Abstract
Background
Conducted by highly experienced investigators with abundant time and resources, phase III studies of continuous glucose sensing (CGS) may lack generalizability to everyday clinical practice.
Method
Community or academic practices in six Central and Eastern European or Mediterranean countries prospectively established an anonymized registry of consecutive patients with type 1 insulin-dependent diabetes mellitus starting CGS-augmented insulin pump therapy with the Paradigm® X22 (Medtronic MiniMed, Northridge, CA) under everyday conditions, without prior CGS with another device. We compared glycosylated hemoglobin (GHb) values before and after 3 months of CGS and assessed relationships between insulin therapy variables and glycemia-related variables at weeks 1, 4, and 12 of CGS.
Results
Of 102 enrolled patients, 85 (83%) with complete weeks 1, 4, and 12 sensor data and baseline/3-month GHb data were evaluable. Evaluable patients were ∼54% male and ∼75% adult (mean age, 33.2 ± 16.9 years) with longstanding diabetes and high personal/family education levels. Mean GHb declined significantly after 3 months of CGS (7.55 ± 1.33% at baseline to 6.81 ± 1.08% after 12 weeks, 0.74% absolute decrease, P < 0.001). The absolute GHb reduction correlated significantly (P < 0.0005) with baseline GHb: larger absolute reductions tended to occur when baseline levels were higher. An increased basal insulin dose as a percentage of the total daily insulin dose and a decreased daily bolus count from week 1 to week 12 of CGS predicted GHb improvement from baseline to week 12.
Conclusions
CGS-augmented insulin pump therapy appears to improve glycemic control in type 1 diabetes in varied everyday practice settings.
Keywords: continuous glucose sensing, continuous subcutaneous insulin infusion, everyday practice, glycemic control, hypoglycemic excursions, patient registry
Introduction
Since becoming available in everyday practice in 2006, real-time continuous glucose sensing (CGS) has been considered an important addition to the care of patients intensively treated for type 1 diabetes.1,2 At the same time, however, concerns have been raised regarding the impact of this technology on glycemic control and patient self-management.3,4 Experts have questioned whether access to continuous glucose data will enable patients to improve their pattern recognition skills, thereby avoiding hypo- or hyperglycemic excursions, or will lead patients to overbolus in overreaction to short-lived glycemic fluctuations.5
In recent studies, real-time CGS showed promise by decreasing glycemic excursions and duration of hypoglycemia and, ultimately, by improving glycosylated hemoglobin (GHb) values.6–17 However, important negative studies18 and critical appraisals of the impact of CGS on diabetes health outcomes4 suggest that this technology cannot yet be considered fully established in everyday clinical practice.
One difficulty in evaluating complex technological interventions such as CGS-augmented insulin pump therapy lies in a limitation of randomized trials, namely, a frequent lack of generalizability to everyday clinical settings. Often, randomized trial results reflect not only the technology tested but the somewhat rarified phase III setting itself, wherein highly qualified health care teams have ample skill, time, and resources to assist patients in implementing the technology and to encourage adherence to relevant protocols.
Prospective data from a multicenter, multinational patient registry, including a wide variety of community as well as academic practices outside the phase III setting, thus may provide a perspective lacking in randomized trials. We therefore formed such a registry to assess the impact of CGS-augmented insulin pump therapy on glycemic control and the effect of CGS on treatment practices in patients with type 1 diabetes. We now present data from the first 3 months of treatment of the first evaluable cohort from the registry.
Methods
Registry and Patients
This investigator-initiated, prospective, anonymized registry includes consecutive patients from the Central and Eastern Europe, Greece, and Israel (CEEGI) CGS Collaborative Study Group, which is made up of clinicians from community or academic practices in six Central or Eastern European or Mediterranean countries. Because patients were treated under everyday practice conditions rather than according to a centralized protocol, this was an effectiveness study rather than an efficacy study. To be enrolled in the registry, patients had to meet three inclusion criteria: (1) they had a diagnosis of type 1 insulin-dependent diabetes mellitus, (2) they were starting CGS-augmented insulin pump therapy with the Paradigm® X22 (Medtronic MiniMed, Northridge, CA), and (3) they had not previously received CGS-augmented insulin pump therapy with another device. Registry exclusion criteria were pregnancy or plans to attempt to become pregnant during the first 3 months of CGS-augmented insulin pump therapy.
Practices were recruited to participate in the registry based on the belief that a practice had at least one eligible patient. No practice was refused enrollment of any eligible patient. Patients were entered into the registry after the 4-week download, thus excluding patients who discontinued CGS-augmented insulin pump therapy after less than a month. The registry data collection protocol was approved by the Chaim Sheba Medical Center institutional review board. At the time that the registry was formed, CGS-augmented insulin pump therapy was not reimbursed in any participating country.
Patient Education
Patients were instructed in CGS-augmented pump use according to standard local protocols by one or more of physicians, nurses, or pump manufacturer staff; the registry did not query the participating practices on the specifics of their protocols. In all patients, at the start of CGS-augmented pump therapy, the sensors were prescribed to be used continuously.
Data Collection
Four categories of data were collected:
Baseline patient/disease characteristics: gender, age, weight, diabetes duration, previous insulin administration method, education level, and, for pediatric patients (age <18 years), height and parent/guardian education level.
GHb at connection to the Paradigm X22 and after 3 months of CGS-augmented pump therapy: GHb was determined locally. All countries but the Czech Republic used Diabetes Control and Complications Trial (DCCT)-normalized values. GHb values from the Czech Republic, which were International Federation of Clinical Chemistry and Laboratory Medicine (IFCC) standardized, were converted to DCCT equivalents using the following formula: DCCT GHb = 0.915 × IFCC GHb + 2.15.19
Frequency of sensor use during months 1 and 3: Based on sensor data, sensor use was classified as continuous (operated ≥3 weeks/month), intermittent (operated ≤2 weeks, 6 days/month), or stopped (no use during the month; month 3 only).
Additional glycemia data and insulin therapy data for weeks 1, 4, and 12 on CGS-augmented insulin pump therapy: Additional glycemia data included weekly mean (BG) and standard deviation glucose (SDEV) levels, the glucose areas <70 mg% (AUCL) or >140 mg% (AUCH) normalized to 7 days of the download, and the number of excursions below 70 mg% (LEX) or above 140 mg% (HEX). Insulin therapy data included the total daily insulin dose (INS), the basal insulin dose as a percentage of the total daily insulin dose (BAS), and the number of daily boluses (BOL; both meal and correction boluses).
Data on baseline patient/disease characteristics and on baseline GHb were collected immediately before connection to the CGS-augmented pump. Week 1 and week 4 sensor and pump data were collected at the end of week 4, and remaining data were collected at the end of week 12. Data were anonymized and uploaded by the treating physician or a Paradigm X22 manufacturer's representative using the Internet-based electronic data capture system enCapture™ (PEC International, Frankfurt, Germany).
Manufacturer's Role
The role of the Paradigm X22 manufacturer and its staff in this study was limited to:
collecting and uploading data for some patients
financially supporting use of the data capture system, statistical analyses by an independent statistician, and editorial assistance by an independent medical editor. The lead author chose the statistician and medical editor.
The manufacturer had no access to the overall database, which was assembled by the lead author and the statistician using the data capture system. The manufacturer also had no prepublication access to the statistical results. All manuscript submission decisions were made by the lead author in consultation with the other investigators, without input from the Paradigm X22 manufacturer.
Statistical Analyses
Only patients with baseline and 3-month GHb data and week 1, week 4, and week 12 downloads were included in the statistical analyses. Values were expressed in means ± standard deviations (SDs). The change in mean GHb levels from baseline (i.e., pre-CGS-augmented pump therapy) to the end of week 12 was analyzed using a paired samples t test. The impact of continuous versus intermittent sensor use on the 3-month change in GHb was assessed with an analysis of covariance (ANCOVA) with the baseline GHb level as a covariate. Changes in mean glycemia and insulin therapy variables from week 1–week 4 or from week 1–week 12 were evaluated using a multivariate analysis of variance (MANOVA) for repeated measures. To predict changes in glycemia variables from week 1–week 4 or week 1–week 12 according to insulin therapy variables, we used linear multiple regression analyses. Each of the two time deltas of each dependent (glycemia) variable was regressed on the relevant delta level of each insulin therapy variable, while the analysis controlled for the week 1 level of the dependent variable.
Statistical calculations were performed on SPSS 15.0 for Windows (SPSS Inc., Chicago, IL). Statistical results were considered significant at P < 0.05.
Results
Patient Characteristics
In total, the registry enrolled 102 patients from six countries. Of the 102 patients, 85 (83.3%) were eligible for the study analysis; selected characteristics of the 85 are summarized in Table 1. Compared to the group with complete data, the 17 patients for whom data collection was incomplete were significantly younger (23 ± 14 years, P < 0.05) and had a significantly shorter duration of diabetes (9.6 ± 9.3 years, P = 0.02), but had a similar baseline GHb (7.19 ± 1.04%, P = 0.2) and gender distribution.
Table 1.
Selected Characteristics of 85 Patients Included in the Study Analysisa
| Characteristic | Value |
|---|---|
| Country of origin, % (n) | |
| Hungary | 27.0% (23) |
| Czech Republic | 27.0% (23) |
| Greece | 16.5% (14) |
| Israel | 15.2% (13) |
| Russia | 11.7% (10) |
| Slovakia | 2.4% (2) |
| Sex, % (n) | |
| Male | 54.1% (46) |
| Female | 45.9% (39) |
| Age, years | |
| Mean ± SD | 33.2 ± 16.9 |
| Percentage (n): | |
| <12 years | 10.6% (9) |
| 12–18 years | 14.1% (12) |
| >18 years | 70.6% (60) |
| ≥65 years | 2.4% (2) |
| Not reported | 2.4% (2) |
| Weight, kg, mean ± SD (patients age >18 years) | 72.9 ± 13.4 |
| Diabetes duration, years, mean ± SD | 14.1 ± 10.1 |
| Highest educational level, % (n) | |
| Primary school or less | 3.5% (3) |
| Secondary school | 31.7% (27) |
| Higher than secondary school | 38.8% (33) |
| Undisclosed | 26.0% (22) |
Because of rounding, percentages may not add up to 100%.
Of the evaluable cohort, a slight majority comprised males, and a larger majority were adults. Unsurprisingly in a group starting CGS-augmented insulin pump therapy, patients tended to have longstanding diabetes. At baseline, all patients were receiving insulin pump therapy. Educational levels of the adult patients and the pediatric patients' parents/guardians were generally high: some 70% had at least secondary school education and nearly 40% had university education.
Glycosylated Hemoglobin
Mean ± SD GHb was 7.55 ± 1.33% at baseline and declined to 6.81 ± 1.08% after 12 weeks. This change represented an absolute decrease of 0.74% in the mean GHb value and was statistically significant (P < 0.001). Three-quarters of patients had an absolute GHb reduction, which exceeded one percentage point in 30 patients (35.3%). Sixteen patients (18.8%) had a slight increase in GHb; these patients' mean ± SD baseline GHb (6.50 ± 1.08%) was substantially lower than that of the overall evaluable patient sample. The remaining 5 patients (5.8%) had no change in GHb.
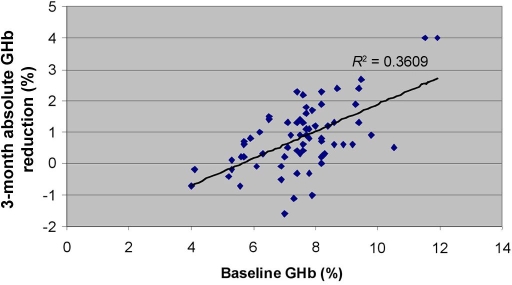
As seen in Figure 1, the size of the absolute GHb reduction correlated significantly (P < 0.0005) with baseline levels of this analyte: greater absolute reductions tended to occur when baseline levels were higher.
Figure 1.
Absolute decrease in GHb (%) after 12 weeks by baseline GHb (%).
Frequency of Sensor Use and Relationship with GHb Changes
During month 1, 56.4 and 43.4% of patients used sensors continuously and intermittently, respectively. During month 3, 48.1 and 50.6% of patients used sensors continuously and intermittently, respectively, while one patient (1.3%) stopped sensor use.
An ANCOVA on GHb reduction from baseline to week 12, with frequency of sensor use (continuous versus intermittent) as an independent variable and baseline GHb as a covariate, found a significant association between continuous sensor use during month 1 and 3-month GHb improvement (F1,73 = 4.36, P = 0.04). However, the sensor use pattern during month 3 did not predict a change in GHb (data not shown).
Other Glycemia and Insulin Therapy Data
Table 2 presents glycemia and insulin therapy data for week 1, week 4, and week 12. A MANOVA for repeated measures revealed no statistically significant changes from week 1 to week 4 in any mean variable, except significant increases in AUCL (F1,85 = 4.12, P = 0.045) and in INS (F1,85 = 5.61, P = 0.022).
Table 2.
Glycemia and Insulin Therapy Data for Weeks 1, 4, and 12 of CGS-Augmented Insulin Pump Therapy in 85 Patients with Type 1 Diabetes in the CEEGI CGS Collaborative Study Group Registry
| Variable | Mean ± SD weekly value | |||
|---|---|---|---|---|
| Week 1 | Week 4 | Week 12 | ||
| Glucose level, mgl/dl | 146.40 ± 35.70 | 144.29 ± 36.58 | 144.95 ± 39.26 | |
| SD in glucose level, mg/dl | 46.52 ± 11.71 | 47.30 ± 13.04 | 47.71 ± 14.01 | |
| Glucose area <70 mg%, mg/dl/24 hours | 24.75 ± 26.54 | 24.43 ± 26.96 | 25.52 ± 29.69 | |
| Glucose area >140 mg%, mg/dl/24 hours | 0.47 ± 1.41 | 0.56 ± 1.42 | 0.67 ± 1.40 | |
| Excursions >150 mg%, n | 15.51 ± 6.68 | 15.65 ± 6.64 | 15.72 ± 6.21 | |
| Total daily insulin dose, units | 38.56 ± 19.71 | 42.44 ± 18.36 | 39.95 ± 16.16 | |
| Total basal insulin dose/total daily insulin dose, % | 51.21 ± 13.13 | 50.28 ± 11.94 | 50.45 ± 12.02 | |
| Daily boluses, n | 6.63 ± 3.10 | 6.89 ± 1.08 | 7.38 ± 5.81 | |
A separate MANOVA for repeated measures detected no significant changes from week 1 to week 12 in any mean variable (F1,79 < 2.43, P > 0.12).
Insulin Therapy Variables as Determinants of Glycemia Variable Changes
Linear multiple regression analyses found that changes in three out of four mean glycemia variables from week 1 to week 4 each correlated significantly with changes in at least two mean insulin therapy variables during that time. First, decreased BG was associated with decreased INS (β = 0.374, R2 change = 0.139, F1,83= 15.50, P = 0.00017), decreased BOL (β = 0.275, R2 change = 0.07, F1,83= 7.41, P = 0.008), and increased BAS (β = -0.420, R2 change = 0.17, F1,83 = 19.64, P < 0.0001). Second, an increase in SDEV, that is, in variability of serum glucose levels, was associated with increased INS (β = 0.469, R2 change = 0.22, F1,83 = 27.49, P < 0.0001) and increased BOL (β = 0.207, R2 change = 0.04, F1,83 = 4.10, P = 0.046). Finally, an increase in AUCH, that is, total time under hyperglycemic conditions, was related to increased INS (β = 0.368, R2 change = 0.13, F1,83 = 16.03, P < 0.0001), decreased BAS (β = -0.431, R2 change = 0.179, F1,83 = 22.70, P < 0.0001), and increased BOL (β = 0.281, R2 change = 0.077, F1,83 = 8.44, P = 0.005).
For the week 1 to week 12 interval, linear multiple regression analyses found a significant relationship only between increased SDEV and increased INS (β = 0.255, R2 change = 0.06, F1,77 = 5.97, P = 0.017).
Discussion
Our experience provides a heretofore largely unpublished perspective, that of everyday community and academic practice, regarding the impact on glycemic outcomes of the introduction of CGS-augmented insulin pump use in type 1 diabetes. Our 85-patient cohort showed a statistically significant improvement in glycemic control during 12 weeks of intensive insulin treatment using CGS-augmented pump therapy in the form of a reduction in mean GHb of 0.74%, that is, from 7.55 ± 1.33% to 6.81 ± 1.08% (P < 0.0001).
Somewhat under a third of our cohort (24 patients, 28.2% of the evaluable sample) were well controlled at baseline (GHb <7.0%) and received CGS augmentation of pump therapy to reduce their frequency and duration of hypoglycemia. In this subgroup, improved glycemic control was demonstrated by decreased time under hypoglycemic conditions: mean ± SD AUCL fell from 0.8 ± 1.4 mg/dl/24 hours during week 1 to 0.7 ± 0.7 mg/dl/24 hours during week 4 and 0.7 ± 1.0 mg/dl/24 hours during week 12. The change in AUCL from week 1 to week 12 represented a 16.4% relative reduction (P = 0.71). Consequently, this subgroup had a nonsignificant change in mean ± SD GHb from baseline to 3 months (6.06 ± 0.7% to 5.94 ± 0.9%). Taken together, our findings in the overall sample and in the well-controlled subgroup support the results of real-time CGS studies showing decreased GHb levels when baseline GHb exceeded 7%.12,15,16,20
For our overall cohort, none of the mean insulin therapy or glycemia variables other than GHb changed significantly from week 1 to week 12. The seeming discrepancy between this observation and the significant 3-month improvement in mean GHb can be explained by the fact that unlike baseline GHb values, initial insulin therapy and non-GHb glycemia variable values were not obtained just before CGS-augmented pump therapy. Rather, the latter values were obtained during the first week of such treatment. Patients' awareness of continuous glucose data affects their behavior very rapidly, especially during initial exposure to the new technology.10 As our registry was not a formal clinical trial and was established with minimal industry support, no pretreatment download was available. Future patient registry studies incorporating pretreatment insulin therapy and glycemia data will better define changes in these variables induced by CGS.
Attempting to identify predictors for improved glycemic control, specifically for reduced GHb or BG, yielded two interesting observations. First, 12-week GHb improvement was significantly associated with continuous sensor use for the first month, but not the third month of CGS-augmented therapy. This suggests a strategy for sensor use: continuously when starting CGS augmentation, when major pump feature adjustment takes place, and then intermittently as needed to stabilize glycemic control or prevent hypoglycemia, as noted in the GuardControl trial.12 This strategy might optimize cost efficiency, but its effects on longer term glycemic control should be studied further.
Second, increased BAS and decreased BOL predicted improved GHb. The increase in the number of boluses from 6.6 to 7.4 during the 12-week observation period was nonsignificant (P = 0.34). This finding highlights the controversy regarding the optimal basal:bolus ratio.21,24 This controversy revolves around whether an increase in BAS versus an increase in the percentage of INS delivered by bolus or an increase in BOL is associated with improved glucose control in intensive insulin pump therapy of type 1 diabetes. A previous study24 demonstrated that the daily bolus number was a significant though low (R2 = 0.13; P < 0.001) predictor of a GHb value <7.5%. The cohort of the aforementioned study consisted of pediatric patients (mean 11.9 ± 4.2 years) with a shorter duration of diabetes (5.9 ± 3.6 years) and a shorter period on insulin pumps. The answer to this question apparently depends on patients' age, carbohydrate intake, or weight: better glycemic control is achieved with increased BOL when patients are younger or have higher carbohydrate intake, but is achieved with increased BAS when they are more obese.
This study was designed to assess the impact of the introduction of real-time CGS on patients' glycemic control and insulin therapy interventions. As in any study, one cannot rule out a contribution of the observation itself to patient behavior and thus to study results—the so-called “Hawthorne (study) effect.”22 Longer observation periods are necessary to decrease the possibility of this effect. Of interest in this context, glycemic control persisted in a subgroup of Greek patients (n = 14) in this registry followed for 6 months.23
Data of 83.3% of patients were available for evaluation. This is not to say that most patients who initiate CGS-augmented insulin pump therapy continue such therapy for 3 months, but rather that those patients who downloaded data after 4 weeks of usage almost always continued such therapy for at least an additional 2 months of follow-up. The study design was not intended to assess technology acceptance, but to evaluate the effect of treatment on those who adopt the technology.
Our overall cohort had relatively good baseline glycemic control (baseline GHb <7% in 24, 28.2% of patients) of fairly long-standing diabetes, and all patients were on continuous subcutaneous insulin infusion therapy prior to implementing CGS augmentation. Thus our patients were on best intensive insulin practices prior to the study intervention, but improved glycemic control nonetheless. This result may be explained partly by high education levels of the patients or their parents/guardians—over 70% had completed at least secondary school—and by the affluence and high motivation that may be inferred from the absence of reimbursement for CGS equipment during the study period in any registry country. Nonetheless, our findings would appear to be of general clinical relevance to the care of patients with type 1 diabetes on intensive insulin regimens.
Conclusions
In this uncontrolled, prospective patient registry-based study, CGS-augmented insulin pump therapy appeared to improve glycemic control in patients with type 1 diabetes. Improvement in GHb was statistically significant in patients with baseline GHb ≥7% and occurred even when patients were on best intensive insulin practices prior to the addition of CGS. The improved glycemic control was associated with continuous sensor use for at least the first 4 weeks and was predicted by an increase in BAS and a slight decrease in BOL. We hypothesized that the increase in GHb in some patients was secondary to a decrease in hypoglycemic events. We cannot, however, substantiate this hypothesis, as no data on the incidence of such events are available from the time prior to the use of CGS. Others have shown that patients adapt rapidly to continuous data and decrease hypoglycemia events already in the first days of CGS use. A comparison of the hypoglycemic event incidence rate and magnitude pre- and post-CGS is therefore important for a future design of clinical trials and registry-based studies with CGS.
These observations occurred in a multinational study involving community as well as academic practices and thus appear to be applicable to a wide variety of health care environments.
Acknowledgments
The authors acknowledge the assistance of Dr. Elisheva Ben-Artzi with statistical analysis and of Robert J. Marlowe with editing the manuscript. Data capture system use, statistical analysis, and editorial assistance on this manuscript were supported by an unrestricted grant from Medtronic, Tolochenaz, Switzerland.
Abbreviations
- ANCOVA
analysis of covariance
- AUCH
glucose area >140 mg% normalized to 7 days of the continuous glucose sensor download
- AUCL
glucose area <70 mg% normalized to 7 days of the continuous glucose sensor download
- BAS
basal insulin dose as a percentage of the total daily insulin dose
- BG
weekly mean glucose level
- BOL
number of daily boluses
- CEEGI
Central and Eastern Europe, Greece, and Israel
- CGS
continuous glucose sensing
- DCCT
Diabetes Control and Complications Trial
- GHb
glycosylated hemoglobin
- HEX
number of glucose excursions above 140 mg%
- IFCC
International Federation of Clinical Chemistry and Laboratory Medicine
- INS
total daily insulin dose
- LEX
number of glucose excursions below 70 mg%
- MANOVA
multivariate analysis of variance
- SD
standard deviation
- SDEV
standard deviation glucose
References
- 1.Klonoff DC. A review of continuous glucose monitoring technology. Diabetes Technol Ther. 2005;7(5):770–775. doi: 10.1089/dia.2005.7.770. [DOI] [PubMed] [Google Scholar]
- 2.Bromberg JS, LeRoith D. Diabetes cure–is the glass half full? N Engl J Med. 2006;355(13):1372–1374. doi: 10.1056/NEJMe068183. [DOI] [PubMed] [Google Scholar]
- 3.Garg SK. Health impact from frequent and continuous glucose monitoring. Diabetes Technol Ther. 2004;6(4):523–524. doi: 10.1089/1520915041706009. [DOI] [PubMed] [Google Scholar]
- 4.Reach G. Continuous glucose monitoring and diabetes health outcomes: a critical appraisal. Diabetes Technol Ther. 2008;10(2):69–80. doi: 10.1089/dia.2007.0261. [DOI] [PubMed] [Google Scholar]
- 5.Wentholt IM, Hart AA, Hoekstra JB, Devries JH. How to assess and compare the accuracy of continuous glucose monitors? Diabetes Technol Ther. 2008;10(2):57–68. doi: 10.1089/dia.2007.0216. [DOI] [PubMed] [Google Scholar]
- 6.Bode BW, Gross TM, Thornton KR, Mastrototaro JJ. Continuous glucose monitoring used to adjust diabetes therapy improves glycosylated hemogloba pilot study. Diabetes Res Clin Pract. 1999;46(3):183–190. doi: 10.1016/s0168-8227(99)00113-8. [DOI] [PubMed] [Google Scholar]
- 7.Kaufman FR, Gibson LC, Halvorson M, Carpenter S, Fisher LK, Pitukcheewanont P. A pilot study of the continuous glucose monitoring system: clinical decisions and glycemic control after its use in pediatric type 1 diabetic subjects. Diabetes Care. 2001;24(12):2030–2034. doi: 10.2337/diacare.24.12.2030. [DOI] [PubMed] [Google Scholar]
- 8.Kaufman FR, Austin J, Neinstein A, Jeng L, Halvorson M, Devoe DJ, Pitukcheewanont P. Nocturnal hypoglycemia detected with the Continuous Glucose Monitoring System in pediatric patients with type 1 diabetes. J Pediatr. 2002;141(5):625–630. doi: 10.1067/mpd.2002.129175. [DOI] [PubMed] [Google Scholar]
- 9.Ludvigsson J, Hanas R. Continuous subcutaneous glucose monitoring improved metabolic control in pediatric patients with type 1 diabetes: a controlled crossover study. Pediatrics. 2003;111:933–938. doi: 10.1542/peds.111.5.933. (5 Pt 1) [DOI] [PubMed] [Google Scholar]
- 10.Garg SK, Schwartz S, Edelman SV. Improved glucose excursions using an implantable real-time continuous glucose sensor in adults with type 1 diabetes. Diabetes Care. 2004;27(3):734–738. doi: 10.2337/diacare.27.3.734. [DOI] [PubMed] [Google Scholar]
- 11.Chase HP, Beck R, Tamborlane W, Buckingham B, Mauras N, Tsalikian E, Wysocki T, Weinzimer S, Kollman C, Ruedy K, Xing D. A randomized multicenter trial comparing the GlucoWatch Biographer with standard glucose monitoring in children with type 1 diabetes. Diabetes Care. 2005;28(5):1101–1106. doi: 10.2337/diacare.28.5.1101. [DOI] [PubMed] [Google Scholar]
- 12.Deiss D, Bolinder J, Riveline JP, Battelino T, Bosi E, Tubiana-Rufi N, Kerr D, Phillip M. Improved glycemic control in poorly controlled patients with type 1 diabetes using real-time continuous glucose monitoring. Diabetes Care. 2006;29(12):2730–2732. doi: 10.2337/dc06-1134. [DOI] [PubMed] [Google Scholar]
- 13.Garg S, Zisser H, Schwartz S, Bailey T, Kaplan R, Ellis S, Jovanovic L. Improvement in glycemic excursions with a transcutaneous, real-time continuous glucose sensor: a randomized controlled trial. Diabetes Care. 2005;29(1):44–50. doi: 10.2337/diacare.29.01.06.dc05-1686. [DOI] [PubMed] [Google Scholar]
- 14.Garg S, Jovanovic L. Relationship of fasting and hourly blood glucose levels to HbA1c values: safety, accuracy, and improvements in glucose profiles obtained using a 7-day continuous glucose sensor. Diabetes Care. 2006;29(12):2644–2649. doi: 10.2337/dc06-1361. [DOI] [PubMed] [Google Scholar]
- 15.Bailey TS, Zisser HC, Garg SK. Reduction in hemoglobin A1C with real-time continuous glucose monitoring: results from a 12-week observational study. Diabetes Technol Ther. 2007;9(3):203–210. doi: 10.1089/dia.2007.0205. [DOI] [PubMed] [Google Scholar]
- 16.Garg SK, Kelly WC, Voelmle MK, Ritchie PJ, Gottlieb PA, McFann KK, Ellis SL. Continuous home monitoring of glucose: improved glycemic control with real-life use of continuous glucose sensors in adult subjects with type 1 diabetes. Diabetes Care. 2007;30(12):3023–3025. doi: 10.2337/dc07-1436. [DOI] [PubMed] [Google Scholar]
- 17.Tamborlane WV, Beck RW, Bode BW, Buckingham B, Chase HP, Clemons R, Fiallo-Scharer R, Fox LA, Gilliam LK, Hirsch IB, Huang ES, Kollman C, Kowalski AJ, Laffel L, Lawrence JM, Lee J, Mauras N, O'Grady M, Ruedy KJ, Tansey M, Tsalikian E, Weinzimer S, Wilson DM, Wolpert H, Wysocki T, Xing D Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group. Continuous glucose monitoring and intensive treatment of type 1 diabetes. N Engl J Med. 2008;359(14):1464–1476. doi: 10.1056/NEJMoa0805017. [DOI] [PubMed] [Google Scholar]
- 18.Hirsch I, Abelseth J, Bode BW, Fischer JS, Kaufman FR, Mastrototaro J, Parkin CG, Wolpert HA, Buckingham BA. Sensor augmented pump therapy: results of the first treat-to-target study. Diabetes Technol Ther. 2008;10(50):377–383. doi: 10.1089/dia.2008.0068. [DOI] [PubMed] [Google Scholar]
- 19.Manley SE. Estimated average glucose derived from HbA1c eAG: report from European Association for the Study of Diabetes (EASD), Amsterdam 2007. Diabet Med. 2008;25(20):126–128. doi: 10.1111/j.1464-5491.2008.02383.x. [DOI] [PubMed] [Google Scholar]
- 20.Mastrototaro JJ, Cooper KW, Soundararajan G, Sanders JB, Shah RV. Clinical experience with an integrated continuous glucose sensor/insulin pump platform: a feasibility study. Adv Ther. 2006;23(5):725–732. doi: 10.1007/BF02850312. [DOI] [PubMed] [Google Scholar]
- 21.Danne T, Battelino T, Kordonouri O, Hanas R, Klinkert C, Ludvigsson J, Barrio R, Aebi C, Gschwend S, Mullis PE, Schumacher U, Zumsteg U, Morandi A, Rabbone I, Cherubini V, Toni S, de Beaufort C, Hindmarsh P, Sumner A, van Waarde WM, van den Berg N, Phillip M. A cross-sectional international survey of continuous subcutaneous insulin infusion in 377 children and adolescents with type 1 diabetes mellitus from 10 countries. Pediatr Diabetes. 2005;6(4):193–198. doi: 10.1111/j.1399-543X.2005.00131.x. [DOI] [PubMed] [Google Scholar]
- 22.Adair G. The Hawthorne effect: a reconsideration of the methodological artifact. J Appl Psychol. 1984;69:334–345. [Google Scholar]
- 23.Zoupas CS, Kefaloyannis N, Pappas A, Kepaptzologou O, Kyrlaki E, Giannakopolous TF, Garoutsou P, Vasilopolous H. Evaluation of continuous use of real-time glucose monitoring, alone and after combined insulin pump therapy, as a means to normalise glycaeic control in IDDM [abstract] Diabetologia. 2007;50:s418. [Google Scholar]
- 24.Danne T, Battelino T, Jarosz-Chobot P, Kordonouri O, Pánkowska E, Ludvigsson J, Schober E, Kaprio E, Saukkonen T, Nicolino M, Tubiana-Rufi N, Klinkert C, Haberland H, Vazeou A, Madacsy L, Zangen D, Cherubini V, Rabbone I, Toni S, de Beaufort C, Bakker-van Waarde W, van den Berg N, Volkov I, Barrio R, Hanas R, Zumsteg U, Kuhlmann B, Aebi C, Schumacher U, Gschwend S, Hindmarsh P, Torres M, Shehadeh N, Phillip M. Establishing glycaemic control with continuous subcutaneous insulin infusion in children and adolescents with type 1 diabetes: experience of the PedPump Study in 17 countries. Diabetologia. 2008;51(9):1594–1601. doi: 10.1007/s00125-008-1072-2. PedPump Study Group. [DOI] [PubMed] [Google Scholar]