Abstract
Personalized medicine represents a new model in how the medical community approaches disease management. Rather than managing those with a particular diagnosis according to an established guideline, the personalized medicine model seeks to identify unique characteristics within each patient that can serve as a basis for disease characterization and specialized treatment. This article reviews several circulating biomarkers of glycemia that are used in the medical management of diabetes, to include hemoglobin A1c, fructosamine, and 1,5-anhydroglucitol. Within the discussion, specific attention is paid to areas in which biomarker results do not correlate with anticipated results based on actual mean glycemia. Variability between actual and anticipated results of the various biomarker tests represents opportunities to identify previously undefined subcategories of diabetes and groups of patients that fit into these subcategories. Finally, research areas are proposed for these subcategories that would further promote the field of personalized medicine in diabetes.
Keywords: 1,5-anhydroglucitol; diabetes biomarkers; glycation gap; hemoglobin A1c variability; hemoglobin glycation index; personalized medicine
Introduction
Personalized medicine is a new concept in the practice of medicine. Traditional practice diagnoses patient ailments in terms of common disease processes. Disease management is based on standardized guidelines. Individual patient characteristics are not considered in this paradigm. Personalized medicine represents a departure from this methodology in that an attempt is made to understand patient characteristics as disease is encountered, with the notion that these characteristics impact the progression of disease as well as most appropriate therapies. While certainly there are many factors in patients with diabetes that would lead to the true personalization of therapy, it is the purpose of this article to review three circulating biomarkers of diabetes management and propose use of these biomarkers to define diabetes subgroups. The sub-groups can then represent future research projects that can advance the field of personalized medicine in diabetes.
Hemoglobin A1c
Hemoglobin A1c (HbA1c) is the predominant biomarker used in diabetes management. Several discoveries in the 1960s and 1970s found that HbA1c could be used as a reliable indicator of glycemic control in the preceding 2–3 months.1 Over the 120-day lifespan of the erythrocyte, HbA1c is formed when glucose attaches permanently to hemoglobin A.2 The HbA1c test reports the ratio of hemoglobin HbA1c to total hemoglobin A. Nondiabetes patients have a normal level under 6%, while uncontrolled diabetes patients can have levels exceeding 10%. Virtually every clinical trial assessing diabetes outcomes incorporates the HbA1c test as the key determinant of glucose control.
The first important clinical trial was the Diabetes Control and Complications Trial (DCCT).3 In this trial, 1441 type 1 diabetes patients were randomized into two groups and followed for an average 6.5 years. The conventional therapy group received usual care in that era and maintained an HbA1c in the 9.0% range. The intensive therapy group was placed on an aggressive insulin regimen and achieved an HbA1c average of 7.1%. The intensive therapy group had reduced incidence of retinopathy, nephropathy, and neuropathy by 76%, 54%, and 60%, respectively. Thus this clinical trial demonstrated that reducing HbA1c levels correlated with reducing diabetes complications.
The second important clinical trial was the United Kingdom Prospective Diabetes Study.4 In this trial, 3867 type 2 diabetes patients were randomized to an intensive group that included use of a sulfonylurea or insulin regimen or to a conventional diet-only regimen and were followed for 10 years. Hemoglobin A1c separation between the two groups was achieved, and a similar reduction in micro-vascular complications was observed. Subsequent analysis determined the benefit of a 1% reduction in HbA1c to be associated with significant reductions in a variety of macrovascular complications, to include myocardial infarction, stroke, amputation, and heart failure.5
These trials provided the basis for professional organizations to incorporate HbA1c targets in their diabetes guidelines. The American Diabetes Association has promoted a goal HbA1c < 7.0%, while the European Association for the Study of Diabetes and the American Association of Clinical Endocrinologists have endorsed a tighter goal HbA1c < 6.5%. Trials have called into question how low the HbA1c goal should be pursued. In particular, patients with advanced diabetes and cardiovascular disease may not benefit from an intensive diabetes management approach.6–8
There are several shortcomings with the HbA1c test. One important shortcoming is that the HbA1c test does not capture glycemic variability. Derr and colleagues studied 256 patients by comparing self-monitoring of blood glucose (SMBG) data, calculated mean glucose levels, and measured HbA1c levels.9 Some patients had low glucose variability of SMBG data (standard deviation [SD] 8.1 mg/dl), while others had very high glucose variability (SD 152.5 mg/dl). This level of glucose variability, however, had no appreciable effect on the correlation between mean glucose levels and HbA1c.
Another shortcoming of the HbA1c test is related to erythrocyte and hemoglobin function. The accuracy of the test depends upon a constant 120-day average erythrocyte lifespan. Anemias that lengthen or shorten the average lifespan impact test reliability by affecting the timeframe for erythrocyte glycosylation. Also, several laboratory techniques produce unexpected results when patients with hemoglobin variants (hemoglobin S, hemoglobin C, hemoglobin E) were tested. Fortunately, efforts to standardize laboratory techniques have overcome this problem. Only 5% of laboratories are still using methods with significant hemoglobinopathy interference.10
A broader problem related to the multitude of laboratory techniques was the differing reference ranges assigned to each technique. Different laboratories reported different HbA1c results for the same patients. The National Glycohemoglobin Standardization Program (NGSP) has represented an important step in standardizing the various techniques to a common reference, that of the original DCCT-based high-performance liquid chroma-tography assay.11
The International Federation of Clinical Chemists (IFCC) has adopted a different worldwide reference standard based on mass spectroscopy and capillary electrophoresis techniques that generate an HbA1c result that is 1.5–2.0% lower than the NGSP value. A master regression equation (NGSP = [0.915 × IFCC] + 2.15) allows translation between the two standards.11 A consensus statement was recently published, stating that IFCC and NGSP units should be reported on all HbA1c laboratory results, along with average glucose.12
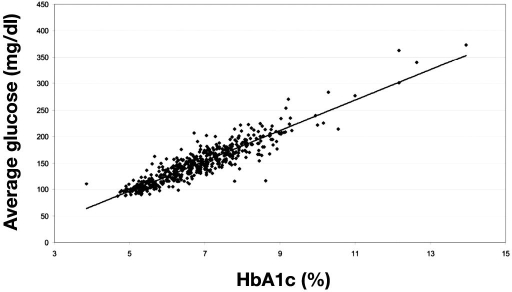
Many patients fail to understand the connection between HbA1c values and glucose levels, which created the impetus for the A1C-Derived Average Glucose Trial.13 In this trial, 2700 glucose values were obtained from 507 adult subjects over a 3-month period to ascertain the relationship between HbA1c and average glucose. The use of continuous glucose monitoring technology allowed for more glucose data points to be collected in this trial. A strong correlation was found between average glucose and HbA1c (Figure 1), such that an estimated average glucose (eAG) can be accurately reported. Table 1 provides the eAG values for incremental HbA1c values.
Figure 1.
Linear regression of HbA1c at the end of month 3 and calculated average glucose during the preceding 3 months. Calculated average glucose (mg/dl) = 28.7 × HbA1c − 46.7; average glucose (mmol) = 1.59 × HbA1c − 2.59; R2 = 0.84, p < .0001. Reprinted with permission from Diabetes Care.13
Table 1.
Calculation of HbA1c into Estimated Average Glucosea
| DCCT-aligned HbA1c % | eAG (mg/dl) | eAG (mmol/liter) |
|---|---|---|
| 5% | 97 | 5.4 |
| 6% | 126 | 7.0 |
| 7% | 154 | 8.6 |
| 8% | 183 | 10.2 |
| 9% | 212 | 11.8 |
| 10% | 240 | 13.4 |
Reprinted with permission from Diabetes Care.13
In subgroup analysis, the relationship between HbA1c and eAG was true regardless of the patient's type of diabetes, presence of diabetes, amount of glucose variability, gender, age, smoking status, and ethnicity. However, there was a trend toward significance (p = .07) in that Africans and African Americans had higher HbA1c values than Caucasians for the same mean glucose levels. If more patients had been enrolled, perhaps this trend would have achieved statistical significance.14
Review of Diabetes Prevention Program data confirmed the presence of ethnic variability in HbA1c testing in prediabetes patients. Hemoglobin A1c levels were higher in Asian, American Indian, Hispanic, and African subjects when compared to Caucasian subjects (p < .001) (Table 2).15 It is unclear why these HbA1c differences exist or whether these differences have clinical significance. Further study could elucidate whether a higher HbA1c for the same glucose levels translates to worse clinical outcomes. If there are subsets of patients that have an elevated HbA1c relative to what is expected, and if this elevated value correlates to an elevated risk for complications, then these subsets represent a target for more aggressive intervention.
Table 2.
Ethnic Variation in HbA1c in Patients with Impaired Glucose Tolerance15
| Race | Mean HbA1c level (adjusted for fasting glucose, glucose area under the curve, and other factors) |
|---|---|
| Caucasian | 5.78% |
| Hispanic | 5.93% |
| Asian | 6.00% |
| American Indian | 6.12% |
| African/African American | 6.18% |
The concept of a hemoglobin glycation index (HGI), defined as actual HbA1c minus predicted HbA1c, was proposed in 2004 by McCarter and associates based on a longitudinal multiple regression model developed from mean blood glucose and HbA1c in DCCT participants. This study reported that increased HGI correlated with increased risk for both retinopathy and nephropathy.16 Lachin and coworkers rebutted these findings, claiming that the HGI level correlates with HbA1c, providing an alternative explanation for increased complications.17 Regardless, the DCCT mean glucose data is based on only seven discrete glucose levels in the 24 h day. Further research with continuous glucose monitoring would help better define the presence and significance of HGI. Twin studies suggest that HbA1c has genetic determinants and is not solely determined by mean glucose.18
Fructosamine
Fructosamine is a second biomarker of glycemia, used less commonly than HbA1c. It is a measurement of glycated serum proteins, the most common of which is albumin. The fructosamine level correlates best with average glucose levels in the previous 10–14 days. Lindsey and colleagues conducted a trial of 72 subjects and determined that, in addition to HbA1c testing, weekly fructosamine testing did not provide a clinical benefit over blood glucose monitoring alone.19 Clinically, fructosamine is used in patients who are known to have a condition that makes HbA1c testing unreliable or to detect short-term changes in a patient's glucose control. There is less fructosamine data when compared to HbA1c data, but mathematical correlation can be made between fructosamine, HbA1c, and average glucose values.
Cohen and associates published two interesting studies that compared fructosamine, HbA1c, and average glucose values that are relevant to the concept of personalized medicine. In these articles, the presence of a glycosylation gap (GG) is defined as actual HbA1c minus HbA1c predicted from fructosamine. Measurements of HbA1c and fructosamine on the same sample in 153 people generated a broad GG distribution range (−3.2% to 5.5%). A 1% increase in GG was associated with a 2.9-fold increase in the risk of nephropathy stage (p = .0014).20
Cohen and associates subsequently evaluated the potential heritability of GG, noting previously cited evidence for genetic determination of HbA1c level in healthy twins and twins with diabetes.18,21 Glycosylation gap was more strongly correlated between monozygotic (r = .65) than dizygotic (r = .48) twins, and 69% of population variance in GG was heritable. Additionally, the GG heritability accounted for about one-third of the HbA1c heritability previously described.
1,5-anhydroglucitol
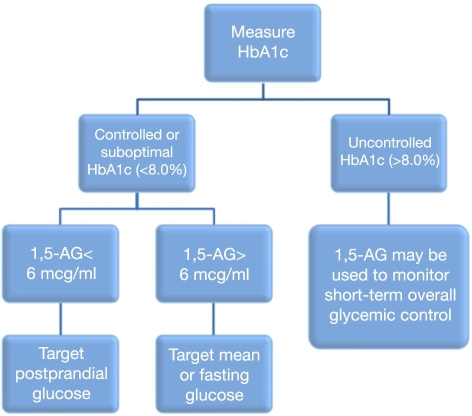
1,5-anhydroglucitol (1,5-AG) is a third circulating biomarker that is being used more commonly. It is not actually a measure of mean glycemia, but rather a measure of hyperglycemic excursions. 1,5- anhydroglucitol has a chemical structure similar to glucose, with one hydroxyl group removed from the 5 position. It predictably accumulates in the bloodstream from the diet. Like glucose, it is filtered by the kidney glomerulus and is reabsorbed completely from the filtrate back into the bloodstream. However, when plasma glucose levels exceed 180 mg/dl, reabsorption of glucose and 1,5-AG is impaired, and both are excreted in the urine. As a result, serum levels of 1,5-AG decrease, and a significant change is detectable in 1–3 days. Therefore, low serum 1,5-AG levels are a short-term indicator of hyperglycemia > 180 mg/dl, usually postprandial hyperglycemia in an otherwise well-controlled diabetes patient.22 Yamanouchi and colleagues reported mean 1,5-AG values in the following patient groups: normal, 24.7 ± 7.5 μg/ml; impaired glucose tolerance, 19.6 ± 8.4 μg/ml; and diabetes mellitus, 8.5 ± 7.3 μg/ml.22,23Dungan and coworkers assessed 40 diabetes patients using a continuous glucose monitoring system and found that mean 1,5-AG levels correlated very well with area under the curve for glucose above 180 mg/dl (r = −0.45, p = .006).24Dungan proposed use of a clinical algorithm incorporating the use of both HbA1c and 1,5-AG in the management of diabetes patients (Figure 2).25 In this algorithm, HbA1c is obtained to understand the overall level of glycemic control. In patients with HbA1c > 8.0%, 1,5-AG can be used to monitor short-term progress. In patients with HbA1c < 8.0%, a threshold 1,5-AG level of 6 mcg/ml can be used to further divide patients into those experiencing postprandial hyperglycemia and those experiencing fasting hyperglycemia. Appropriate therapies that target these patterns can then be prescribed. This type of algorithm represents personalized medicine at a very basic level. It divides patients into disease categories and targets appropriate therapies for those categories.
Figure 2.
Proposed algorithm for 1,5-AG. Reprinted with permission from Expert Review of Molecular Diagnostics.
Implications for Personalized Medicine and Proposed Research Opportunities
Circulating diabetes biomarkers can be used to categorize groups of diabetes patients for further study. Opportunity arises when a group of patients does not have an expected biomarker result. Several examples have already been described here, to include (1) HbA1c–mean glucose discordance based on ethnicity, (2) HbA1c–mean glucose discordance, or HGI, independent of ethnicity, (3) HbA1c–fructosamine discordance, or GG, and (4) hyperglycemic excursions as identified by 1,5-AG. Once a patient group is defined, further characterization can begin by asking the following questions: How can the group be best defined in terms of biomarker results? What is the cause of the unexpected results? Are there underlying genetic traits that define the group? What are the potential environmental causes? Is the group at a higher risk for diabetic complications? Will the group respond better to specific medications or to a specific level of therapeutic intensity? Designing research studies to help answer these questions will provide more insight to the management of diabetes and will ultimately advance the field of personalized medicine in diabetes.
Abbreviations
- 1,5-AG
1,5-anhydroglucitol
- DCCT
Diabetes Control and Complications Trial
- eAG
estimated average glucose
- GG
glycosylation gap
- HbA1c
hemoglobin A1c
- HGI
hemoglobin glycation index
- IFCC
International Federation of Clinical Chemists
- NGSP
National Glycohemoglobin Standardization Program
- SD
standard deviation
- SMBG
self-monitoring of blood glucose
References
- 1.Kilpatrick ES. Haemoglobin A1c in the diagnosis and monitoring of diabetes mellitus. J Clin Pathol. 2008;61(9):977–982. doi: 10.1136/jcp.2007.054304. [DOI] [PubMed] [Google Scholar]
- 2.Goldstein DE, Little RR, Lorenz RA, Malone JI, Nathan D, Peterson CM, Sacks DB. Tests of glycemia in diabetes. Diabetes Care. 2004;27(7):1761–1773. doi: 10.2337/diacare.27.7.1761. [DOI] [PubMed] [Google Scholar]
- 3.The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329(14):977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 4.United Kingdom Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352(9131):837–853. [PubMed] [Google Scholar]
- 5.Stratton IM, Adler AL, Neil HA, Matthews DR, Manley SE, Cull CA, Hadden D, Turner RC, Holman RR. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321(7258):405–412. doi: 10.1136/bmj.321.7258.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gerstein HC, Miller ME, Byington RP, Goff DC, Jr, Bigger JT, Buse JB, Cushman WC, Genuth S, Ismail-Beigi F, Grimm RH, Jr, Probstfield JL, Simons-Morton DG, Friedewald WT The Action to Control Cardiovascular Risk in Diabetes Study Group. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358(24):2545–2559. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patel A, MacMahon S, Chalmers J, Neal B, Billot L, Woodward M, Marre M, Cooper M, Glasziou P, Grobbee D, Hamet P, Harrap S, Heller S, Liu L, Mancia G, Mogensen CE, Pan C, Poulter N, Rodgers A, Williams B, Bompoint S, de Galan BE, Joshi R, Travert F ADVANCE Collaborative Group. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Eng J Med. 2008;358(24):2560–2572. doi: 10.1056/NEJMoa0802987. [DOI] [PubMed] [Google Scholar]
- 8.Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, Zieve FJ, Marks J, Davis SN, Hayward R, Warren SR, Goldman S, McCarren M, Vitek ME, Henderson WG, Huang GD. VADT Investigators. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360(2):129–139. doi: 10.1056/NEJMoa0808431. [DOI] [PubMed] [Google Scholar]
- 9.Derr R, Garrett E, Stacy GA, Saudek CD. Is HbA(1c) affected by glycemic instability? Diabetes Care. 2003;26(10):2728–2733. doi: 10.2337/diacare.26.10.2728. [DOI] [PubMed] [Google Scholar]
- 10.National Diabetes Information Clearinghouse. Sickle cell trait and other hemoglobinopathies and diabetes: important information for physicians. Updated November 2007. http://diabetes.niddk.nih.gov/dm/pubs/hemovari-A1C/. Accessed March 10, 2009.
- 11.National Glycohemoglobin Standardization Program. Updated February 2009. http://www.ngsp.org. Accessed March 10, 2009.
- 12.The American Diabetes Association, European Association for the Study of Diabetes, International Federation of Clinical Chemistry and Laboratory Medicine, and the International Diabetes Federation Consensus Committee. Consensus statement on the worldwide standardization of the hemoglobin A1c measurement. Diabetes Care. 2007;30(9):2399–2400. doi: 10.2337/dc07-9925. [DOI] [PubMed] [Google Scholar]
- 13.Nathan DM, Kuenen J, Borg R, Zheng H, Schoenfeld D, Heine RJ. A1c-Derived Average Glucose Study Group. Translating the A1C assay into estimated average glucose values. Diabetes Care. 2008;31(8):1473–1478. doi: 10.2337/dc08-0545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.American Diabetes Association. Use of the estimated average glucose (eAG) in patient care. Updated February 2009. http://professional.diabetes.org/Content/eAGPowerpointSlides.ppt. Accessed March 10, 2009.
- 15.Herman WH, Ma Y, Uwaifo G, Haffner S, Kahn SE, Horton ES, Lachin JM, Montez MG, Brenneman T, Barrett-Connor E. Diabetes Prevention Program Research Group. Differences in A1C by race and ethnicity among patients with impaired glucose tolerance in the diabetes prevention program. Diabetes Care. 2007;30(10):2453–2457. doi: 10.2337/dc06-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McCarter RJ, Hempe JM, Gomez R, Chalew SA. Biological variation in HbA1c predicts risk of retinopathy and nephropathy in type 1 diabetes. Diabetes Care. 2004;27(6):1259–1264. doi: 10.2337/diacare.27.6.1259. [DOI] [PubMed] [Google Scholar]
- 17.Lachin JM, Genuth S, Nathan DM, Rutledge BN. The hemoglobin glycation index is not an independent predictor of the risk of microvascular complications in the Diabetes Control and Complications Trial. Diabetes. 2007;56(7):1913–1921. doi: 10.2337/db07-0028. [DOI] [PubMed] [Google Scholar]
- 18.Snieder H, Sawtell PA, Ross L, Walker J, Spector TD, Leslie RD. HbA(1c) levels are genetically determined even in type 1 diabetes: evidence from healthy and diabetic twins. Diabetes. 2001;50(12):2858–2863. doi: 10.2337/diabetes.50.12.2858. [DOI] [PubMed] [Google Scholar]
- 19.Lindsey CC, Carter AW, Mangum S, Greene D, Richardson A, Brown SJ, Essary JL, McCandless B. A prospective, randomized, multicentered controlled trial to compare the annual glycemic and quality outcomes of patients with diabetes mellitus monitored with weekly fructosamine testing versus usual care. Diabetes Technol Ther. 2004;6(3):370–377. doi: 10.1089/152091504774198070. [DOI] [PubMed] [Google Scholar]
- 20.Cohen RM, Holmes YR, Chenier TC, Joiner CH. Discordance between HbA1c and fructosamine: evidence for a glycosylation gap and its relation to diabetic nephropathy. Diabetes Care. 2003;26(1):163–167. doi: 10.2337/diacare.26.1.163. [DOI] [PubMed] [Google Scholar]
- 21.Cohen RM, Snieder H, Lindsell CJ, Beyan H, Hawa MI, Blinko S, Edwards R, Spector TD, Leslie RD. Evidence for independent heritability of the glycation gap (glycosylation gap) fraction of HbA1c in nondiabetic twins. Diabetes Care. 2006;29(8):1739–1743. doi: 10.2337/dc06-0286. [DOI] [PubMed] [Google Scholar]
- 22.Buse JB, Freeman JL, Edelman SV, Jovanovic L, McGill JB. Serum 1,5-anhydroglucitol (GlycoMark): a short-term glycemic marker. Diabetes Technol Ther. 2003;5(3):355–363. doi: 10.1089/152091503765691839. [DOI] [PubMed] [Google Scholar]
- 23.Yamanouchi T, Akanuma Y, Toyota T, Kuzuya T, Kawai T, Kawazu S, Yoshioka S, Kanazawa Y, Ohta M, Baba S. Comparison of 1,5-anhydroglucitol, HbA1c, and fructosamine for detection of diabetes mellitus. Diabetes. 1991;40(1):52–57. doi: 10.2337/diab.40.1.52. [DOI] [PubMed] [Google Scholar]
- 24.Dungan KM, Buse JB, Largay J, Kelly MM, Button EA, Kato S, Wittlin S. 1,5-anhydroglucitol and postprandial hyperglycemia as measured by continuous glucose monitoring system in moderately controlled patients with diabetes. Diabetes Care. 2006;29(6):1214–1219. doi: 10.2337/dc06-1910. [DOI] [PubMed] [Google Scholar]
- 25.Dungan KM. 1,5-anhydroglucitol (GlycoMark) as a marker of short-term glycemic control and glycemic excursions. Expert Rev Mol Diagn. 2008;8(1):9–19. doi: 10.1586/14737159.8.1.9. [DOI] [PubMed] [Google Scholar]