Abstract
People on insulin therapy are challenged with evaluation of numerous factors affecting the blood glucose in order to select the optimal dose for reaching their glucose target. Following medical recommendations precisely still results in considerable blood glucose unpredictability, often resulting in frustration in the short term due to hypoglycemia and hyperglycemia, and, in the long term, will likely result in complications.
The kinetics of insulin do indeed vary significantly and have become an important focus when developing new insulin analogues and delivery systems; however, numerous of other factors impact glycemic variability. These have different dependences and interactions and are therefore difficult to characterize. Some of the factors are highly dependent and influenced by the type of insulin and devices used in therapy. Development of future therapy products is therefore highly focused on how to minimize glycemic variability.
Keywords: diabetes, glucose prediction, insulin, variability
Introduction
Glycemic stability is the major issue for people on insulin therapy, especially since studies have shown how lowering variability can reduce long-term complications.1,2 However, we have learned that following medical recommendations strictly does not guarantee perfect glycemic stability and that strict therapy has a negative effect on convenience.
Numerous factors influence glycemic variability of people on insulin therapy. They are exposed to variability through physiology and through insulin delivery, which increases uncertainty of their insulin level. Glucose meters have limited accuracy. Also, manual estimation of meal carbohydrates is difficult and adds variability, and lifestyle changes and compliance difficulties are major contributors. Although the different variability effects are interacting, for clarity, these have been divided into five independent categories as illustrated at Figure 1.
Figure 1.
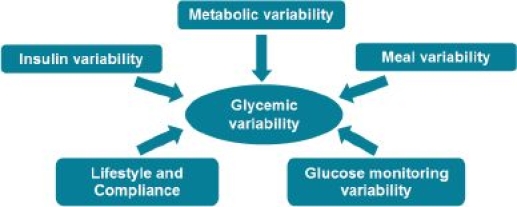
Five different factors that influence glycemic variability.
The motivation for writing this paper is the need to illustrate some of the obstacles having influence on glycemic variability and in parallel to describe the direction in which insulin, devices, and therapy are developed in order to facilitate this.
Insulin Variability
With modern insulin and delivery systems, insulin therapy has greatly improved glycemic predictability, and the industry is continuously focusing on improving products in these areas.
Insulin Preparation
Insulin preparations have changed from porcine to human to recombinant insulin. Also, the technology for prolongation of action has changed from crystal insulin preparations to insulin binding to albumin, like detemir.3 Pharmacokinetic (PK) predictability is important in order to treat as close to glucose target as possible. Figure 2 shows the PK interindividual variability of different insulin preparations. Intraindividual variability follows the pattern, however, with slightly smaller values. The coefficient of variation (CV) is defined as the ratio of the standard deviation to the mean.
Figure 2.
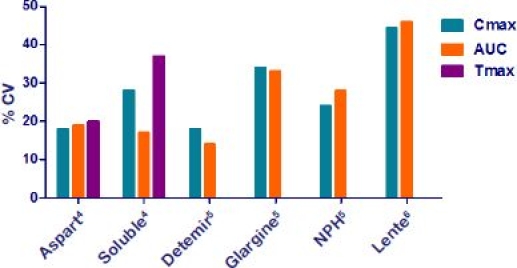
Pharmacokinetic interindividual variability of different insulin preparations.4–6 Long-acting insulin (detemir, glargine, NPH, and lente) is considered not to have a well-defined peak. Tmax, time of the peak concentration; Cmax, maximal concentration; AUC, area under the curve.
Fast-acting insulin analogues are modified to have a faster rate of diffusion subcutaneously than human insulin. Figure 2 shows how the traditional crystal insulin, neutral protamine Hagedorn (NPH), and lente typically introduce more variability than in analogues, illustrated here with Aspart and detemir.7
Insulin analogues have been developed primarily to create long-acting basal profiles and fast-acting prandial choices. This trend is likely to continue. Also, the reduced variability has become a key selling point for novel insulin.
Insulin Delivery
Insulin pens have been shown to be more accurate than syringes, with a % CV around 2 compared to around 5 for syringes when delivering 5?U of insulin.8,9 However, insulin pens still have limited accuracy. When investigating delivery at 5, 10, and 30?IU, the standard deviation of the mean dose delivered by FlexPen were 0.19, 0.27, and 0.34, respectively, and were 0.30, 0.52, and 0.47, respectively, for SoloStar.10
Insulin pumps have demonstrated several treatment advances, as it is a flexible platform for tailoring personalized insulin profiles.11 Pumps are still considered expensive for the majority;11 however, the number of commercially available insulin pumps is growing, and focus exists on providing less expensive options in the future.
The exact use of the delivery system is another issue, with depth, anatomical site, and delay before withdrawing the needle giving rise to poor reproducibility.4,12 Time delay before withdrawing the needle causes loss of insulin. In one study,13 rapid withdrawal of the needle was investigated and led to loss of insulin in 25% of the patients. In 13.8% of the patients, the loss of insulin was 10% of the dose. Also, accidental intramuscular injections of NPH have shown to reduce T50 from 10.3 to 5.3?h.4,14 Therefore, there are many obstacles to avoid by following therapy guidelines, and simply using the insulin delivery method correctly can improve therapy outcome. These problems are another reason for switching patients to pumps in the future because of the reduced variability that exist between injection boli. Pump therapy eliminates some of the variability factors from injection, although shorter PK peak and duration of action have been observed from insulin catheter aging.15
Metabolic Variability
A number of internal factors influence glucose metabolism. Fluctuations in insulin sensitivity and insulin action have been known to be important, considerable contributions to glycemic variability.16,17 Variability of insulin sensitivity under clinical conditions have shown an average interday CV of 20.2 +/- 3.2%.18
Also, hypoglycemia triggers counter-regulatory mechanisms that give rise to insulin resistance.19 Hypo-glycemia is a complex therapy area regarding rules and is considered, by some, the largest contributor to glycemic variability.20
Tissue sensitivity to insulin is affected by outside conditions, for instance, accelerated blood flow as a result of exercise.21 Although the concept of some of these effects is simple, the consequences in the short term are unpredictable for most people, because it involves dynamic effect of multiple hormones. For example, minor differences in exercise have shown significant changes in blood glucose dynamics.22,23
Some of the metabolic fluctuations are known to follow daily patterns.24 For instance, a significant decrease of insulin sensitivity the luteal phase of the normal menstrual cycle has been observed.25 Diurnal variation in glycemia and insulin sensitivity have been observed; however, this is still poorly understood.26 People on insulin therapy are usually aware of the daily patterns, and novel insulin pumps have options to set different insulin gain factors throughout the day. Hence there is a need for technology-assisted retrospective analysis in order to identify these daily fluctuations and adjust the insulin delivery accordingly. As a result, it is important to integrate future delivery systems with glucose monitoring and online/offline analysis capabilities.
Meal Variability
Meal content and effect estimation are some of the least studied areas regarding contribution to glycemic variability. Correct estimation of carbohydrate contents of meals is, however, crucial if glycemic variability is to be minimized. People on insulin therapy are encouraged to focus on counting carbohydrates, especially when changing diet. Figure 3A shows the results from a study we did at Steno Diabetes Hospital in 2005, where 10 people with diabetes had to estimate carbohydrate contents of meals they had not self-prepared.27 The % CV has been calculated compared to the predicted average and shows that people estimate very differently. The result showed a fairly high % CV of the different meal types, meaning that people were especially different in how to count carbohydrates in lunch, dinner, and snacks. Not surprisingly, breakfast had the least variability, probably due to its simple composition.
Figure 3.
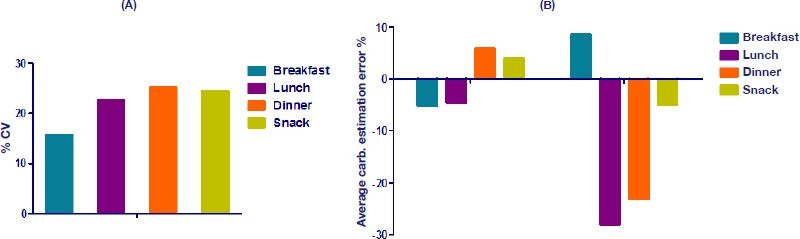
(A) Percent CV of estimated carbohydrate content in meals.27 (B) Percent average error of estimated carbohydrate content in meals; the data on the left-hand side of the graph represent the results of Kildegaard and associates,27 and the data on the right-hand side of the graph represent the results of Graff and coworkers.28
Figure 3B shows the average percentage error for two different carbohydrate estimation studies. Clearly, the study by Graf and colleagues28 shows a large bias toward underestimation of most meals. The same pattern was not seen in our study. These studies show how difficult meal estimation can be and are one reason why people on bolus insulin have a tendency not to change their diet.
Studies showing variability in repeated meal responses with an exact carbohydrate count have been performed.29 This implies that, even given an exact carbohydrate content, there can be a large difference in glycemic response to foods with different glycemic indexes. Besides this, day-to-day meal absorption is influenced by other factors, for instance, hunger.
No successful automatic system for carbohydrate estimation exists; however, some insulin pumps contain functionality for calculating the optimal meal bolus based on meal carbohydrate content. This is likely to be further developed in the future.
Lifestyle and Compliance
Being on insulin therapy requires focus and consistence in order to avoid and correct for glycemic fluctuations. This can, however, be a very difficult task. Exercise is, for instance, an important part of diabetes treatment, but even small intervals with changes in intensity can have significant effects for many hours.23
Even though modern delivery systems have improved in many ways, forgetting boluses does happen. In a study of children using insulin pumps, an average of 2.1 missed boluses per week was observed.30 Another similar study on children showed how 38% of all children had missed more than 15% of their boli.31 Obviously, this can explain hyperglycemic episodes; equally complicated, this sometimes calls for a correcting bolus, which can be difficult to predict.
Also, reporting food intake has shown to be difficult. People <120% ideal body weight miss reporting 10% of their diet, and people >120% Ideal body weight miss reporting 33%.32 Another study showed how less than 59% of people report accurate diary intake defined by more than 90%.33
Some insulin pens include memory for storage of prior doses. Even though the idea of assisting people to remember injections is good, these systems have not been successful. The electronic capabilities of insulin pumps provide an ideal platform for electronic diaries, with possibilities for integration with other systems.
Glucose Monitoring Variability
The glucose monitor is one of the most important tools for glycemic regulation, with clear effects on long-term metabolic control.34 Although laboratory tests have shown glucose meters to be accurate,35,36 some studies have shown how accuracy can be a significant issue when in the hands of patients in their daily setting, as shown at Figure 4.22,36 This is mostly because people contaminate the blood samples or have difficulties handling the monitors.22
Figure 4.
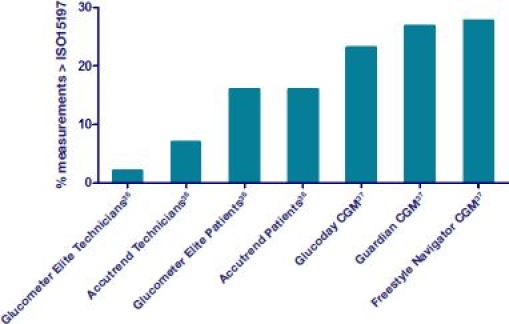
The percentage of measurements falling outside the ISO 15197 requirements for a number of strip-based glucose meters and CGM devices.
Figure 4 shows the error of several strip-based systems and continuous glucose monitors (CGMs) tested in two studies.36,37 Numerous methods for evaluation of glucose measurement accuracy exist; however, for self-testing devices for managing diabetes, they have to be measured against the ISO15197 standard.38 This standard states that (1) at a blood glucose level below 75?mg/dl, 95% of data should be ±15?mg/dl; and (2) at above 75?mg/dl, 95% of glucose should be ±20%. For system accuracy by least squares regression, 95% of the data should agree with the reference method and slope can only deviate by 65%.
Although CGM shows a larger error on Figure 4, it does provide additional information compared to strip-based systems and enables better glycemic control in people with type 1 and 2 diabetes.39 For instance, CGM systems may be used for hypo alarms and could prevent hypos by turning off pumps.40 One of the most beneficial outcomes of using CGM devices is that they have helped people better understand their blood glucose.39
Discussion
It is important to discriminate the involved variabilities in order to pinpoint therapy changes that can have important impact on glycemic predictability. Some of the variabilities are constant, some can be minimized, and some can be avoided.
Insulin variability is largely caused by intrinsic factors that cannot be easily changed. Minimized insulin variability is achieved by moving to analogs combined with insulin pens or, even better, insulin pumps.
Even though people are taught to count carbohydrates, this is one of the most difficult tasks to do right and an area where no tools are available to provide an exact carbohydrate content of a meal in an easy way. If successfully invented, such a tool probably has the greatest potential to minimize glycemic variability.
Much can clearly be gained in glycemic predictability with increased focus on more frequent glucose readout, as it has been shown how hemoglobin A1c is reduced with the number of daily glucose measurements.34 Continuous glucose monitor integration and analysis capability is therefore likely to become some of the most important future features in future insulin delivery systems.
The intrinsic factors influencing glycemic variability are more or less unalterable. People should be trained to be aware of the effect of counter-regulating mechanisms, as the dynamics can last for multiple hours. Exercise has many positive effects such as improved insulin sensitivity and loss of weight. Nevertheless, the effect of hard exercise can significantly trigger glucose dynamics and should therefore be considered.
Better compliance and a more stable lifestyle are key elements to improved glycemic stability. Compliance is often difficult because people themselves have to remember to take all injections and be aware of all snacks and meals. Fortunately, these problems have been recognized by the industry, and tools are appearing on the market to help, and pumps, glucose meters, and pedometers are beginning to integrate into electronic diaries and effective insulin management tools.41
Greater glycemic predictability can be achieved by modern diabetes technology; however, many elements can be further developed for great improvement in patient care.
Abbreviations
- CGM
continuous glucose monitor
- CV
coefficient of variation
- NPH
neutral protamine Hagedorn
- PK
pharmacokinetic
References:
- 1.UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352(9131):837–853. [PubMed] [Google Scholar]
- 2.The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329(14):977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 3.Klein O, Lynge J, Endahl L, Damholt B, Nosek L, Heise T. Albumin-bound basal insulin analogues (insulin detemir and NN344): comparable time-action profiles but less variability than insulin glargine in type 2 diabetes. Diabetes Obes Metab. 2007;9(3):290–299. doi: 10.1111/j.1463-1326.2006.00685.x. [DOI] [PubMed] [Google Scholar]
- 4.Heinemann L, Weyer C, Rauhaus M, Heinrichs S, Heise T. Variability of the metabolic effect of soluble insulin and the rapid-acting insulin analog insulin aspart. Diabetes Care. 1998;21(11):1910–1914. doi: 10.2337/diacare.21.11.1910. [DOI] [PubMed] [Google Scholar]
- 5.Heise T, Nosek L, Rønn BB, Endahl L, Heinemann L, Kapitza C, Draeger E. Lower within-subject variability of insulin detemir in comparison to NPH insulin and insulin glargine in people with type 1 diabetes. Diabetes. 2004;53(6):1614–1620. doi: 10.2337/diabetes.53.6.1614. [DOI] [PubMed] [Google Scholar]
- 6.Galloway JA, Spradlin CT, Nelson RL, Wentworth SM, Davidson JA, Swarner JL. Factors influencing the absorption, serum insulin concentration, and blood glucose responses after injections of regular insulin and various insulin mixtures. Diabetes Care. 1981;4(3):366–376. doi: 10.2337/diacare.4.3.366. [DOI] [PubMed] [Google Scholar]
- 7.Chen JW, Christiansen JS, Lauritzen T. Limitations to subcutaneous insulin administration in type 1 diabetes. Diabetes Obes Metab. 2003;5(4):223–233. doi: 10.1046/j.1463-1326.2003.00266.x. [DOI] [PubMed] [Google Scholar]
- 8.Keith K, Nicholson D, Rogers D. Accuracy and precision of low-dose insulin administration using syringes, pen injectors, and a pump. Clin Pediatr (Phila) 2004;43(1):69–74. doi: 10.1177/000992280404300109. [DOI] [PubMed] [Google Scholar]
- 9.Lteif AN, Schwenk WF. Accuracy of pen injectors versus insulin syringes in children with type 1 diabetes. Diabetes Care. 1999;22(1):137–140. doi: 10.2337/diacare.22.1.137. [DOI] [PubMed] [Google Scholar]
- 10.Asakura T, Seino H, Kageyama M, Yohkoh N. Dosing accuracy of two insulin pre-filled pens. Curr Med Res Opin. 2008;24(5):1429–1434. doi: 10.1185/030079908x297394. [DOI] [PubMed] [Google Scholar]
- 11.Bode BW, Tamborlane WV, Davidson PC. Insulin pump therapy in the 21st century. Strategies for successful use in adults, adolescents, and children with diabetes. Postgrad Med. 2002;111(5):69–77. doi: 10.3810/pgm.2002.05.1200. [DOI] [PubMed] [Google Scholar]
- 12.Heinemann L. Variability of insulin absorption and insulin action. Diabetes Technol Ther. 2002;4(5):673–682. doi: 10.1089/152091502320798312. [DOI] [PubMed] [Google Scholar]
- 13.Stewart NL, Darlow BA. Insulin loss at the injection site in children with type 1 diabetes mellitus. Diabet Med. 1994;11(8):802–805. doi: 10.1111/j.1464-5491.1994.tb00356.x. [DOI] [PubMed] [Google Scholar]
- 14.Vaag A, Handberg A, Lauritzen M, Henriksen JE, Pedersen KD, Beck-Nielsen H. Variation in absorption of NPH insulin due to intramuscular injection. Diabetes Care. 1990;13(1):74–76. doi: 10.2337/diacare.13.1.74. [DOI] [PubMed] [Google Scholar]
- 15.Swan KL, Dziura JD, Steil GM, Voskanyan GR, Sikes KA, Steffen AT, Martin ML, Tamborlane WV, Weinzimer SA. Effect of age of infusion site and type of rapid-acting analog on pharmacodynamic parameters of insulin boluses in youth with type 1 diabetes receiving insulin pump therapy. Diabetes Care. 2009;32(2):240–244. doi: 10.2337/dc08-0595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bergman RN, Finegood DT, Ader M. Assessment of insulin sensitivity in vivo. Endocr Rev. 1985;6(1):45–86. doi: 10.1210/edrv-6-1-45. [DOI] [PubMed] [Google Scholar]
- 17.Heinemann L. Variability of insulin absorption and insulin action. Diabetes Technol Ther. 2002;4(5):673–682. doi: 10.1089/152091502320798312. [DOI] [PubMed] [Google Scholar]
- 18.Steil GM, Murray J, Bergman RN, Buchanan TA. Repeatability of insulin sensitivity and glucose effectiveness from the minimal model. Implications for study design. Diabetes. 1994;43(11):1365–1371. doi: 10.2337/diab.43.11.1365. [DOI] [PubMed] [Google Scholar]
- 19.Spyer G, Hattersley AT, MacDonald IA, Amiel S, MacLeod KM. Hypoglycaemic counter-regulation at normal blood glucose concentrations in patients with well controlled type-2 diabetes. Lancet. 2000;356(9246):1970–1974. doi: 10.1016/s0140-6736(00)03322-5. [DOI] [PubMed] [Google Scholar]
- 20.Cryer PE. Hypoglycaemia: the limiting factor in the glycaemic management of type I and type II diabetes. Diabetologia. 2002;45(7):937–948. doi: 10.1007/s00125-002-0822-9. [DOI] [PubMed] [Google Scholar]
- 21.Karpe F, Fielding BA, Ilic V, MacDonald IA, Summers LK, Frayn KN. Impaired postprandial adipose tissue blood flow response is related to aspects of insulin sensitivity. Diabetes. 2002;51(8):2467–2473. doi: 10.2337/diabetes.51.8.2467. [DOI] [PubMed] [Google Scholar]
- 22.Alto WA, Meyer D, Schneid J, Bryson P, Kindig J. Assuring the accuracy of home glucose monitoring. J Am Board Fam Pract. 2002;15(1):1–6. [PubMed] [Google Scholar]
- 23.Guelfi KJ, Jones TW, Fournier PA. The decline in blood glucose levels is less with intermittent high-intensity compared with moderate exercise in individuals with type 1 diabetes. Diabetes Care. 2005;28(6):1289–1294. doi: 10.2337/diacare.28.6.1289. [DOI] [PubMed] [Google Scholar]
- 24.Bolli GB, Gerich JE. The “dawn phenomenon”—a common occurrence in both non-insulin-dependent and insulin-dependent diabetes mellitus. N Engl J Med. 1984;310(12):746–750. doi: 10.1056/NEJM198403223101203. [DOI] [PubMed] [Google Scholar]
- 25.Valdes CT, Elkind-Hirsch KE. Intravenous glucose tolerance test-derived insulin sensitivity changes during the menstrual cycle. J Clin Endocrinol Metab. 1991;72(3):642–646. doi: 10.1210/jcem-72-3-642. [DOI] [PubMed] [Google Scholar]
- 26.Van Cauter E, Polonsky KS, Scheen AJ. Roles of circadian rhythmicity and sleep in human glucose regulation. Endocr Rev. 1997;18(5):716–738. doi: 10.1210/edrv.18.5.0317. [DOI] [PubMed] [Google Scholar]
- 27.Kildegaard J, Randløv J, Poulsen JU, Hejlesen OK. Non-model related variations profoundly impact blood glucose prediction. Proceedings of the Sixth Annual Diabetes Technology Meeting; November 2–4, 2006; Atlanta, GA. [Google Scholar]
- 28.Graff MR, Gross TM, Juth SE, Charlson J. How well are individuals on intensive insulin therapy counting carbohydrates? Diabetes Res Clin Pract. 2000;50(Suppl 1):238–239. [Google Scholar]
- 29.Mohammed NH, Wolever TM. Effect of carbohydrate source on post-prandial blood glucose in subjects with type 1 diabetes treated with insulin lispro. Diabetes Res Clin Pract. 2004;65(1):29–35. doi: 10.1016/j.diabres.2003.11.018. [DOI] [PubMed] [Google Scholar]
- 30.Burdick J, Chase HP, Slover RH, Knievel K, Scrimgeour L, Maniatis AK, Klingensmith GJ. Missed insulin meal boluses and elevated hemoglobin A1c levels in children receiving insulin pump therapy. Pediatrics. 2004;113(3 Pt 1):e221–e224. doi: 10.1542/peds.113.3.e221. [DOI] [PubMed] [Google Scholar]
- 31.Olinder AL, Kernell A, Smide B. Missed bolus doses: devastating for metabolic control in CSII-treated adolescents with type 1 diabetes. Pediatr Diabetes. 2009;10(2):142–148. doi: 10.1111/j.1399-5448.2008.00462.x. [DOI] [PubMed] [Google Scholar]
- 32.Alemazade R, Goldberg T, Fort P, Recker B, Lifshitz F. Reported dietary intakes of patients with insulin-dependent diabetes mellitus: limitations of dietary recall. Nutrition. 1992;8(2):87–93. [PubMed] [Google Scholar]
- 33.Kalergis M, Nadeau J, Pacaud D, Yared Z, Yale JF. Accuracy and reliability of reporting self-monitoring of blood glucose results in adults with type 1 and type 2 diabetes. Canadian J Diabetes. 2006;30(3):241–247. [Google Scholar]
- 34.Schütt M, Kern W, Krause U, Busch P, Dapp A, Grziwotz R, Mayer I, Rosenbauer J, Wagner C, Zimmermann A, Kerner W, Holl RW DPV Initiative. Is the frequency of self-monitoring of blood glucose related to long-term metabolic control? Multicenter analysis including 24,500 patients from 191 centers in Germany and Austria. Exp Clin Endocrinol Diabetes. 2006;114(7):384–388. doi: 10.1055/s-2006-924152. [DOI] [PubMed] [Google Scholar]
- 35.Chen ET, Nichols JH, Duh SH, Hortin G. Performance evaluation of blood glucose monitoring devices. Diabetes Technol Ther. 2003;5(5):749–768. doi: 10.1089/152091503322526969. [DOI] [PubMed] [Google Scholar]
- 36.Skeie S, Thue G, Nerhus K, Sandberg S. Instruments for self-monitoring of blood glucose: comparisons of testing quality achieved by patients and a technician. Clin Chem. 2002;48(7):994–1003. [PubMed] [Google Scholar]
- 37.Kovachev B, Anderson S, Heinemann L, Clarke W. Comparison of the numerical and clinical accuracy of four continuous glucose monitors. Diabetes Care. 2008;31(6):1160–1164. doi: 10.2337/dc07-2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.In vitro diagnostic test systems: requirements for blood-glucose monitoring systems for self-testing in managing diabetes mellitus (ISO 15197) Geneva: International Organization for Standardization; 2003. International Organization for Standardization. [Google Scholar]
- 39.Bode B, Silver M, Weiss R, Martin K. Evaluation of a continuous glucose monitoring system for home-use conditions. Manag Care. 2008;17(8):40–45. [PubMed] [Google Scholar]
- 40.Buckingham B, Cobry E, Clinton P, Gage V, Caswell K, Kunselman E, Cameron F, Chase HP. Preventing hypoglycemia using predictive alarm algorithms and insulin pump suspension. Diabetes Technol Ther. 2009;11(2):93–97. doi: 10.1089/dia.2008.0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gross TM, Kayne D, King A, Rother C, Juth S. A bolus calculator is an effective means of controlling postprandial glycemia in patients on insulin pump therapy. Diabetes Technol Ther. 2003;5(3):365–369. doi: 10.1089/152091503765691848. [DOI] [PubMed] [Google Scholar]


