. Also in Sweden, Nordfelt and Ludvigsson10 reported that, in children with type 1 diabetes, there was a seasonal variation with higher values in autumn and winter and lower values in summer and spring. They noted that patients showing larger seasonal variation reported severe hypoglycemia, whereas patients with lower seasonal variation did not show severe hypoglycemia. In the United Kingdom, Carney and colleagues11 found an increase in June as well as the winter months. In the United States, Tseng and associates12 noted a difference of 0.22 between summer and winter HbA1c values, with the highest mean HbA1c values in January/February and the lowest HbA1c values in September/October. They also noted that the differences with colder winters had larger winter/summer variation than those with warmer winters. In contrast, Garde and coworkers13 from Copenhagen, Denmark, noted that, in healthy women, the HbA1c was higher in September and October and lower in December and January.
All these studies were performed in the northern hemisphere in locations with significant differences in temperatures between winter and summer. We wished to investigate if similar differences existed in the southern hemisphere in a location where there is minimal difference between summer and winter temperatures.
Methods and Materials
The mean maximum temperature for each month and location is shown in Table 1 and was found from meteorological data published by the National Meteorological Institutes (or equivalent) of the country of each location.
Table 1.
Mean Monthly Maximum Temperatures (in Degrees Celsius) for Melbourne, Singapore, Calgary, Edmonton, and Marshfield.
| January | February | March | April | May | June | July | August | September | October | November | December | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Melbourne | 26.2 | 26.4 | 24.0 | 20.2 | 16.6 | 13.6 | 13.0 | 14.4 | 16.5 | 19.1 | 21.7 | 24.4 |
| Singapore | 29.9 | 31.0 | 31.4 | 31.7 | 31.6 | 31.2 | 30.8 | 30.8 | 30.7 | 31.1 | 30.5 | 29.6 |
| Edmonton | - 8.2 | - 4.2 | 1.1 | 10.5 | 17.5 | 21.3 | 23.0 | 22.1 | 16.6 | 11.3 | - 0.1 | - 6.3 |
| Calgary | - 3.6 | - 0.5 | 3.3 | 10.6 | 16.4 | 20.6 | 23.2 | 22.7 | 17.4 | 12.6 | 2.9 | - 2.3 |
| Marshfield, Wisconsin | - 5.5 | - 2.8 | - 3.3 | 12.8 | 20.0 | 25.0 | 27.2 | 25.6 | 21.1 | 14.4 | 5.0 | - 3.3 |
Two years of HbA1c data were supplied by the participants. Some participants supplied more than two years of data, so data were selected to give maximum overlap between participants. For Singapore, the data were from January 2006 through December 2007. The Melbourne data period was from October 2003 through September 2005. For Edmonton, the period was from February 2002 through January 2004, Marshfield from October 2005 through September 2007, and Calgary from March 2005 through February 2007. The required elements of the data consisted of analysis date and the HbA1c value. No attempt was made to track changes in an individual's HbA1c over the data period. The following HbA1c methods were used in the participant's laboratories: Edmonton, Bio Rad VARIANT II; Melbourne and Singapore, Bio Rad VARIANT II Turbo; Marshfield, Tosoh 2+2; and Calgary, Roche Tina-quant performed on a Roche Integra 700 analyzer.
The mean and median HbA1c for each location was calculated using Microsoft Excel and expressed in graphical form in Figures 1 and 2.
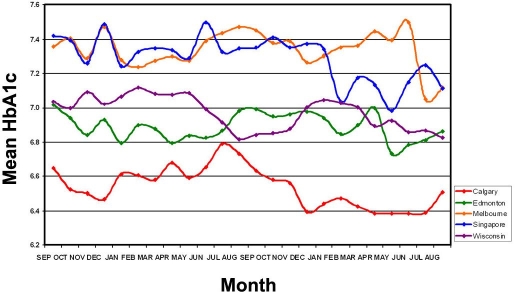
Figure 1.
Graphical representation of mean HbA1c values over a 2-year period for Calgary, Edmonton, Marshfield (Wisconsin), Singapore, and Melbourne.
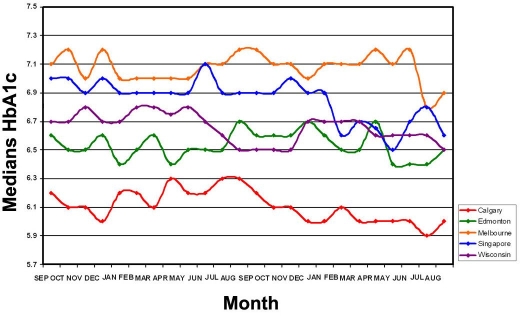
Figure 2.
Graphical representation of the median HbA1c values over a 2-year period for Calgary, Edmonton, Marshfield (Wisconsin), Singapore, and Melbourne.
Results
There appears to be three distinct groupings of mean HbA1c data. Overall, the mean data from Singapore and Melbourne are very close (p = .29) and exhibit the highest mean HbA1c values. Both these locations show a marked drop in HbA1c values during the time of the study, with the drop in Singapore HbA1c occurring in January 2007 and in Melbourne in July 2005. In both cases, the daily quality control values did not indicate a shift in HbA1c values nor was there a change in method. The data from Marshfield and Edmonton show similar means (p = .05), especially after the second June data point, and these are lower than the Singapore/Melbourne means (p < .0001). The mean of the HbA1c data from Calgary is lower than the other two groups (p < .0001). This difference in mean values between the North America and Australasia may be interpreted with the ordering of HbA1c in Singapore and Melbourne occurring mainly in individuals with diabetes having a HbA1c mean of the means of 7.3% (range 7.1% to 7.5%). In Marshfield, the HbA1c mean of means is 7.0% (range 6.8% to 7.2%), and in Edmonton, it is 6.9% (range 6.7% to 7.0%), indicating that HbA1c testing is mainly ordered on individuals with diabetes, with some ordering on nondiabetes patients. The HbA1c mean of the means in Calgary is 6.6% (range 6.4% to 6.8%), which may indicate that HbA1c is ordered on many individuals without diabetes.
For median HbA1c values, there appears to be the same grouping of data as noted for the mean HbA1c data. The Calgary median data (range of 5.9% to 6.3%) are different (p < .001) from the Singapore (range 6.9% to 7.1%), Melbourne (range 7.0% to 7.2%), Edmonton (range 6.4% to 6.7%), and Marshfield data (range 6.5% to 6.8%).
Inspection of the median data from Singapore shows very little variation The maximum HbA1c values in Melbourne is noted in the winter months of June though September, with lower values observed December through March. In Edmonton and Marshfield, the higher values are noted November through March, with lower values June through October. In Calgary, the highest HbA1c value appears in July.
Discussion
The 2-year study reported here is longer than that of 1 year reported in many of the papers on seasonal variation of HbA1c. The finding by Garde and colleagues13 of higher HbA1c values in a location in the northern hemisphere in September and October with lower values in December and January appears at variance with the reported literature. One possible explanation, although it is a weak argument, is that the subjects in the study were patients without diabetes, while those in other studies were previously identified patients with diabetes.
The clear seasonal variation described by others is not seen in this study except for the Marshfield data. Calgary shows no seasonal variation over the study period, and other locations show marked declines in median and mean HbA1c toward the end of the 2-year period. The reasons for this decline are not obvious, as there is no change in quality control in the period of the study. There could be a change in study population, with an increase in patients without diabetes.
The difference between the low and highest HbA1c mean or median value is 0.4 for Marshfield, 0.3 for Edmonton, 0.2 for Melbourne, and 0.1 for Singapore. A possible explanation for this is the variation in temperature over the course of the year. Singapore has very little variation (2.0 °C) in mean monthly high temperature. The variation in Melbourne is greater (13.4 °C), whereas Marshfield, Calgary, and Edmonton show significant temperature changes (33.7, 26.8, and 31.2 °C, respectively) over the course of a year. Tseng and associates12 in East Orange, New Jersey, with a temperature difference of 26.2 °C, reported a difference in HbA1c values of 0.22 over the course of a year and commented that the difference in HbA1c values was temperature related and that the HbA1c difference over the year was dependent on the difference in temperature for the year, with greater HbA1c changes noted when there were larger temperature differences.
The cyclical pattern is most pronounced with the Marshfield data and may be related to a number of factors, including a stable analysis population of individuals receiving therapy for their diabetes and low analytical variation in the HbA1c method.14 In previous work it has been noted that a significant proportion of testing in Edmonton and Calgary is performed on asymptomatic individuals as a screen for diabetes and this may skew the data.15
Conclusion
Data from our and other studies show that temperature differences play a role in HbA1c values. However, the difference in HbA1c is not as obvious as in other studies. It is important for both laboratorians and clinicians to be aware of the influence of temperature on the HbA1c value, especially in the context of meeting treatment target goals for HbA1c.
Abbreviations
- HbA1c
hemoglobin A1c
References
- 1.The Canadian Diabetes Clinical Practice Association Expert Committee. Canadian Diabetes Association 2003 clinical practice guidelines for the prevention and management of diabetes in Canada. Can J Diabetes. 2003;27(suppl 2):S18–S20. [Google Scholar]
- 2.Sacks DB, Bruns DE, Goldstein DE, Maclaren NK, McDonald JM, Parrott M. Guidelines and recommendations for laboratory analysis in the diagnosis and management of diabetes mellitus. Clin Chem. 2002;48(3):436–472. [PubMed] [Google Scholar]
- 3.Philipou G, Phillips PJ. Intraindividual variation of glycohemoglobimplications for interpretation and analytical goals. Clin Chem. 1993;39:2305–2308. (11 Pt 1) [PubMed] [Google Scholar]
- 4.Rohlfing C, Wiedmeyer HM, Little R, Grotz VL, Tennill A, England J, Madsen R, Goldstein D. Biological variation of glycohemoglobin. Clin Chem. 2002;48(7):1116–1118. [PubMed] [Google Scholar]
- 5.Mortensen HB. Glycated hemoglobin. Reaction and biokinetic studies. Clinical application of hemoglobin A1c in the assessment of metabolic control in children with diabetes mellitus. Dan Med Bull. 1985;32(6):309–328. [PubMed] [Google Scholar]
- 6.Ishii H, Suzuki H, Baba T, Nakamura K, Watanabe T. Seasonal variation of glycemic control in type 2 diabetic patients. Diabetes Care. 2001;24(8):1503. doi: 10.2337/diacare.24.8.1503. [DOI] [PubMed] [Google Scholar]
- 7.Sohmiya M, Kanazawa I, Kato Y. Seasonal changes in body composition and blood HbA1c levels without weight change in male patients with type 2 diabetes treated with insulin. Diabetes Care. 2004;27(5):1238–1239. doi: 10.2337/diacare.27.5.1238. [DOI] [PubMed] [Google Scholar]
- 8.Chen HS, Jap TS, Chen RL, Lin HD. A prospective study of glycemic control during holiday time in type 2 diabetic patients. Diabetes Care. 2004;27(2):326–330. doi: 10.2337/diacare.27.2.326. [DOI] [PubMed] [Google Scholar]
- 9.Asplund J. Seasonal variation of HbA1c in adult diabetic patients. Diabetes Care. 1997;20(2):234. doi: 10.2337/diacare.20.2.234a. [DOI] [PubMed] [Google Scholar]
- 10.Norfeldt S, Ludvigsson J. Seasonal variation of HbA1c in intensive treatment of children with type 1 diabetes. J Pediatr Endocrinol Metab. 2000;13(5):529–535. doi: 10.1515/jpem.2000.13.5.529. [DOI] [PubMed] [Google Scholar]
- 11.Carney TA, Guy SP, Helliwell CD. Seasonal variation in HbA1c in patients with type 2 diabetes mellitus. Diabet Med. 2000;17(7):554–555. doi: 10.1046/j.1464-5491.2000.00311-5.x. [DOI] [PubMed] [Google Scholar]
- 12.Tseng CL, Brimacombe M, Xie M, Rajan M, Wang H, Kolassa J, Crystal S, Chen TC, Pogach L, Safford M. Seasonal patterns in monthly A1c values. Am J Epidemiol. 2005;161(6):565–574. doi: 10.1093/aje/kwi071. [DOI] [PubMed] [Google Scholar]
- 13.Garde AH, Hansen AM, Skovgaard LT, Christensen JM. Seasonal and biological variation of blood concentrations of total cholesterol, dehydroepiandrosterone sulfate, hemoglobin A1c, IgA, prolactin and free testosterone in healthy women. Clin Chem. 2000;46(4):551–559. [PubMed] [Google Scholar]
- 14.Tran DV, Lyon AW, Higgins TN, Wesenberg JC, Vandergouwe L, Wiley CL, Cembrowski GS. Use of serial patient hemoglobin a1c differences to determine long-term imprecision of immunoassay and high-performance liquid chromatography analyzers. J Diabetes Sci Technol. 2009;3(3):418–423. doi: 10.1177/193229680900300304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lyon AW, Higgins TN, Wesenberg JC, Tran DV, Cembrowski GS. Variation in the fequency of hemoglobin A1c (HbA1c) testing: population studies used to assess compliance with clinical practice guidelines and use of HbA1c to screen for diabetes. J Diabetes Sci Technol. 2009;3(3):411–417. doi: 10.1177/193229680900300302. [DOI] [PMC free article] [PubMed] [Google Scholar]