Abstract
Exercise causes profound changes in glucose homeostasis. For people with type 1 diabetes, aerobic exercise usually causes blood glucose concentration to drop rapidly, while anaerobic exercise may cause it to rise, thereby making glycemic control challenging. Having the capacity to know their glucose levels and the direction of change during exercise increases self-efficacy in these persons who are prone to hypo- and hyperglycemia. For people with type 2 diabetes, learning first hand that regular exercise improves glucose levels may be a motivating factor in getting them to be more active. Continuous glucose monitoring is a potentially useful adjunct to diabetes management for the active person with either forms of diabetes. This review aims to guide the reader to use this technology to its maximum advantage by providing an overview of technical features, performance characteristics, and clinical utility, all balanced against the limitations that may be more prominent during physical activity.
Keywords: physical activity, hypoglycemia, hyperglycemia, blood glucose, self-monitoring, energy metabolism/physiology
Introduction
Regular physical activity, which can include structured exercise in a variety of forms, offers a net benefit for most individuals with diabetes. The benefits for individuals with type 2 diabetes are undisputed. Regular physical activity enhances insulin sensitivity, increases cardiorespiratory fitness, improves glycemic control, reduces the risk of cardiovascular mortality, and enhances psychosocial well-being.1 However, a paradox exists in the therapeutic lifestyle management of type 1 diabetes. Although important for maintaining or improving overall health and fitness, most exercise training studies in this population fail to show objective improvements in glycemic control.2,3 The evidence for this paradox in the literature extends to the observation that athletes with type 1 diabetes have impaired metabolic control even as compared to sedentary individuals with type 1 diabetes.4 Fear of hypoglycemia underlies this paradox, which generally leads to overcompensation of additional carbohydrate intake for exercise and excessive basal and bolus insulin reductions.5,6
Many experts in the field believe that continuous glucose monitoring (CGM) will increase the likelihood that physically active people with diabetes can actually improve overall metabolic control. First, becoming more aware of glycemic excursions during exercise may empower the individual with type 1 diabetes to make clinical decisions at the time of exercise. Second, knowledge of long-term trends in blood sugar prior to, during, and after exercise should help the individual plan for future exercise by estimating extra carbohydrate intake for exercise and more effective adjustment of basal and bolus insulin doses.7 Finally, for people with type 2 diabetes, CGM appears to hold promise as a motivational tool to become more physically active, which in turn will enhance diabetes management. Despite potential advantages of CGM, this tool has limitations in accuracy and acceptability, and therefore all benefits need to be weighed against these risks. This review aims to guide the reader to use this technology to its maximum advantage by providing an overview of technical features, performance characteristics, and clinical utility, all balanced against the limitations that may be more prominent during physical activity.
Exercise Physiology
Exercise Challenges Normal Glucose Homeostasis
Exercise and sport are common physiological stressors that cause perturbation to glucose homeostasis and energy needs. Exercise can be categorized into two different types: aerobic and anaerobic, depending on the speed and force of muscle contraction and the utilization of energy substrates. These two categories of exercise have diverging effects on blood glucose levels in persons with diabetes.8 A brief overview of the physiology behind glucose control is useful to understand these differences in glycemic response and how CGM may be a useful tool to track blood glucose levels during and after exercise.
The amount of glucose that normally circulates in the blood of a person weighing 70 kg is only estimated to be 4 g, and this glucose is critical for normal function in many tissues.9 At the onset of moderate-intensity aerobic exercise, glucose production by the liver increases 5- to 10-fold to match peripheral glucose disposal into working muscle, or if not done, circulating glucose levels will drop. In healthy, nondiabetes patients, glucose production can be up to 10 mg/kg body mass/min during high-intensity aerobic exercise (i.e., 50–70% of maximal aerobic capacity, VO2max) with very minimal changes in circulating glucose concentration.10 During intense anaerobic exercise that typically lasts only seconds to minutes, hepatic glucose production may reach 15 mg/kg body mass/min, an amount that exceeds muscular glucose disposal.11 Control of glucose homeostasis during exercise is dictated by a complex interaction between multiple hormonal regulators (e.g., insulin, glucagon, catecholamines, and glucocorticoids), the nervous system, and various molecular regulators within skeletal muscle and liver, allowing for precise control of glucose concentration during most activities. In persons with type 1 diabetes, however, control of glucose homeostasis during exercise is extremely challenging, as insulin levels cannot change rapidly in response to exercise, and there may be deficiencies or exaggerations in other hormonal responses.8 As a result of a variety of unpredictable factors, exercise may cause either hypoglycemia or hyperglycemia in persons with type 1 diabetes.
Hypoglycemia and Hyperglycemia and Exercise
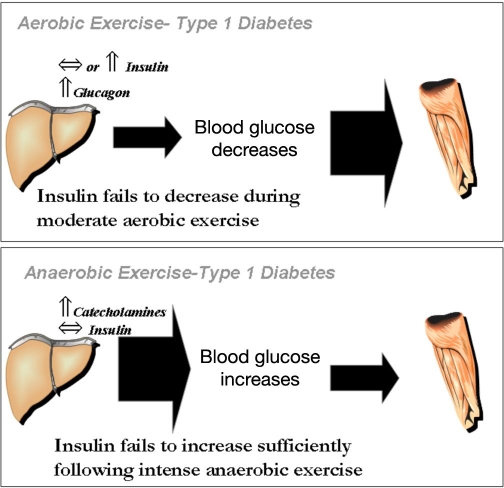
A schematic representation of the effects of aerobic and anaerobic exercise on blood glucose levels in persons with type 1 diabetes is shown in Figure 1. Typically, prolonged moderate-intensity aerobic exercise (i.e., 30–70% of one's VO2max) causes a reduction in glucose concentrations because of a failure in circulating insulin levels to decrease at the onset of exercise.12 During this type of physical activity, glucose utilization may be as high as 1.5 g/min in adolescents with type 1 diabetes13 and exceed 2.0 g/min in adults with type 1 diabetes,14 an amount that quickly lowers circulating glucose levels. Persons with type 1 diabetes have large interindividual differences in blood glucose responses to exercise, although some intraindividual reproducibility exists.15 The wide ranging glycemic responses among individuals appears to be related to differences in pre-exercise blood glucose concentrations, the level of circulating counterregulatory hormones and the type/duration of the activity.2
Figure 1.
Mechanisms for exercise-associated hypoglycemia and hyperglycemia in type 1 diabetes. During aerobic exercise, a failure in circulating insulin levels to decrease in individuals with type 1 diabetes limits glucose production by the liver while facilitating an increase in glucose disposal into skeletal muscle. Because of the mismatch in glucose production and utilization, circulating glucose levels drop and hypoglycemia can occur (upper panel). Prior exposure to either aerobic exercise or hypoglycemia also blunts glucose production during subsequent exercise by lowering glucose counterregulatory responses (i.e., glucagon and catecholamines). This makes the active person susceptible to frequent exposure to hypoglycemia. In contrast, during anaerobic exercise, a rise in catecholamines and a failure in circulating insulin levels to increase at the end of vigorous exercise in individuals with type 1 diabetes increases glucose production by the liver while limiting glucose disposal into skeletal muscle (lower panel). Because of the mismatch in glucose production and utilization, circulating glucose levels rise and hyperglycemia can occur.
Anaerobic exercise, on the other hand, frequently causes increases in blood glucose levels in persons with type 1 diabetes because of elevations in catecholamine levels that are not offset by an increase in insulin availability.16 Exercise-induced hyperglycemia may last for hours after the end of the activity and can compromise overall glycemic control and subsequent exercise performance. Corrective measures for exercise-induced hyperglycemia usually requires additional insulin,17 however, guidelines are not currently available to assist patients, and the risk for postexercise, late-onset hypoglycemia increases if too much insulin is administered. In reality, many sporting activities are a combination of both aerobic and anaerobic phases, making blood glucose control in active individuals with type 1 diabetes particularly challenging.18 For some, just the anticipatory stress of competition can cause hyperglycemia.
Late-Onset Postexercise Hypoglycemia
Most patients with type 1 diabetes quickly realize that exercise in all forms can increase the risk of hypoglycemia for several hours after the end of activity. In a laboratory setting used to quantify the periods of elevated insulin sensitivity caused by prior exercise, glucose disposal was found to be heightened immediately after the end of exercise and then again 7–11 h later,19 perhaps as a biphasic response to muscle and liver glycogen deposition. Moreover, the hormonal responses to exercise and to hypoglycemia are similar and appear to promote a viscous cycle of repeated autonomic failure during either exercise or insulin-induced hypoglycemia.20 The length of time for the effect of prior exercise on subsequent counterregulation failure to hypoglycemia is unknown but may last for at least 24 h.21 Thus active people with type 1 diabetes may be at particular risk for autonomic failure and hypoglycemia unawareness. It is well-known that exercise masks some of the symptoms of hypoglycemia, such as sweating, dizziness, and tiredness, and young people with type 1 diabetes are unable to estimate their glucose levels accurately or sense hypoglycemia during exercise.6
Continuous Glucose Monitoring Technology
Given the problems of exercise-associated disturbances in glycemia described earlier and the risk for hypoglycemia unawareness, frequent monitoring of glucose is essential for active individuals with type 1 diabetes. Most exercise-related guidelines recommend self-monitoring of blood glucose (SMBG) with capillary blood at least twice before exercise and every 30 min during the exercise as well as hours into recovery.2 This recommendation for frequent SMBG is difficult to adhere to for some, because it requires a pause in activity, a limitation that would be ameliorated with CGM. Moreover, a fear of exercise associated hypoglycemia is a major barrier to exercise participation in adults5 and in youth,6 and CGM might help increase self-efficacy during sport. Finally, CGM has the potential to assist active persons with diabetes by recording exercise-associated changes in blood glucose levels, and this information may be useful in developing appropriate insulin and carbohydrate modifications during times of increased activity. A brief overview of CGM technology is provided in the next section.
Continuous glucose monitoring devices have been available since the late 1990s and were developed for the measurement of interstitial glucose levels in subcutaneous tissue as a reflection of circulating glucose concentrations. The main components of CGM include a transcutaneous sensor inserted into the abdomen or arm, a transmitter, and a receiver that is typically worn on a belt or carried in a pocket. With this technology, the implanted sensor is exposed to glucose within the interstitial fluid of subcutaneous tissue (arm or abdomen). The glucose in the interstitial fluid reacts with the glucose oxidase enzyme, creating an electrical charge that is transmitted to the recorder, which estimates circulating blood glucose concentration based on an algorithm and some assumptions about the equilibrium of these two regions. Readings are transmitted every few minutes to the receiver, and data can be downloaded and stored on a PC. After the initial setup, a period of calibration follows that requires regular finger-prick measurements and data entry into the CGM system. Therefore, CGM must be used as an adjunct to, and does not replace, SMBG. Continuous glucose monitoring requires calibration by SMBG, and any corrective action based on CGM values requires confirmation by SMBG.
Initially, CGM technology was limited to retrospective data, but newer units now have the ability to provide data in real time with the intent that patients can be more readily prepared to take action against impending hypoglycemia and hyperglycemia. The potential benefits of using CGM for patients include a better understanding of their dynamic pattern of glycemic control and their being made aware of significant glucose excursions. In real-time units, trends in glucose can become more evident, either with graphs or with directional arrows, showing the user which direction their glucose level is heading and how quickly it is rising or falling. These are designed to allow the patient to make more informed decisions about managing their glucose compared to what information they receive from a finger-stick sample, which does not indicate the direction or rate of change.
Despite their availability, CGM use is somewhat limited for a number of reasons: (1) expense of the components (receiver/transmitter and disposable sensors), (2) issues of accuracy in reflecting blood glucose levels, (3) a paucity of data from large-scale clinical trials that have shown their utility in reducing glycemic excursions and improving hemoglobin A1c (HbA1c), and (4) the technology is evolving rapidly and patients may be waiting for when they feel that sufficient advancement has been made in accuracy, cost, and features to warrant their use.
Continuous Glucose Monitoring Devices on the Market
Several “professional” and “personal” CGM devices are currently available, and these differ somewhat in their technical characteristics. The professional devices are intended for use by clinics and physicians as tools to detect asymptomatic hypoglycemia, posthypoglycemic hyperglycemia, causes for suboptimal glycemic control and to assess trends in glycemic patterns. The professional CGM device is loaned to the patient for a short period (days to weeks), after which time the health care provider makes therapy adjustments based on the gathered information. Professional use systems include Medtronic's CGMS® iPro™ Recorder, Medtronic's CGMS System Gold™ (www.medtronic.com), and Menarini's GlucoDay®S (www.menarini.com). A major difference between these two manufacturers is that the GlucoDayS consists of a micropump and a biosensor coupled with a micro-dialysis system, while the Medtronic units use the implanted enzyme-based technology described in the previous section.
Personal CGM devices are owned by the patient and used on an on-going basis to help make immediate therapy adjustments based on real-time glucose information (Table 1). All personal CGM devices have the ability to illustrate glucose concentrations in “real time” for the user. Some provide trend arrows and/or graphs, and all have “alerts” and/or “alarms” that allow for proactive measures to limit exposure to hypoglycemia and hyperglycemia. An alert is designed to warn the user of a drop in glycemia that is approaching the hypoglycemic range (or a rise in glucose is occurring that is approaching hyperglycemia), while alarms are designed to notify if the user is outside their targeted glucose range. Historical CGM data recorded by the device can also be reviewed by both the patient and the health care provider to make future therapy adjustments. Currently, there are three personal CGM (real time) devices available: DexCom SEVEN® CGM System™ (www.dexcom.com), Medtronic Guardian® Real-Time and Paradigm® Real-Time (www.medtronic.com), and the Abbott FreeStyle Navigator® (http://www.abbottdiabetescare.com). Real-time CGM devices are designed for patients to use, either intermittently or continuously, as an adjunct to their SMBG. Medtronic Minimed's Paradigm Real-Time system integrates the sensor and the transmitter with an insulin pump that also serves as the monitor so that a separate device is not necessary. This product is often referred to as a “sensor augmented insulin pump.”
Table 1.
Personal Continuous Glucose Monitoring Currently Available
| Features | Guardian-RT (Medtronic Diabetes) | DexCom Seven (DexCom) | FreeStyle Navigator (Abbott Diabetes Care) |
|---|---|---|---|
| Update frequency (minutes) | 5 | 5 | 1 |
| Sensor lifespan approved for (days) | 3 | 7 | 5 |
| Start-up time (hours) | 2 | 2 | 10 |
| Customized hypoglycemic and hyperglycemic alarms | Yes | Yes | Yes |
| Trend arrows | Yes | No | Yes |
| Glucose graphs | Yes (3, 6, 12, and 24- h) | Yes (1, 3, and 9- h) with custom trend lines | Yes (2, 4, 6, and 24- h) |
| Predictive alerts | Yes | No | Yes |
| Approved for ages (years) | 7+ | 18+ | 18+ |
| Event markers for exercise | Yes | No | Yes |
All CGM technology has some limitations that need to be understood by the user and his/her health care provider. One of the major limitations is the difference due to lag time between blood glucose levels and subcutaneous glucose levels, which is estimated to be between 5 and 15 min, depending on the rate of glucose change.22 Thus, when glucose is rapidly falling, as can be the case during exercise, interstitial glucose lags behind blood, and there may be a failure to document the true drop in blood glucose. Because of this limitation, there may be a tendency for new users to “over bolus” insulin when interstitial glucose is still rising, even though blood glucose may, in fact, be leveling off or even dropping. Similarly, users may consume more carbohydrates than necessary when treating hypoglycemia, because they fail to observe an immediate rise in their CGM glucose values. In practical terms, patients need to be made aware that, because of the physiologic lag time when the glucose is either rising or falling rapidly, there can be a marked difference between the sensor reading and finger-stick measurements. Another related and important consideration when using CGM technology is that sensor calibration needs to be performed when blood (and interstitial) glucose is in a steady state. It has been recommended that calibrations not be entered if the glucose is changing by more than 2 mg/dl/min.23
Issues of accuracy and the ability to “alarm” appropriately to hyperglycemia and hypoglycemia must be considered when recommending and counseling patients on CGM. The best assessment of CGM accuracy is to test the device against a gold standard [such as Yellow Springs Instrument (YSI) assessment of plasma glucose] and plot the data on a Clarke's Error Grid analysis.24 This analysis makes use of a scatter plot of the CGM device on the Y axis and the reference standard (i.e., YSI plasma glucose analyzer) on the X axis. The scatter plot is divided into five regions: region A are those values within 20% of the reference sensor, region B contains points that are outside 20% but would not lead to inappropriate treatment, region C are those points leading to unnecessary treatment, region D are those points indicating a potentially dangerous failure to detect hypo- or hyperglycemia, and region E are those points that would confuse treatment of hypoglycemia for hyperglycemia and vice versa. In a study of 16 individuals contributing over 1000 CGM and plasma glucose data pairs, a vast majority of readings (93.7%) occupied regions A and B, while the remaining 6.3% occupied region D (representing failures to correct unforeseen hypoglycemia).25 Although representing a relatively small proportion of paired readings, this suggests that the technology is not fully reliable for identifying hypoglycemia. Although the reasons are unclear, there are also reports of falsely detecting nocturnal hypoglycemia in tightly controlled patients with type 1 diabetes.26 Although randomized clinical studies demonstrated less exposure to hypoglycemia when using (unblinded) continuous glucose monitors, there seems to be general agreement that the accuracy of these systems is not sufficient to provide perfectly reliable hypoglycemia alarms without substantial false positive and false negative alarms.26 Patients are sometimes frustrated by false positive “alarms” that wake them up at night and that are subsequently not confirmed by capillary testing. However, an appropriate pending hypoglycemia “alert” is obviously of major benefit. Unlike an alarm, which is meant to indicate that the blood glucose is in the hypoglycemia range at the moment of the alarm, an alert is signaled when the glucose level is dropping quickly, indicating that the risk of subsequent hypoglycemia is high. In this way, a patient can be alerted to a dropping glucose level, examine the visual graph of glucose trends on the monitor, and take corrective action if necessary. Clearly, the alert benefits the patient who has a good working knowledge of target blood glucose levels and an understanding of peak insulin action.
Some recent evidence using glucose clamp methodology in 34 adults with type 1 diabetes suggests that the accuracy of the Navigator and GlucoDay is superior to the Guardian and DexCom during hypoglycemia, while all units are comparable during euglycemia.27 Despite shortcomings in accuracy, CGM tends to have good precision28 and thus the ability to track the direction of change of blood glucose concentration.
Research is ongoing on the utility of CGM to improve glycemic control as are the changes in the units themselves, and readers are encouraged to seek other articles for a full review of this literature.29,30 A few short-term CGM studies demonstrate reductions in HbA1c levels and reduced time spent in hypoglycemic and hyperglycemic ranges.31–33 In a multinational randomized control study, compared to patients who did not use CGM, continual use of the CGM was associated with lower HbA1c at 1 and 3 months in adolescents with type 1 diabetes.34,35 Several additional well-designed randomized controlled clinical trials have been launched with the intention of testing the hypothesis that using real-time CGM results in improved metabolic control and less glycemic variability in type 1 diabetes. Compared with just an insulin pump and SMBG, the sensor-augmented pump lowers the occurrence of hypoglycemia, and those with greater sensor utilization show a greater improvement in HbA1c.36 To date, no specific studies have been conducted to determine if this technology is particularly useful for physically active persons with type 1 diabetes, although a number of small trials have shown the utility of the device during and after exercise (discussed later). Some recent studies also show that CGM is a useful adjunct to help increase exercise adherence in individuals with type 2 diabetes. These studies are also highlighted in the following section.
Summary of Clinical Research on Continuous Glucose Monitoring in the Context of Exercise
A number of relatively small clinical trials have investigated the utility of using CGM with exercise in diabetes. One of the first questions to be answered was whether CGM could report glucose levels during exercise and whether the units could measure accurately the drop in blood glucose associated with prolonged moderate intensity exercise. One of the first CGM technologies tested in an exercise setting was the GlucoWatch Biographer (Cygnus Inc., Redwood City, CA), the first Food and Drug Administration-approved CGM unit available on the market. This was a wristwatch-like device that created an electrical charge to stimulate the movement of interstitial glucose to the skin surface, which in turn could be sampled by an abrasive sensor on the underside of the watch. (Animas Corporation, a Johnson & Johnson company, purchased the intellectual property and all the assets of the original manufacturer of the GlucoWatch and is no longer offering it for sale.) In a study designed specifically to test the utility of the GlucoWatch Biographer during exercise in nine subjects (only five had diabetes), Nunnold and colleagues37 reported that the device worked best under resting conditions and that the effectiveness fell as the exercise intensity increased. During vigorous exercise, in fact, the GlucoWatch failed to capture glucose data at all, likely because of movement artifact and increased sweating rates.37 A similarly designed study of the GlucoWatch G2TM Biographer in children showed an overall 60% skip rate during exercise, with half of the skip rates due to perspiration.38 Thus the GlucoWatch was deemed to have limited utility to detect changes in blood glucose during exercise.
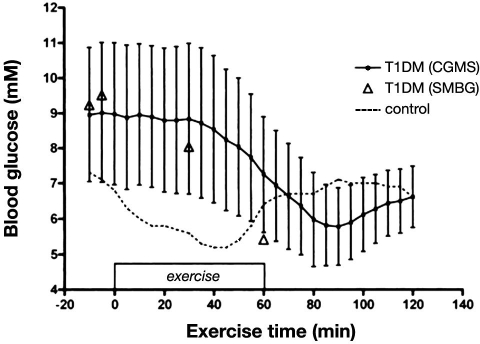
Now with implanted sensors, the utility of CGM is again being tested in an exercise setting. A small pilot study of five adults with diabetes was among the first to show that active individuals with type 1 have a high level of self-efficacy when using CGM (GuardianRT, Medtronic) and that the unit is able to track reasonably the drop in glycemia during 45 min of vigorous cycling exercise, with no missing data.39 However, CGM tended to overestimate glucose levels during aerobic exercise compared to measures made simultaneously in capillary blood,39 perhaps because of the 10–20 min time delay in equilibrium between interstitial fluid and capillary glucose (Figure 2). This sort of delay observed with exercise was attributed to the rapid fall in circulating glucose levels and was similar to the 4–10 min delay that these units have consistently shown at rest when glucose is changing rapidly.28 The DirecNet group also reported that CGM (FreeStyle Navigator) accurately tracked the magnitude of drop in blood glucose induced by exercise in 19 children and adolescents with type 1 diabetes with about a 10 min delay.40 However, as expected, because the sensor glucose lagged behind blood glucose in the response, the device underestimated the true rate of fall in most subjects.40 In one study, designed to determine if the number of daily calibrations influence the accuracy of the CGM (CGMS, Medtronic), it was shown that the unit correctly detected only 65% of cases of exercise-induced hypoglycemia (reference glucose ≤ 70mg/dl) when three calibrations per day were used and only 69% of exercise-induced hypoglycemia when four calibrations per day were used.23 Interestingly, nighttime accuracy of the CGMS improved when daytime calibrations (prelunch and predinner) were removed, leaving only calibrations at 9:00 pm and 6:00 am (i.e., twice per day). Taken together, the lag in CGM in detecting the rapid drop in blood glucose during exercise and thus the failure to accurately assess exercise-induced hypoglycemia somewhat limit the utility of currently available real-time units. During vigorous anaerobic activity when blood glucose levels typically climb rapidly (see earlier discussion), CGM also lags in its response time and thus underestimates true plasma concentrations.41 Impressively, the accuracy of these sensors does not appear to be affected by reductions in body pH that occur with very vigorous exercise.41
Figure 2.
Glucose levels during 60 min of moderate-intensity “spin class” cycling using CGM and SMBG. Glucose values are shown for five subjects with type 1 diabetes using CGM (continuous line) and capillary glucose (triangles). Continuous glucose monitoring values are also shown for one nondiabetes control during exercise. Values for CGM values in the subjects with diabetes are shown in means and standard errors of the mean. T1DM, type 1 diabetes. Reprinted by permission of Diabetes Technology & Therapeutics.39
Some work has shown that CGM may be very useful in certain exercise situations in which vigilant SMBG is just not practical. For example, team type 1 cyclists with diabetes use the FreeStyle Navigator during their race across America to monitor their glucose continuously (www.freestylenavigator.com). In one of the best examples of the utility of CGM, the Medtronic Guardian was shown to be very useful for scuba diving when symptoms of hypoglycemia may be masked and SMBG is impossible.42
Since CGM reports glucose values in real time and these data may be stored and examined retrospectively, the tools might be useful to show patients how exercise alters glucose levels. By using a CGM satisfaction scale, the DirecNet group reported that the use of the Navigator helped some children (about 45% of the users) learn how exercise affected their glucose levels.33 A majority of the youth in that study found that CGM was helpful in making insulin adjustments and helped to prevent low blood glucose from happening. However, nearly half of the subjects (46%) felt that CGM interfered with sports and playing outside.33
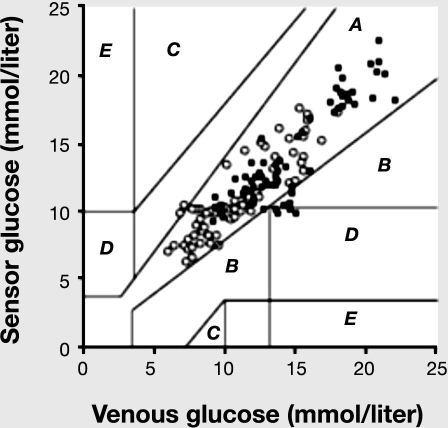
The use of the GlucoDay device in subcutaneous tissue also tracks the drop in glucose during light and heavy exercise with good accuracy.43 Clarke's Error Grid analysis of these data showed that ∼90% of glucose paired values fell within range A and 6.4% within range B, while only one pair of values (0.7%) obtained during high-intensity exercise fell on the edge of range D (Figure 3).
Figure 3.
Clarke's Error Grid analysis for GlucoDay sensor glucose concentration and venous glucose concentration as measured by standard laboratory practice (Beckman analyzer, Fullerton, CA) before, during, and in 1 h recovery from low- and high-intensity exercise (n = 141). Accuracy of the GlucoDay during and after exercise was deemed acceptable as 92.9% of glucose paired values fell within region A and 6.4% within region B, while only one pair of values (0.7%) obtained during high-intensity exercise fell on the edge of region D. Open circles, low-intensity exercise; full squares, high-intensity exercise. Reprinted by permission of Diabetes & Metabolism.43
The use of CGM to measure glycemia in patients with type 2 diabetes during exercise and to determine if it is a useful motivational tool is also of research interest. In one study, CGMS (blinded) overestimated blood glucose levels in a small group of newly diagnosed patients with type 2 diabetes during 60 min of moderate-intensity cycling, again likely because of the delay in detecting the drop in glycemia.44 In that same study, however, cycling exercise improved next day glucose concentrations as measured by the time spent within 10% of fasting glucose concentrations.44 In a randomized control trial (RCT) of 57 patients with type 2 diabetes, Allen and associates45 found that the introduction to CGM along with physical activity counseling improved self-efficacy around exercise, increased physical activity behaviors, and lowered HbA1c, blood pressure, and body mass index. This is an important observation, as it shows that the combination of two behavior modification tools (CGM and physical activity counseling) work synergistically to increase physical activity levels and thereby reduce the risk of diabetes-related complications. In line with this, in a RCT of 65 patients with poorly controlled type 2 diabetes, the use of real-time CGM (3 days at a time for 3 months) increased physical activity patterns and improved HbA1c from 9.1% to 8.0%, proving much more effective than SMBG alone.46 Specifically, in the real-time CGM group, there were significant reductions in total daily calorie intake, body mass, body mass index, and postprandial glucose levels and a significant increase in total exercise time per week by the end of 3 months of use.46 Thus real-time CGM may be particularly useful in modifying a patient's dietary and physical activity habits, thereby ultimately contributing to an improvement in their glycemic control.
Another potentially important use of CGM for active people with diabetes is the ability to track and alarm during nocturnal hypoglycemia or hyperglycemia. As mentioned earlier, late-onset, postexercise hypoglycemia is common in active individuals with type 1 diabetes, with events frequently occurring at night while the patient may be sleeping and the symptoms of hypoglycemia masked (i.e., nocturnal hypoglycemia).39,47 Continuous glucose monitoring (Guardian RT and CGMS Gold, Medtronic) was used to document high rates of nocturnal hypoglycemia in a sports camp for athletes with type 1 diabetes.48 In this regard, the location of the sensor may impact the accuracy of detecting glucose levels at night, as the abdominal site (versus the arm site) tends to underestimate venous values and increases the potential for false hypoglycemic alarms.43
Other Considerations for the Current Technology for the Active Person with Diabetes
As mentioned earlier, the current CGM technology has a significant time delay in detecting changes in glucose, and these changes can be particularly rapid during exercise. Thus not all episodes of exercise-induced hypoglycemia are detected quickly enough when using the current technology found in real-time units. Related to this is the temptation for patients to over bolus during periods of hyperglycemia and the potential failure of treating an impending hypoglycemic event.22 In addition, the size of these devices and the requirement for an implanted sensor and externally worn transmitter may be a barrier for some activities that require vigorous movement and/or physical contact. The Medtronic MiniLink Real-Time transmitter is the smallest on the market and may be worn during swimming. The systems can also be labor intensive in that they currently require insertion of a subcutaneous sensor every 3–7 days and that they do not eliminate the need for capillary blood glucose testing. Using CGM initially can create a situation of information overload, both with the amount of data they generate and the technical maneuvers that they require, which some patients and caregivers are unable to overcome. Finally, the immediate cost of the monitors and the sensors far outweigh the current costs of conventional glucose monitoring, and this particular issue may be a major barrier if it is not reconciled with insurance providers.
Conclusion
In summary, CGM devices are useful as adjunct tools for active people with diabetes, although they are not yet capable of being used as a replacement for capillary blood glucose testing. As adjunct tools, they can have an impact by reducing exposure to hypoglycemia and hyperglycemia and by reducing glycosylated hemoglobin A1c in those with suboptimal control. Continuous glucose monitoring can be used for the evaluation of the effects of exercise on glucose levels and to strategize the timing of insulin adjustments and extra carbohydrates relative to activity, but they may not be useful as tools to detect exercise-induced hypoglycemia because of the time delay associated with the equilibrium between interstitial fluid and the capillary blood. Active patients with type 1 diabetes may feel a higher level of self-efficacy and sense of reassurance, however, in knowing what direction their blood glucose is heading toward during exercise and the ability to respond rapidly to glycemic excursions may improve their exercise recovery. It is currently unclear if CGM technology can help to improve the poor metabolic control that is sometimes observed in athletes with type 1 diabetes. In persons with type 2 diabetes, this technology appears to be a useful adjunct to exercise counseling and lifestyle intervention. This may be because many patients may not be able to sufficiently apply their diabetes knowledge on account of the limited feedback that episodic capillary blood glucose monitors provide. Additional improvements in sensor technology, user interface features, algorithms designed to help detect rapid changes in glycemia, and a reduction in cost will likely further enhance their acceptance into routine care.
Abbreviations
- CGM
continuous glucose monitoring
- HbA1c
hemoglobin A1c
- RCT
randomized control trial
- SMBG
self-monitoring of blood glucose
- YSI
Yellow Springs Instrument
References
- 1.Sigal RJ, Kenny GP, Wasserman DH, Castaneda-Sceppa C, White RD. Physical activity/exercise and type 2 diabetes: a consensus statement from the American Diabetes Association. Diabetes Care. 2006;29(6):1433–1438. doi: 10.2337/dc06-9910. [DOI] [PubMed] [Google Scholar]
- 2.Wasserman DH, Zinman B. Exercise in individuals with IDDM. Diabetes Care. 1994;17(8):924–937. doi: 10.2337/diacare.17.8.924. [DOI] [PubMed] [Google Scholar]
- 3.Sigal R, Kenny G, Oh P, Perkins BA, Plotnikoff RC, Prud’homme D, Riddell MC. Physical activity and diabetes. Canadian Diabetes Association Clinical Practice Guidelines Expert Committee. Canadian Diabetes Association 2008 clinical practice guidelines for the prevention and management of diabetes in Canada. Can J Diab. 2008;32(1):S37–S39. [Google Scholar]
- 4.Ebeling P, Tuominen JA, Bourey R, Koranyi L, Koivisto VA. Athletes with IDDM exhibit impaired metabolic control and increased lipid utilization with no increase in insulin sensitivity. Diabetes. 1995;44(4):471–477. doi: 10.2337/diab.44.4.471. [DOI] [PubMed] [Google Scholar]
- 5.Brazeau AS, Rabasa-Lhoret R, Strychar I, Mircescu H. Barriers to physical activity among patients with type 1 diabetes. Diabetes Care. 2008;31(11):2108–2109. doi: 10.2337/dc08-0720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Riddell MC, Iscoe KE. Physical activity, sport, and pediatric diabetes. Pediatr Diabetes. 2006;7(1):60–70. doi: 10.1111/j.1399-543X.2006.00146.x. [DOI] [PubMed] [Google Scholar]
- 7.Perkins BA, Riddell MC. Type 1 diabetes and exercise. Part II: using the insulin pump to maximum advantage. Can J Diab. 2006;30:72–80. [Google Scholar]
- 8.Riddell MC, Perkins BA. Type 1 diabetes and exercise. Part I: applications of exercise physiology to patient management during vigorous activity. Can J Diab. 2006;30:63–71. [Google Scholar]
- 9.Wasserman DH. Four grams of glucose. Am J Physiol Endocrinol Metab. 2009;296(1):E11–E21. doi: 10.1152/ajpendo.90563.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raguso CA, Coggan AR, Gastaldelli A, Sidossis LS, Bastyr EJ, III, Wolfe RR. Lipid and carbohydrate metabolism in IDDM during moderate and intense exercise. Diabetes. 1995;44(9):1066–1074. doi: 10.2337/diab.44.9.1066. [DOI] [PubMed] [Google Scholar]
- 11.Sigal RJ, Fisher SJ, Manzon A, Morais JA, Halter JB, Vranic M, Marliss EB. Glucoregulation during and after intense exercise: effects of alpha-adrenergic blockade. Metabolism. 2000;49(3):386–394. doi: 10.1016/s0026-0495(00)90374-3. [DOI] [PubMed] [Google Scholar]
- 12.Camacho RC, Galassetti P, Davis SN, Wasserman DH. Glucoregulation during and after exercise in health and insulin-dependent diabetes. Exerc Sport Sci Rev. 2005;33(1):17–23. [PubMed] [Google Scholar]
- 13.Riddell MC, Bar-Or O, Hollidge-Horvat M, Schwarcz HP, Heigenhauser GJ. Glucose ingestion and substrate utilization during exercise in boys with IDDM. J Appl Physiol. 2000;88(4):1239–1246. doi: 10.1152/jappl.2000.88.4.1239. [DOI] [PubMed] [Google Scholar]
- 14.Robitaille M, Dubé MC, Weisnagel SJ, Prud’homme D, Massicotte D, Péronnet F, Lavoie C. Substrate source utilization during moderate intensity exercise with glucose ingestion in type 1 diabetic patients. J Appl Physiol. 2007;103(1):119–124. doi: 10.1152/japplphysiol.01462.2006. [DOI] [PubMed] [Google Scholar]
- 15.Temple MY, Bar-Or O, Riddell MC. The reliability and repeatability of the blood glucose response to prolonged exercise in adolescent boys with IDDM. Diabetes Care. 1995;18(3):326–332. doi: 10.2337/diacare.18.3.326. [DOI] [PubMed] [Google Scholar]
- 16.Sigal RJ, Purdon C, Fisher SJ, Halter JB, Vranic M, Marliss EB. Hyperinsulinemia prevents prolonged hyperglycemia after intense exercise in insulin-dependent diabetic subjects. J Clin Endocrinol Metab. 1994;79(4):1049–1057. doi: 10.1210/jcem.79.4.7962273. [DOI] [PubMed] [Google Scholar]
- 17.Marliss EB, Vranic M. Intense exercise has unique effects on both insulin release and its roles in glucoregulation: implications for diabetes. Diabetes. 2002;51(Suppl 1):S271–S283. doi: 10.2337/diabetes.51.2007.s271. [DOI] [PubMed] [Google Scholar]
- 18.Guelfi KJ, Jones TW, Fournier PA. New insights into managing the risk of hypoglycaemia associated with intermittent high-intensity exercise in individuals with type 1 diabetes mellitus: implications for existing guidelines. Sports Med. 2007;37(11):937–946. doi: 10.2165/00007256-200737110-00002. [DOI] [PubMed] [Google Scholar]
- 19.McMahon SK, Ferreira LD, Ratnam N, Davey RJ, Youngs LM, Davis EA, Fournier PA, Jones TW. Glucose requirements to maintain euglycemia after moderate-intensity afternoon exercise in adolescents with type 1 diabetes are increased in a biphasic manner. J Clin Endocrinol Metab. 2007;92(3):963–968. doi: 10.1210/jc.2006-2263. [DOI] [PubMed] [Google Scholar]
- 20.Ertl AC, Davis SN. Evidence for a vicious cycle of exercise and hypoglycemia in type 1 diabetes mellitus. Diabetes Metab Res Rev. 2004;20(2):124–130. doi: 10.1002/dmrr.450. [DOI] [PubMed] [Google Scholar]
- 21.Briscoe VJ, Tate DB, Davis SN. Type 1 diabetes: exercise and hypoglycemia. Appl Physiol Nutr Metab. 2007;32(3):576–582. doi: 10.1139/H07-025. [DOI] [PubMed] [Google Scholar]
- 22.Wolpert HA. The nuts and bolts of achieving end points with real-time continuous glucose monitoring. Diabetes Care. 2008;31(Suppl 2):S146–S149. doi: 10.2337/dc08-s238. [DOI] [PubMed] [Google Scholar]
- 23.Buckingham BA, Kollman C, Beck R, Kalajian A, Fiallo-Scharer R, Tansey MJ, Fox LA, Wilson DM, Weinzimer SA, Ruedy KJ, Tamborlane WV Diabetes Research In Children Network (DirecNet) Study Group. Evaluation of factors affecting CGMS calibration. Diabetes Technol Ther. 2006;8(3):318–325. doi: 10.1089/dia.2006.8.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clarke WL. The original Clarke Error Grid Analysis (EGA) Diabetes Technol Ther. 2005;7(5):776–779. doi: 10.1089/dia.2005.7.776. [DOI] [PubMed] [Google Scholar]
- 25.Clarke WL, Anderson S, Farhy L, Breton M, Gonder-Frederick L, Cox D, Kovatchev B. Evaluating the clinical accuracy of two continuous glucose sensors using continuous glucose-error grid analysis. Diabetes Care. 2005;28(10):2412–2417. doi: 10.2337/diacare.28.10.2412. [DOI] [PubMed] [Google Scholar]
- 26.McGowan K, Thomas W, Moran A. Spurious reporting of nocturnal hypoglycemia by CGMS in patients with tightly controlled type 1 diabetes. Diabetes Care. 2002;25(9):1499–1503. doi: 10.2337/diacare.25.9.1499. [DOI] [PubMed] [Google Scholar]
- 27.Kovatchev B, Anderson S, Heinemann L, Clarke W. Comparison of the numerical and clinical accuracy of four continuous glucose monitors. Diabetes Care. 2008;31(6):1160–1164. doi: 10.2337/dc07-2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boyne MS, Silver DM, Kaplan J, Saudek CD. Timing of changes in interstitial and venous blood glucose measured with a continuous subcutaneous glucose sensor. Diabetes. 2003;52(11):2790–2794. doi: 10.2337/diabetes.52.11.2790. [DOI] [PubMed] [Google Scholar]
- 29.Hirsch IB, Armstrong D, Bergenstal RM, Buckingham B, Childs BP, Clarke WL, Peters A, Wolpert H. Clinical application of emerging sensor technologies in diabetes management: consensus guidelines for continuous glucose monitoring (CGM) Diabetes Technol Ther. 2008;10(4):232–246. doi: 10.1089/dia.2008.0016. [DOI] [PubMed] [Google Scholar]
- 30.Chetty VT, Almulla A, Odueyungbo A, Thabane L. The effect of continuous subcutaneous glucose monitoring (CGMS) versus intermittent whole blood finger-stick glucose monitoring (SBGM) on hemoglobin A1c (HBA1c) levels in type I diabetic patients: a systematic review. Diabetes Res Clin Pract. 2008;81(1):79–87. doi: 10.1016/j.diabres.2008.02.014. [DOI] [PubMed] [Google Scholar]
- 31.Garg S, Zisser H, Schwartz S, Bailey T, Kaplan R, Ellis S, Jovanovic L. Improvement in glycemic excursions with a transcutaneous, real-time continuous glucose sensor: a randomized controlled trial. Diabetes Care. 2006;29(1):44–50. doi: 10.2337/diacare.29.01.06.dc05-1686. [DOI] [PubMed] [Google Scholar]
- 32.Bailey TS, Zisser HC, Garg SK. Reduction in hemoglobin A1C with real-time continuous glucose monitoring: results from a 12-week observational study. Diabetes Technol Ther. 2007;9(3):203–210. doi: 10.1089/dia.2007.0205. [DOI] [PubMed] [Google Scholar]
- 33.Buckingham B, Beck RW, Tamborlane WV, Xing D, Kollman C, Fiallo-Scharer R, Mauras N, Ruedy KJ, Tansey M, Weinzimer SA, Wysocki T Diabetes Research in Children Network (DirecNet) Study Group. Continuous glucose monitoring in children with type 1 diabetes. J Pediatr. 2007;151(4):388–393. doi: 10.1016/j.jpeds.2007.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Deiss D, Bolinder J, Riveline JP, Battelino T, Bosi E, Tubiana-Rufi N, Kerr D, Phillip M. Improved glycemic control in poorly controlled patients with type 1 diabetes using real-time continuous glucose monitoring. Diabetes Care. 2006;29(12):2730–2732. doi: 10.2337/dc06-1134. [DOI] [PubMed] [Google Scholar]
- 35.Deiss D, Hartmann R, Schmidt J, Kordonouri O. Results of a randomised controlled cross-over trial on the effect of continuous subcutaneous glucose monitoring (CGMS) on glycaemic control in children and adolescents with type 1 diabetes. Exp Clin Endocrinol Diabetes. 2006;114(2):63–67. doi: 10.1055/s-2006-923887. [DOI] [PubMed] [Google Scholar]
- 36.Hirsch IB, Abelseth J, Bode BW, Fischer JS, Kaufman FR, Mastrototaro J, Parkin CG, Wolpert HA, Buckingham BA. Sensor-augmented insulin pump therapy: results of the first randomized treat-to-target study. Diabetes Technol Ther. 2008;10(5):377–383. doi: 10.1089/dia.2008.0068. [DOI] [PubMed] [Google Scholar]
- 37.Nunnold T, Colberg SR, Herriott MT, Somma CT. Use of the noninvasive GlucoWatch Biographer during exercise of varying intensity. Diabetes Technol Ther. 2004;6(4):454–462. doi: 10.1089/1520915041705848. [DOI] [PubMed] [Google Scholar]
- 38.Tsalikian E, Beck RW, Kalajian A, Janz KF, Tansey MJ Diabetes Research in Children Network (DirecNet) Study Group. Function of the GlucoWatch G2 Biographer during exercise. Diabetes Technol Ther. 2005;7(1):230. doi: 10.1089/dia.2005.7.230. [DOI] [PubMed] [Google Scholar]
- 39.Iscoe KE, Campbell JE, Jamnik V, Perkins BA, Riddell MC. Efficacy of continuous real-time blood glucose monitoring during and after prolonged high-intensity cycling exercise: spinning with a continuous glucose monitoring system. Diabetes Technol Ther. 2006;8(6):627–635. doi: 10.1089/dia.2006.8.627. [DOI] [PubMed] [Google Scholar]
- 40.Wilson DM, Beck RW, Tamborlane WV, Dontchev MJ, Kollman C, Chase P, Fox LA, Ruedy KJ, Tsalikian E, Weinzimer SA DirecNet Study Group. The accuracy of the FreeStyle Navigator continuous glucose monitoring system in children with type 1 diabetes. Diabetes Care. 2007;30(1):59–64. doi: 10.2337/dc06-1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Davey RJ, Ferreira LD, Jones TW, Fournier PA. Effect of exercise-mediated acidosis on determination of glycemia using CGMS. Diabetes Technol Ther. 2006;8(4):516–518. doi: 10.1089/dia.2006.8.516. [DOI] [PubMed] [Google Scholar]
- 42.Adolfsson P, Örnhagen H, Jendle J. The benefits of continuous glucose monitoring and a glucose monitoring schedule in individuals with type 1 diabetes during recreational diving. J Diabetes Sci Technol. 2008;2(5):778–784. doi: 10.1177/193229680800200505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fayolle C, Brun JF, Bringer J, Mercier J, Renard E. Accuracy of continuous subcutaneous glucose monitoring with the GlucoDay in type 1 diabetic patients treated by subcutaneous insulin infusion during exercise of low versus high intensity. Diabetes Metab. 2006;32(4):313–320. doi: 10.1016/s1262-3636(07)70285-9. [DOI] [PubMed] [Google Scholar]
- 44.MacDonald AL, Philp A, Harrison M, Bone AJ, Watt PW. Monitoring exercise-induced changes in glycemic control in type 2 diabetes. Med Sci Sports Exerc. 2006;38(2):201–207. doi: 10.1249/01.mss.0000183852.31164.5a. [DOI] [PubMed] [Google Scholar]
- 45.Allen NA, Fain JA, Braun B, Chipkin SR. Continuous glucose monitoring counseling improves physical activity behaviors of individuals with type 2 diabetes: a randomized clinical trial. Diabetes Res Clin Pract. 2008;80(3):371–379. doi: 10.1016/j.diabres.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yoo HJ, An HG, Park SY, Ryu OH, Kim HY, Seo JA, Hong EG, Shin DH, Kim YH, Kim SG, Choi KM, Park IB, Yu JM, Baik SH. Use of a real time continuous glucose monitoring system as a motivational device for poorly controlled type 2 diabetes. Diabetes Res Clin Pract. 2008;82(1):73–79. doi: 10.1016/j.diabres.2008.06.015. [DOI] [PubMed] [Google Scholar]
- 47.Tsalikian E, Mauras N, Beck RW, Tamborlane WV, Janz KF, Chase HP, Wysocki T, Weinzimer SA, Buckingham BA, Kollman C, Xing D, Ruedy KJ Diabetes Research In Children Network (DirecNet) Study Group. Impact of exercise on overnight glycemic control in children with type 1 diabetes mellitus. J Pediatr. 2005;147(4):528–534. doi: 10.1016/j.jpeds.2005.04.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Iscoe KE, Corcoran M, Riddell MC. High rates of nocturnal hypoglycemia in a unique sports camp for athletes with type 1 diabetes: lessons learned from continuous glucose monitoring systems. Can J Diab. 2008;32(3):182–198. [Google Scholar]