Abstract
Diabetic retinopathy is one of the leading causes of blindness in the United States and other parts of the world. Historically, laser photocoagulation and vitrectomy surgery have been used for the treatment of diabetic retinopathy, including diabetic macular edema. Both procedures have proven to be useful under certain conditions but have their limitations. New pathways and processes that promote diabetic retinopathy have been identified, and several new therapeutic approaches are under investigation. These new therapies may be beneficial in the treatment of diabetic retinopathy and include antivascular endothelial growth factor agents, corticosteroids, and therapies that may potentially target a number of additional diabetic retinopathy-related factors and processes, including antisense oligonucleotides. Second-generation antisense oligonucleotides, such as iCo-007, may offer a significant advantage in the treatment of diabetic retinopathy by downregulating the signal pathways of multiple growth factors that seem to play a critical role in the process of ocular angiogenesis and vascular leakage. Benefits of such molecules are expected to include the specificity of the kinase target and an extended half-life, resulting in less frequent intravitreal drug administration, resistance to molecule degradation, and a good safety profile.
Keywords: antisense oligonucleotides, c-Raf kinase, diabetic macular edema, diabetic retinopathy, iCo-007, multiple proangiogenic factors, neovascular growth, vascular leakage
Diabetic Retinopathy
Diabetic retinopathy (DR) is characterized by microvascular changes in the retina and remains one of the leading causes of blindness in the United States, often affecting working-aged adults.1 As the incidence of diabetes mellitus has been on the rise for many years, there has also been a significant impact on health economics, not only in the United States but also around the world. For decades, in the absence of therapeutic options, there have been only two procedures used for the treatment of DR: laser photocoagulation and vitrectomy.2,3
Laser photocoagulation has been used for the treatment of nonproliferative diabetic retinopathy, macular edema, and proliferative diabetic retinopathy since the 1960s. The Diabetic Retinopathy Study was one of the first randomized clinical trials in ophthalmology. This study showed the advantage of laser photocoagulation for patients with proliferative retinopathy. The Early Treatment Diabetic Retinopathy Study (ETDRS) guideline for laser treatment of macular edema was published in the 1980s. Vitrectomy surgery, a procedure used commonly to treat nonclearing vitreous hemorrhage, vitreomacular traction, epiretinal membranes, and retinal detachment, was introduced around the same time. Recent advances have led to identification of the pathways and processes that promote the development of DR. As a result, several novel therapeutic treatments are under investigation.2 These new therapies may offer a significant advantage in the treatment of DR and include antivascular endothelial growth factor agents, corticosteroids, and therapies that may potentially target a number of additional DR-related factors and processes, such as antisense oligonucleotides. They may be utilized as a solo treatment or in combination with laser or surgery. Although the ETDRS showed an improvement in vision over the observation period, a marked improvement of vision was not seen. It is conceivable that these newer treatments, alone or in combination, may improve the visual results and reduce the need for surgery and the potential complications associated with a focal laser.
Pathophysiology of Diabetic Retinopathy
The pathophysiology of DR is a complex process and a full description is beyond the scope of this review article. Based on the extent of microvascular changes in the retina, DR can be broadly divided into nonproliferative and proliferative.2,4,5 Hyperglycemia-induced pericyte death and thickening of the basement membrane lead to incompetence of the vascular walls. As a consequence of this process, the blood–retinal barrier is altered, resulting in an increased permeability of retinal vessels.2 Hyperglycemia in patients with poorly controlled diabetes may cause an alteration of hemodynamics of the retinal vasculature, resulting in chronic hypoxia. As a result, compensatory mechanisms will cause an upregulation of the vascular endothelial growth factor (VEGF),5 as well as other DR-related growth factors and inflammatory factors [e.g., erythropoietin (EPO), hepatocyte growth factor (HGF), basic fibroblast growth factor (bFGF), insulin-like growth factor, interleukin-6]. These effects result in pathologic processes such as retinal capillary microaneurysms (Figure 1), vascular permeability, macular edema, vascular occlusion or closure, and, in the proliferative form of DR (Figure 2), neovascularization, vitreous hemorrhage, and tractional detachment of the retina caused by fibrovascular proliferation (Figure 3).6 Neuronal function alterations may be one of the first detectable defects in DR, and evidence shows that inflammatory processes may contribute to these changes as well. Processes leading to DR-associated vasculopathy include the entrapment of leukocytes, new capillary formation, and capillary dropout resulting in local hypoxia. The number of neutrophils may also be increased significantly in the retinal and choroidal vasculature of patients with diabetes. Increased levels of intercellular adhesion molecule-1 (ICAM-1) have been found in patients with diabetes compared to nondiabetic controls.7 Damage from inflammatory changes seems to affect mainly retinal vasculature. Therefore, anti-inflammatory agents may be a useful part of DR treatment.
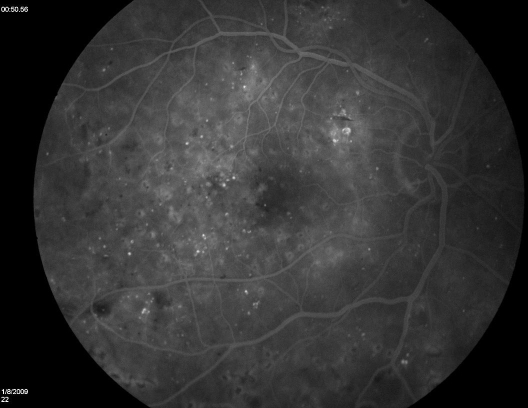
Figure 1.
Fluorescein angiogram (midphase) demonstrating multiple retinal microaneurysms in a nonproliferative DR.
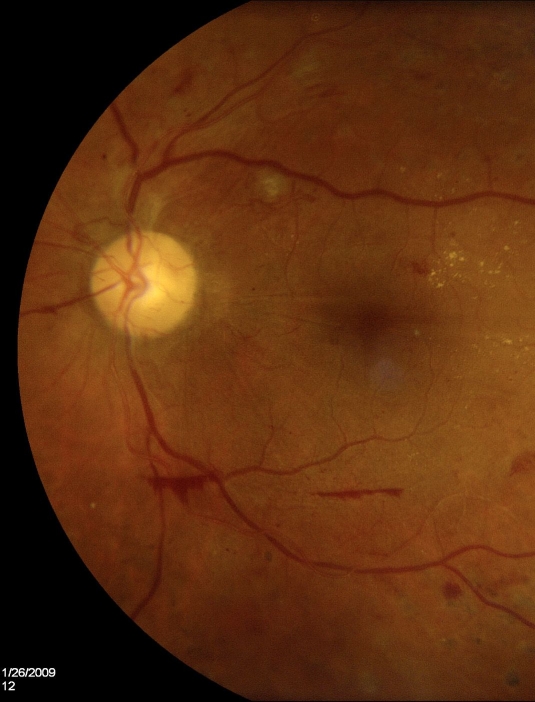
Figure 2.
Fundus photography demonstrating proliferative diabetic retinopathy with preretinal hemorrhage and slight new vessel development.
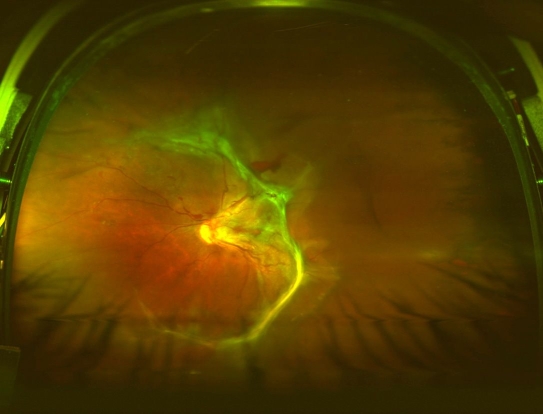
Figure 3.
Wide-field Optos photograph demonstrating proliferative retinopathy with fibroglial changes and early tractional retinal detachment.
Macular edema, a retinal thickening resulting from the accumulation of fluid, is a frequent cause of vision decrease in patients with DR.6 The breakdown of endothelial tight junctions and loss of the blood–retinal barrier, typically seen in macular edema, can occur in DR at any stage.5 The prevalence of macular edema after 15 years of known diabetes is between 15 and 25% regardless of age and depending on the type of diabetes and insulin dependency.6 Retinal thickening, measured by optical coherence tomography, is the consequence of an increase in endothelial cell fenestrations, which leads to an increase in vascular permeability and a subsequent leakage of fluid in the subretinal space. When the thickening occurs in the area of the fovea, the result is visual impairment.8
Angiogenesis and the Role of Various Growth Factors on Vascular Leakage and Neovascularization
Ischemia and hypoxia result in the upregulation of molecules that promote angiogenesis, including proangiogenic growth factors. Retinal cells start producing vasoproliferative growth factors, which lead to the formation of new blood vessels. Neovascularization is a hallmark of proliferative DR.9 New vessels, which tend to grow on the surface of the retina and optic disc, are surrounded by fibrous tissue. The fibrovascular tissue becomes attached to the vitreous surface. Contraction of the vitreous surface causes traction on the new vessels, resulting in preretinal or vitreous hemorrhage or in retinal detachment,6 which is the direct cause of most cases of severe visual loss in DR.9
Angiogenesis is a multistep process, including the production of angiogenic growth factors by the hypoxic tissue, binding of angiogenic factors to receptors on existing vascular endothelial cells, activation of endothelial cell gene expression of proangiogenic molecules, endothelial cell invasion of surrounding tissue, migration and proliferation, formation of vascular tubes, and, eventually, stabilization of new blood vessels.6
In healthy tissue, there is a balance between inducers (VEGF and other factors) and inhibitors (e.g., pigment epithelium-derived factor) of angiogenesis.9 When hypoxia causes an increase in the level of inducers and a decrease in inhibitors, the resulting imbalance is believed to lead to angiogenesis.6 An important mediator is hypoxia-inducible factor-1.10 This factor activates the transcription of several proangiogenic growth factors, including VEGF, EPO, placental growth factor, angiopoietin-2 (Ang-2), and platelet-derived growth factor. Proangiogenic growth factors bind to receptors on preexisting endothelial cells, resulting in activation of these cells, followed by the formation of vascular tubes. At the final stage, remodeling will take place with subsequent formation of a new basement membrane, as well as the recruitment of mural cells (pericytes and smooth muscle cells) to form mature vessels.9 Drugs interfering with any of these steps may be potentially useful in the treatment of DR.
Vascular Endothelial Growth Factor
Vascular endothelial growth factor, also known as vascular permeability factor, is a glycoprotein that has an impact on both angiogenesis and vasopermeability. VEGF is produced by many cells within the eye, including retinal pigment epithelium, pericytes, endothelial cells, glial cells, Müller cells, and ganglion cells.9 Typically, VEGF concentrations are increased in the vitreous and aqueous fluids of patients with DR, especially in those with a proliferative form of DR compared to patients with no neovascular disorder.12 VEGF is involved in a number of steps in the angiogenic process, including endothelial cell migration, proliferation, tubulogenesis, vascular permeability, and neuroprotection.9,10 It is a very important growth factor in the process of retinal neovascularization. At the same time, it is also highly likely that additional growth factors play important roles in this process as well. These growth factors either may have synergistic effects with VEGF or may be involved in other steps of the neovascular process.9
Other Factors Important in Retinal Neovascularization and Vascular Leakage in DR
It has been shown that a number of additional proangiogenic factors are major contributors responsible for pathologic changes in DR. These include, but are not limited to, insulin-like growth factor, bFGF, EPO, HGF, Ang-2, and integrins.13–16
Insulin-like growth factor and bFGF have been shown to activate endothelial cells and are thought to be the growth factors responsible for the initiation of angiogenesis.16
Erythropoetin is a glycoprotein that stimulates the formation of red blood cells and displays angiogenic activity in vascular endothelial cells by stimulating cell proliferation and migration through erythropoietin receptors. It is interesting that vitreous EPO levels have been shown to be more strongly associated with the presence of proliferative DR than VEGF.14,17
Hepatocyte growth factor levels in vitreous and aqueous fluids were found to be significantly elevated in patients with proliferative DR. HGF increases the migratory and invasive properties of endothelial cells. In addition, it has also been shown to have an impact on retinal vascular permeability at relevant concentrations with a magnitude similar to VEGF, but without changing retinal hemodynamics.17,18 For all these reasons, HGF is likely to play a role in both retinal angiogenesis and blood–retinal barrier disruption; therefore, downregulating its activity may prove to be useful in the treatment of angiogenesis.
Angiopoietins are ligands for a class of endothelial-specific tyrosine kinase receptors that have been shown to play an important role in vascular growth and development. Blocking of these receptors resulted in a decrease in retinal neovascularization in treated animal retinas. Angiopoietin-2 increases the sensitivity of retinal vessels to the angiogenic effects of VEGF. Significantly increased vitreous levels of Ang-2 have been found in patients with proliferative DR compared to patients with nondiabetic ocular disease.17
Therapeutic Oligonucleotides
Targeting these molecules and other factors important for the development of DR may result in new effective treatments for retinal neovascularization and leakage in patients with DR, including second-generation antisense oligonucleotides, such as iCo-007, targeting c-Raf kinase. Defects in cell signaling pathways have been associated with many human diseases, including ocular diseases (e.g., diabetic retinopathy). Understanding the complex mechanisms by which cells transduce extracellular signals into cellular responses has come a long way in recent years.19 Oligonucleotides represent one of the new treatment entities targeting specific links in the disease process. There are two main categories of oligonucleotide therapeutic agents: antisense oligonucleotides, including short interfering RNA (siRNA), and oligonucleotide aptamers.
Antisense oligonucleotides are novel therapeutics designed to bind to specific messenger RNA (mRNA) that result in the degradation of the message encoding the targeted protein, thus affecting a decrease in the production of a particular protein associated with the targeted disease (Figure 4).20Fomivirsen sodium (Vitravene™, Isis Pharmaceuticals, Inc., and Novartis Pharmaceuticals, Switzerland) is the only approved antisense molecule and is a representative of the “first generation” of this class of compounds. Fomivirsen was approved for the treatment of cytomegalovirus (CMV) retinitis as an intravitreous injection.21 Since the approval of fomivirsen, additional chemical modifications created a “second-generation” antisense oligonucleotide with significant improvements in potency, pharmacologic properties (e.g., longer half-life), and tolerability profile. The modified oligonucleotides that have progressed the most toward therapeutic application are the 2'-O-methoxy-ethyl (2'-MOE) antisense oligonucleotides. Incorporation of 2'-MOE modifications resulted in an increased hybridization affinity and a concomitant increase in potency, increased resistance to nuclease cleavage, and extended residence time of oligonucleotides in tissue. This may result in less frequent treatment administration. Additionally, these modifications show a decrease in proinflammatory effects, resulting in less toxicity. An example of a “second-generation” antisense drug currently under clinical investigation is iCo-007, which targets c-Raf kinase. It is currently in a phase I trial for the treatment of diffuse diabetic macular edema (DME). siRNA inhibitors, such as siRNA-027 and Cand5, also belong to the category of antisense inhibitors, but these are double-stranded RNA and function through the RNA-induced silencing complex mechanism. The half-life of siRNA appears to be far shorter than for “second-generation” antisense compounds because siRNA molecules are not stabilized against nuclease degradation to the same degree. However, the duration of action may be longer in a clinical setting.22 These siRNA inhibitors may work at Toll-like receptor 3 (TLR3) on the cell membrane.23,24 TLR3 is the pattern recognition receptor that recognizes double-stranded RNA. This molecular pattern is associated with viral infection because it is produced by most viruses at some point during their replication. Activation of TLR3 induces the activation of nuclear factor-κB and the production of type I interferons, which signal other cells to increase their antiviral defenses. There are several second-generation antisense compounds in clinical trials for various cancers, hypercholesterolemia, diabetes, asthma, and so on, some in combination with already approved therapy and others with chemistry variations.
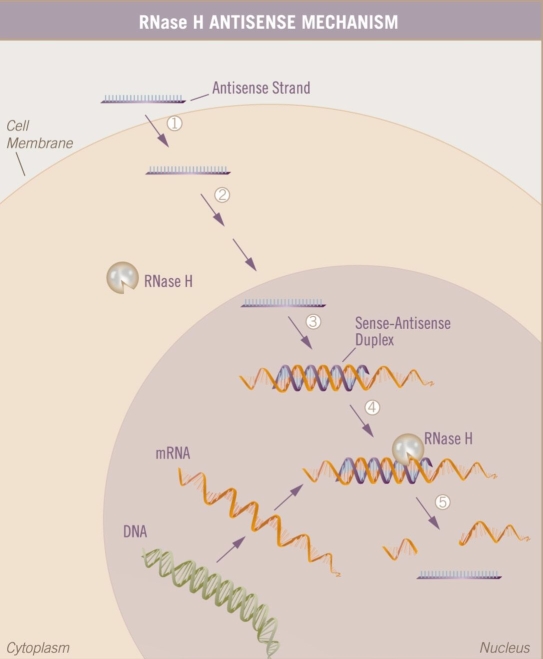
Figure 4.
A simplified view of the antisense mechanism. The single-stranded antisense oligonucleotide enters the cell and interacts with its specific target mRNA in the cytoplasm or nucleus. There, it recruits RNase H, which cleaves the mRNA target in the mRNA–DNA duplex. Thus, the message in the mRNA is intercepted and no longer readable.
The mode of action of oligonucleotide aptamers is through selection of an oligonucleotide that will interact with a specific soluble protein or cell surface receptor rather than through hybridization with mRNA. The interaction between aptamer and target is dependent on the conformational structure of the oligonucleotide. Aptamer oligonucleotides do not need to enter the cells and instead interact outside of the cells, binding to receptors or soluble factors such as cytokines or growth factors. The presumed attraction of using oligonucleotide aptamers is the added resistance to nucleases and the fact that these are not linear molecules carrying no genetic information. Because they function through binding to a target protein rather than to mRNA, aptamers do not interrupt the translation of genetic information. Pegaptanib sodium (Macugen®, Pfizer), approved for the treatment of age-related macular degeneration (AMD), is an oligonucleotide aptamer.22
Ocular Clinical Targets for Antisense Drugs
Because both diabetic retinopathy (retinal neo-vascularization) and neovascular age-related macular degeneration (choroidal neovascularization) are the leading causes of blindness in the Western world, ocular neovascularization has been a target for the research of molecular mechanisms.8,22 So far, most of the attention has been focused on the role of VEGF in neovascular disease. Although VEGF plays an important role in this process, additional factors (multiple growth factors) and complex processes are involved in this condition.22 Pegaptanib was the first anti-VEGF drug to be approved for neovascular AMD, followed by the VEGF antibody fragment ranibizumab (Lucentis™, Genentech). Since then, intravitreous injections have become a routine procedure for chronic ocular diseases and are part of everyday retinal practice.
A novel approach to decrease vascular leakage and neovascularization uses an antisense mechanism to inhibit a downstream component of the mitogen-activated protein (MAP) kinase signaling cascade. A number of growth factors critical for neovascularization and leakage signal through the MAP kinase cascade, which includes c-Raf kinase. c-Raf kinase is involved in cellular processes regulating proliferation, differentiation, and apoptosis.25 It belongs to the class of signaling proteins that participate in growth factor and extracellular matrix signal transduction.16
c-Raf kinase activation following VEGF stimulation was confirmed in human endothelial cells. Other growth factors, such as erythropoietin, hepatocyte growth factor, basic fibroblast growth factor, and others, may play important roles in the etiology of diabetic retinopathy and DME. Inhibiting a downstream target such as c-Raf kinase may prove to be a more effective treatment strategy by downregulating multiple growth factors than targeting individual growth factors only.22 “Second-generation” antisense compounds have been investigated in a pig model of venous-occlusion retinal neovascularization and showed a decrease of c-Raf kinase expression,16 improvement in the neovascularization severity score, and good ocular tolerability.26
Antisense oligonucleotide delivery via an intravitreous injection seems to be a reasonable strategy in the treatment of retinal diseases. Alternative options for the drug delivery of antisense and other oligonucleotides have been under investigation, including periorbital administration, iontophoresis, and sustained release formulations (likely not needed for an antisense compound modified for a long half-life). Treatment of ocular conditions other than DR with antisense oligonucleotides may include other conditions associated with neovascularization and leakage [e.g., AMD, branch retinal vein occlusion (BRVO), central retinal vein occlusion (CRVO)], viral infections (including CMV retinitis, herpes simplex, varicella-zoster, Epstein–Barr, and adenovirus), glaucoma (activity in retinal ganglion cells, adjuvant to filtration surgery), and intraocular inflammation (blocking expression or function of adhesion molecules, ICAM-1, ICAM-2).22
Conclusion
iCo-007 is under investigation in a phase I clinical trial in patients with diffuse DME. The recent development of this second-generation antisense oligonucleotide may show a significant advantage in the treatment of DR compared to approved treatments (e.g., focal photocoagulation, vitrectomy), as well as experimental treatments targeting VEGF, by downregulating multiple growth factors that seem to play a critical role in the process of ocular angiogenesis and leakage (e.g., VEGF, EPO, HGF, bFGF). This may result in a more potent anatomical effect (retinal thickness), as well as a functional effect (vision). Benefits of such molecules are expected to include an extended half-life, resulting in less frequent intravitreal drug administration, resistance to molecule degradation, a good safety profile, and, due to a differing mechanism of action, the possibility of adjunct therapy to other agents or procedures. Additional ocular retinal indications, including AMD, BRVO, and CRVO, have been implemented in the developmental programs.
Abbreviations
- AMD
age-related macular degeneration
- Ang-2
angiopoietin
- bFGF
basic fibroblast growth factor
- BRVO
branch retinal vein occlusion
- CMV
cytomegalovirus
- CRVO
central retinal vein occlusion
- DME
diabetic macular edema
- DR
diabetic retinopathy
- ETDRS
Early Treatment Diabetic Retinopathy Study
- EPO
erythropoietin
- HGF
hepatocyte growth factor
- ICAM
intercellular adhesion molecule
- MAP
mitogen-activated protein
- 2'-MOE
2'-O-methoxy-ethyl
- mRNA
messenger RNA
- siRNA
short interfering RNA
- TLR3
toll-like receptor 3
- VEGF
vascular endothelial growth factor
References
- 1.Henry SP, Miner RC, Drew WL, Fitchett J, York-Defalco C, Rapp LM, Levin AA. Antiviral activity and ocular kinetics of antisense oligonucleotides designed to inhibit CMV replication. Invest Ophthalmol Vis Sci. 2001;42(11):2646–2651. [PubMed] [Google Scholar]
- 2.Ciulla TA, Amador AG, Zinman B. Diabetic retinopathy and diabetic macular edema. Diabetes Care. 2003;26(9):2653–2664. doi: 10.2337/diacare.26.9.2653. [DOI] [PubMed] [Google Scholar]
- 3.Duh E. Diabetic retinopathy. Totowa (NJ): Humana Press; 2008. [Google Scholar]
- 4.Fong DS, Aiello LP, Ferris FL, 3rd, Klein R. Diabetic retinopathy. Diabetes Care. 2004;27(10):2540–2553. doi: 10.2337/diacare.27.10.2540. [DOI] [PubMed] [Google Scholar]
- 5.Meyerle CB, Chew EY. Duh E, editor. Diabetic retinopathy. Totowa (NJ): Humana Press; 2008. Ferris FL 3rd. Nonproliferative diabetic retinopathy; pp. 4–10. [Google Scholar]
- 6.Davis MD, Aiello LM Ferris FL 3rd. Treatment of diabetic retinopathy. N Engl J Med. 1999;341(9):667–678. doi: 10.1056/NEJM199908263410907. [DOI] [PubMed] [Google Scholar]
- 7.Swenarchuk LE, Whetter LE, Adamis AP. Duh E, editor. Diabetic retinopathy. Totowa (NJ): Humana Press; 2008. The role of inflammation in the pathophysiology of diabetic retinopathy; pp. 303–332. [Google Scholar]
- 8.Frank RN. Diabetic retinopathy. N Engl J Med. 2004;350(1):48–58. doi: 10.1056/NEJMra021678. [DOI] [PubMed] [Google Scholar]
- 9.Duh E. Retinal neovascularization and the role of VEGF. In: Duh E, editor. Diabetic retinopathy. Totowa (NJ): Humana Press; 2008. pp. 353–374. [Google Scholar]
- 10.Saint-Geniez M, Maharaj AS, Walshe TE, Tucker BA, Sekiyama E, Kurihara T, Darland DC, Young MJ, D'Amore PA. Endogenous VEGF is required for visual function: evidence for a survival role on müller cells and photoreceptors. PLoS ONE. 2008;3(11):e3554. doi: 10.1371/journal.pone.0003554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Treins C, Giorgetti-Peraldi S, Murdaca J, Monthouel-Kartmann MN, Van Obberghen E. Regulation of hypoxia-inducible factor (HIF)-1 activity and expression of HIF hydroxylases in response to insulin-like growth factor-1. Mol Endocrinol. 2005;19(5):1304–1317. doi: 10.1210/me.2004-0239. [DOI] [PubMed] [Google Scholar]
- 12.Miller JW. Vascular endothelial growth factor and ocular neovascularization. Am J Pathol. 1997;151(1):13–23. [PMC free article] [PubMed] [Google Scholar]
- 13.Khan ZA, Chakrabarti S. Growth factors in proliferative diabetic retinopathy. Exp Diabesity Res. 2003;4(4):287–301. doi: 10.1155/EDR.2003.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Watanabe D, Suzuma K, Matsui S, Kurimoto M, Kiryu J, Kita M, Suzuma I, Ohashi H, Ojima T, Murakami T, Kobayashi T, Masuda S, Nagao M, Yoshimura N, Takagi H. Erythropoietin as a retinal angiogenic factor in proliferative diabetic retinopathy. N Engl J Med. 2005;353(8):782–792. doi: 10.1056/NEJMoa041773. [DOI] [PubMed] [Google Scholar]
- 15.Eliceiri BP, Cheresh DA. The role of alpha integrins during angiogenesis. Mol Med. 1998;4(12):741–750. [PMC free article] [PubMed] [Google Scholar]
- 16.Henry S. Carlsbad (CA): Isis Pharmaceuticals; 2002. Intravitreous anti-raf-1 kinase antisense oligonucleotide as an angioinhibitory agent in porcine preretinal neovascularization [Study No.: 13650-IP01] [DOI] [PubMed] [Google Scholar]
- 17.Das A, Navaratna D, McGuire P. Beyond VEGF. Other factors important in retinal neovascularization: potential targets in proliferative diabetic retinopathy. In: Duh E, editor. Diabetic retinopathy. Totowa (NJ): Humana Press; 2008. pp. 375–398. [Google Scholar]
- 18.Clermont AC, Cahill M, Salti H, Rook SL, Rask-Madsen C, Goddard L, Wong JS, Bursell D, Bursell SE, Aiello LP. Hepatocyte growth factor induces retinal vascular permeability via MAP-kinase and PI-3 kinase without altering retinal hemodynamics. Invest Ophthalmol Vis Sci. 2006;47(6):2701–2708. doi: 10.1167/iovs.05-0071. [DOI] [PubMed] [Google Scholar]
- 19.Monia BP, Koller E, Gaarde WA. A role for antisense technology in the discovery of highly specific and versatile signal transduction inhibitors. In: Crooke ST, editor. Antisense drug technology: principles, strategies, and applications. New York: Marcel Dekker, Inc.; 2001. pp. 83–106. [Google Scholar]
- 20.Monteith DK, Geary RS, Johnston J, Monia BP, Levin AA. Preclinical evaluation of the effects of a novel antisense compound targeting C-raf kinase in mice and monkeys. Toxicol Sci. 1998;46(2):365–375. doi: 10.1006/toxs.1998.2527. [DOI] [PubMed] [Google Scholar]
- 21.Online U.S. National Library of Medicine and National Institutes of Health. San Francisco: FDA approves fomivirsen, famciclovir, and thalidomide. Food and Drug Administration; 1998 [cited 2009 Jan 29]. Available from: http://www.ncbi.nlm.nih.gov/pubmed/. [PubMed] [Google Scholar]
- 22.Grillone LR, Henry SP. Potential therapeutics: applications of antisense oligonucleotides in ophthalmology. In: Crooke ST, editor. Antisense drug technology: principles, strategies, and applications. 2nd ed. New York: CRC Press Taylor & Francis Group; 2008. pp. 585–600. [Google Scholar]
- 23.Yang Z, Stratton C, Francis PJ, Kleinman ME, Tan PL, Gibbs D, Tong Z, Chen H, Constantine R, Yang X, Chen Y, Zeng J, Davey L, Ma X, Hau VS, Wang C, Harmon J, Buehler J, Pearson E, Patel S, Kaminoh Y, Watkins S, Luo L, Zabriskie NA, Bernstein PS, Cho W, Schwager A, Hinton DR, Klein ML, Hamon SC, Simmons E, Yu B, Campochiaro B, Sunness JS, Campochiaro P, Jorde L, Parmigiani G, Zack DJ, Katsanis N, Ambati J, Zhang K. Toll-like receptor 3 and geographic atrophy in age-related macular degeneration. N Engl J Med. 2008;359(14):1456–1463. doi: 10.1056/NEJMoa0802437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kleinman ME, Yamada K, Takeda A, Chandrasekaran V, Nozaki M, Baffi JZ, Albuquerque RJ, Yamasaki S, Itaya M, Pan Y, Appukuttan B, Gibbs D, Yang Z, Karikó K, Ambati BK, Wilgus TA, DiPietro LA, Sakurai E, Zhang K, Smith JR, Taylor EW, Ambati J. Sequence-and-target-independent angiogenesis suppression by siRNA via TLR3. Nature. 2008;452(7817):543–545. doi: 10.1038/nature06765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hagemann C, Rapp UR. Isotype-specific functions of raf kinases. Exp Cell Res. 1999;253(1):34–46. doi: 10.1006/excr.1999.4689. [DOI] [PubMed] [Google Scholar]
- 26.Danis RP, Criswell MH, Orge F, Wancewicz EW, Stecker K, Henry SP, Monia BP. Intravitreous anti-raf-1 kinase antisense oligonucleotide as an angioinhibitory agent in porcine preretinal neovascularization. Current Eye Research. 2003;26(1):45–54. doi: 10.1076/ceyr.26.1.45.14252. [DOI] [PubMed] [Google Scholar]