Abstract
With the rising number of individuals affected with diabetes and the significant health care costs of treatment, the emphasis on prevention is key to controlling the health burden of this disease. Several genetic and genomic studies have identified genetic variants associated with increased risk to diabetes. As a result, commercial testing is available to predict an individual's genetic risk. Although the clinical benefits of testing have not yet been demonstrated, it is worth considering some of the ethical implications of testing for this common chronic disease. In this article, I discuss several issues that should be considered during the translation of predictive testing for diabetes, including familial implications, improvement of risk communication, implications for behavioral change and health outcomes, the Genetic Information Nondiscrimination Act, direct-to-consumer testing, and appropriate age of testing.
Keywords: ethics, genetic testing, risk
Introduction
Type 2 diabetes mellitus (T2DM) is a prevalent, chronic condition associated with extensive morbidity, decreased quality of life, and increased utilization of health services.1 Approximately 23 million people in the United States are affected with diabetes, and more than twice that number are prediabetic.2 The annual risk of developing T2DM for the average person living in the United States with normal glucose levels is approximately 0.7% per year.3
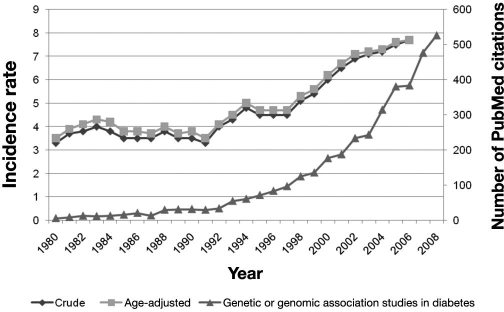
The polygenic nature of T2DM has been a major challenge to identifying genes involved in the pathogenesis of this disease—knowledge that could give rise to new treatments and tests. However, following the completion of the Human Genome Project and HapMap and the development of high-throughput technologies, scientists are in a much better position to tackle the complex genetic underpinnings of T2DM.4 The rise of genetic and genomic studies has aligned with the increasing incidence rate of T2DM (Figure 1). A number of commercial tests have already been developed that assay a panel of genetic variants in several genes identified from genome-wide association studies of T2DM. Among the best studied of these are two very closely linked single nucleotide polymorphisms (SNPs) in the transcription factor 7-like 2 (TCF7L2) gene.5 More than 20 studies have replicated the association between these two SNPs in TCF7L2 and increased T2DM risk. The largest pooled analysis reported an overall odds ratio of 1.37 with a single copy of the higher-risk allele at one of the TCF7L2SNPs.6 In comparison, individuals with a positive family history for T2DM are at a 2–6 times increased risk compared to those without a family history.7–10
Figure 1.
U.S. incidence rate of diabetes (1980–2006)11 and the number of PubMed-cited genetic or genomic association studies on diabetes (1980–2008).
Unlike single-gene testing for Mendelian disorders that produce a relatively certain prediction of disease, genomic testing for complex diseases like T2DM will generate disease riskinformation. Some of the ethical issues of genome risk profiling or predispositional testing overlap with single-gene testing used primarily for diagnosis, although additional issues related to predispositional testing include challenges of communicating risk information (particularly low risks), uncertainty of disease risk and psychosocial impact of “at-risk” status, and ensuring patient comprehension. Of substantial importance is that individuals are informed about these and other issues when they are deciding if the test is appropriate for them. Although written informed consent may not be warranted, a discussion with a physician or other professional such as a genetic counselor can serve to educate and encourage careful consideration of the benefits and risks of testing as well as alternatives to testing. This article presents an overview of several issues that should be considered as genome risk profiling for T2DM becomes integrated into clinical care.
Familial Implications
As with any type of genetic testing, it is important to consider the impact of testing on family members. Predisposition testing for T2DM and other chronic diseases raises familial implications on two levels. The implication of test results for biological family members raises the issue of whether and how to discuss the results with other family members.12 Tested individuals may be reluctant to share the results due to fear it will disrupt relationships, be hesitant of having to contact estranged and distant family members, and feel guilt.13–16 Those who opt to share the results with family members may have difficulty accurately communicating the results17,18 or minimize the seriousness of the finding.19 Although a positive test result could be inferred from changes in lifestyle and preventive medical procedures, individuals undergoing testing should ascertain the wishes of other family members prior to discussing their test results.20 Furthermore, as many individuals choose to undergo genetic testing for the sake of their children, they will need to understand when and how best to discuss the results with their children.21,22 Family members who decide to learn of their relative's results must also decide how they'll act upon them (e.g., getting themselves tested), if at all.
Second, given that environment can substantially influence risk for T2DM and other complex diseases, a positive result of one individual can affect the lifestyle of the entire family. For example, adoption of healthy eating habits may be better achieved if the entire family is involved in promoting healthy living.23–26 Special treatment of a child found to be at increased genetic risk may lead to feelings of ostracism, stigmatization, and inferiority.
A related issue of the familial implications of genetic testing is the duty of physicians to disclose genetic test results to family members when their patient chooses not to do so. Studies have identified a subset of patients who declined to inform at-risk family members of their genetic test result.27,28 In these situations, physicians may feel somewhat obligated to contact family members, although the practice is not common.28 A handful of legal decisions have ruled that, under circumstances where a disease may be prevented, a physician has an obligation to warn relatives at risk.29,30 Experts recommend physicians should encourage their patients to share test results with at-risk family members during the pretest and posttest counseling sessions.31,32 The American Society of Human Genetics (ASHG) recommends that “the legal and ethical norm of patient confidentiality should be respected” and that the harms of nondisclosure must be weighed against breaching patient confidentiality.33 It is unlikely that knowledge of the genetic risk of T2DM would satisfy the four ASHG criteria for disclosure, particularly the criterion of imminent harm.33
Risk Communication
Communicating and understanding risk or probabilities has been an ongoing challenge for health professionals and patients, respectively. Misunderstanding genetic risks may lead to psychosocial harms or familial implications and significantly impact life decisions (e.g., family planning).34–37 Unfamiliarity with genetic concepts and terminology as well as preconceived perceptions of personal and familial risk may pose barriers to understanding genetic test results.36 For instance, individuals with a family history of heart disease did not always perceive themselves at increased risk since they felt “different” in crucial ways from affected relatives.38 While there appears to be a tendency to overestimate risk for inherited cancers,39 some studies have found individuals who test positive underestimate their risk.40 Furthermore, some individuals may interpret their risk as an absolute prediction of disease (fatalism), which may affect their likelihood to engage in preventive steps due to reduced perception of personal controllability to reduce disease risk.41–43 However, this does not appear to be a typical response,44 and often individuals will undergo genetic testing in order to gain a sense of control.45,46
To maximize patient understanding, a combination of numeric, verbal, and pictorial approaches may be warranted to effectively communicate genetic risk.47 The personal meaning of a test result is further framed by the ethnic and cultural environments of the individual and community.48–52 Small to moderate risks revealed by testing can also pose a challenge to communication. Some patients may struggle with the concept of being “at risk” for a disease.53 The concept of a singular, static general population ignores the fact that societies are highly diverse with different experiential influences and attitudes that can change over time.51,54–56 Therefore, health professionals will need to be sensitive to these additional factors that may influence patient understanding and application of risk information.
Implications for Behavioral Change and Improved Health Outcomes
The clinical utility of T2DM risk information to prevent disease or reduce disease severity will depend on the likelihood of individuals to modify behaviors. The Diabetes Prevention Program demonstrated that an intensive program of lifestyle change (healthy eating and daily exercise) or initiation of metformin can delay diabetes onset.57 Lifestyle changes and treatment with metformin have been shown to reduce the risk of progression of prediabetes back to baseline in individuals with an increased genetic risk, suggesting that preventive interventions in genetically at-risk individuals may prevent or delay T2DM onset.58 However, data on the impact of genetic information for positive behavior have been conflicting, suggesting that such information may not serve as a strong motivator for behavior change.59 For example, genetic testing was not found to motivate smoking cessation60 but has been found to increase regular cancer screenings43,61–64 and other positive health behaviors regardless of efficacy65 in high-risk individuals. The relationship between family history, genetic testing, and behavioral change has also been shown to be ambiguous.66,67
The determination of whether knowledge of perceived health risk motivates individuals to adopt risk-reducing behaviors is a complex process involving both cognitive and emotional responses.68–71 The motivation for behavior change has been linked to an individual's underlying perception of disease risk and disease-related worry.70,72,73 Individuals with a higher perception of risk prior to testing have been shown to have greater intention to modify their behavior to reduce risk.7,74 Beliefs in genetic fatalism may influence perceptions of personal controllability and ability to take action against a gene threat.42 However, this does not appear to be a typical response,44 and often, individuals will undergo genetic testing in order to gain a sense of control.45,46 In addition, information-seeking behavior has been linked with health behavior with respect to establishing knowledge and as part of the coping mechanism.75,76 Individuals who do not seek health information are less likely to take preventive actions.77,78 Clinical studies are urgently needed to assess likelihood of behavior change based on genetic risk information compared to standard clinical risk factors, including family history.
Discrimination
Genetic discrimination has been a long-standing concern regarding the use of genetic tests and participation in genetic research.79–81 Although only a few cases of employment or health insurance discrimination have been documented,79 empirical evidence suggests the occurrence may be more widespread.82,83
In 2008, the Genetic Information Nondiscrimination Act (GINA) was signed into law,84,85 13 years after the first federal bill was introduced to prohibit discrimination by health insurers or employers. Health insurers (group, individual, and Medicare issuers) are prohibited from adjusting premiums or contribution amounts, requesting or requiring an individual or a family member of an individual to undergo a genetic test, obtaining and using genetic test results in making a determination regarding payment, or requesting, requiring, or purchasing genetic information for underwriting purposes.
With some exceptions, employers are prohibited from using genetic information to discriminate against applicants or employees based on their genetic information (hiring, firing, or any personnel decisions), to “limit, segregate, or classify” employees on this basis, and to “request, require, or purchase genetic information with respect to an employee or a family member of an employee.” Regulations will be developed by the appropriate federal agencies for implementation in 2009. As a majority of states have legislation prohibiting genetic discrimination by employers and health insurers, the new federal law will not preempt state laws with broader protections but rather will establish a minimum level of protection for all.
While GINA provides comprehensive protections against employment and health insurance discrimination, the law does not prohibit use of genetic information by long-term care, disability, and life insurers.86 Given the range of complications and high mortality (seventh leading cause of death2) of T2DM, individuals at risk for T2DM or other chronic diseases may consider purchasing or increasing their coverage provided by these groups.87 In addition, the health insurance protections do not apply to members of the U.S. military or individuals who receive their health care through the Department of Veterans Affairs or Indian Health Service. Patients considering genetic testing should be informed of state and federal protections and be advised of noncovered groups.
Direct-to-Consumer Testing
Several companies offer genetic testing for a range of diseases directly to consumers without the need to obtain physician authorization. At least three companies (23andMe, Inc., deCodeMe, and Navigenics) currently provide whole genome profiling services from 10 to more than 100 diseases and traits. While direct-to-consumer testing may increase awareness of genetic testing in general and increase accessibility and convenience of testing,88–90 the lack of involvement of a health professional may increase the potential for inappropriate testing and misinterpretation and misapplication of results.91,92 Furthermore, consumers may experience confusion, anxiety, and possible discrimination/stigmatization, depending on the confidentiality of results.91,93–95
Each of these companies includes T2DM in their panel of diseases or offers stand-alone testing (deCode). However, each company tests for a different combination of genes (Table 1). The characteristics of test performance with respect to analytical and clinical validity (including predictive value) and clinical utility are difficult for health professionals, let alone the public, to discern and make an informed decision about the “best” test for them.
Table 1.
Comparison of Genes/Variants Tested between Three Companies Providing Direct-to-Consumer Marketing Testing for Type 2 Diabetes Mellitus as a Stand-Alone Service or Part of a Genomic Risk Profile (as of Feb 2009)
Although some companies provide access to genetic counseling services by phone, the online communication of genomic risk information introduces a new means for individuals to learn of their testing results. Both genotype and risk information are included in the test report along with information about the disease, the role of genes and environment in disease risk, and links to additional resources, including the scientific literature as well as general health information. Consumers of these services may seek assistance from their health practitioner to interpret and apply the results to reduce their risk of disease.
Appropriate Age of Testing
Many professional groups strongly discourage genetic testing for children unless immediate clinical benefit can be gained.96–99 Based on these guidelines, predictive testing for T2DM would likely be discouraged and testing delayed until adulthood. Potential harms include the risk of stigmatization, discrimination, and other adverse psychosocial impacts. However, several commercial genetic laboratories permit testing of children,100 providing an alternative option for parents interested in learning of their child's risks. Despite the absence of immediate clinical benefit in the prevention of T2DM, children may benefit by reducing their risk for a range of diseases from simple modifications to their lifestyle such as healthy eating and regular exercise and thereby maintain a healthy weight at a young age. Physicians should discuss the risks and benefits of testing children for T2DM with the entire family and, when possible, obtain the assent of the child.
Conclusion
As new predictive genetic tests for common, complex diseases such as T2DM are developed and commercialized, it will be critical to the safe and appropriate use of these new applications to consider the potential ethical implications they raise and steps to prevent or ameliorate harms. Although risk-based genetic testing for common diseases raise similar ethical issues to more traditional genetic testing for rare diseases, new challenges are raised due to the type of information revealed and access to tests. With thoughtful deliberation with health professionals, patients and families, test developers and laboratories, insurers and other stakeholders, these issues can be addressed to ensure the safe and appropriate use of these promising new clinical applications.
Acknowledgments
I thank Ms. Geneviève Tindall for her technical assistance in the preparation of this manuscript.
Abbreviations
- ASHG
American Society of Human Genetics
- GINA
Genetic Information Nondiscrimination Act
- SNP
single nucleotide polymorphism
- T2DM
type 2 diabetes mellitus
- TCF7L2
transcription factor 7-like 2
References
- 1.American Diabetes Association. Economic consequences of diabetes mellitus in the U.S. in 1997. Diabetes Care. 1998;21(2):296–309. doi: 10.2337/diacare.21.2.296. [DOI] [PubMed] [Google Scholar]
- 2.Atlanta: U.S. Department of Health and Human Services. 2008. Centers for Disease Control and Prevention. National diabetes fact sheet: general information and national estimates on diabetes in the United States, 2007. [Google Scholar]
- 3.Centers for Disease Control and Prevention. Incidence of diabetes: crude and age-adjusted incidence of diagnosed diabetes per 1,000 population aged 18-79 years, United States, 1980–2007. http://www.cdc.gov/diabetes/statistics/incidence/fig2.htmAccessed October 2008.
- 4.O'Rahilly S, Barroso I, Wareham NJ. Genetic factors in type 2 diabetes: the end of the beginning? Science. 2005;307(5708):370–373. doi: 10.1126/science.1104346. [DOI] [PubMed] [Google Scholar]
- 5.Freely associating. Nat Genet. 1999;22(1):1–2. doi: 10.1038/8702. [DOI] [PubMed] [Google Scholar]
- 6.Zeggini E, Weedon MN, Lindgren CM, Frayling TM, Elliott KS, Lango H, Timpson NJ, Perry JR, Rayner NW, Freathy RM, Barrett JC, Shields B, Morris AP, Ellard S, Groves CJ, Harries LW, Marchini JL, Owen KR, Knight B, Cardon LR, Walker M, Hitman GA, Morris AD, Doney AS. Wellcome Trust Case Control Consortium (WTCCC), McCarthy MI, Hattersley AT. Replication of genome-wide association signals in UK samples reveals risk loci for type 2 diabetes. Science. 2007;316(5829):1336–1341. doi: 10.1126/science.1142364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hariri S, Yoon PW, Qureshi N, Valdez R, Scheuner MT, Khoury MJ. Family history of type 2 diabetes: a population-based screening tool for prevention? Genet Med. 2006;8(2):102–108. doi: 10.1097/01.gim.0000200949.52795.df. [DOI] [PubMed] [Google Scholar]
- 8.Brownson RC, Remington PL, Davis JR. 2nd ed. Washington DC: American Public Health Association; 1998. Chronic disease epidemiology and control. [Google Scholar]
- 9.Harrison TA, Hindorff LA, Kim H, Wines RC, Bowen DJ, McGrath BB, Edwards KL. Family history of diabetes as a potential public health tool. Am J Prev Med. 2003;24(2):152–159. doi: 10.1016/s0749-3797(02)00588-3. [DOI] [PubMed] [Google Scholar]
- 10.Valdez R, Yoon PW, Liu T, Khoury MJ. Family history and prevalence of diabetes in the US population: 6-year results from the National Health and Nutrition Examination Survey (NHANES, 1999 2004) Diabetes. 2007 doi: 10.2337/db07-0720x. [Epub ahead of print.] [DOI] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention. Crude and age-adjusted incidence of diagnosed diabetes per 1,000 population aged 18–79 years, United States, 1980–2006. http://www.cdc.gov/diabetes/statistics/incidence/fig2.htm.
- 12.Gaff CL, Clarke AJ, Atkinson P, Sivell S, Elwyn G, Iredale R, Thornton H, Dundon J, Shaw C, Edwards A. Process and outcome in communication of genetic information within families: a systematic review. Eur J Hum Genet. 2007;15(10):999–1011. doi: 10.1038/sj.ejhg.5201883. [DOI] [PubMed] [Google Scholar]
- 13.Julian-Reynier C, Eisinger F, Vennin P, Chabal F, Aurran Y, Noguès C, Bignon YJ, Machelard-Roumagnac M, Maugard-Louboutin C, Serin D, Blanc B, Orsoni P, Sobol H. Attitudes towards cancer predictive testing and transmission of information to the family. J Med Genet. 1996;33(9):731–736. doi: 10.1136/jmg.33.9.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Green J, Richards M, Murton F, Statham H, Hallowell N. Family communication and genetic counseling: the case of hereditary breast and ovarian cancer. J Genet Couns. 1997;6(1):45–60. doi: 10.1023/A:1025611818643. [DOI] [PubMed] [Google Scholar]
- 15.McInerney-Leo A, Biesecker BB, Hadley DW, Kase RG, Giambarresi TR, Johnson E, Lerman C, Struewing JP. BRCA1/2 testing in hereditary breast and ovarian cancer families II: impact on relationships. Am J Med Genet A. 2005;133A(2):165–169. doi: 10.1002/ajmg.a.30566. [DOI] [PubMed] [Google Scholar]
- 16.Stoffel EM, Ford B, Mercado RC, Punglia D, Kohlmann W, Conrad P, Blanco A, Shannon KM, Powell M, Gruber SB, Terdiman J, Chung DC, Syngal S. Sharing genetic test results in Lynch syndrome: communication with close and distant relatives. Clin Gastroenterol Hepatol. 2008;6(3):333–338. doi: 10.1016/j.cgh.2007.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Claes E, Evers-Kiebooms G, Boogaerts A, Decruyenaere M, Denayer L, Legius E. Communication with close and distant relatives in the context of genetic testing for hereditary breast and ovarian cancer in cancer patients. Am J Med Genet A. 2003;116A(1):11–19. doi: 10.1002/ajmg.a.10868. [DOI] [PubMed] [Google Scholar]
- 18.Wagner Costalas J, Itzen M, Malick J, Babb JS, Bove B, Godwin AK, Daly MB. Communication of BRCA1 and BRCA2 results to at-risk relatives: a cancer risk assessment program's experience. Am J Med Genet C Semin Med Genet. 2003;119C(1):11–18. doi: 10.1002/ajmg.c.10003. [DOI] [PubMed] [Google Scholar]
- 19.Ayme S, Macquart-Moulin G, Julian-Reynier C, Chabal F, Giraud F. Diffusion of information about genetic risk within families. Neuromuscul Disord. 1993;3(5-6):571–574. doi: 10.1016/0960-8966(93)90118-4. [DOI] [PubMed] [Google Scholar]
- 20.Daly MB, Barsevick A, Miller SM, Buckman R, Costalas J, Montgomery S, Bingler R. Communicating genetic test results to the family: a six-step, skills-building strategy. Fam Community Health. 2001;24(3):13–26. doi: 10.1097/00003727-200110000-00004. [DOI] [PubMed] [Google Scholar]
- 21.Tercyak KP, Hughes C, Main D, Snyder C, Lynch JF, Lynch HT, Lerman C. Parental communication of BRCA1/2 genetic test results to children. Patient Educ Couns. 2001;42(3):213–224. doi: 10.1016/s0738-3991(00)00122-1. [DOI] [PubMed] [Google Scholar]
- 22.Tercyak KP, Peshkin BN, Demarco TA, Patenaude AF, Schneider KA, Garber JE, Valdimarsdottir HB, Schwartz MD. Information needs of mothers regarding communicating BRCA1/2 cancer genetic test results to their children. Genet Test. 2007;11(3):249–255. doi: 10.1089/gte.2006.0534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nader PR, Sellers DE, Johnson CC, Perry CL, Stone EJ, Cook KC, Bebchuk J, Luepker RV. The effect of adult participation in a school-based family intervention to improve children's diet and physical activity: the Child and Adolescent Trial for Cardiovascular Health. Prev Med. 1996;25(4):455–464. doi: 10.1006/pmed.1996.0077. [DOI] [PubMed] [Google Scholar]
- 24.Cookson S, Heath A, Bertrand L. The HeartSmart Family Fun Pack: an evaluation of family-based intervention for cardiovascular risk reduction in children. Can J Public Health. 2000;91(4):256–259. doi: 10.1007/BF03404283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Golan M, Weizman A. Familial approach to the treatment of childhood obesity: conceptual mode. J Nutr Educ. 2001;33(2):102–107. doi: 10.1016/s1499-4046(06)60173-5. [DOI] [PubMed] [Google Scholar]
- 26.Kardia SL, Modell SM, Peyser PA. Family-centered approaches to understanding and preventing coronary heart disease. Am J Prev Med. 2003;24(2):143–151. doi: 10.1016/s0749-3797(02)00587-1. [DOI] [PubMed] [Google Scholar]
- 27.Falk MJ, Dugan RB, O'Riordan MA, Matthews AL, Robin NH. Medical geneticists' duty to warn at-risk relatives for genetic disease. Am J Med Genet A. 2003;120A(3):374–380. doi: 10.1002/ajmg.a.20227. [DOI] [PubMed] [Google Scholar]
- 28.Dugan RB, Wiesner GL, Juengst ET, O'Riordan M, Matthews AL, Robin NH. Duty to warn at-risk relatives for genetic disease: genetic counselors' clinical experience. Am J Med Genet C Semin Med Genet. 2003;119C(1):27–34. doi: 10.1002/ajmg.c.10005. [DOI] [PubMed] [Google Scholar]
- 29.Pate v. Threlkel. 661 So.2d 278 (Fla. 1995) [Google Scholar]
- 30.Safer v. Estate of Pack. 677 A.2d 1188 (N.J. Super. Ct. App. Div. 1996) [Google Scholar]
- 31.Offit K, Groeger E, Turner S, Wadsworth EA, Weiser MA. The “duty to warn” a patient's family members about hereditary disease risks. JAMA. 2004;292(12):1469–1473. doi: 10.1001/jama.292.12.1469. [DOI] [PubMed] [Google Scholar]
- 32.Chan-Smutko G, Patel D, Shannon KM, Ryan PD. Professional challenges in cancer genetic testing: who is the patient? Oncologist. 2008;13(3):232–238. doi: 10.1634/theoncologist.2007-0203. [DOI] [PubMed] [Google Scholar]
- 33.The American Society of Human Genetics Social Issues Subcommittee on Familial Disclosure. ASHG statement. Professional disclosure of familial genetic information. Am J Hum Genet. 1998;62(2):474–483. [PMC free article] [PubMed] [Google Scholar]
- 34.Fanos JH. The missing link in linkage analysis: the well sibling revisited. Genet Test. 1999;3(3):273–278. doi: 10.1089/109065799316581. [DOI] [PubMed] [Google Scholar]
- 35.Fanos JH, Johnson JP. Perception of carrier status by cystic fibrosis siblings. Am J Hum Genet. 1995;57(2):431–438. [PMC free article] [PubMed] [Google Scholar]
- 36.Saukko PM, Ellard S, Richards SH, Shepherd MH, Campbell JL. Patients' understanding of genetic susceptibility testing in mainstream medicine: qualitative study on thrombophilia. BMC Health Serv Res. 2007;7:82. doi: 10.1186/1472-6963-7-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harrison HF, Harrison BW, Walker AP, Lohman K, Ellis SD, Hall MA, Reiss J, Adams PC, Holup J, Acton RT, Bent T, Rivers C, Fadojutimi-Akinsiku M. Screening for hemochromatosis and iron overload: satisfaction with results notification and understanding of mailed results in unaffected participants of the HEIRS study. Genet Test. 2008;12(4):491–500. doi: 10.1089/gte.2008.0004. [DOI] [PubMed] [Google Scholar]
- 38.Hunt K, Emslie C, Watt G. Lay constructions of a family history of heart disease: potential for misunderstandings in the clinical encounter? Lancet. 2001;357(9263):1168–1171. doi: 10.1016/S0140-6736(00)04334-8. [DOI] [PubMed] [Google Scholar]
- 39.Croyle RT, Lerman C. Risk communication in genetic testing for cancer susceptibility. J Natl Cancer Inst Monogr. 1999;25:59–66. doi: 10.1093/oxfordjournals.jncimonographs.a024210. [DOI] [PubMed] [Google Scholar]
- 40.Aktan-Collan K, Haukkala A, Mecklin JP, Uutela A, Kääriäinen H. Comprehension of cancer risk one and 12 months after predictive genetic testing for hereditary non-polyposis colorectal cancer. J Med Genet. 2001;38(11):787–792. doi: 10.1136/jmg.38.11.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shiloh S. Illness representations, self-regulation, and genetic counseling: a theoretical review. J Genet Couns. 2006;15(5):325–337. doi: 10.1007/s10897-006-9044-5. [DOI] [PubMed] [Google Scholar]
- 42.DeVries H, Mesters I, van de Steeg H, Honing C. The general public's information needs and perceptions regarding hereditary cancer: an application of the Integrated Change Model. Patient Educ Couns. 2005;56(2):154–165. doi: 10.1016/j.pec.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 43.Frosch DL, Mello P, Lerman C. Behavioral consequences of testing for obesity risk. Cancer Epidemiol Biomarkers Prev. 2005;14(6):1485–1489. doi: 10.1158/1055-9965.EPI-04-0913. [DOI] [PubMed] [Google Scholar]
- 44.Marteau T, Senior V, Humphries SE, Bobrow M, Cranston T, Crook MA, Day L, Fernandez M, Horne R, Iversen A, Jackson Z, Lynas J, Middleton-Price H, Savine R, Sikorski J, Watson M, Weinman J, Wierzbicki AS, Wray R. Genetic Risk Assessment for FH Trial Study Group. Psychological impact of genetic testing for familial hypercholesterolemia within a previously aware population: a randomized controlled trial. Am J Med Genet A. 2004;128A(3):285–293. doi: 10.1002/ajmg.a.30102. [DOI] [PubMed] [Google Scholar]
- 45.Rose AL, Peters N, Shea JA, Armstrong K. Attitudes and misconceptions about predictive genetic testing for cancer risk. Community Genet. 2005;8(3):145–151. doi: 10.1159/000086757. [DOI] [PubMed] [Google Scholar]
- 46.Rose A, Peters N, Shea JA, Armstrong K. The association between knowledge and attitudes about genetic testing for cancer risk in the United States. J Health Commun. 2005;10(4):309–321. doi: 10.1080/10810730590950039. [DOI] [PubMed] [Google Scholar]
- 47.Lipkus IM. Numeric, verbal, and visual formats of conveying health risks: suggested best practices and future recommendations. Med Decis Making. 2007;27(5):696–713. doi: 10.1177/0272989X07307271. [DOI] [PubMed] [Google Scholar]
- 48.Catz DS, Green NS, Tobin JN, Lloyd-Puryear MA, Kyler P, Umemoto A, Cernoch J, Brown R, Wolman F. Attitudes about genetics in underserved, culturally diverse populations. Community Genet. 2005;8(3):161–172. doi: 10.1159/000086759. [DOI] [PubMed] [Google Scholar]
- 49.Bates BR, Lynch JA, Bevan JL, Condit CM. Warranted concerns, warranted outlooks: a focus group study of public understandings of genetic research. Soc Sci Med. 2005;60(2):331–344. doi: 10.1016/j.socscimed.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 50.Brunk CG. Public knowledge, public trust: understanding the “knowledge deficit. Community Genet. 2006;9(3):178–183. doi: 10.1159/000092654. [DOI] [PubMed] [Google Scholar]
- 51.Cunningham-Burley S. Public knowledge and public trust. Community Genet. 2006;9(3):204–210. doi: 10.1159/000092658. [DOI] [PubMed] [Google Scholar]
- 52.Bates BR. Public culture and public understanding of genetics: a focus group study. Public Underst Sci. 2005;14(1):47–65. doi: 10.1177/0963662505048409. [DOI] [PubMed] [Google Scholar]
- 53.Scott S, Prior L, Wood F, Gray J. Repositioning the patient: the implications of being “at risk. Soc Sci Med. 2005;60(8):1869–1879. doi: 10.1016/j.socscimed.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 54.Van der Sanden MCA, Meijman FJ. Dialogue guides awareness and understanding of science: an essay on different goals of dialogue leading to different science communication approaches. Public Understanding Sci. 2008;17(1):89–103. [Google Scholar]
- 55.Wynne B. Public engagement as a means of restoring public trust in science—hitting the notes, but missing the music? Community Genet. 2006;9(3):211–220. doi: 10.1159/000092659. [DOI] [PubMed] [Google Scholar]
- 56.Gottweis H. Gene therapy and the public: a matter of trust. Gene Ther. 2002;9(11):667–669. doi: 10.1038/sj.gt.3301752. [DOI] [PubMed] [Google Scholar]
- 57.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM. Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Florez JC, Jablonski KA, Bayley N, Pollin TI, de Bakker PI, Shuldiner AR, Knowler WC, Nathan DM, Altshuler D. Diabetes Prevention Program Research Group. TCF7L2 polymorphisms and progression to diabetes in the Diabetes Prevention Program. N Engl J Med. 2006;355(3):241–250. doi: 10.1056/NEJMoa062418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Marteau TM, Lerman C. Genetic risk and behavioural change. BMJ. 2001;322(7293):1056–1059. doi: 10.1136/bmj.322.7293.1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McBride CM, Bepler G, Lipkus IM, Lyna P, Samsa G, Albright J, Datta S, Rimer BK. Incorporating genetic susceptibility feedback into a smoking cessation program for African-American smokers with low income. Cancer Epidemiol Biomarkers Prev. 2002;11(6):521–528. [PubMed] [Google Scholar]
- 61.Phillips KA, Jenkins MA, Lindeman GJ, McLachlan SA, McKinley JM, Weideman PC, Hopper JL, Friedlander ML, kConFab Investigators Risk-reducing surgery, screening and chemoprevention practices of BRCA1 and BRCA2 mutation carriers: a prospective cohort study. Clin Genet. 2006;70(3):198–206. doi: 10.1111/j.1399-0004.2006.00665.x. [DOI] [PubMed] [Google Scholar]
- 62.Meiser B, Dunn S, Dixon J, Powell LW. Psychological adjustment and knowledge about hereditary hemochromatosis in a clinic-based sample: a prospective study. J Genet Couns. 2005;14(6):453–463. doi: 10.1007/s10897-005-6192-y. [DOI] [PubMed] [Google Scholar]
- 63.Peshkin BN, Schwartz MD, Isaacs C, Hughes C, Main D, Lerman C. Utilization of breast cancer screening in a clinically based sample of women after BRCA1/2 testing. Cancer Epidemiol Biomarkers Prev. 2002;11 (10 Pt 1):1115–8. [PubMed] [Google Scholar]
- 64.Antill YC, Reynolds J, Young MA, Kirk JA, Tucker KM, Bogtstra TL, Wong SS, Dudding TE, Di Iulio JL, Phillips KA. Screening behavior in women at increased familial risk for breast cancer. Fam Cancer. 2006;5(4):359–368. doi: 10.1007/s10689-006-0006-8. [DOI] [PubMed] [Google Scholar]
- 65.Chao S, Roberts JS, Marteau TM, Silliman R, Cupples LA, Green RC. Health behavior changes after genetic risk assessment for Alzheimer disease: The REVEAL Study. Alzheimer Dis Assoc Disord. 2008;22(1):94–97. doi: 10.1097/WAD.0b013e31815a9dcc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sanderson SC, Michie S. Genetic testing for heart disease susceptibility: potential impact on motivation to quit smoking. Clin Genet. 2007;71(6):501–510. doi: 10.1111/j.1399-0004.2007.00810.x. [DOI] [PubMed] [Google Scholar]
- 67.Hicken B, Tucker D. Impact of genetic risk feedback: perceived risk and motivation for health protective behaviours. Psychol Health Med. 2002;7(1):25–36. [Google Scholar]
- 68.Moss-Morris R, Petrie KJ. Cognitive distortions of somatic experiences: revision and validation of a measure. J Psychosom Res. 1997;43(3):293–306. doi: 10.1016/s0022-3999(97)00020-2. [DOI] [PubMed] [Google Scholar]
- 69.Rees G, Fry A, Cull A, Sutton S. Illness perceptions and distress in women at increased risk of breast cancer. Psychol Health. 2004;19(6):749–765. [Google Scholar]
- 70.Kaptein AA, van Korlaar IM, Cameron LD, Vossen CY, van der Meer FJ, Rosendaal FR. Using the common-sense model to predict risk perception and disease-related worry in individuals at increased risk for venous thrombosis. Health Psychol. 2007;26(6):807–812. doi: 10.1037/0278-6133.26.6.807. [DOI] [PubMed] [Google Scholar]
- 71.Marteau TM, Weinman J. Self-regulation and the behavioural response to DNA risk information: a theoretical analysis and framework for future research. Soc Sci Med. 2006;62(6):1360–1368. doi: 10.1016/j.socscimed.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 72.Cameron LD, Diefenbach MA. Responses to information about psychosocial consequences of genetic testing for breast cancer susceptibility: influences of cancer worry and risk perceptions. J Health Psychol. 2001;6(1):47–59. doi: 10.1177/135910530100600104. [DOI] [PubMed] [Google Scholar]
- 73.Cameron LD, Reeve J. Risk perceptions, worry, and attitudes about genetic testing for breast cancer susceptibility. Psychol Health. 2006;21(2):211–230. doi: 10.1080/14768320500230318. [DOI] [PubMed] [Google Scholar]
- 74.Smerecnik CM, Mesters I, van Keulen H, Scheffers I, Beeks E, De Leeuw PW, de Vries NK, de Vries H. Should individuals be informed about their salt sensitivity status? First indications of the value of testing for genetic predisposition to low-risk conditions. Genet Test. 2007;11(3):307–314. doi: 10.1089/gte.2007.0008. [DOI] [PubMed] [Google Scholar]
- 75.Johnson JD, Meischke H. A comprehensive model of cancer-related information seeking applied to magazines. Human Commun Res. 1993;19(3):343–367. [Google Scholar]
- 76.Perry CF, Bauer KD. Effect of printed tailored messaging on cancer risk behavior. Top Clin Nutr. 2001;16(2):42–52. [Google Scholar]
- 77.Ramanadhan S, Viswanath K. Health and the information nonseeker: a profile. Health Commun. 2006;20(2):131–139. doi: 10.1207/s15327027hc2002_4. [DOI] [PubMed] [Google Scholar]
- 78.Rutten LJ, Squiers L, Hesse B. Cancer-related information seeking: hints from the 2003 Health Information National Trends Survey (HINTS) J Health Commun. 2006;11(Suppl 1):147–156. doi: 10.1080/10810730600637574. [DOI] [PubMed] [Google Scholar]
- 79.Baruch S, Hudson K. Civilian and military genetics: nondiscrimination policy in a post-GINA world. Am J Hum Genet. 2008;83(4):435–444. doi: 10.1016/j.ajhg.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hudson KL, Rothenberg KH, Andrews LB, Kahn MJ, Collins FS. Genetic discrimination and health insurance: an urgent need for reform. Science. 1995;270(5235):391–393. doi: 10.1126/science.270.5235.391. [DOI] [PubMed] [Google Scholar]
- 81.Offit K, Thom P. Ethical and legal aspects of cancer genetic testing. Semin Oncol. 2007;34(5):435–443. doi: 10.1053/j.seminoncol.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 82.Secretary's Advisory Committee on Genetics, Health, and Society. Bethesda: Department of Health and Human Services; 2004. Public perspectives on genetic discrimination. http://oba.od.nih.gov/oba/sacghs/reports/Public_Perspectives_GenDiscrim.pdf. [Google Scholar]
- 83.Penziner E, Williams JK, Erwin C, Bombard Y, Wallis A, Beglinger LJ, Hayden MR, Paulsen JS. Perceptions of discrimination among persons who have undergone predictive testing for Huntington's disease. Am J Med Genet B Neuropsychiatr Genet. 2008;147(3):320–325. doi: 10.1002/ajmg.b.30600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Public Law 110-233. Genetic Information Nondiscrimination Act of 2008 [Google Scholar]
- 85.Slaughter LM. The Genetic Information Nondiscrimination Act: why your personal genetics are still vulnerable to discrimination. Surg Clin North Am. 2008;88(4):723–738. doi: 10.1016/j.suc.2008.04.004. Vi. [DOI] [PubMed] [Google Scholar]
- 86.Hudson KL, Holohan MK, Collins FS. Keeping pace with the times—the Genetic Information Nondiscrimination Act of 2008. N Engl J Med. 2008;358(25):2661–2663. doi: 10.1056/NEJMp0803964. [DOI] [PubMed] [Google Scholar]
- 87.Zick CD, Mathews CJ, Roberts JS, Cook-Deegan R, Pokorski RJ, Green RC. Genetic testing for Alzheimer's disease and its impact on insurance purchasing behavior. Health Aff (Millwood) 2005;24(2):483–490. doi: 10.1377/hlthaff.24.2.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Centers for Disease Control and Prevention. Genetic testing for breast and ovarian cancer susceptibility: evaluating direct-to-consumer marketing—Atlanta, Denver, Raleigh-Durham, and Seattle, 2003. MMWR Morb Mortal Wkly Rep. 2004;53(27):603–606. [PubMed] [Google Scholar]
- 89.Mouchawar J, Hensley-Alford S, Laurion S, Ellis J, Kulchak-Rahm A, Finucane ML, Meenan R, Axell L, Pollack R, Ritzwoller D. Impact of direct-to-consumer advertising for hereditary breast cancer testing on genetic services at a managed care organization: a naturally-occurring experiment. Genet Med. 2005;7(3):191–197. doi: 10.1097/01.gim.0000156526.16967.7a. [DOI] [PubMed] [Google Scholar]
- 90.Mouchawar J, Laurion S, Ritzwoller DP, Ellis J, Kulchak-Rahm A, Hensley-Alford S. Assessing controversial direct-to-consumer advertising for hereditary breast cancer testing: reactions from women and their physicians in a managed care organization. Am J Manag Care. 2005;11(10):601–608. [PubMed] [Google Scholar]
- 91.Hogarth S, Javitt G, Melzer D. The current landscape for direct-to-consumer genetic testing: legal, ethical, and policy issues. Annu Rev Genomics Hum Genet. 2008;9:161–182. doi: 10.1146/annurev.genom.9.081307.164319. [DOI] [PubMed] [Google Scholar]
- 92.Goddard KA, Robitaille J, Dowling NF, Parrado AR, Fishman J, Bradley LA, Moore CA, Khoury MJ. Health-related direct-to-consumer genetic tests: a public health assessment and analysis of practices related to Internet-based tests for risk of thrombosis. Public Health Genomics. 2009;12(2):92–104. doi: 10.1159/000176794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gollust SE, Hull SC, Wilfond BS. Limitations of direct-to-consumer advertising for clinical genetic testing. JAMA. 2002;288(14):1762–1767. doi: 10.1001/jama.288.14.1762. [DOI] [PubMed] [Google Scholar]
- 94.Hull SC, Prasad K. Reading between the lines: direct-to-consumer advertising of genetic testing. Hastings Cent Rep. 2001;31(3):33–35. [PMC free article] [PubMed] [Google Scholar]
- 95.Williams-Jones B. Where there's a web, there's a way: commercial genetic testing and the Internet. Community Genet. 2003;6(1):46–57. doi: 10.1159/000069538. [DOI] [PubMed] [Google Scholar]
- 96.American Society of Human Genetics Board of Directors, American College of Medical Genetics Board of Directors. Points to consider: ethical, legal, and psychosocial implications of genetic testing in children and adolescents. Am J Hum Genet. 1995;57(5):1233–1241. [PMC free article] [PubMed] [Google Scholar]
- 97.Clarke A. The genetic testing of children. Working Party of the Clinical Genetics Society (UK) J Med Genet. 1994;31(10):785–797. doi: 10.1136/jmg.31.10.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nelson RM, Botkjin JR, Kodish ED, Levetown M, Truman JT, Wilfond BS, Harrison CE, Kazura A, Krug E, 3rd, Schwartz PA, Donovan GK, Fallat M, Porter IH, Steinberg D. Committee on Bioethics. Ethical issues with genetic testing in pediatrics. Pediatrics. 2001;107(6):1451–1455. doi: 10.1542/peds.107.6.1451. [DOI] [PubMed] [Google Scholar]
- 99.American Medical Association. Genetic testing in children (Policy E-2.138). http://www.ama-assn.org/ama/pub/physician-resources/medical-ethics/code-medical-ethics/opinion2138.shtml.
- 100.Borry P, Howard HC, Sénécal K, Avard D. Direct-to-consumer genome scanning services. Also for children? Nat Rev Genet. 2009;10(1):8. doi: 10.1038/nrg2501. [DOI] [PubMed] [Google Scholar]